Abstract
Rice is one of the dominant staple foods of Southeast Asia; its production has been affected by a number of abiotic and biotic factors. Oxides of nitrogen, carbon monoxide and volatile organic compounds produced from vehicles and industries interact with ultraviolet light and form tropospheric ozone. Excessive ozone in the troposphere and anoxia caused by submergence of plants are two important abiotic stresses causing extensive damage to the rice crop. The physiology and growth of rice is extremely susceptible to ozone stress, which can cause reduction in productivity of the crop. Therefore, it is a call of the time to address these abiotic stresses to safeguard the rice production system. Recent advancements in genomics facilitate the identification of various quantitative trait loci (QTLs) and single nucleotide polymorphisms related with tolerance to both these stressors. It is assumed that the trait is controlled by multiple medium-effect loci rather than by a single large-effect locus. However, the underlying mechanism of stress tolerance is yet to be investigated, or in other words, the target genes that could be engineered in high-yielding varieties are yet to be fished out. The present chapter summarises the available knowledge on molecular physiology of hyper ozone concentration and anoxia tolerance in rice plant.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Ozone is present in small amounts in the Earth’s stratosphere and absorbs ultraviolet radiation. It is used in many industries as an oxidant and disinfecting agent. It has bactericidal effects against a broad array of organisms like gram-positive and -negative bacteria as well as spores and vegetative cells. Ozone is harmful when present in the troposphere and the majority of tropospheric ozone formation occurs when oxides of nitrogen (NOx), carbon monoxide (CO) and volatile organic compounds (VOCs) react in the atmosphere in presence of sunlight. Ozone enters the plant through stomatal opening and causes chlorosis, necrosis and leaf bronzing, which result in low yield from the crop (Felzer et al. 2007). Ozone pollution is one of the most important abiotic factors that affect the rice production system. The photosynthesis process is highly affected by ozone exposure along with decrease in the stomatal conductance and the rate of transpiration (Banerjee and Roychoudhury 2018).
Ozone is quickly degraded to reactive oxygen species (ROS) in the apoplast (Rao and Davis 2001; Baier et al. 2005). These ROS lead to direct necrosis or induce programmed cell death (Kangasjarvi et al. 2005; Frei et al. 2010a). Ozone tolerance can be developed by two ways, with the help of antioxidants or antioxidant enzymes. The response of rice plant to ozone varies from cultivar to cultivar, and the regulation of these traits takes place at gene, transcriptome, proteome and metabolome levels. There are various approaches for the development of ozone-tolerant rice varieties. Rice is also badly affected by environmental stresses such as anoxia and oxygen deficiency. Ethanolic fermentation is the principal pathway of anaerobic carbohydrate catabolism in rice during anoxic condition. Advance functional genomics studies, QTL mapping and genetic engineering approaches can lead to the development of ozone- and anoxia-tolerant rice varieties. The present chapter is an attempt to summarise the molecular mechanisms of ozone and anoxia tolerance and the genetic engineering approach to develop tolerant rice varieties.
2 Ozone Pollution and Mechanism of Hyper Ozone Tolerance
2.1 Ozone and Ozone Pollution
Ozone is the tri-atomic allotrope of oxygen, i.e. three atoms of oxygen (O) form a bent molecule with an O–O–O angle of 116.78°. It is mainly present in the stratosphere of the earth’s atmosphere at a range of around 10–50 km from the ground at a concentration of about 8–10 ppm. This region of the earth’s atmosphere is known as the ozone layer, and it is the layer that absorbs most of the ultraviolet radiations (wavelength range of 200–315 nm) of the sunlight and protects all life forms from its harmful effects. Due to its highly unstable structure and high oxidisation property, it is used in many industries as an oxidising agent including pharmaceutical and lubricant industries. It reacts with iron and manganese in water and precipitates it, which can be filtered to make water drinkable. It is a highly reactive molecule and has bactericidal property; thus, it is used as a disinfectant.
Despite a wide range of beneficial effects and industrial applications, it has many harmful effects on all life forms including plants, animals and humans. In case of animals, ozone has been found to affect a wide range of metabolic functions, especially lung functions; in all the species including humans, rat, mouse, sheep, pigs, monkeys, and dogs, rapid and shallow breathing develops on exposure to ozone. Ozone exposure also causes inflammation and increases the susceptibility of animals to infections. Apart from this, it has been found to be having DNA mutational effects.
Ozone also causes devastating effects on plants, and it has been claimed that ozone causes more damage to plants than all other air pollutants combined (https://www.ars.usda.gov/southeast-area/raleigh-nc/plant-science-research/docs/climate-changeair-quality-laboratory/ozone-effects-on-plants/). Ozone enters the plants through the stromatal opening and causes several symptoms such as chlorosis, necrosis, flecks, bronzing and reddening, leading to loss of yield.
