Abstract
Anaerobic extremophiles are generally obligate anaerobes that strictly require anoxic environment; however, facultative anaerobe can tolerate both anaerobic and aerobic environmental conditions. Some of the anaerobic microbes are found in extreme habitat such as deep in the ocean or earth’s crust; others are present in parts of varied landscape such as marshes, bogs, sewers, polar lakes, tundra, calderas, geothermal submarine vents, hot springs, deep-sea sediments, and deep subsurface rock. Further, mammalian, ruminant, and arthropod digestive tracts are also the houses of the anaerobic extremophiles. Besides facultative anaerobes and oxygen-tolerant anaerobes, obligate anaerobes require strictly anoxic environment for their existence. This chapter defines the different approaches required to understand the growth and isolation of the anaerobes and large-scale production of the most economically important biomolecules produced by obligate anaerobes. The physiological and biochemical factors accounting for the relative resistance of many strict anaerobes to oxygen and products of incomplete reduction are also described in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
One can understand the concept origin of life through the complex processes involved in “early microbial evolution.” The first appearance of the life and diversification among primary microbial lineage during the primitive anaerobic condition of the earth lead to the complex eukaryotic organisms (Martin and Sousa 2016; Subhashini et al. 2017). Exergonic reactions without involving living organism lead to the release of geochemical methane near hydrothermal vents. This concept of methane production and biological production fits a methanogenic root for archaea and an autotrophic origin of microbial physiology (Martin and Sousa 2016). It is believed that photosynthetic organism’s cyanobacteria were found approximately 3.5 billion years ago, which in turn is very complex in physiology to be the primary prokaryotes (Des Marais 2000). Cyanobacteria plays an important role to change the primitive earth atmosphere as anaerobic to aerobic due to its photosynthetic capabilities, further resulted in the evolution of other aerobic, anaerobic, and facultative organism.
According to Oparin-Haldane theory, life originated in the sea under reducing (free of oxygen) atmosphere. Hence, it is assumed that anaerobic bacteria are too close to the primitive forms of life. Many researchers have successfully demonstrated that the synthesis of organic matter is possible under such conditions in their laboratory experiments (Oro et al. 1990). The primary requirement for such synthesis included HCN, CO2, and H2S. The nature of these compounds is basically toxic for aerobic organisms and creates the environment for the escalation of anaerobic microbial species. Further, evolution of anaerobic organisms leads to the origination of anoxic phototrophs, and the studies of anaerobic bacteria regarding continued diversification revealed that it appeared before the development of aerobic photosynthesis. Later on, aerobic photosynthesis gave rise to an oxygen-rich atmosphere. However, the current book chapter highlights those anaerobes which live in CO2-rich environment or anaerobic growth conditions and their physiological adaptation.
The extreme environmental condition leads to the diversity of anaerobic microorganisms. Places/regions with extreme temperature play an immense and major role in the diversity and ecology of anaerobes among other factors because such temperate places lead to the oxygen-deficient/oxygen-free environment. During 1979, the first thermo anaerobe was reported in thermal spring. Ever since, hyperthermophile anaerobes have been isolated from continental and submarine volcanic areas, such as solfatara fields, volcano vents, hydrothermal vents, and geothermal power plants.
2 Anaerobic Thermophiles
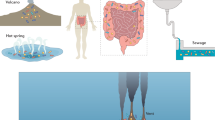
Most of the anaerobic microorganisms (anaerobic thermophiles, acidophiles, alkanophiles, psychrophiles, radiation-resistant) exist under geographically extreme conditions and do not require oxygen for their growth. These are known as anaerobic extremophiles (Fig. 11.1). The extremophiles are mainly prokaryotes (bacteria and archaea) but also some eukaryotes (Table 11.1). Based on the temperature in which anaerobic microorganisms exist, they may be classified in categories as optimum thermophiles (an optimal temperature 50–64 °C), extreme thermophiles (optimum temperature 65–80 °C), and Hyperthermophiles (optimum temperature +80 °C).
Anaerobic extreme thermophilic Bacteria and Archaea are widely distributed in all sorts of thermobiotic environments. Anaerobic extreme thermophiles living near hydrothermal vent tolerate the additional pressure generated by the water column pie and are known as piezophilic (Alain et al. 2002). The microorganisms exist near hydrothermal vent sites include Thermodiscus, Archaeoglobus, Thermoproteus, Pyrococcus, Thermococcus, and Desulfurococcus, which reduce sulfur or sulfate. The utilization of organic and inorganic compounds such as elemental sulfur, methane, and iron is used to classify anaerobic extreme thermophilic bacteria (Table 11.1).
