Abstract
Microscopic thymic rests are not uncommon in the lateral neck and upper mediastinum, especially in the pediatric population. The undescended thymus is almost always asymptomatic; however, it may give rise to ectopic thymic tumors. Thyroid and intrathoracic thymic tumors mirror the morphology of their orthotopic counterparts but a diagnosis of ectopic thymic tumor is always challenging owing to extreme rarity and unusual location. This chapter provides an illustrative overview of the benign and malignant ectopic thymic tumors with a special focus on the intrathyroidal location.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Ectopic Thymic Tissue
The path taken by the developing thymus during embryogenesis determines an area in the lateral neck and upper mediastinum which may harbor ectopic thymic rests. Aberrant thymic tissue is found in the neck in > 20% of the general population and is much more common in the prepubertal pediatric population [1]. The residual cervical thymus is usually <15 mm, paratracheal, with a marked left-sided predilection [1]. The undescended thymus is rarely symptomatic; however, it may mimic solid or cystic neck lesions when found incidentally on imaging. More importantly, thymic rests in ectopic locations may give rise to tumors.
The closely related descent of the thyroid and parathyroid glands and thymus explains their ectopic location within each other. Portions of thymic tissue are present in 70% of normal thyroid glands of infants studied by serial sectioning, which implies that the presence of thymus and parathyroid tissue within the thyroid is so common as to be classified as normal [2]. Ultrasound (US) screening detected intrathyroidal thymus in 1% of the general pediatric population [3]. Ultrasonography is a sensitive tool because of the unique echogenicity of thymic tissue appeared as an irregular, triangular, polygonal hypoechoic area with punctate, granular, or linear bright internal echoes (Fig. 11.1) [4].
Grossly, aberrant thymic tissue is often unremarkable (small size), whitish yellow (Fig. 11.2), soft (associated adipose tissue), and rarely cystic. In intrathyroidal location, it may be confused with the parathyroid gland, if lying superficially.
11.1.1 Microscopic Findings
-
Ectopic thymic rests repeat a histological appearance of orthotopic thymic tissue, e.g., a combination of mature lymphoid tissue and thymic epithelial cells with Hassall corpuscles (Figs. 11.3 and 11.4).
-
Extent may vary from a small area of thymic tissue containing one or two Hassall corpuscles to complete well-differentiated glands with distinct cortex and medulla.
-
Marked adipose involution in adults.
-
Ectopic parathyroid can be found in the vicinity, forming a so-called parathyroid-thymic complex (Figs. 11.3a and 11.4b).
-
Cystic changes (Fig. 11.4b).
-
Preoperative fine-needle aspiration cytology may yield epithelial cells and Hassall corpuscles on a background of abundant small- and medium-sized lymphocytes with a mature chromatin pattern.
Histology of intrathyroidal thymic tissue. A parathyroid-thymic complex completely enclosed by thyroid tissue (a). Intrathyroidal thymic tissue composed of band-like aggregations of predominantly lymphoid cells within the thyroid (b). Small cluster of thymic tissue embedded in adipose pad within the thyroid gland (c). Hassall corpuscle (yellow arrowhead) identified on high power (d). Hematoxylin and eosin, ×40 (a–c), ×200 (d)
Ectopic thymus in perithyroidal location. Cervical thymic tissue embedded in the perithyroidal fat (a). Papillary thyroid carcinoma invading ectopic thymus (b). Note cyst formation and attached parathyroid (upper right). The cyst is lined by thymic epithelium positive for p63 immunostain (inset). Hematoxylin and eosin, ×5 (a: bar, 2 mm), ×20 (b: bar, 0.5 mm), ×400 (b, inset)
11.1.2 Differential Diagnosis
-
Lymph node: absence of Hassall corpuscles and epithelial cells
-
Hashimoto thyroiditis: no Hassall corpuscles
-
Lymphoma: monoclonal proliferation
-
Thymic tumors: rich epithelial component
11.2 Ectopic Thymoma
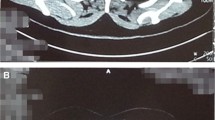
Over 95% of thymomas are originated from the orthotopic thymus in the anterosuperior mediastinum. The vast majority of rare ectopic thymomas are developed in the cervical region (including thyroid) and thorax (lung, pleura, pericardium) [5]. Cervical ectopic thymomas are usually located in the anterolateral neck close to the lower poles of the thyroid gland. Most patients are females manifested in the fifth–sixth decade with a growing mass in the neck (thyroid nodule) or lung. Autoimmune syndromes (myasthenia gravis) are uncommon. Ultrasound and computed tomography are the imaging modalities of choice for cervical (Fig. 11.5a) and intrapulmonary thymomas, respectively. The gross appearance of ectopic thymomas is essentially similar to their anterior mediastinal prototype, i.e., circumscribed/encapsulated and lobulated (Fig. 11.5b).