2.1.1 Sources of Ozone Pollution
Ozone is not directly synthesised or emitted from any natural or anthropogenic activity; rather, it is synthesised as a by-product of the photochemical reaction of many primary pollutants originating from various anthropogenic activities involving oxygen. These primary pollutants include oxides of nitrogen (NOx), methane, carbon monoxide and other VOCs. Fossil fuels are the primary sources of both nitrogen oxides and carbon monoxide. Apart from that, nitrogen oxides are generated from bio-mass burning, lightning, soils and other sources, whereas VOCs are produced from natural vegetation. These gases in the presence of sunlight react with oxygen to produce ozone. Thus, solar radiations play an important role in ozone production and its production is higher in stagnant high pressure systems in summer when the temperature is high (Fig. 1).
Ozone is also transported into a region by local winds and downward from the stratosphere. The different spatial distributions of NOx and VOC production, often result in the largest ozone concentrations downwind of urban centres, rather than in urban areas themselves (Felzer et al. 2007).
2.1.2 Effect of Ozone Pollution on Rice Plants
Rice is the most important staple food worldwide, especially in Asia, and one of the agricultural commodities with the highest production. In the year 2017, about 769 million metric tonne of rice was produced worldwide. China, followed by India, is the top lister among the rice-producing countries. China and India together produced around 382 million tonne of rice, which is approximately 50% of the total rice production worldwide. The importance of rice in human nutrition can be understood from the fact that about 20% of the calories consumed by humans worldwide come from rice.
Rice production has grown steadily in the recent years from 538 metric tonne in 1994 to 769 million tonnes in 2017 due to better management practices including water, fertiliser and pest management. However, the rice production system is facing constant challenges, both biotic and abiotic stressors being the foremost challenges. Abiotic stressors include drought, submergence (excessive rain), salinity, and ozone pollution, whereas the abiotic stressors mainly include pests and pathogens.
Ozone pollution is one of the most important abiotic factors that affect the rice production system. It has been found that for an additional day with the ozone concentration of >120 ppb, there could be loss of about 1.12% in rice yield compared to a day with ozone concentration of <60 ppb (https://www.ucdavis.edu/news/surface-ozone-pollution-damages-rice-production-china/).
The physiological effects of ozone pollution on plants include reduced photosynthesis, increased turnover of antioxidant systems, damage to reproductive processes, increased dark respiration and lowered carbon transport to roots (Felzer et al. 2007; Ariyaphanphitak et al. 2005). The physiological aberrations ultimately lead to decline in productivity. Zong-wei have reported 49.1%, 26.1% and 8.2% decrease in grain yield per rice plant when exposed to 200 ppb, 100 ppb and 50 ppb of ozone. Ariyaphanphitak et al. reported a whopping 78% reduction in filled seed per ear in Pathumthani 1 rice cultivar when exposed to 150 ppb of ozone. Similarly, Shi et al. reported yield loss of 17.5% and 15% in SY63 and LYPJ rice varieties of China, respectively, when exposed to 50% more ozone than ambient.
2.1.2.1 Physiological and Molecular Effects on Rice Plants
The effect of high ozone concentration may vary from cultivar to cultivar. Leaf injury leading to severe necrosis and chlorosis is one of the primary visible effects of high ozone exposure. The appearance of brown spots on rice leaves or otherwise called “leaf bronzing” is also observed in ozone-exposed plants. The photosynthesis process is highly affected by ozone exposure as synthesis of many of the proteins related with light harvesting and electron transport systems decline. The stomatal conductance and the rate of transpiration decline, which results in decline in photosynthesis, ultimately leading to chlorosis (Banerjee and Roychoudhury 2018). Along with the photosynthetic activity, there is a decline in grain yield. Even though these two are often simultaneous processes in ozone-exposed rice plants, the degree of visible leaf injury does not correlate well with loss in grain yield (Tsukahara et al. 2015; Sawada and Kohno 2009).
Ozone enters through the stomatal opening and it is quickly degraded to ROS such as the superoxide anion (O2 −) and hydrogen peroxide (H2O2) in the apoplast (Rao and Davis 2001; Baier et al. 2005). These ROS can have dual effects; it may lead to direct necrotic tissue damage or may induce programmed cell death (PCD) (Kangasjarvi et al. 2005; Frei et al. 2010a).