3 Anaerobic Psychrophiles
Anaerobic psychrophiles are those organisms which can survive and metabolize in low temperature ranging from −20 to +10 °C like glaciers, polar ice, permafrost, and sea ice waters (Clarke et al. 2013). The anaerobic psychrophiles control changes within macromolecules (protein) to retain the necessary fluidity or flexibility which is required to survive at low temperature. Major groups related to psychrophiles include alpha, beta, and gamma Proteobacteria in addition to relatives of the Bacteroides, Thermus, Eubacterium, and Clostridium (Segawa et al. 2003).
4 Anaerobic Ionizing Radiation-Resistant Microorganisms
Anaerobic ionizing radiation-resistant microorganisms can survive under the exposure of high gamma radiation. Archaeon isolated from a Northern Pacific hydrothermal vent, Thermococcus gammatolerans sp., showed resistance against ionizing radiation, during exposure of gamma rays at a dose of 30 kGy (Jolivet et al. 2003).
5 Anaerobic Thermoacidophiles
The Anaerobic thermoacidophiles have the ability to grow below 4.0 pH and high temperature 65–80 °C, such as genera Thermoanaerobacter and Thermoanaerobacterium found in the deep-sea vent (Prokofeva et al. 2005).
6 Anaerobic Alkalithermophiles
Alkalithermophiles represent those groups which are adapted to grow under high temperature and high alkaline conditions. Alkalithermophiles are representative of both archaea as well as bacteria. Growth of anaerobic Clostridium thermoalcaliphilum usually required pH ranging from 7.0 to 11.0, whereas the optimum pH was recorded as 9.6 to 10.1 (Engle et al. 1994).
7 Physiology of Anaerobic Archaea and Bacteria
The physiology of prokaryotic microorganisms differ from eukaryotic microbes and in part resulted in their survivability at extreme growth conditions like above 100 °C temperature (>100 °C), extreme salinity (saturated NaCl), pH (less than 2.0 and more than 10), and substrate stress. More importantly they do not utilize oxygen for respiration. This kind of extreme microbial growth conditions was found to be prevalent during primitive times but is limited in today’s world. Some of the important and well-studied anaerobes are described given below.
7.1 Methanogen
The bacteria produce methane as a metabolic by-product in the anoxic condition known as methanogens. These methanogenic bacteria belong to a large and diverse group characterized by mainly three features: methane as the major by-product, strictly anaerobic, and belong to archaebacteria.
The prokaryotic methanogens are commonly found in wet wood in living trees, optimum pH for the growth ranges from 7.5–8.5 (Zeikus and Henning 1975). The sole source of carbon and energy for the growth and development of methanogenic bacteria depends upon production of methane. Methane can be utilized by few other aerobic bacteria as sole source of carbon, which ultimately breakdown the methane into carbon dioxide and water (Kunkel et al. 1998); hence, they are termed as methane-oxidizing bacteria.
7.1.1 Adaptation as Anaerobes and Biochemistry of Methane Production
The Methane-producing bacteria don’t require oxygen for the respiration; in fact oxygen act as a growth limiting factor of methanogens. Therefore, carbon is the terminal electron acceptor instead of oxygen during methanogenesis. Carbon can occur in a small number of organic compounds, all with low molecular weights. The two well-defined pathways showed the use of inorganic carbon dioxide or acetic acid as terminal electron acceptors are as given below:
Hydrogen acts as intermediates in the processes of methanogenic degradation of organic matter and serves as substrates for methanogenic archaea. However, it only contributes 33% during methanogenesis, while carbohydrates or associated forms of organic matter are degraded (Conrad 1999). However, during methanogenesis on the basis of temperature and pH, other small organic compounds have been also utilized as carbon source, like formate, methylamines, methanol, dimethyl sulfide, and methanethiol. The degradation of the methyl compounds is mediated by methyltransferases to give methyl-coenzyme M (Thauer 1998).
7.1.2 Methane-Oxidizing and Methane-Producing Bacteria
The methane is a primary component of oxygen-free water, marshes, soil, rumen of cattle, and gastrointestinal tract of mammals. Bacterial methanogenesis is a generalized process in most anaerobic environments. Thus, natural methane gas production can often be attributed to the growth of methanogens on specific energy sources that are formed as a result of microbial degradation of simple or complex organic matter or with the involvement of geochemical activities.