11.2.1 Histopathology
-
Similar to orthotopic/mediastinal thymoma (see Chap. 7).
-
Jigsaw puzzle-like lobules separated by sclerotic bands [6].
-
Circumscribed/encapsulated, capsular invasion should be evaluated.
-
Biphasic tumor with a combination of neoplastic epithelial cells and small lymphocytes (Fig. 11.6).
-
The ratio of epithelial cells to lymphocytes and shape of the tumor cells (spindle or ovoid) define the WHO type [7].
-
Residual ectopic thymus and entrapment of native tissue, depending on the primary site, may be found [5].
11.2.2 Cytology
-
Type A thymoma is characterized by individual and cohesive groups of spindle cells (bland nuclei, scant or absent cytoplasm) with variable proportions of small mature lymphocytes in background.
-
Type B thymoma displays prominent numbers of uniform small lymphocytes (Fig. 11.7c) and clusters of polygonal epithelial cells (bland round nuclei, moderate/abundant cytoplasm).
11.2.3 Immunohistochemistry
-
Consistent with orthotopic thymoma (see Chap. 7).
-
The cytoplasm of epithelial cells is positive for cytokeratins (Fig. 11.8), including pancytokeratin (AE1/AE3), low molecular weight cytokeratin (CAM5.2), high molecular weight cytokeratin (34 beta E12), and CK5/6 [5].
-
Nuclei of epithelial cells react with p63 or p40 and polyclonal PAX8.
-
Nonneoplastic lymphocytes are positive for T cell markers (CD1a, CD99, TdT, etc.).
Immunohistochemical study of cervical thymoma. Pancytokeratin staining with AE1/AE3 antibody demonstrated positively stained spindle epithelial cells in the background of negatively stained lymphocytes (a). High proliferation activity predominantly in lymphoid cells detected by Ki-67 (MIB-1) immunostaining (b). Immunohistochemistry, ×100 (a), ×200 (b)
11.2.4 Differential Diagnosis
-
Lymphocyte-rich variants may mimic lymphoma or Hashimoto thyroiditis: cytokeratin staining disclose neoplastic epithelial cells in thymoma.
-
Spindle cell-type thymoma resembles mesenchymal tumors, which are negative for cytokeratin and positive for CD34 and/or S100.
-
Epithelial-type thymoma should be differentiated from poorly differentiated squamous cell carcinoma (SCC): evident atypia.
-
Ectopic hamartomatous thymoma: exceedingly rare benign tumor in the soft tissues of the lower neck, which is not a genuine thymoma [9], characterized by haphazardly intermingled spindle epithelial cells, solid or cystic epithelial islands, and adipose cells, lack of lobulation, and lymphocytic background.
11.3 Intrathyroid Thymic Carcinoma
Thymic carcinoma arising outside the mediastinum is extremely rare, and thyroid gland is the major location. Intrathyroid thymic carcinoma (ITC) is a low-grade malignant epithelial tumor of the thyroid gland with thymic epithelial differentiation [6]. It is a malignant counterpart of ectopic thymoma. ITC was previously known as intrathyroid epithelial thymoma or carcinoma showing thymus-like differentiation/elements (CASTLE). This tumor affects middle-aged adults with a slight female preponderance. Most of the cases were reported from Asia [10].
