Abstract
Energy consumption in the form of fossil fuels has increased continuously. Because of its lightweight, simple carrying and low price, the modern world utilizes many products with waste plastic and waste tyre as a main source. As a result of this, there is an issue of dumping plastic and tyres into areas of land due to the drastic rise in these reserves each year, municipal waste posses many environmental problems. The latest trend toward converting waste into quality fuels is waste to energy. This present article introduces the use of waste plastic pyrolysis oil and waste tyre pyrolysis oil as an alternative fuel for testing the four-stroke single-cylinder VCR water-cooled diesel engine. Performance and emissions characteristics for mixing waste plastic and waste tyre pyrolysis oil together with diesel mixtures with different blending ratios such as D100, B5-D95, B10-D90, B15-D85, B20-D80, B25-D75, and B30-D70 at a speed of 1500 rpm with loads of 2, 4, 6, 8, and 10 kg at rated brake power are investigated. Compared to diesel and all other blends, the B20-D80 blend found decent effects. For B20-D80 blend, brake thermal efficiency has increased compared to pure diesel, and brake-specific fuel consumption is reduced. With enhanced blend ratios, CO emissions were increased, NOx reduced with increase in blend ratios, and UHC emissions and smoke are decreased than diesel fuel.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Energy, environment, and economy are the main contributors to the country’s development. Daily, fossil fuels are depleting, resulting in rising prices of petroleum products around the globe. This shows the need for a cheaper alternative fuel to meet the transportation needs and electric power generation in the rural area. On the other hand, waste product generated with growth of population and its management plays essential role to maintain economic and healthy country. Among many types of solid waste, plastic waste is a major problem as its non-biodegradable in nature. Due to their lightweight, durability, non-perishability, quicker production rate, and flexibility in design and commodity supply, plastic use has become a vital component of today’s world [1, 2]. Venkatesan [1] reported that, based on the experimental study into the combustion and performance features of a four-stroke compression ignition engine driven with diesel and waste plastic oil blends, the BTE showed small differences at low load, but noticeable changes were observed at complete load which might be due to increased calorific value of WPPO blends, and mechanical efficiency was observed to have minimal impact on all fuel mixtures at all loads, but BSFC was reported to be higher for all non-load fuel mixtures, and fuel consumption declined with load increases. Varma [2] researched the experimental inquiry on the addition of waste plastic oil resulting in increased brake thermal efficiency (BTE) and decreased BSFC. Muhammed Qasim and Ansari [3] conducted experimental study on a diesel engine operated with fuel blends extracted from a combination of Pakistani waste tyre oil and soybean oil biodiesel revealed that all the fuel blends tested showed reduced BTE, smoke emissions, but increased NOx emissions. Ventaka subbaiah and Durga Prasad [4] have noticed that the greater deposition of plastic waste in the earth’s pyrolysis cycle leads to a better transformation of plastic to effective hydrocarbon fuels such as petrol and diesel. The cost of manufacturing per liter of fuel reduces when compared to crude oil refining when performing this method effectively. This method showed an alternative way of generating fuel that could satisfy the worldwide demand for fuel due to enhanced fuel demand. The plastic oil blend [B20] generates the same power as diesel. Plastic oil indicates slightly greater emission values than diesel when absorbing the emission trait graphs. It is feasible to distinguish better fuel quality from the alteration in manufacturing such as pressure heating of the acquired crude type of fuel. If manufacturing is carried out effectively with defined parameters, quality fuel can be produced, and this will take the place of standard diesel. Mani [5] conducted the test on performance, emission, and combustion characteristics of a DI diesel engine using waste plastic oil and reviles that engine fueled with waste plastic oil exhibits greater heat efficiency up to 75% of the rated energy and smoke emission decreases by about 40%, but CO and NOx emissions increase when the engine is operating at complete load circumstances. percentnoted that the brake thermal efficiency of the engine was 3–4% smaller for PPO90 and 2–3% lower for PPO70 compared to diesel, and all measured emissions (NOx, UHC, CO, and CO2) were greater for PPO70 and PPO90 compared to all measured emissions (PPO70 and