Abstract
Acute respiratory distress syndrome (ARDS) is a critical syndrome consisting of acute respiratory failure, which is characterized by injury to the alveolar capillary barrier that leads to the influx of protein-rich fluid infiltrates into alveolar spaces and subsequent devastating lung fibrosis. This deadly respiratory condition can be caused by various pulmonary (e.g., pneumonia, aspiration) or non-pulmonary (e.g., sepsis, pancreatitis, trauma) injuries, leading to the development of nonhydrostatic pulmonary edema. Despite various seminal events in the understanding of molecular mechanisms underpinning the ARDS, the armamentarium of therapies aimed at the underlying pathology remains limited and highly ineffective. ARDS has an enormous mortality rate of approximately 40%. Till today, ARDS management remains mostly supportive with lung-protective mechanical ventilation. Here, we deduce the recent advances in the understanding of the biology of the disease, the diagnosis, and the treatment of ARDS. Moreover, this review also provides a historical background and highlights areas of future perspectives in therapeutics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
The syndrome of acute respiratory distress (ARDS) is a life-threatening condition that is characterized by intense lung inflammation, diffuse alveolar damage, progressive micro-atelectasis, increased pulmonary vascular permeability, increased lung weight, and loss of aerated tissue [1, 2]. ARDS was first described in 1967 by Ashbaugh et al. [3] in a case series of 12 patients who shared the common features of unusually persistent tachypnea and hypoxemia, opacification on chest radiographs, and poor lung compliance, despite different underlying causes [2,3,4].
Following the initial description of acute respiratory distress syndrome (ARDS) by Ashbaugh et al. in 1967, multiple definitions were proposed and used until the 1994 publication of the American-European Consensus Conference (AECC) definition. The AECC defined ARDS as the acute onset of hypoxemia (arterial partial pressure of oxygen to a fraction of inspired oxygen [PaO2/FIO2] 200 mmHg) with bilateral infiltrates on frontal chest radiographs, with no evidence of left atrial hypertension. A new overarching entity—acute lung injury (ALI)—was also described, using similar criteria but with less severe hypoxemia (PaO2/FIO2 300 mmHg) [5].
Initially, ARDS was described as an “adult” respiratory distress syndrome to differentiate it from infant respiratory distress syndrome. Presently, this has been changed to acute respiratory distress syndrome due to the fact that both adults and children develop ARDS. Lung development increases linearly with age and height until the adolescent growth spurt at 10 years in girls and 12 years in boys. Therefore, there are significant differences between adult and child ARDS pathophysiology due to remodeling, growth of the lung parenchyma, and progressive maturation of the immune system [5,6,7,8,9,10]. Until recently, the most widely accepted definition was that from the American European Consensus Conference (AECC) in 1994. The AECC defined ARDS as the acute onset of hypoxemia with bilateral infiltrates on a frontal chest radiograph, with no clinical evidence of left atrial hypertension (or pulmonary artery wedge pressure of greater than or equal to 18 mmHg when measured) and a P/F ratio of less than or equal to 200 mmHg. Acute lung injury (ALI) was also defined using the same criteria but with a P/F threshold of 300 mmHg; thus, ARDS was a subset of patients with ALI. Although this consensus definition better enabled comparative studies of epidemiology and mortality and enrollment in clinical trials, it was hampered by many limitations. Key among the limitations were the uncertainty of timing of the insult leading to ARDS, confusion surrounding the ALI category, ambiguity surrounding the use of P/F ratio relative to the application of PEEP, which may modify this ratio, marked inter-observer variation in the interpretation of chest radiography, and controversies in excluding volume overload or heart failure as the primary cause of respiratory failure. The current Berlin definition of ARDS addresses these limitations. It specifies an acute time frame for the development of ARDS (within 1 week of known clinical insult or new or worsening respiratory symptoms), stipulates minimum ventilator settings (PEEP of 5 cm H2O or more), and clarifies chest radiography criteria as well as the adjudication of respiratory failure from volume overload or heart failure. Furthermore, the Berlin definition removes the ALI term and classifies ARDS into three categories (mild, moderate, and severe) to facilitate prognostication [11, 12].
Despite nearly five decades of study into ARDS, there are still no specific therapies beyond low tidal mechanical ventilation to reduce the mechanical stress placed on the injured lung. However, even with the advent of protective ventilation ARDS, mortality has not significantly declined below 40% [12, 13].