ITC is usually located in the lower poles of lateral lobes or attached to them. On ultrasound, the tumor appears as a hypoechoic solid mass with irregular borders and no calcification (Fig. 11.9a). Despite locally invasive behavior with relatively frequent lymph node metastases (20–50%), patients with ITC have a favorable outcome after surgical resection usually followed by radiation therapy [10, 11].
Grossly, the tumor is solid, usually well-circumscribed but not encapsulated, tan to yellowish on a cut. Lobulation characteristic of thymic tumors can be noted (Fig. 11.9b).
11.3.1 Histopathology
-
Identical to orthotopic/mediastinal thymic carcinoma (see Chap. 9).
-
From well-demarcated from adjacent thyroid (Fig. 11.10) to locally invasive (Fig. 11.11).
-
Lobulated and anastomosing large nests of squamoid cells separated by dense fibrous septa in a lymphocytic background (Figs. 11.10, 11.11, and 11.12).
-
Neoplastic epithelial cells with large nuclei featured prominent small nucleoli (Fig. 11.12b) and cytoplasm ranged from abundant to sparse (Fig. 11.12b–c).
-
Rarely seen high-grade features, such as necrosis (Figs. 11.10c and 11.12d), increased mitotic activity (Fig. 11.12e), and pleomorphism (Fig. 11.12f).
-
Three histological subtypes have been reported, including squamous cell carcinoma type (keratinization), lymphoepithelioma type (basaloid cells with lymphocyte-rich stroma), and neuroendocrine carcinoma type [12].
Low-power view of ITC. The tumor (right) is demarcated from benign thyroid (left) but there is no capsule formation (a). Lobulated appearance due to irregular invasion and compression of the outer fibrous tissue (b). Sometimes necrosis can be prominent, up to geographic and confluent (c). Hematoxylin and eosin, ×10 (a–b: bar, 1 mm), ×40 (c)
Histological features of ITC. Fibrous bands between solid nests of cancer cells (a). Invasion into the adjacent thyroid (b). Associated lymphoid infiltrate can be abundant, with the formation of lymphoid follicles (c). Perineural invasion (d). Local invasion (b, d) is a characteristic feature of ITC. Hematoxylin and eosin, ×100
High-power appearance of ITC. Solid epithelial growth in a background of lymphocyte-rich stroma (a). Enlarged nuclei with prominent nucleoli (b). Densely packed neoplastic epithelial cells (c). Cell appearance can be ranged from squamoid with abundant eosinophilic cytoplasm (b) to basaloid (c). Coagulative necrosis (lower left) (d). Mitotic activity (arrowheads) with atypical mitoses (e). Cancer cells with pleomorphism (f). Hematoxylin and eosin, ×400 (a, e), ×600 (b–c), ×200 (d, f)
11.3.2 Cytology
-
Cellular smear with a mixture of dyshesive and large cohesive solid/trabecular cell clusters (Fig. 11.13).
-
No papillary/follicular structures and colloid.
-
Round to short spindled nuclei with vesicular or finely granular chromatin and distinct nucleoli.
-
Cytoplasm varies from sparse and clear to abundant and dense suggesting keratinization (Fig. 11.13c).
-
A small to moderate amount of lymphocytes in the background (Fig. 11.13b).
-
Cytologic features alone are not distinctive of ITC but sufficient to render a diagnosis of malignancy and allow for the inclusion of ITC in the differential diagnosis [13,14,15].
Fine-needle aspiration cytology of ITC. Large trabecular cluster with no follicular or papillary patterns (a). The spindle-shaped carcinoma cells with sparsely scattered small lymphocytes in the background (b). Tightly arranged tumor cells with dense and wide cytoplasm suggesting squamous differentiation (c). Loosely cohesive tumor cells have small round nuclei and cyanophilic narrow cytoplasm (d). Papanicolaou stain, ×100 (a), ×400 (b–d). [Fig. 11.13b is reproduced from Fig. 41.3 in https://doi.org/10.1007/978-981-13-1897-9_41 published by Springer]
11.3.3 Immunohistochemistry
-
Main immunohistochemical markers are CD5, CD117 (c-Kit), and p63 (Fig. 11.14) [6].