PPO90). Subramanyam [6] researched the performance investigation of diesel engine using waste plastic pyrolysis oil and diesel blends that waste to energy is the current trend that will concentrate on different research fields. Waste plastic oil blend’s (WPO20) brake thermal efficiency BTE of 26.24% at 3.67 kw bp was higher than diesel (24.85%). Specific fuel consumption was slightly greater than diesel fuel consumption. Plastic oil’s exhaust gas temperature with diesel blends is growing owing to longer plastic oil ignition delay. Carbon monoxide emissions increase with increased brake power under load circumstances at 3.67 kw. The CO emissions for the blends like WPO15, WPO20, WPO30, WPO40, and WPO50 are 3.69, 3.63, 3.72, 3.96, 4.18, and 4.18%. Carbon dioxide emissions from plastic oil blends with diesel rise significantly at WPO20 brake power with 2.94 kw lower than diesel emissions owing to full fuel combustion. Hydrocarbon emission plastic oil at all brake powers blends more than diesel emission. The emissions of 3.67 kw bp hc were 1606, 1610, 1609, 1614, 1616, and 1618 ppm for diesel, WPO15, WPO20, WPO30, WPO40, and WPO50. Paramar [7] operates on emission analysis of CI engine using tyre pyrolysis oil and diesel blend suggesting that CO emissions are increased with TPO mix in exhaust emissions. The D75T25 mix provides the required impact. The emission of HC, CO, and CO2 decreases with the TPO blend and the optimum values of the D95T5 blend. With the rise in separate TPO blend D75T25, the emission of NOx and O2 provides the desired impact than other blends and diesel. Senthil Kumar [9] investigated a DI naturally aspirated engine using tire pyrolysis oil–diesel blends as a biodiesel and realized that pyrolysis blends’ brake thermal efficiencies are lesser than diesel. This may be due to reduced heating value and reduced pyrolysis combustion. Due to reduced viscosity, the rise in thermal efficiency for 50% compared to 75% can be ascribed to improved fuel atomization. Reduction in thermal efficiency by approximately 14% is greater than that of the other pyrolysis oil blends which is eventually closer to blending the distilled method to fix the distinct aims, and NOx values for 50 and 75% of the pyrolysis oil are lower than diesel, and CO and HC emissions for diesel can be high owing to the existence of unsaturated oil. Smoke is greater than diesel for tyre pyrolysis blends. Mohammad Younus [10] researched the performance and emission attributes of diesel engine fueled with tyre pyrolysis oil and diesel blends with additives and reported that BTE is improved for all blends compared to standard diesel fuel, and BSFC is lowered for all blends compared to diesel fuel. CO, CO2, and HC emissions are considerably reduced with the blends, and lastly, the blend TPE 20 demonstrates better efficiency compared to other blends (TP10, TP20, TP30, TPE10, and TPE30) and diesel. Pawan conducted his research work on the performance and emission characteristics of tyre pyrolysis oil (TPO) blend with diesel for weird composition and revealed that the engine’s brake thermal efficiency decreases with increased concentration of TPO blend than diesel. BTE for the operation of diesel is at varying loads. In case of TPO 10% blending, it is 22.47%. In the event of a 20% mix, it is 15.83%. It is 18.55 for 30% mix, and the TPO–DF mix demonstrates greater BSFC value than diesel because of reduced TPO–DF blend calorific value. As the TPO–DF’s BSFC load increases, it reduces. TPO–DF’s mechanical efficiency improves as the load rises. The highest efficiency achieved is 21.64% for 10%. In the event of a 20% blend, it is 53.2%. And it is 49.7% in 30%. At greater loads, the CO emission is greater and grows with the blend proportion (TPO–diesel). Similarly, the HC emission is large for greater loads. The emission concentration is high in all load circumstances for the CO2 emission mix at 30%.
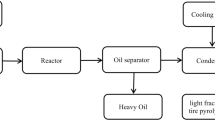
2 Production Process of WPPO and WTPO
2.1 Pyrolysis
In the lack of oxygen, pyrolysis is usually described as the monitored heating method of a material. The macro-molecular structures of polymers are split into lower molecules and sometimes monomer units in plastics and tyre pyrolysis. Further degradation of these subsequent molecules is dependent on a number of different conditions including (and not restricted to) temperature, residence time, catalyst presence, and other process circumstances. In a cylindrical chamber or reactor, plastic waste is continually handled. At 300–500 °C, the plastic and tyre oil is pyrolized.
2.1.1 Condenser
It cools the reactor’s entire heated vapor. The cold water to pass through its exterior region enters and exits the outlet through the inlet. This is used for vapor cooling. The gaseous hydrocarbons are condensed to about 30–35 °C at a temperature of around 35 °C.