On a biochemical level, ozone induces the synthesis of salicylic acid, jasmonic acid, abscisic acid and ethylene, which play important roles in plant defence mechanism including cell death (Rao and Davis 2001). Apart from these, the synthesis of other antioxidative enzymes like superoxide dismutase (SOD), catalases and ascorbate peroxidase have also been found to be increasing (Banerjee and Roychoudhury 2018). These biochemicals act on various interdependent or independent and sometimes antagonistic pathways related to the plant defence mechanism. For instance, salicylic acid has an inhibitory effect on enzymes such as catalase and ascorbate peroxidise. Catalase and ascorbate peroxidase are enzymes that are involved in the metabolism of H2O2; when there is increased synthesis of SA, it inhibits the synthesis of catalase and ascorbate peroxidise, leading to cell death of leaves (Rao and Davis 2001). Similarly, jasmonic acid and ethylene act synergistically and may antagonise or synergise the effects of salicylic acid depending upon the stimulus and rice variety. Ozone exposure has been found to induce the biosynthesis of ethylene, which is known to induce organ senescence.
Abscisic acid (ABA) is another plant hormone that has been found to have protective effects against ozone. Lin et al. (2001) have showed that treatment of rice plants with ABA significantly reduced H2O2 content in leaves of ozone-exposed plants. It also effectively reduced stomatal conductance and the degree of injury. They have suggested that ABA induced tolerance to ozone could be associated more with stomatal movement than on the modulation of antioxidant enzymes.
2.1.2.2 Effect of Ozone on Rice Yield
Ozone when present in the troposphere is called as bad ozone. This ozone is produced from methane, VOCs and nitric oxides (Ainsworth et al. 2012). Troposphere ozone significantly affects the growth of rice; the number of main stem leaves decrease and there is marked decrease in the plant height of rice. Significant reduction in the percentage of filled spikelet and individual grain mass in rice is due to bad ozone (Shi et al. 2009). Due to the increased concentration of ozone, carbon dioxide assimilation at the leaf level decreases, which significantly affects the yield of crop (Fiscus et al. 2005). High level of ozone leads to stomata closing and affect the yield of rice (Imai and Kobori 2008). Grain yield is reduced significantly due to the exposure of rice to ozone (Pang et al. 2009). Exposure of plant to high concentration of ozone causes oxidative damage that leads to tissue damage, which can be subsequently detected from the damaged leaves (Baier et al. 2005; Fiscus et al. 2005; Rao and Davis 2001).
High concentration of ozone affects the quality of rice grain and straw. In case of grain, starch concentration decreases and protein content increases (Frei et al. 2012a, b; Wang et al. 2012; Zheng et al. 2013). Ozone enhances senescence, so nitrogen remobilisation takes place in the grain. High protein content is a good nutritional property but the quantity of rice grain decreases significantly so protein yield also decreases (Frei et al. 2012a, b; Zheng et al. 2013). It also affects the texture of rice as the grain becomes tougher and chewier (Hamaker 1994; Singh et al. 2011). High level of ozone induces grain chalkiness, which leads to change in the visual appearance of rice grain, ultimately affecting consumers’ acceptance (Wang et al. 2014a, b). Frei et al. (2012a, b) found that lipid concentration increases in rice grain but the grain size becomes smaller.
Rice straw contains high amount of lignin and phenolics under ozone exposure than normal grown rice. Lignin and phenolics are antinutrients for ruminants. Straw quality is degraded on exposure to ozone, which is not suitable to feed herbivores (Devendra and Sevilla 2002; Frei et al. 2010b, 2011; Frei 2013; Wang and Frei 2011).
Ozone at a molecular level causes toxicity. It produces ROS like hydrogen peroxide, superoxide radicals and hydroxyl radicals. These radicals damage the lipids and proteins of the cell membrane. Membrane function and fluidity changes as a consequence of lipid damage caused due to ozone toxicity (Luwe et al. 1993). ROS also degrades proteins and DNA (Sharma et al. 2012). Ozone along with primary ROS produces secondary ROS, which degrades biomolecules (Choudhury et al. 2013). High ozone concentration induces chlorosis and necrotic lesions at a cellular level (Schlagnhaufer et al. 1995; Rao et al. 2000, 2002).
2.2 Mechanism of Ozone Tolerance
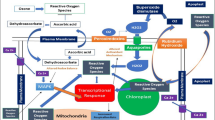
Ozone produces ROS in the apoplast region of plant. The ozone signalling mechanism in plants and its possible pathways are depicted in Fig. 2.