-
Epithelial neoplastic cells are positive for various keratins, e.g., pancytokeratin, high molecular weight cytokeratin 34 beta E12 (Fig. 11.15a), CK5/6, CK7, and others.
-
Chromogranin A and synaptophysin expression in scattered cells.
-
Negative for thyroid-specific markers (TTF-1, thyroglobulin), except PAX8 (Fig. 11.15e).
-
EBER and p16 negative.
-
Other markers could be diffusely or focally expressed in ITC (Fig. 11.15); however, these are of limited diagnostic significance [16].
Main immunohistochemical markers of ITC. CD5 immunoreactivity well-appreciated on low magnification (a). A delicate membranous staining pattern with CD5 in carcinoma cells (b). CD117 (c-Kit) revealed membranous expression (c). Nuclear positivity of cancer cells with p63 antibody (d). Immunohistochemistry, ×100 (a, d), ×400 (b), ×200 (c)
Extended immunophenotype of ITC. High molecular weight cytokeratin antibody (clone 34 beta E12) revealed diffuse expression in carcinoma cells (a). Strong expression of bcl-2 in large neoplastic epithelial cells and small-sized lymphocytes (b). Focal expression of EMA (c) and CEA (d). Heterogeneous nuclear expression of PAX8 (e). Diffuse positivity for p53 (f). Immunohistochemistry, ×100 (a–d, f), ×200 (e)
11.3.4 Differential Diagnosis
11.3.4.1 Histology
-
Metastatic head-neck SCC: negative for CD5/CD117, high Ki-67 labeling index (> 50%), positive for EBER (nasopharyngeal carcinoma) and p16 (HPV-associated oropharyngeal carcinoma).
-
Medullary thyroid carcinoma (MTC): negative for thymic markers (CD5/CD117), positive for calcitonin.
-
Anaplastic thyroid carcinoma: evident pleomorphism, high Ki-67 labeling index (> 50%), CD5/CD117 negative, clinical correlation (rapid growth, history of thyroid nodules).
-
Poorly differentiated thyroid carcinoma: CD5 negative, thyroid follicular cell markers (TTF-1, thyroglobulin) positive.
-
Most of the carcinomas from the differential diagnosis list, either thyroid origin or metastatic, are high-grade malignancies while ITC is a morphologically and clinically low-grade carcinoma.
-
Ectopic thymoma: no pleomorphism and cytologic features of malignancy, abundant lymphocytic component, absent/rare expression of CD117.
11.3.4.2 Cytology
-
Poorly differentiated thyroid carcinoma: close mimicker, immunostaining (thyroid and thymic markers) can be helpful.
-
MTC: dyscohesive, salt-and-pepper chromatin, no lymphocytes, calcitonin.
-
Anaplastic thyroid carcinoma: prominent pleomorphism.
-
Metastatic SCC: more atypical.
-
Lymphoma: no epithelial clusters.
-
SETTLE: more spindled, no lymphocytes.
11.4 Spindle Epithelial Tumor with Thymus-Like Differentiation (SETTLE)
Spindle epithelial tumor with thymus-like differentiation or elements (SETTLE) is a rare biphasic malignant tumor of the thyroid composed of spindle epithelial cells that merge into glandular structures [9]. Histogenesis of SETTLE is uncertain with speculations revolving around a branchial pouch or thymic origin. It is a low-grade malignancy manifested as thyroid or neck mass in children and adolescents, with a male predilection [6]. Cervical lymph nodes involvement is infrequent, reported in 10% of cases. It may also develop delayed blood-borne metastases with latency from few to 20–35 years after the primary diagnosis [17]. Surgical resection added by chemoradiotherapy for metastatic cases is usually effective in controlling disease and achieving long-term survival [18].
The tumor is grossly encapsulated, partially circumscribed, or infiltrative, with a mean size of 4 cm. The cut surface is firm, greyish white to tan, and vaguely lobulated (Fig. 11.16).
11.4.1 Histopathology
-
Highly cellular biphasic tumor with fibrous septa producing a vaguely lobulated pattern.