2.1.2 Reactor
It is a 1000 mm long cylindrical stainless steel container with an inner diameter of 300 mm, an outer diameter of 320 mm secured on one end and an outlet tube on the other. Inside, the reactor is put into the entire cylindrical container. External heating is carried out from below the container and inside the reactor using the raw material such as coal, wood, and coke. The reactor for lagging is produced of stainless steel, mild steel, and clay. The reactor is heated at about 1450 °C and higher temperatures.
2.2 Process Description
The method of thermal cracking is used to convert waste plastic and tyre into liquid fuel. For this specific experiment, only one sort of waste plastic is chosen, i.e., low-density polyethylene. Waste plastic is collected and cleaned using liquid soap and water as strong smooth forms. Washed plastics and tyre are cut to a size of 3–5 cm to fit conservatively into the reactor. The experiment is performed with no vacuum applied during the thermal cracking process under a closed system. In a batch process system, we used polyethylene plastics of low density due to the relatively low conversion temperatures for these plastics. At the beginning of melting, the waste plastics and tyre, heat is applied from 100 °C, the melted waste plastic and tyre transform into liquid slurry when temperature increases gradually. When the temperature rises to 570 °C, liquid slurry becomes vapor, and then, the vapor passes through a condenser unit. We obtain liquid fuel at the end of this phase. Between 100 and 250 °C, about 20–30% of the fuel is gathered, and then, the next 40% is obtained when raised to 325 °C, and the output is lastly finished when retained at 400 °C. Plastic and tyre pieces are not immediately broken down during the thermal cracking phase because plastics have distinct chain hydrocarbons. Only the straight chain hydrocarbon is applied in the starting mode heat. The temperature profile that slowly breaks this plastic and tyre carbon bond is further improved. The long chains’ molecules are dissolved step by step with the temperature rise.
3 Engine Setup and Procedure
For experimental testing, Kirloskar single-cylinder water-cooled variable compression ratio diesel engine is used. To apply loads to the engine, the Eddy current dynamometer is connected to the flywheel. To inject the petrol, a 200 bar injection pressure is retained (Fig. 1).
The pressure of the cylinder is evaluated by the piezo-sensor installed on the head of the engine cylinder and the angle of the crank encoded on the fly wheel. The conventional engine is supplied with 0° to 25° BDC injection point variation. The HC, CO, CO2, UBHC and NOx emissions are evaluated using the fire gas analyzer AVL-DIGAS 444. AVL smoke meter is used to measure the opacity of the smoke (Fig. 2; Table 1).
3.1 Test Procedure
This research focuses on the use of WPPO and WTPO as fuel in the conventional diesel direct injection (DI) engine. Some of these two biodiesel characteristics are closer to diesel fuel. As a result, WPPO and WTPO biodiesel are selected as an alternative fuel, and the highest possible diesel substitution is explored in a DI diesel engine. However, the combination of these two biodiesel mixed with diesel has a reduced amount of cetane number. We used these WPPO and WTPO oil by reducing its viscosity. Also engine will be made to run with blends of diesel and WPPO and WTPO biodiesel consisting of 5, 10, 15, 20, 25, and 30% into the diesel fuel (Table 2).
5 Results and Discussion
5.1 Performance Analysis
5.1.1 Brake-Specific Fuel Consumption (BSFC)
The differences in brake-specific fuel consumption with regard to the brake power of distinct blends are shown in Fig. 3. The plot reveals that the BSFC is diminishing as the load rises. The BSFC obtained are 0.39, 0.366, 0.361, 0.36, 0.34, 0.36, and 0.38 kg/kw h for diesel fuels, B5-D95, B10-D90, B15-D85, B20-D80, B25-D75, and B30-D70, respectively, under maximum load conditions. BSFC’s percentage variation of all diesel blends at different load circumstances is declining at 0.938, 0.925, 0.923, 0.871, 0.924, and 0.974%. Increased BSFC was noted at low load, which is due to greater aromatic compounds with enhanced viscosity and volatility. This results in inadequate air–fuel mixture, particularly during lean operations. For the B20-D80 mix, which is 0.34 kg/kw h, the minimum BSFC is acquired, i.e., 0.871% reduces with 10 kg than diesel fuel. The BSFC for B20-D80 is achieved lower compared to all oil blends, but for B5-D95 and B30-D70, the BSFC is closer to diesel.