7.2 Epidemiology/Morbidity
Various studies have attempted to identify the incidence, outcomes, and population metrics of ARDS. But, the epidemiology of ARDS suffers from the lack of a true diagnostic test. Despite standardized definitions, most are hampered by inter-observer variability in ascertainment of cases, variations in validity of case definitions, geographic differences in availability of medical resources such as intensive care unit (ICU) beds, heterogeneity of risk factors among populations, and problems with determining mortality that can be directly attributed to ARDS, leading to inconsistencies of epidemiological measures [11].
The Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNG-SAFE) is the latest cross-sectional study that provides data on the epidemiology of ARDS. Globally, ARDS accounts for 10% of intensive care unit admissions, representing more than three million patients with ARDS annually [11, 14]. Of the 12,906 patients who received mechanical ventilation, 23.4% fulfilled the Berlin definition of ARDS [11].
ARDS affects approximately 200,000 patients each year in the United States, resulting in nearly 75,000 deaths annually, more than breast cancer or HIV infection [15, 16]. Mortality from ARDS remains high, ranging from 35% to 46%, with higher mortality being associated with greater degrees of lung injury severity at onset. Survivors may have substantial and persistent physical, neuropsychiatric, and neurocognitive morbidity that has been associated with significantly impaired quality of life, as long as 5 years after the patient has recovered from ARDS.
7.3 Cause/Symptoms
ARDS is characterized by impaired oxygenation and bilateral infiltrates on a chest radiograph. It is caused by various conditions like pneumonia, burns, sepsis, trauma, acute pancreatitis, aspiration, toxic inhalation transfusion, and cardiopulmonary bypass surgery (Table 7.1) [1, 5, 6]. The clinical hallmarks are hypoxemia and bilateral radiographic opacities, associated with increased venous admixture, increased physiological dead space, and decreased lung compliance. The morphological hallmark of the acute phase is diffuse alveolar damage (i.e., edema, inflammation, hyaline membrane, or hemorrhage) [5].
A variety of clinical risk factors have been associated with the development of ARDS and fall into two broad categories: direct and indirect lung injury (Table 7.1).
The symptoms of ARDS are potentially underrecognized by clinicians. Early recognition and subsequent optimal treatment of patients with ARDS may be facilitated by usage of biomarkers. Several biomarkers have been examined with regard to their discriminatory ability for the diagnosis of ARDS (Table 7.2). Surfactant protein D (SP-D) has been proposed as such a candidate. Lung inflammation and injury affect the synthesis and secretion of SP-D from lung epithelial cells into the systematic circulation [17,18,19]. Infants and children are vulnerable to severe respiratory insult as compared to adults. Therefore, adult-based clinical definitions and parameters of ARDS are not applicable in pediatrics due to anatomic and physiologic differences. Pediatric acute lung injury consensus conference (PALICC), defined the first pediatric-focused characterization of ARDS. In pediatric ARDS, the oxygen index (OI) or oxygen saturation index defines the stratification of the severity of the disease based on the oxygen deficit [20,21,22,23].
7.4 Pathophysiology of ARDS
ARDS, a clinical syndrome of noncardiogenic pulmonary edema, is characterized by hypoxemia, radiographic infiltrates, decreased functional residual capacity, and decreased lung compliance [5, 23].
The most common pathophysiology of ARDS are the following:
-
1.
Disruption of pulmonary endothelial and alveolar epithelial integrity
-
2.
Alveolar Epithelial injury and dysfunction
-
3.
Disruption of Lung endothelial homeostasis
-
4.
Inflammatory dysfunction
-
5.
Surfactant dysfunction
-
6.
Coagulation and fibrinolysis dysfunction
7.4.1 Disruption of Pulmonary Endothelial and Alveolar Epithelial Integrity
Alveolar epithelial cells (AECs) collaboratively form a tight barrier between atmosphere and fluid-filled tissue to enable normal gas exchange. The tight junctions of AECs provide intercellular sealing and are integral to the maintenance of the AEC barrier integrity [25]. The alveolar epithelium is coated with a thin layer of alveolar wall liquid, which is necessary for dispersion of surfactant, transfer of gases, and host defense against inhaled pathogens. Integrity of this barrier is critical for gas exchange, and separation of the aqueous and gaseous compartments. Disruption and failure of reconstitution of the AEC barrier result in catastrophic consequences, leading to alveolar flooding with protein-rich edema fluid and subsequent devastating fibrotic scarring. Cytokines (interleukin-1 [IL-1], IL-8, tumor necrosis factor-a [TNF-a]) and lipid mediators (leukotriene B4) are attracted to alveoli and, in response to these proinflammatory mediators, neutrophils are recruited into the pulmonary interstitium and alveoli. The presence of protein, fibrinogen, and fibrin degradation products in the alveolar fluid results in surfactant degradation [6, 21, 22, 25].