Mechanism of ozone tolerance is associated with the scavenging of ROS generated by oxidative stress. ROS detoxification involves antioxidants and antioxidant enzymes (Mittler 2002; Neill et al. 2002; Dietz 2003). Antioxidants are mainly ascorbic acid, glutathione and polyphenols. Antioxidant enzymes are SOD, catalase and peroxidase (Blokhina et al. 2003). The ascorbate-glutathione cycle works with the help of enzymes that use ascorbic acid and glutathione as substrate. Scavenging of ROS takes place and the reduced state of antioxidants is restored (Noctor and Foyer 1998). Ascorbic acid level and its reduced state are a critical factors for ozone detoxification (Conklin and Barth 2004). Polyamines and putrescine help in the scavenging of intercellular ROS (Langebartels et al. 1991; Navakoudis et al. 2003; Nali et al. 2006; Bussotti et al. 2007). Similarly, isoprene has significant role in scavenging hydrogen peroxides and revert the effect of lipid peroxidation of membranes (Loreto et al. 2001; Loreto and Velikova 2001). SOD and monodehydroascorbate reductase play important roles in the protection of plants from ozone damage and provide tolerance to ozone (Eltayeb et al. 2007; Van Camp et al. 1994).
2.2.1 Molecular Responses of Rice Plant Against Ozone Toxicity
The response of rice plant to ozone varies from cultivar to cultivar; some of the varieties are tolerant, whereas others have been found to be susceptible. So, it is obvious to expect the regulation of these traits at the gene, transcriptome, proteome and metabolome level. There have been numerous studies on the mechanism of hyper ozone tolerance in plants; these studies are not limited to rice. In fact, only few studies have been carried out in rice. Some of such studies are discussed here.
Cho et al. (2008) have extensively studied the transcriptomic, proteomic and metabolomic alterations in rice plants to survey ozone response in leaves of rice seedling. Microarray-based transcriptomics study has, shown upregulation of 1266 genes and suppression of 267 genes involved in a wide range of pathways including photosynthesis, MAPK cascades, and jasmonic acid, ethylene and tryptophan biosynthesis. These results were also corroborated by proteomic and metabolomic investigations. One of the most highly represented genes were those of the WRKY domain proteins. OsWRKY1, 11, 24, 26, 28, 42, 68, 69, 71, 45, 55, 72, 76 and 77 were among the WRKY genes that were upregulated in one or other time points of ozone exposure. Many of these WRKY proteins have antagonising effects. For instance, OsWRKY72 and -77 activate the abscisic acid-inducible HVA22 promoter, whereas OsWRKY24 and 45 repress it. This study puts light on the molecular mechanism of the ozone-induced enhancement on the abscisic acid synthesis as discussed in the previous section. Further, the findings of the study suggested that ABA signalling is controlled by the competitive mechanism of positive regulators and negative regulators.
A number of previous studies have determined genetic factors associated with ozone tolerance in rice. Rice is among the very few crop plants for which putative QTLs for ozone tolerance have been identified (Frei et al. 2010a). It has been suggested that ozone tolerance is controlled by multiple medium-effect loci rather than by a single large-effect locus (Frei 2013; Ueda et al. 2015). Frei et al. (2008) have identified two QTLs for ozone tolerance through screening 23 rice varieties with a leaf bronzing score (leaf bronzing is one of the symptoms of ozone toxicity). Out of the 23 varieties, the indica variety ‘Kasalath’ showed no visible symptoms, whereas the japonica variety ‘Nipponbare’ was found to be moderately susceptible. Subsequent backcrosses identified two QTLs associated with leaf bronzing, i.e. OzT3 and OzT9. These two QTLs have been found to be having contrasting effects; while OzT3, located at 2 cM on chromosome 3, has been found to be enhancing LBS, the OzT9 has been found to be reducing it. Further, they characterised the QTLs through comparative gene expression analysis, which revealed that these genes are involved in PCD and antioxidative ROS generation synthesis (Frei et al. 2008).
Wang et al. (2014a, b) have shown that pyramiding the two QTLs OzT9 and OzT8 provides improved tolerance to ozone exposure. They created lines with tolerance alleles at both loci by crossing two chromosome segment substitution lines containing individual ozone tolerance QTLs OzT8 and OzT9. It was found that the lines containing both the QTLs performed better than the Nipponbare parents SL46 (line with OzT8) and SL41 (line with OzT9) in terms of leaf bronzing, lipid peroxidation, chlorophyll level and stomatal conductance. These physiological advantages also led to better yield. It was observed that pyramiding of both the QTLs provided better yield as compared to a single QTL (Wang et al. 2014a, b).
An apoplastic protein, namely Ozone-Responsive Apoplastic Protein1 (OsORAP1), has been found to induce cell death in ozone-stressed rice plants. This gene is associated with the QTL OzT9 and has a sequence similar to ascorbate oxidase. A knockout rice line of OsORAP1 has been found to have enhanced tolerance to ozone stress as evidenced from less formation of visible leaf symptoms (i.e. cell death), less lipid peroxidation and lower NADPH oxidase activity, which indicates reduced production of ROS (Ueda et al. 2015).