5.1.2 Brake Thermal Efficiency (BTE)
The brake thermal efficiency variations regarding brake power is shown in Fig. 4. The brake thermal efficiency generally improves with increased load. As the load increases, the brake power increases and reduces the bsfc which improves the brake thermal efficiency. At low loads, it was hard to atomize WPPO and WTPO mixed fuel particles, leading in lower brake thermal efficiency. Higher BTE was noted for enhanced concentration of WPPO and WTPO at part and complete load conditions, which is due to better preparing of air–fuel mixture at high loads. All the fuel blends showed a rising trend in brake thermal efficiency with load. Brake thermal efficiency is 22.08, 23.45, 23.68, 23.18, 24.73, 23.78, and 22.67% for fuel blends such as diesel, B5-D95, B10-D90, B15-D85, B20-D80, B25-D75, and B30-D70, respectively, from graphs at 10 kg load conditions. The percentage variation of brake thermal efficiency of all diesel blends at different load conditions is improved by 1.062, 1.072, 1.049, 1.12, 1.076, and 1.02%. The maximum brake thermal efficiency for the B20-D80 blend is 24.73%, i.e., 1.12% higher than diesel fuel, and blends such as B25-D75 and B30-D70 reduced as compared to B20-D80 owing to a rise in the proportion of WPPO and WTPO in diesel fuel.
5.1.3 Mechanical Efficiency (Mechη)
Mechanical efficiency is the measure of the engine’s useful work with fuel and energy input. The graph 5 demonstrates mechanical efficiency variations for all fuel mixtures under different load conditions, and it is noted that with increasing loads for pure diesel to all blends, there is an increase in mechanical efficiency. At 10 kg load conditions, the mechanical efficiency values for fuel blends such as diesel, B5-D95, B10-D90, B15-D85, B20-D80, B25-D75, and B30-D70 were 52.50, 53.47, 53.52, 53.71, 54.10, 54.32, and 56.94%. The percentage variation in the mechanical efficiency of all blends at different load circumstances is improved by 1.018, 1.019, 1.023, 1.030, 1.034, and 1.08%. The mechanical efficiency is improved from the diesel fuel to the blend B30-D70 due to rise in the mixing ratios. Higher ignition delay results in increased premixed combustion and lower fuel atomization at elevated loads. The maximum mechanical efficiency is achieved by 56.94% for the blend B30-D70, i.e., 1.08% higher than diesel (Fig. 5).
5.2 Emission Analysis
5.2.1 Oxides of Nitrogen Emissions (NOx)
Figure 6 shows the variation of oxides of nitrogen with distinct blends at varying brake power. The increase in temperature causes the formation of nitrogen oxides in the exhaust. But in this case, the engine temperature is equivalent to normal diesel for B15-D85 and B20-D80, and the blend B30-D70 is nearer to diesel. This is because pyrolyzed fuel contains more oxygen content than standard diesel fuel, although the engine temperature is less. This provides excess oxygen, resulting in surplus nitrogen oxide release, and enhanced delay in the ignition of WPPO, promoting premixed combustion by providing more time for fuel to be injected for ignition, may also be another reason to increase NOx. That is the reason we received that much NOx emissions. It is portrayed from the graph that all blends such as B5-D95, B10-D90, B15-D85, B20-D80, B25-D75, and B30-D70 yield greater NOx emissions. Among them, B25-D75 and B30-D70 produce NOx emissions, i.e., 479 and 442 ppm, which is much closer to diesel fuel, and the B5-D95 blend provides maximum NOx emissions, i.e., 534 ppm.
5.2.2 Carbon Monoxide Emissions
Carbon monoxide (CO) is a slightly less dense, colorless, odorless, and tasteless gas than air. Figure 7 compares the emissions of carbon monoxide (CO) to the brake power. In practice, when there is incomplete fuel burning, carbon monoxide can be seen in the exhaust. For the obtained pyrolysis oil, there is a presence of aromatics in both WPPO and WTPO. This element does not exhibit for complete combustion. From the graph at different load conditions, the blends B25-D75 and B30-D70 give higher CO emissions, and B20-D80 emits lesser CO% by volume compared to all blends and diesel because of having required quantity of oxygen for complete combustion. The CO emissions obtained are 0.12%, 0.12%, 0.12%, 0.13%, 0.11%, 0.14%, and 0.14% for the blends like diesel, B5-D95, B10-D90, B15-D85, B20-D80, B25-D75, and B30-D70, respectively.