7.1.3 Oxidation and Production of Methane
Natural groupings of microorganisms, like those designated as parts in Bergey’s Manual of Determinative Bacteriology (Buchanan and Gibbons 1974), are morphologically different as the methanogenic bacteria. However, all methanogenic species share few distinct unique or combine physiological properties. The world of methanogens no longer remained as a mysterious group due to the recent researches and advancements in the field of methanogen studies. The demand of energy in current scenario paves the way to better understand bacteria that produces natural gas; it could be the source of cheap energy as bioenergy at large scale (biogas).
The bacteria which produce methane play the major role in regulating the breakdown (fermentation) of food under anoxic condition. The bacteria remove hydrogen gas through reduction of carbon dioxide to form methane. The low level of hydrogen favors the other microorganisms to grow faster due to the production of methane. Hence, collectively these consortia of microbes enhance the processes of fermentation more efficiently (Fig. 11.2).
8 Representative Life in Stress of Oxygen Concentration
8.1 Clostridium
The genus Clostridium belongs to the family Clostridiaceae, and it includes 203 species and 5 subspecies, out of which only few species are being reported as human pathogen. Among these species, 21 species have been placed to other genera, 5 have been reclassified within the genus, and 1 has been removed (Euzeby 2013).
The genus Clostridium have great genetic diversity such as strictly anaerobes, heterofermentative, spore-forming, and motile by peritrichous flagella. Generally Clostridium appear as Gram-positive but can be decolorized to show the appearance as Gram-negative or Gram-variable, with or without spore former, shape varies from either rods or cocci, and strictly anoxic bacteria. Generally, pathogenic strains are Gram-positive rods, but few are Gram variable; size varies from 0.3–2.0 × 1.5–20.0 μm which are more often arranged as pairs or short chains, with rounded or pointed ends. They are pleomorphic and differ significantly in their demand of oxygen (Finegold et al. 2002). The biotin and 4-aminobenzoate are crucially important as growth factors for the genus Clostridium at 37 °C temperature for optimum growth (Dolly et al. 2000).
Genus Clostridium can be isolated by following methods. These are (1) ability or inability to form endospores, (2) anoxic metabolism, (3) lack of ability to dissimilate sulfate reduction, and (4) presence of a Gram-positive cell wall (Andreesen et al. 1989).
Number of Clostridia species are saccharolytic in nature; hence, it can be easily isolated from acidic soils, because slightly acidic pH provides good opportunity for the growth of solvent-producing bacteria (Dolly et al. 2000).
The spores of Clostridium spp. play an immense important role in the field of medical and health sectors. For eample, two of the Clostridium spp. (C. tetani and C. difficile) enhances the chance of hospital-acquired infections further resulted in severe helath complications (McFarland 1995).
However, few nonpathogenic species of Clostridia have great significance in gene therapy which provides the treatment of cancer by targeting the tumor cells. Bio-engineered strain of Clostridia proved to be good in targeting cancer cells and safe antitumor delivery system (Mellaert et al. 2008).
On the other hand, due to the anaerobic nature of Clostridia, it provides many economically important fermentation products, proteins, and enzymes. C. acetobutylicum is the most exploited microorganism for the production of acetone/butanol at large scale. Acetogenic clostridia (e.g., C. thermoaceticum, C. thermoautotrophicum, and C. formicoaceticum) have been studied as potential producers for calcium magnesium acetate as deicer. C. thermohydrosulfuricum, C. thermocellum, and C. saccharolyticum prove to be producer of alcohol. Some Clostridium species offer high-quality source of stable enzymes due to the tolerance of extreme conditions. Such as the main product, thermostable amylases and ethanol of starch fermentation by C. thermosulfurogenes and C. thermohydrosulfuricum. Fermentation of starch by C. thermosaccharolyticum resulted into pullalanase beside the production of thermostable amylases and ethanol beside this pullalanase also produce during this reaction. An enzyme Pullalanase is economically important industrial product generally used in sugar syrup production. C. thermocellum is the common cellulose-degrading species, accountable for the bioconversion of cellulose to useful economically important products like ethanol and biofuel (Minton and Clarke 1989).
8.2 Propionibacterium
Propionibacterium sp. are Gram-positive bacilli, non-motile, non-spore-forming, and catalase-positive, and size varies from 1 to 5 μm. Propionibacterium are considered as either anaerobic or facultative anaerobic bacteria. The studies of propionic acid bacteria (PAB) under anoxic conditions revealed that they are tiny and spherical (cocci) in shape. Moreover, in the presence of oxygen, it acts like pleomorphic bacteria (keep changing their shape). The optimum pH for the growth of PAB is reported around 7.0 (oscillate between 4.5 and 8.0) suitable for the production of propionic acid and vitamin B12. Generally, Propionibacterium sp. are mesophiles, though they can resist higher temperatures for some extent (few strains survive temperatures of up to 76 °C for 10 s). Nature of tolerance adopted by PAB to abovementioned stress may lead to the development of resistance to other factors (Benjelloun et al. 2007; Daly et al. 2010).