2.3 Genetic Engineering for Ozone-Tolerant Rice Variety
Given the emission of greenhouse gases from a wide range of anthropogenic activities, the atmospheric ozone concentration will surely increase in days to come. This would adversely affect all crop plants, especially rice plants, and thus, there is an urgent need to look for mitigation strategies. Even though multiple QTLs for ozone tolerance have been identified, there are speculations regarding their stable expression after the incorporation of these QTLs into high-yielding varieties through conventional breeding. Incorporation of novel genes through genetic engineering is one of the feasible strategies that could be employed to develop ozone-tolerant rice varieties.
Even though the molecular pathways involved in the process of ozone tolerance have not been extensively studied, there are some report on the transcriptomic and proteomic alterations in tolerant rice varieties in response to ozone. These studies have shown that the antioxidative enzyme systems and the pathways involved in ABA, JA, SA and ethylene metabolism play important role in ozone tolerance. Thus, genes involved in these metabolism processes are possible targets that could be engineered and overexpressed for providing ozone tolerance. For example, the wrky genes have been found to play an important role in ozone tolerance. The wrky gene identified in rice, OsWRKY89, known for its ROS scavenging activity, could possibly be overexpressed for ozone tolerance. Similarly, gene encoding enzymes associated with the AsA-GSH cycle like APX, dehydroascorbate reductase, monodehydroascorbate reductase and GR can be overexpressed to promote O3 tolerance (Frei et al. 2012a, b). Downstream proteins like late embryogenesis abundant proteins can also be expressed to confer O3 tolerance (Banerjee and Roychoudhury 2018).
3 Anoxia and Mechanism of Anoxia Tolerance
Plants usually face anoxia as an ecological challenge during their life cycle due to certain natural conditions like amalgamation of freezing and flooding stresses including spring floods and ice encasement. Aquatic plants have developed many adaptive metabolic structures to combat hypoxia or anoxia (Blokhina et al. 2003). Energy crisis is the major concern during anoxia so anoxia-tolerant plants develop mechanisms to adapt to energy crisis. Plant tissues enhance carbohydrate catabolism during the initial period and downregulate the rate of carbohydrate catabolism with time (Gibbs and Greenway 2003). Energy production is totally through glycolysis concurrent to ethanolic fermentation during the initial 3–4 h of anoxia in rice (Menegus et al. 1991; Ricard et al. 1994). There are reports that supply of high concentrations of exogenous glucose improves anoxia tolerance in rice coleoptile tips, as they do not lose K+, P and Cl− even up to 120 h of anoxia, indicating that these tips did not suffer injury. Energy-dependent solute transport also has priority during an energy crisis. During anoxia, the energy distribution in rice coleoptiles favours increase in fresh weight instead of protein synthesis (Alpi and Beevers 1983).
Rice coleoptiles reacted to anoxia considerably at the level of changed metabolite pools. A set of enzymes, i.e. alcohol dehydrogenase (ADH), phosphoenolpyruvate carboxykinase (PCK), pyruvate decarboxylase (PDC) and pyruvate orthophosphate dikinase (PPDK), showed increased abundance in anoxic rice. There are reports of accumulation of certain amino acids such as Ser, Gly and Ala in anoxic rice coleoptiles. Many genes have been found to be downregulated under anoxia, especially those genes that code for enzymes requiring oxygen for their activity, indicating the existence of an energy-saving strategy in the regulation of gene expression. Similarly, various genes coding for signal transduction components (Baxter-Burrell et al. 2002), transcription factors (Liu et al. 2005), nitrogen metabolism (Mattana et al. 1994), nonsymbiotic haemoglobin (Dordas et al. 2004), ethylene biosynthesis (Vriezen et al. 1999), and cell wall loosening (Saab and Sachs 1996) were upregulated at low oxygen concentration. Anaerobic proteins like enzymes involved in sugar metabolism, glycolysis and fermentation pathways are overexpressed during anoxia (Huang et al. 2005).
3.1 Genetic Engineering for Anoxia-Tolerant Rice Varieties
Rice (Oryza sativa L.) is extremely sensitive to anoxia during germination and early growth of the embryo (Yang et al. 2019). Genetic engineering of novel genes into intolerant rice varieties could successfully resolve the limitations of anaerobic germination. Siangliw et al. (2003) reported that the Sub1 locus strongly affects the survival of rice flooding tolerance varieties by synthesising ethylene response factors. Incorporation of the Sub1 locus into the ‘japonica’ (intolerant; Toojinda et al. 2003) significantly increased its flooding tolerance (Fukao et al. 2006).