5.2.3 Carbon Dioxide Emissions
The variation of carbon dioxide with different blends with varying brake power is represented graphically in Fig. 8. Complete combustion occurs when adequate supply of oxygen makes carbon dioxide which is seen in engine exhaust. The oils we used, WPPO and WTPO, may have excess oxygen. This leaves excess oxygen that eventually releases large amounts of exhaust carbon dioxide. In reality, carbon dioxide in the diesel engine was seen as pollution accompanied lived green house gas. From the graph at different load conditions, the blend B30-D70 and diesel emit lesser co2 emissions of about 5.84 and 6.1%, whereas the blend B20-D80 gives 6.62% of CO2 emissions due to more amount of oxygen present in the fuel which makes to burn the fuel completely than other fuel mixtures.
5.2.4 Unburnt Hydrocarbons Emissions (UHC)
Figure 9 shows the variation of unburned hydrocarbons with distinct blends at varying brake power. It is generally possible to see hydrocarbons in the exhaust when the fuel has a greater amount of cetane number, and there is not enough time to perform combustion process. Compared to standard diesel, the cetane number of WPPO and WTPO pyrolyzed oil is lower, and hence, the level of combustion rises and hydrocarbon emissions reduces. From the graph, it is noted that all blends such as B5-D95, B10-D90, B15-D85, B20-D80, B25-D75, and B30-D70 produce lower UHC emissions. Among them, B15-D85 and B20-D80 give lesser UHC emissions, i.e., 36 and 35 ppm.
5.2.5 Smoke Opacity
Opacity of smoke rises with load increases. Figure 10 demonstrates a graph plotted between the opacity of smoke and the engine brake power fueled with mixtures, i.e., diesel, B5-D95, B10-D90, B15-D85, B20-D80, B25-D75, and B30-D70. In general, smoke opacity of diesel fuel is greater than other fuels. Due to more oxygen content, smoke opacity reduces with a rise in the percentage of the blends. The minimum opacity of smoke is for the blend B15-D85 (47.8BSU) while considering the fuel and the maximum in the blend B25-D75 (68.6BSU). Opacity of smoke in diesel fuel is noted at 71.8 BSU. It can be observed that the amount of smoke is lesser than diesel for all blends. The reason for this decreased smoke is the availability of oxygen within the engine cylinder with premixed and homogeneous charge well before the beginning of combustion. The few reasons for decreased smoke are higher combustion temperature, prolonged combustion duration, and rapid flame propagation. However, there was a noticeable white smoke emission at higher load owing to lack of adequate oxygen and abnormal combustion.
6 Conclusions
The following conclusions were drawn based on the experimental investigation on performance and emission characteristics of a four-stroke single-cylinder diesel engine fueled with straight diesel and blends of WPPO and WTPO with diesel in a blending ratios of D100, B5-D95, B10-D90, B15-D85, B20-D80, B25-D75, and B30-D70, respectively.
-
1.
The physio-chemical characteristics of WPPO and WTPO such as kinematic viscosity, density, dynamic viscosity, flash point, fire point, and calorific value and its diesel blends were found.
-
2.
The engine’s brake thermal efficiency improves as the blending proportions increase from B5-D95 to B20-D80 and subsequently reduces for B25-D75 to B30-D70. The maximum brake thermal efficiency of the B20-D80 blend is 24.73% and the minimum for diesel (D100) approximately 22.08%.
-
3.
Brake-specific fuel consumption reduces with load which increases from B5-D95 to B20-D80 in all blending ratios and increased later for B25-D75 to B30-D70. At the B20-D80 blend, the minimum BSFC is 0.34 kg/kw h, and a maximum value of 0.39 kg/kw h is obtained.
-
4.
The mechanical efficiency increased with increase in fuel blends from diesel, B5-D95 to B30-D70. The maximum mechanical efficiency is obtained at blend B30-D70 is 56.94%, and minimum values are obtained at diesel fuel of about 52.50%.
-
5.
The CO emission decreases with increase in the blending proportions, whereas at higher loads, the CO emission is increased for the blends B25-D75 and B30-D70, i.e., 0.14 ppm. The minimum CO emissions occurred at B20-D80 blend is about 0.11 ppm.
-
6.
Because of presence of oxygen content in both WPPO and WTPO, the CO2 emissions are more for all blending proportions than the diesel fuel. B20-D80 blend gives 6.62% of CO2 emissions.
-
7.