7.4.2 Alveolar Epithelial Injury and Dysfunction
The alveolar wall is lined by two types of AECs: type I and type II AECs. The flat type I AECs and capillary endothelial cells constitute the blood–air barrier that facilitates efficient gas exchange. The cuboidal type II AECs secrete a surfactant that is believed to contribute to the lowering of alveolar surface tension. Apart from this, it is also responsible for the removal of excess alveolar fluid through sodium-dependent intracellular transport. Type II AECs proliferate and differentiate to Type I cells after injury.
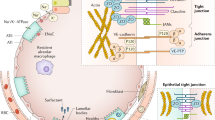
Decreased alveolar fluid clearance is associated with severity and worse clinical outcomes and increased mortality in ARDS. Direct alveolar epithelial injury and indirect alveolar capillary injury (Table 7.1) cause barrier breakdown and inability of the alveolar epithelium to remove excess alveolar fluid. Surfactant protein D (SP-D), a marker of alveolar epithelial injury, has been proposed as a potentially useful biomarker for diagnosis of ARDS in a few studies [17] (Fig. 7.1).
Schematic representation of healthy alveolus and pathophysiology in ARDS: In the healthy alveolus (Top), the alveolar epithelium and capillary endothelium are intact, which maintains the air-filled, fluid-free alveoli. The alveolar wall liquid formation is required for gas exchange, which is the medium for dispersal of the surfactant and alveolar macrophages, thereby maintaining alveolar stability and host defenses. (Bottom) In contrast, the alveolar space in ARDS looks highly disturbed with severe flooding with solutes and large molecules such as albumin. This fluid accumulation is due to the loss of epithelial and endothelial barrier integrity, leading to severe pulmonary edema. The endothelium of pulmonary micro-vessels gets activated; there is high influx of proinflammatory mediators. This leads to the recruitment of leukocytes into the pulmonary interstitium and alveoli. There are also high amounts of fibrin deposition due to increased concentration of fibrinogen and fibrin degradation products in the edema fluid. Depletion of surfactant leads to increase in surface tension and loss of alveolar shape and integrity. There is also fibroblast proliferation, ROS generation, and NET (neutrophil extracellular traps) in the diseased alveoli
7.4.3 Disruption of Lung Endothelial Homeostasis
Dysregulated endothelial activation and the resultant loss of homoeostatic mechanisms are aspects of ARDS pathobiology [6, 26]. Pulmonary endothelium is a dynamic, metabolically active organ that modulates several key regulatory functions including leucocyte diapedesis, intravascular coagulation, vasomotor tone, and solute and fluid trafficking via regulation of barrier permeability. Reactive oxygen species (ROS) and reactive nitrogen species production by activated cells saturate local antioxidants and contribute to tissue injury directly via downregulation of VE-cadherin, upregulation of neutrophil adhesion molecule expression, and release of neutrophil chemotactic factors. Endothelial-specific proteins, such as von Willebrand factor and angiotensin-converting enzyme activity, correlate with ARDS mortality. Moreover, activated endothelial cells also assume a procoagulant phenotype by increasing the expression of platelet adhesion molecules, intra-alveolar and intravascular fibrin deposition, and release of activators of the extrinsic coagulation cascade [1, 26,27,28].
7.4.4 Inflammatory Dysfunction
Several studies have demonstrated evidence for dysregulation of inflammatory pathways in ARDS and their association with outcome. There is a strong correlation between mortality and upregulated levels of proinflammatory (IL-6, IL-8, IL-18, MIP-1b, TNF-a) and anti-inflammatory cytokines (IL-1RA, IL-10, and TNF-R2). These cytokines were associated with ARDS illness severity, including the P/F ratio, OI, pediatric risk of mortality score (PRISM-3), ICU morbidity, and biochemical evidence of endothelial injury, including elevated plasma angiopoietin 2 and soluble thrombomodulin [4, 6, 29].