Very few papers have been published on the genetic engineering of anoxia-tolerant recombinant lines. Quimio et al. (2000) reported overexpression of PDC (pdc1) in the rice cultivar ‘Taipei 309’ that improved anoxia tolerance as a result of an increase in alcohol metabolism. Kretzschmar et al. (2015) explained that the expression of trehalose-6-phosphate (T6P) phosphatase gene (OsTPP7) increases starch mobilisation and the elongation of coleoptile, as a result of enhanced anoxia tolerance. However, the precise mechanism of anoxia tolerance is still not clear and merits further investigation. QTL mapping and genome-wide association studies were recently used to identify some QTLs associated with anoxia tolerance. Even though numerous tolerance loci have been identified, only one QTL (qAG-9-2) has been fine mapped and cloned as OsTPP7.
4 Conclusion
The chapter summarises the molecular mechanisms developed by rice to cope with ozone and anoxia. Recent developments in molecular biology and genetics have enhanced our understanding of mechanisms adapted by rice plant to survive under anoxia and ozone stress conditions. Further efforts are needed to develop transgenic rice varieties that can solve the global food demand in the era of climate change.
References
Ainsworth EA, Yendrek CR, Sitch S, Collins WJ, Mberson LD (2012) The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu Rev Plant Biol 63:637–661
Alpi A, Beevers H (1983) Effect of O2 concentrations on rice seedlings. Plant Physiol 71:30–34
Ariyaphanphitak W, Sarobo CE, Bashkin N, Towprayoon S (2005) Effects of elevated ozone concentrations on Thai jasmine rice cultivars (Oryza Sativa L.). Water Air Soil Pollut 167(1–4):179–200
Baier M, Kandlbinder A, Golldack D, Dietz KJ (2005) Oxidative stress and ozone: perception, signaling and response. Plant Cell Environm 28:1012–1020
Banerjee A, Roychoudhury A (2018) Rice responses and tolerance to elevated ozone. In: Hasanuzzaman M, Fujita M, Nahar K, Biswas JK (eds) Advances in rice research for abiotic stress tolerance. Academic Press/Elsevier, Amsterdam, pp 399–411
Baxter-Burrell A, Yang ZB, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296:2026–2028
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91(2):179–194
Bussotti F, Strasser RJ, Schaub M (2007) Photosynthetic behavior of woody species under high ozone exposure probed with the JIP-test: a review. Environ Pollut 147(3):430–437
Cho K, Shibato J, Agrawal GK, Jung YH, Kubo A, Jwa NS et al (2008) Integrated transcriptomics, proteomics, and metabolomics analyses to survey ozone responses in the leaves of rice seedling. J Proteome Res 7(7):2980–2998
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8:e23681. https://doi.org/10.4161/psb.23681
Conklin PL, Barth C (2004) Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ 27:959–970
Devendra C, Sevilla CC (2002) Availability and use of feed resources in crop animal systems in Asia. Agric Syst 71:59–73
Dietz KJ (2003) Redox control, redox signalling and redox homeostasis in plant cells. Int Rev Cytol 228:141–193
Dordas C, Hasinoff BB, Rivoal J, Hill RD (2004) Class-1 hemoglobins, nitrate and NO levels in anoxic maize cell-suspension cultures. Planta 219:66–72
Eltayeb AM, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225:1255–1264
Felzer BS, Cronin T, Reilly JM, Melillo JM, Wang X (2007) Impacts of ozone on trees and crops. C R Geosci 339:784–798
Fiscus EL, Booker FL, Burkey KO (2005) Crop responses to ozone: uptake, modes of action, carbon assimilation and partitioning. Plant Cell Environ 28:997–1011
Frei M (2013) Lignin: characterization of a multifaceted crop component. Sci World J 2013:436517. https://doi.org/10.1155/2013/436517
Frei M, Tanaka JP, Wissuwa M (2008) Genotypic variation in tolerance to elevated ozone in rice: dissection of distinct genetic factors linked to tolerance mechanisms. J Exp Bot 59:3741–3752
Frei M, Tanaka JP, Chen CP, Wissuwa M (2010a) Mechanisms of ozone tolerance in rice: characterization of two QTLs affecting leaf bronzing by gene expression profiling and biochemical analyses. J Exp Bot 61:1405–1417
Frei M, Makkar HPS, Becker K, Wissuwa M (2010b) Ozone exposure during growth affects the feeding value of rice shoots. Anim Feed Sci Technol 155:74–79