-
Spindle cells arranged in interlacing fascicles or reticular structures are usually observed as a predominant population (Fig. 11.17a–c).
-
The glandular component appears as tubules, papillae, cords, cystic spaces, mucinous and glomeruloid glands (Fig. 11.17d–e).
-
Both components are blended imperceptibly.
-
Spindle epithelial cells are bland and monomorphic; glandular cells are variable in shape (flat, cuboidal, columnar) and are sometimes mucinous or ciliated (Fig. 11.17d–e).
-
Occasionally seen mitotic activity and squamous differentiation (Fig. 11.17f).
-
Stroma is hyalinized, with scant lymphocytes (Fig. 11.17b).
-
Most cases are biphasic, but monophasic variants (spindle or glandular) have been reported.
-
Separated from the adjacent thyroid tissue by a capsule or sclerotic rim (Fig. 11.17a).
-
Invasive growth with vascular invasion can be rarely identified (Fig. 11.17a).
Histological features of SETTLE. Encapsulated tumor demarcated from the adjacent thyroid tissue (left) (a). Stromal hyalinization (b). Monophasic spindle cell pattern resembling monophasic synovial sarcoma (c). Biphasic pattern with spindle cells and pseudoglandular structures (d). Uniform spindle cells without evident atypia (e). Tubular duct with squamous metaplasia (f). Hematoxylin and eosin, ×100 (a–c), ×200 (d–f). Figure 11.17 a, b, c are reproduced from Fig. 1 g, h, j in https://doi.org/10.1111/cyt.12742 Ref. [19]
11.4.2 Cytology
-
Cellular smear with large cohesive solid/trabecular cell clusters.
-
Spindle cells are less cohesive, with scanty cytoplasm and uniform elongated nuclei (Fig. 11.18).
-
Cohesive clusters of glandular/epithelial cells with moderate-to-abundant cytoplasm and variable-sized mildly pleomorphic oval nuclei.
-
Fine-needle aspiration biopsy is rarely diagnostic for SETTLE [18, 19].
11.4.3 Immunohistochemistry
-
Both spindle and glandular cells diffusely express high molecular weight cytokeratin and CK7, but only focally low molecular weight cytokeratin (Fig. 11.19) [6].
-
Nuclear expression of p63 and p40, predominantly in spindle cells (Fig. 11.19b, d).
-
Variable expression of vimentin, CD5/CD117 (CD117 > CD5), myoepithelial and neuroendocrine markers.
-
Negative for thyroid-specific markers (TTF-1, thyroglobulin) and calcitonin.
-
Different series and case reports provide contradictory results regarding immunoexpression of ancillary (vimentin, CD5/CD117) and even main (cytokeratins, p63) markers
11.4.4 Differential Diagnosis
-
Synovial sarcoma (main mimicker): high-grade morphology (pleomorphism, necrosis), frequent mast cells, no diffuse expression of cytokeratins, SS18-SSX translocation
-
Ectopic thymoma: prominent lobulation, abundant T lymphocytes
-
ITC: squamoid morphology, lymphocyte-rich background, higher mitotic activity, essentially CD5 positive
-
Ectopic hamartomatous thymoma: prominent anastomosing syringoma-like networks, squamoid features, myoepithelial component, admixed adipocytes
-
MTC, spindle cell variant: amyloid, expression of TTF-1, and calcitonin
-
Anaplastic thyroid carcinoma: high-grade morphology, high Ki-67 labeling index (> 50%), clinical correlation (rapid growth)
References
Prabhu AV, Kale HA, Branstetter BFT. Residual cervical thymus: a normal CT finding that may be present throughout patients’ lives. AJNR Am J Neuroradiol. 2015 Aug;36(8):1525–8.
Carpenter GR, Emery JL. Inclusions in the human thyroid. J Anat. 1976 Sep;122(Pt 1):77–89.
Fukushima T, Suzuki S, Ohira T, Shimura H, Midorikawa S, Ohtsuru A, et al. Prevalence of ectopic intrathyroidal thymus in Japan: the Fukushima health management survey. Thyroid. 2015 May;25(5):534–7.