When alveolar macrophages are activated, they recruit neutrophils and circulating macrophages to the alveoli. Neutrophils communicate between the vessel wall and platelets, which results in endothelial injury and releases neutrophil extracellular traps, which may also cause damage to the lung [30, 31].
7.5 Current Therapies for ARDS
Despite decades of research, treatment options for ARDS are limited. Supportive care with mechanical ventilation remains the mainstay of management [32]. Most advances in ARDS therapeutics have been through changes in mechanical ventilation methods, culminating in a 2017 International Clinical Practice Guideline for mechanical ventilation on adults with ARDS [14, 15, 24, 32]. The guidelines address six interventions:
-
1.
low tidal volume and inspiratory pressure ventilation
-
2.
prone positioning
-
3.
high-frequency oscillatory ventilation
-
4.
higher versus lower positive end-expiratory pressure
-
5.
lung recruitment maneuvers and
-
6.
extracorporeal membrane oxygenation
Most of the current treatment is supportive and palliative, with no current disease-modifying therapies available [24].
7.5.1 Management-Based Therapy for ARDS
A standard therapy for ARDS is mainly based and reliant on management of pulmonary dysfunction; however, the identification involves non-pulmonary organ dysfunction also.
7.5.1.1 Treatment of the Inciting Clinical Disorder
ARDS is an outcome of several disease processes, which mainly include trauma, ischemia reperfusion, pneumonia, multiple transfusions, sepsis, and aspiration of gastric contents [33, 34]. Several of these have a direct treatment based on antibiotics/antimicrobials, which however needs a careful examination for the type of pathogen involved in inciting the disease such as bacteria (Mycoplasma or Legionella) or fungi (Pneumocystis carinii). Sometimes ALI/ARDS is incited by extrapulmonary infections, e.g., urinary tract, pulmonary infection, or biliary tract infection, which hence warrants a thorough clinical examination. The clinical treatment should take the clinical history of the patient into account. In some situations, like transfusions or gastric aspirations, stopping the recurrence is the optimal treatment method along with good supportive care [33,34,35,36].
7.5.1.2 Mechanical Ventilation
ARDS generates an alveolar dead space, an impaired pulmonary compliance. The required amount of breathing is too high a work for the voluntary pulmonary system, which may lead to a ventilatory failure pulmonary acidosis and hypercapnia [37]. Overall, there is a huge respiratory burden, which demands an extra support to be functional. In such situations, mechanical ventilation serves as a backbone to respiration and hence is an essential supplementary care. Mechanical ventilation not only acts as a mainstay of supportive care but also provides sufficient time for natural healing and drug treatments [36, 38].
7.5.1.3 Effective Oxygenation (PEEP and FIO2)
An ARDS patient needs effective oxygenation at the start of mechanical ventilation, which is a high priority measure. During mechanical ventilation, positive end-expiratory pressure (PEEP) and fraction of inspired oxygen (FiO2) provide effective oxygenation and support arterial oxygenation and hence are crucial. PEEP and FiO2 are associated with serious limitations, which can be avoided by keeping the FiO2/PEEP index in check. For human subjects, FiO2 <50% is considered safe [39,40,41,42]. PEEP improves arterial oxygenation and decreases intrapulmonary shunt. It keeps a check on FiO2 while allowing adequate arterial oxygenation [43, 44].
7.5.1.4 Vasodilators
ARDS patients are known to have modest pulmonary arterial hypertension, which has been demonstrated to progress with the progression of disease. The rise is a multifactorial process and can make the disease severe by affecting the vascular bed and hypoxic vasoconstriction or sometimes cardiac dysfunction. Several studies have attempted to reduce the pulmonary hypertension to evaluate its effect on progression of ARDS. There are reports of using hydralazine in animal models, which has proven effective though [36, 45]. Continuous infusion of prostaglandin E1 has proven effective in clinical cases also [46]. Nitric oxide (NO) is an effective endogenous vasodilator. Encouraged from promised results obtained in animal models, clinicians attempted to use gaseous NO to treat pulmonary hypertension in ARDS patients; however, mild and transient success in clinical cases of ARDS was noted [47,48,49,50,51].