Frei M, Kohno Y, Wissuwa M, Makkar HPS, Becker K (2011) Negative effects of tropospheric ozone on the feed value of rice straw are mitigated by an ozone tolerance QTL. Glob Change Biol 17:2319–2329
Frei M, Wissuwa M, Pariasca-Tanaka J, Chen CP, Sudekum KH, Kohno Y (2012a) Leaf ascorbic acid level-is it really important for ozone tolerance in rice? Plant Physiol Biochem 59:63–70
Frei M, Kohno Y, Tietze S, Jekle M, Hussein MA, Becker T, Becker K (2012b) The response of rice grain quality to ozone exposure during growth depends on ozone level and genotype. Environ Pollut 163:199–206
Fukao T, Xu KN, Ronald PC, Bailey-Serres J (2006) A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 18:2021–2034
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30(1):1–47
Hamaker BR (1994) The influence of rice protein on rice quality. In: Marshall WE, Wadsworth JI (eds) Rice science and technology. Marcel Dekker Inc, New York
Huang S, Ishizawa K, Greenway H, Colmer TD (2005) Manipulation of ethanol production in anoxic rice coleoptiles by exogenous glucose determines rates of ion fluxes and provides estimates of energy requirements for cell maintenance during anoxia. J Exp Bot 56(419):2453–2463
Imai K, Kobori K (2008) Effects of the interaction between ozone and carbon dioxide on gas exchange, ascorbic acid content, and visible leaf symptoms in rice leaves. Photosynthetica 46:387–394
Kangasjarvi J, Jaspers P, Kollist H (2005) Signalling and cell death in ozone-exposed plants. Plant Cell Environ 28:1021–1036
Kretzschmar T, Pelayo MAF, Trijatmiko KR, Gabunada LFM, Alam R, Jimenez R, Mendioro MS, Slamet-Loedin IH, Sreenivasulu N, Bailey-Serres J, Ismail AM, Mackill DJ, Septiningsih EM (2015) A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nat Plants 1:15124
Langebartels C, Kerner K, Leonardi S, Schraudner M, Trost M, Heller W, Sandermann H (1991) Biochemical plant responses to ozone: I. Differential induction of polyamine and ethylene biosynthesis in tobacco. Plant Physiol 95(3):882–889
Lin DI, Lur HS, Chu C (2001) Effects of abscisic acid on ozone tolerance of rice (Oryza sativa L.) seedlings. Plant Growth Regul 35:295–300
Liu FL, VanToai T, Moy LP, Bock G, Linford LD, Quackenbush J (2005) Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol 137:1115–1129
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126:993–1000
Luwe M, Takahama U, Heber U (1993) Role of ascorbate in detoxifying ozone in the apoplast of spinach (Spinacia oleracea L.) leaves. Plant Physiol 101:969–976
Mattana M, Coraggio I, Bertani A, Reggiani R (1994) Expression of the enzymes of nitrate reduction during the anaerobic germination of rice. Plant Physiol 106:1605–1608
Menegus F, Cattaruzza L, Mattana M, Beffagna N, Ragg E (1991) Response to anoxia in rice and wheat seedlings. Changes in the pH of intracellular compartments, glucose-6-phosphate level and metabolic rate. Plant Physiol 95:760–767
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nali C, Francini A, Lorenzini GJ (2006) Biological monitoring of ozone: the twenty-year Italian experience. Environ Monit 8(1):25–32
Navakoudis E, Lutz C, Langebartels C, Lutz-Meindl U, Kotzabasis K (2003) Ozone impact on the photosynthetic apparatus and the protective role of polyamines. Biochim Biophys Acta 1621:160–169
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Pang J, Kobayashi K, Zhu JG (2009) Yield and photosynthetic characteristics of flag leaves in Chinese rice (Oryza sativa L.) varieties subjected to free-air release of ozone. Agric Ecosyst Environ 132:203–211
Quimio CA, Torrizo LB, Setter TL, Ellis M, Grover A, Abrigo EM, Oliva NP, Ella ES, Carpena AL, Ito O, Peacock WJ, Dennis E, Dattal SK (2000) Enhancement of submergence tolerance in transgenic Rice overproducing pyruvate decarboxylase. J Plant Physiol 156:516–521
Rao MV, Davis KR (2001) The physiology of ozone-induced cell death. Planta 213:682–669
Rao MV, Koch JR, Davis KR (2000) Ozone: a tool for probing programmed cell death in plants. Plant Mol Biol 44:345–358
Rao MV, Lee H, Davis KR (2002) Ozone-induced ethylene production is dependent on salicylic acid, and both salicylic acid and ethylene act in sconcert to regulate ozone-induced cell death. Plant J 32:447–456