Kim HG, Kim MJ, Lee MJ. Sonographic appearance of intrathyroid ectopic thymus in children. J Clin Ultrasound. 2012 Jun;40(5):266–71.
Weissferdt A, Moran CA. The spectrum of ectopic thymomas. Virchows Archiv. 2016 Sep;469(3):245–54.
Lloyd RV, Osamura RY, Klöppel GK, Rosai J. WHO classification of tumours. Pathology and genetics of tumours of endocrine organs. 4th ed. Lyon: IARC Press; 2017.
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. 4th ed. Lyon: IARC Press; 2015.
Ali SZ, Cibas ES. The Bethesda system for reporting thyroid cytopathology: definitions, criteria, and explanatory notes. 2nd ed. New York: Springer; 2018. 236 p.
Chan JK, Rosai J. Tumors of the neck showing thymic or related branchial pouch differentiation: a unifying concept. Hum Pathol. 1991 Apr;22(4):349–67.
Ge W, Yao YZ, Chen G, Ding YT. Clinical analysis of 82 cases of carcinoma showing thymus-like differentiation of the thyroid. Oncol Lett. 2016 Feb;11(2):1321–6.
Ito Y, Miyauchi A, Nakamura Y, Miya A, Kobayashi K, Kakudo K. Clinicopathologic significance of intrathyroidal epithelial thymoma/carcinoma showing thymus-like differentiation: a collaborative study with Member Institutes of The Japanese Society of Thyroid Surgery. Am J Clin Pathol. 2007 Feb;127(2):230–6.
Kakudo K, Bai Y, Ozaki T, Homma K, Ito Y, Miyauchi A. Intrathyroid epithelial thymoma (ITET) and carcinoma showing thymus-like differentiation (CASTLE): CD5-positive neoplasms mimicking squamous cell carcinoma of the thyroid. Histol Histopathol. 2013 May;28(5):543–56.
Collins JA, Ping B, Bishop JA, Ali SZ. Carcinoma showing thymus-like differentiation (CASTLE): cytopathological features and differential diagnosis. Acta Cytol. 2016;60(5):421–8.
Hirokawa M, Kuma S, Miyauchi A. Cytological findings of intrathyroidal epithelial thymoma/carcinoma showing thymus-like differentiation: a study of eight cases. Diagn Cytopathol. 2012 May;40(Suppl 1):E16–20.
Rajeshwari M, Singh V, Nambirajan A, Mridha AR, Jain D. Carcinoma showing thymus like elements: report of a case with EGFR T790M mutation. Diagn Cytopathol. 2018 May;46(5):413–8.
Weissferdt A, Moran CA. Immunohistochemistry in the diagnosis of thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol. 2014 Aug;22(7):479–87.
Ippolito S, Bellevicine C, Arpaia D, Peirce C, Ciancia G, Vigliar E, et al. Spindle epithelial tumor with thymus-like differentiation (SETTLE): clinical-pathological features, differential pathological diagnosis and therapy. Endocrine. 2016 Mar;51(3):402–12.
Recondo G Jr, Busaidy N, Erasmus J, Williams MD, Johnson FM. Spindle epithelial tumor with thymus-like differentiation: a case report and comprehensive review of the literature and treatment options. Head Neck. 2015 May;37(5):746–54.
Nambirajan A, Singh V, DVK I, Agarwal S, Jain D. Spindle epithelial tumor with thymus-like differentiation of thyroid presenting with lymph node metastasis: an illustrative case report with review of literature. Cytopathology. 2019 Jun 18;30(6):657–61. https://doi.org/10.1111/cyt.12742.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bychkov, A., Hirokawa, M., Kakudo, K. (2020). Pathology of Ectopic Thymic Tumors. In: Jain, D., Bishop, J.A., Wick, M.R. (eds) Atlas of Thymic Pathology. Springer, Singapore. https://doi.org/10.1007/978-981-15-3164-4_11
Download citation
DOI: https://doi.org/10.1007/978-981-15-3164-4_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3163-7
Online ISBN: 978-981-15-3164-4
eBook Packages: MedicineMedicine (R0)