7.5.1.5 Infection Management in ARDS
Uncontrolled infection is a prominent feature associated with ALI/ARDS. Often, lung infection becomes a causal factor for development of ALI/ARDS [51]. However, infection may occur as an outcome of ARDS development, though reports of non-pulmonary sepsis are also known to cause ARDS. There is a high amount of risk associated with development of pneumonia and catheter-related sepsis also known as nosocomial infections in patients receiving ventilation, which is an area of investigation that is being highly investigated for quite some time [36, 52, 53]. Administration of antibiotics/antimicrobials does not help always. Therefore, infections are managed by frequent washing of hands by medical personnel, nonstop suction of subglottic secretions to avoid their aspiration, and the usage of new endotracheal tubes to avoid making of a bacterial biofilm. Almost all the management strategies for nosocomial pneumonia in ARDS are contentious. One of the important aspects that adds to this is the ambiguity in the diagnosis itself. There is a significant overlap between ARDS diagnosis and clinical data obtained from pneumonia, as the immune cell infiltration is highly similar in both pathologies [54, 55].
7.5.2 Drawbacks of the Current Pharmacological Treatment
Despite several decades of investigation into potential treatment strategies, use of lung-protective ventilation remains the only proven therapy to decrease mortality in ARDS [2]. In patients with ARDS, recruitment maneuvers improve oxygenation, but this is a temporary approach that does not improve mortality. Clinical trials of β2-agonists, statins, surfactants, and keratinocyte growth factor (KGF) have been disappointing. In addition, monoclonal antibodies (anti-TNF) and TNFR fusion protein have also been unconvincing. Glucocorticoids may improve oxygenation and airway pressures in established ARDS, but have failed to demonstrate a role in preventive therapy. In patients with pneumonia, steroids may improve radiological appearances, but again does not improve mortality [24, 56]. Trials conducted on established ARDS investigated different doses and duration of treatment, preventing generalization of the results. However, analysis suggests that if steroids are started 14 days or more after the diagnosis of ARDS, they can be harmful. The combination of inhaled β2-agonists and glucocorticoid administered early in patients at risk of ARDS has recently shown to prevent development of ARDS and improve oxygenation but its effect on mortality has not been demonstrated [24].
Despite intense investigation, no specific pharmacological treatment for ARDS has been shown to affect the mortality, even though preclinical trials in animal models have looked promising. Therefore, targeting a single pathogenetic pathway is not unlikely to be advantageous due to the complexity of the mechanisms involved [24].
7.6 Future Directions in Therapeutics and Perspectives
No pharmacologic treatments aimed at the underlying pathology have been shown to be effective, and management remains supportive with lung-protective mechanical ventilation. Guidelines on mechanical ventilation in patients with acute respiratory distress syndrome can assist clinicians in delivering evidence-based interventions that may lead to improved outcomes [15].
Even after 50 years of study, ARDS still looks like a threatening enemy to defeat. Studies on ARDS incidence consistently show that the disease is not rare, albeit often not recognized by clinicians. ARDS is undertreated and basic ventilator strategies are not yet standardly optimized, despite major advances in the management of mechanical ventilation and non-ventilatory strategies, aimed at preventing the VILI. Although mortality associated with ARDS is decreasing in clinical trials, it remains unchanged at approximately 40% in major observational studies. Various factors contribute to the mortality associated with ARDS. They include patient age and illness severity, country-level socioeconomic status, ventilator management, and ICU organizational method. This suggests the need of a commonly accepted therapeutic strategy for ARDS, which should be the prerogative of all the countries including the less-developed ones [57, 58]. Molecular mechanisms identifying the susceptibility of injury and aberrant activation of alveolar epithelial cells and pulmonary endothelial cells would be pivotal in moving toward the positive outcomes of these intractable diseases.
The characterization of the ARDS sub-phenotype by blood biomarkers may help clinicians to select patients who may benefit from specific therapeutic strategies and ultimately tailor the treatment of our single patient. In fact, it has been proved that a high PEEP strategy in ARDS patients affected the major outcome only in the hyperinflammatory sub-phenotype. Moreover, the restrictive fluid strategy was beneficial in the same selected ARDS patients. More studies are needed to further explore the benefits of different therapies based on a particular ARDS biomarker profile [24, 59]. To further reduce mortality, the therapy of ARDS should possibly take genetic difference among patients and the origin of ARDS into account, such as the primary or the secondary ARDS.
In the future, by understanding the role of biomarkers in the pathophysiology of ARDS and lung injury, it is hoped that this will provide rational therapeutic targets and ultimately improve clinical care.