Genus Propionibacterium has extensive application in the food industry specially cheese industry like production of hard rennet cheese (Swiss Emmental cheese, The French Comte). Fermentation of lactate results in the production of acetic and propionic acid, which ultimately enhance the aroma of the final product and act as natural preservatives (Thierry and Maillard 2002; Thierry et al. 2005). Recent studies conducted by Cousin et al. (2016) reported that strain of P. freudenreichii subsp. shermanii can trigger apoptosis of the colon cancer cells. P. freudenreichii maintains microflora of the gastrointestine, by inspiring the growth of Bifidobacterium, and defends the organism/host by the secretion of bacteriocins, limiting the growth of certain pathogenic microorganisms. This aforementioned property recommends the use of PAB as probiotics for animal feeding.
8.3 Porphyromonas
Bacteria belonging to Porphyromonas sp. are strictly anaerobic, non-spore-forming, Gram-negative, and rod, culture on blood agar, and produce porphyrin pigment (dark brown/black). The Porphyromonas is common microflora of oral cavities of human body belongs to the Bacteroides genus. There are currently 16 valid Porphyromonas species that have been reported so far. The most common pathogenic strains of genus Porphyromonas are P. gingivalis and P. endodontalis that cause infection of periodontitis and endodontic in human, respectively (Kononen et al. 1996). Many studies revealed that fimbriae or fimbriae-like arrangement in bacteria has a significant role in the adhesion to the tooth surface.
8.4 Anaerobic Fungi
Prior to the finding of anaerobic fungi, it was believed that molds are not able to metabolize carbohydrates anaerobically (fermentation). In 1949, W. Foster completely refused the existence of anaerobic fungi, by stating that the difference between bacteria and fungi in the absence of anaerobic molds is either obligate or facultative. This belief held until the first recognition of anaerobic fungi in the mid-1970s (Orpin 1975). Orpin described that certain flagellated cells are zoospores of anaerobic fungi, earlier mistakenly identified as protozoan. Mostly the aquatic fungi posse’s zoospore with single or bi flagella (Sparrow 1960) however, N. frontalis have multiple flagella up to 14 (Orpin 1975). Although, except multiple flagella stage rest of the life cycle of N. frontalis resembles to that of the chytrids. Since then, anaerobic fungi have been broadly identified and well established as gastrointestinal tract microflora of mammalian herbivores (Bauchop 1981; Joblin and Naylor 1989; Trinci et al. 1994). Nowadays, the significance and role of anaerobic fungus seek great attention to many research groups especially the complex mechanism involved in degrading the ingested cellulose and hemicellulose in mammalian herbivores. Such metabolism relies on symbiotic association of anaerobic fungi with another gut microorganism. However, anoxic fungi play the important role in the digestion of lignocellulosic plant material inside the rumen among other microbial consortia (Akin et al. 1988, 1990; Lee et al. 2000a, b).
Anaerobic fungi make some metabolic changes to adapt under the anoxygenic condition such as lack of mitochondria and other biochemicals/molecules which take part in oxidative phosphorylation (Yarlett et al. 1986; Youssef et al. 2013). Besides these hydrogenosomes, a specialized organelle present in anaerobic fungi helps in the glucose metabolism for the production of cellular energy under the anoxygenic condition (Brul and Stumm 1994; Muller et al. 2012).
Neocallimastigomycota represents anaerobic fungi that inhabit gastrointestinal tract and play a major role in plant material degradation. Anaerobic fungi degrade lignocellulosic plant material by producing a range of enzymes, mainly the powerful polysaccharide-degrading enzyme hydrogenase. However, recent research on Orpinomyces sp. toward carbohydrate-degrading enzymes revealed that there is a great diversity among them (Youssef et al. 2013). The study of Orpinomyces sp. genome sequence indicates that these genes come from rumen bacteria through horizontal gene transfer. Recent research pointed out that anaerobic fungi contain free enzymes and multiple enzyme system (Wilson and Wood 1992; Joblin et al. 2010). However, taxa of such phylum further need to be revised according to molecular phylogeny.