The UHC emissions decrease with increase in the blending proportions. The blends B20-D80 and B15-D85 give lesser UHC emissions of about 35 and 36 ppm than other fuel blends.
-
8.
The increase in temperature causes the formation of nitrogen oxides in the exhaust. But in this case, the engine temperature is more than standard diesel for B15-D85 and B20-D80, and the blend B30-D70 is nearer to diesel. Among all, the blends B25-D75 and B30-D70 yield NOx emissions, i.e., 479 and 442 ppm, which is much nearer to diesel fuel, and the B5-D95 blend yields maximum NOx emissions, i.e., 534 ppm.
-
9.
In general, smoke opacity of diesel fuel is greater than other fuels. Due to more oxygen content, smoke opacity reduces with a rise in the percentage of the mixture. For the blends B15-D85 and B20-D80 of approximately 47.8 BSU and 65.4 BSU, the minimum smoke opacity is acquired while considering the fuel. The diesel fuel smoke opacity is noted at 71.8 BSU. The B20-D80 blend provides less opacity smoke than diesel.
As a result, the B20-D80 is chosen as an optimum mixture owing to better results and can be used as a replacement for diesel fuel.
Abbreviations
- WPPO:
-
Waste plastic pyrolysis oil
- WTPO:
-
Waste tyre pyrolysis oil
- CO:
-
Carbon monoxide
- UHC:
-
Unburnt hydrocarbons
- CO2:
-
Carbon dioxide
- NOx:
-
Oxides of nitrogen
- DI:
-
Direct ignition
- CI:
-
Compression ignition
- PPM:
-
Parts per million
References
Venkatesan H, Sivamani S, Bhutoria K, Vora HH (2017) Experimental study on combustion and performance characteristics in a DI CI engine fuelled with blends of waste plastic oil. Alex Eng J. 1110-0168
Verma A, Raghuvansi A, Quraishi MA, Tirkey JV, Verma C Engine fuel production from waste plastic pyrolysis (WPO) and performance evaluation in a CI engine with diesel blend. J Mater Environ Sci. ISSN: 2028;2508 CODEN:JMESCN
Qasim M, Ansari TM, Hussain M (2017) Experimental investigations on a diesel engine operated with fuel blends derived from a mixture of Pakistani waste tyre oil and waste soybean oil biodiesel. Received: 5 May 2017. Accepted: 2 October 2017# Springer, GmbH Germany
Venkata Subbaiah M, Sunil KuarReddy S, Durga Prasad B (2018) Performance and emission characteristics of diesel engine fuelled with waste plastic bio-oil/diesel. In: 2nd international conference on emerging trends in engineering, sciences and management-2018 (ICEESM’18)
Mani M, Subash C, Nagarajan G (2009) Performance, emission and combustion characteristics of a DI diesel engine using waste plastic oil. Appl Therm Eng 29:2738–2744
Vasukumar N, Subramanyam D, Tammineni L (2017) Performance investigation of diesel engine using waste plastic pyrolysis oil and diesel blends. IJESC 7(11)
Paramar MD, Patel SC, Patel TM, Patel P (2016). Emission analysis of C.I engine using tyre pyrolysis oil and diesel blend. IJRTER-2016 (2455-1457)
Bhatt Prathmesh M, Patel Paresh D (2012) Suitability of tyre pyrolysis oil (TPO) as an alternative fuel for internal combustion engine. IJAERS I(IV):61–65
Senthil Kumar R, Prabu M. (2014) Experimental investigation of a di diesel engine using tyre pyrolysis oil-diesel blends as a biodiesel 5(2). ISSN 0976-6340
Mohammad Younus SK, Ranjith Kumar V, Hanumantha Rao YV (2013) Performance and emissions characteristics of diesel engine fueled with tyre pyrolysis oil&diesel blends with additives 2(5):32–37. www.ijesi.org
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Mohammed Riyaz, M., Rangaiah, M.P. (2021). Experimental Investigation on Mixing of Waste Plastic and Tyre Pyrolysis Oil Blends with Diesel in a Single-Cylinder Four-Stroke Diesel Engine. In: Arockiarajan, A., Duraiselvam, M., Raju, R. (eds) Advances in Industrial Automation and Smart Manufacturing. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-4739-3_83
Download citation
DOI: https://doi.org/10.1007/978-981-15-4739-3_83
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4738-6
Online ISBN: 978-981-15-4739-3
eBook Packages: EngineeringEngineering (R0)