References
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Alessandri F, Pugliese F, Ranieri VM (2018) The role of rescue therapies in the treatment of severe ARDS. Respir Care 63:92–101
Ashbaugh DG, Bigelow DB, Petty TL, Levine BE (1967) Acute respiratory distress in adults. Lancet 2:319–323
Roy GB, Lorraine BW, Yves B, Michael AM (2001) Treatment of ARDS. Chest 120:1347–1367
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD et al (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533
Heidemann SM, Nair A, Bulut Y, Sapru A (2017) Pathophysiology and management of acute respiratory distress syndrome in children. Pediatr Clin North Am 64:1017–1037
Sapru A, Flori H, Quasney MW et al (2015) Pathobiology of acute respiratory distress syndrome. Pediatr Crit Care Med 16:S6–S22
Katz R (1987) Adult respiratory distress syndrome in children. Clin Chest Med 8:635–639
Sherrill DL, Camilli A, Lebowitz MD (1989) On the temporal relationships between lung function and somatic growth. Am Rev Respir Dis 140:638–644
Wang X, Dockery DW, Wypij D et al (1993) Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 15:75–88
Nanchal RS, Truwit JD (2018) Recent advances in understanding and treating acute respiratory distress syndrome. F1000Res 7:F1000 Faculty Rev-1322
Villar J, Sulemanji D, Kacmarek RM (2014) The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care 20:3–9
Wang T, Gross C, Desai AA et al (2017) Endothelial cell signaling and ventilator-induced lung injury: molecular mechanisms, genomic analyses, and therapeutic targets. Am J Physiol Lung Cell Mol Physiol 312:L452–L476
Fan E, Brodie D, Slutsky AS (2018) Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA 319:698–710
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG et al (2017) An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 195:1253–1263
Rubenfeld GD, Caldwell E, Peabody E et al (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693
Park J, Pabon M, Choi AMK et al (2017) Plasma surfactant protein-D as a diagnostic biomarker for acute respiratory distress syndrome: validation in US and Korean cohorts. BMC Pulm Med 17:204
Terpstra ML, Aman J, van Nieuw Amerongen GP, Groeneveld AB (2014) Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med 42:691–700
Hermans C, Bernard A (1999) Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med 159:646–678
Flori H, Glidden D, Rutherford G et al (2005) Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 171:995–1001
Yehya N, Servaes S, Thomas NJ (2015) Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med 43:937–946
Zimmerman JJ, Akhtar SR, Caldwell E et al (2009) Incidence and outcomes of pediatric acute lung injury. Pediatrics 124:87–95
Cheifetz IM (2016) Year in review 2015: pediatric ARDS. Respir Care 61:980–985
Spadaro S, Park M, Turrini C et al (2019) Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J Inflamm (Lond) 16:1
Yanagi S, Tsubouchi H, Miura A, Matsumoto N, Nakazato M (2015) Breakdown of epithelial barrier integrity and overdrive activation of alveolar epithelial cells in the pathogenesis of acute respiratory distress syndrome and lung fibrosis. Biomed Res Int 2015:573210
Bachofen M, Weibel ER (1977) Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis 116:589–615
Ware LB, Eisner MD, Thompson BT et al (2004) Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med 170:766–772
Orfanos SE, Armaganidis A, Glynos C et al (2000) Pulmonary capillary endothelium bound angiotensin-converting enzyme activity in acute lung injury. Circulation 102:2011–2018
Parsons PE, Eisner MD, Thompson BT et al (2005) Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 33:1–2
Aggarwal NR, King LS, D’Alessio FR (2014) Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol 306:L709–L725
Caudrillier A, Kessenbrock K, Gilliss BM et al (2012) Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 122:2661–2671
Fan E, Needham DM, Stewart TE (2005) Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA 294:2889–2896
Anderson ID, Fearon KC, Grant IS (1996) Laparotomy for abdominal sepsis in the critically ill. Br J Surg 83:535–539
Sinanan M, Maier RV, Carrico CJ (1984) Laparotomy for intraabdominal sepsis in patients in an ICUs. Arch Surg 119:652–658
Doyle RL, Szaflarski N, Modin GW et al (1995) Identification of patients with acute lung injury: predictors of mortality. Am J Respir Crit Care Med 152:1818–1824