Ricard B, Couee I, Raymond P, Saglio PH, Saint-Ges V, Pradet A (1994) Plant metabolism under hypoxia and anoxia. Plant Physiol Biochem 32:1–10
Saab IN, Sachs MM (1996) A flooding-induced xyloglucan endo-transglycosylase homolog in maize is responsive to ethylene and associated with aerenchyma. Plant Physiol 112:385–391
Sawada H, Kohno Y (2009) Differential ozone sensitivity of rice cultivars as indicated by visible injury and grain yield. Plant Biol 11:70–75. https://doi.org/10.1111/j.1438-8677.2009.00233.x. PMID: 19778370
Schlagnhaufer CD, Glick RE, Arteca RN, Pell EJ (1995) Molecular cloning of an ozone-induced 1-aminocyclopropane-1-carboxylate synthase cDNA and its relationship with a loss of rbcS in potato (Solanum tuberosum L.) plants. Plant Mol Biol 28:93–103
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012: Article ID 217037, 26 pages. https://doi.org/10.1155/2012/217037
Shi G, Yang L, Wang Y, Kobayashi K, Zhu J, Tang H, Pan S, Chen T, Liu G, Wang Y (2009) Impact of elevated ozone concentration on yield of four Chinese rice cultivars under fully open-air field conditions. Agric Ecosyst Environ 131(3–4):178–184
Siangliw M, Toojinda T, Tragoonrung S, Vanavichit A (2003) Thai jasmine rice carrying QTLch9 (SubQTL) is submergence tolerant. Ann Bot 91(2):255–261
Singh N, Pal N, Mahajan G, Singh S, Shevkani K (2011) Rice grain and starch properties: effects of nitrogen fertilizer application. Carbohydr Polym 86:219–225
Toojinda T, Siangliw M, Tragoonrung S, Vanavichit A (2003) Molecular genetics of submergence tolerance in rice: QTL analysis of key traits. Ann Bot (Lond) 91:243–253
Tsukahara K, Sawada H, Kohno Y, Matsuura T, Mori IC, Terao T, Ioki M, Tamaoki M (2015) Ozone-induced rice grain yield loss is triggered via a change in panicle morphology that is controlled by aberrant panicle organization 1 gene. PLoS One 10(4):e0123308. https://doi.org/10.1371/journal.pone.0123308
Ueda Y, Siddique S, Frei M (2015) A novel gene, ozone-responsive apoplastic protein1, enhances cell death in ozone stress in rice. Plant Physiol 169:873–889
Van Camp W, Willekens H, Bowler C, van Montagu M, Inze D, Reupold-Popp P, Sandermann H, Langebartels C (1994) Elevated levels of superoxide dismutase protect transgenic plants against ozone damage. Nat Biotechnol 12:165–168
Vriezen WH, Hulzink R, Mariani C, Voesenek LACJ (1999) 1-Aminocyclopropane-1-carboxylate oxidase activity limits ethylene biosynthesis in Rumex palustris during submergence. Plant Physiol 121:189–195
Wang Y, Frei M (2011) Stressed food e the impact of abiotic environmental stresses on crop quality. Agric Ecosyst Environ 141:271–286
Wang Y, Yang L, Han Y, Zhu J, Kobayashi K, Tang H, Wang Y (2012) The impact of elevated tropospheric ozone on grain quality of hybrid rice: a free-air gas concentration enrichment (FACE) experiment. Field Crops Res 129:81–89
Wang Y, Yang L, Höller M, Zaisheng S, Pariasca-Tanaka J, Wissuwa M, Frei M (2014a) Pyramiding of ozone tolerance QTLs OzT8 and OzT9 confers improved tolerance to season-long ozone exposure in rice. Environ Exp Bot 104:26–33
Wang Y, Song Q, Frei M, Shao Z, Yang L (2014b) Effects of elevated ozone, carbon dioxide, and the combination of both on the grain quality of Chinese hybrid rice. Environ Pollut 189:9–17
Yang J, Sun K, Li D, Luo L, Liu Y, Huang M, Yang G, Liu H, Wang H, Chen Z, Guo T (2019) Identification of stable QTLs and candidate genes involved in anaerobic germination tolerance in rice via high-density genetic mapping and RNA-Seq. BMC Genomics 20(1):355
Zheng F, Wang X, Zhang W, Hou P, Lu F, Du K, Sun Z (2013) Effects of elevated O-3 exposure on nutrient elements and quality of winter wheat and rice grain in Yangtze River Delta, China. Environ Pollut 179:19–26
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Panda, A.K., Mishra, R., Mahanty, A., Lenka, S., Panda, K.K., Bisht, S.S. (2020). Genomics and Genetic Engineering of Rice for Tolerance to Ozone and Anoxia. In: Roychoudhury, A. (eds) Rice Research for Quality Improvement: Genomics and Genetic Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-4120-9_16
Download citation
DOI: https://doi.org/10.1007/978-981-15-4120-9_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4119-3
Online ISBN: 978-981-15-4120-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)