Brower RG, Ware LB, Berthiaume Y, Matthay MA (2001) Treatment of ARDS. Chest 120:1347–1367
Falke KJ, Pontoppidan H, Kumar A et al (1972) Ventilation with end-expiratory pressure in acute lung disease. J Clin Invest 51:2315–2323
Lamy M, Fallat RJ, Koeniger E et al (1976) Pathologic features and mechanisms of hypoxemia in adult respiratory distress syndrome. Am Rev Respir Dis 114:267–284
Royston BD, Webster NR, Nunn JF (1990) Time course of changes in lung permeability and edema in the rat exposed to 100% oxygen. J Appl Physiol 69:1532–1537
Fracica PJ, Knapp MJ, Piantadosi CA, Takeda K, Fulkerson WJ, Coleman RE, Wolfe WG, Crapo JD (1991) Responses of baboons to prolonged hyperoxia: physiology and qualitative pathology. J Appl Physiol 71:2352–2362
Royer F, Martin DJ, Benchetrit G, Grimbert FA (1988) Increase in pulmonary capillary permeability in dogs exposed to 100% O2. J Appl Physiol 65:1140–1146
Fox RB, Hoidal JR, Brown DM, Repine JE (1981) Pulmonary inflammation due to oxygen toxicity: involvement of chemotactic factors and polymorphonuclear leukocytes. Am Rev Respir Dis 123:521–523
Shank JC, Latshaw RF (1980) Adult respiratory distress syndrome. Am Fam Physician 21:107–114
Falke KJ, Pontoppidan H, Kumar A, Leith DE, Geffin B, Laver MB (1972) Ventilation with end-expiratory pressure in acute lung disease. J Clin Invest 51:2315–2323
Harrison WD, Raizen M, Ghignone M, Girling L, Slykerman LJ, Prewitt RM (1983) Treatment of canine low pressure pulmonary edema: nitroprusside versus hydralazine. Am Rev Respir Dis 128:857–861
Bone RC, Slotman G, Maunder R, Silverman H, Hyers TM, Kerstein MD, Ursprung JJ (1989) Randomized double-blind, multicenter study of prostaglandin E1 in patients with the adult respiratory distress syndrome: Prostaglandin E1 Study Group. Chest 96:114–119
Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM (1991) Inhaled nitric oxide: a selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 83:2038–2047
Fratacci MD, Frostell CG, Chen TY, Wain JC Jr, Robinson DR, Zapol WM (1991) Inhaled nitric oxide: a selective pulmonary vasodilator of heparin-protamine vasoconstriction in sheep. Anesthesiology 75:990–999
Shah NS, Nakayama DK, Jacob TD (1994) Efficacy of inhaled nitric oxide in a porcine model of adult respiratory distress syndrome. Arch Surg 129:158–164
Rossaint R, Falke KJ, López F, Slama K, Pison U, Zapol WM (1993) Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med 328:399–405
Rayees S, Joshi JC, Tauseef M, Anwar M, Baweja S, Rochford I, Joshi B, Hollenberg MD, Reddy SP, Mehta D (2019) PAR2-mediated cAMP generation suppresses TRPV4-dependent Ca2+ signaling in alveolar macrophages to resolve TLR4-induced inflammation. Cell Rep 27:793–805
Sutherland KR, Maunder RJ, Milberg JA, Allen DL, Hudson LD (1995) Pulmonary infection during the acute respiratory distress syndrome. Am J Respir Crit Care Med 152:550–556
Meduri GU, Wunderink RG, Leeper KV Jr, Jones CB, Tolley E, Mayhall G (1994) Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest 106:221–235
Seidenfeld JJ, Bell RC, Harris GD, Johanson WG Jr (1986) Incidence, site, and outcome of infections in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 134:12–16
American Thoracic Society (1995) Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventative strategies; a consensus statement. Am J Respir Crit Care Med 153:1711–1725
Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M (2006) National Heart L. Blood institute acute respiratory distress syndrome clinical trials N: efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354:1671–1684
McNicholas BA, Rooney GM, Laffey JG (2018) Lessons to learn from epidemiologic studies in ARDS. Curr Opin Crit Care 24:41–48
Rezoagli E, Fumagalli R, Bellani G (2017) Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med 5:282
Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, Network NA (2014) Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2:611–620
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Vellingiri, V., Thirusangu, P., Din, I. (2020). Acute Respiratory Distress Syndrome: Therapeutics, Pathobiology, and Prognosis. In: Rayees, S., Din, I., Singh, G., Malik, F. (eds) Chronic Lung Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-15-3734-9_7
Download citation
DOI: https://doi.org/10.1007/978-981-15-3734-9_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3733-2
Online ISBN: 978-981-15-3734-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)