9 Techniques and Culture Media for Isolation of Microbes in Oxygen Stresses
Contribution of Hungate (1969) to study the rumen microbiology is immense especially designing of techniques and culture media for anaerobic rumen bacteria. The same culture methods with slight modifications (enzymes and antibiotics) are used to study anaerobic fungi (Bryant 1972; Miller and Wolin 1974). Instead of using oxygen-free N2 as a reducing agent, media were prepared under oxygen-free CO2. Lowe et al. (1985) described two kinds of media (with or without rumen fluid) for the isolation of rumen anaerobic fungi that had been widely accepted. The culture is maintained under an atmosphere of either 100% CO2 or a combination of 85% N2, 10% CO2 and 5% H2: 20–30% N2 at 39 °C. Modified anaerobic glove box and Petri dish methods are significant even today to isolate and study anaerobic fungi (Leedle and Hespell 1980; Lowe et al. 1985). Batch culture explained by Theodorou et al. (1990) demonstrated the importance of antibiotics, range of pH 6.5–6.8, resazurin as a redox indicator, and incubation time from 2 to 10 days. Some other fermentors were also used for anaerobic fungi like continuous cultures (Hillaire and Jouany 1989) and fed-batch cultures (Tsai and Calza 1993). However, the most effective technique for isolation was illustrated by Theodorou et al. (2005) after modifying earlier described methods and techniques.
10 Application of Anaerobic Extremophiles
10.1 Biotechnology
Biotechnology is a technology that utilizes living organism or parts of this to develop different industrial product for betterment of human life. Along with other organism, extremophiles are important players of biotechnology, and therefore they have been used in biotechnology since long time for production of useful product in economic price and time (Fig. 11.3). New technologies and products are developed every year within the areas of medicine, agriculture, or industrial biotechnology (Coker 2016; Singh et al. 2017; Prajakta et al. 2019).
10.2 General Steps of Obtaining Product from Extremophiles at Large Scale
Extreme microorganism responsible for important enzymes/products is isolated and characterized, and particular gene of the respective product is cloned and cultured in laboratory labeled. The product is verified and optimized for the operational condition. The product is verified and optimized for the operational condition subsequently new organism could be use in the bioreactor for further large scale production (Fig. 11.4).
Primary concern of using archaeal enzymes and metabolites at industrial level is the low production of the output of expensive fermentation processes, low biomass yields (Schiraldi et al. 2002; Zhang et al. 2019). Best way to address such problem is to develop extremophile conducive bioreactor with microfiltration. The microfiltration has been proven to be greatly resistant to the extreme working conditions suitable for the thermoacidophilic fermentation. Moreover, due to the high-level cell densities during the fermentation, frequent back flushing was proved to be enough to preserve 75% transmembrane flux of the maximum. The use of microfiltration is far better than earlier used cross-flow filtration, which fail to maintain even 20% flux after 30 h (Hayakawa et al. 1990). The assembled membrane bioreactor allowed a cell production that was 15- to 20-fold greater than the known best batch fermentation (Schiraldi et al. 1999).
10.3 Anaerobic Extremophiles and Its Commercial Exploitation
The major advantage for choosing enzymes from extremophiles are its worth of stability at extreme environment, little chance of contamination and easy to maintain other parameters of bioreactor (fermentation) (Dalmaso et al. 2015). The exploitation of extremophiles increases in current time, with the progress of research, development, and understanding of extremophiles’ metabolic activity.
Biofuel Production
Elevation of temperatures and pH is frequent in several steps of biofuel production (Singh et al. 2014). Therefore, extremophiles are replacing the mesophilic organisms used in traditional methods. Recently certain thermophilic species such as Thermotoga elfii and Caldicellulosiruptor saccharolyticus are used for the larger-scale industrial-based system. The bacteria Thermoanaerobacterium saccharolyticum produces ethanol after the breakdown of complex carbohydrate, hemicellulose. Barnard et al. (2010) have revealed that extremophile fungi such as Cyanidium caldarium and Galdieria sulphuraria contain long-chain hydrocarbons like those found in petroleum.
DNA Polymerases
Polymerase chain reaction is the most important molecular technique comes into existence after the introduction of thermostable Taq polymerase, isolated from extremophile anaerobic T. aquaticus. Recent advancement and research regarding PCR leads to identification of other thermostable polymerase such as Pyrococcus and Thermococcus sp. (optimum temperature 90–100 °C) which have been introduced to the research industries (Antranikian 2009).
Carotenoids and Protease/Lipases
The Carotenoids are very stable molecules that habitually accompany the halophilic archaea and algae and have been isolated from the halophilic archeon such as Halobacterium salinarum. It has been reported for various application ranges from holography, production of artificial retinas, dyes, and more importantly in the renewable energy. Nowadays lipid-soluble canthaxanthin has been studied for use in food industries due to its antioxidant and safe property like additives and food dye. As with bacteriorhodopsin, halophilic archaea are the producers of choice with Haloferax alexandrinus being the preferred strain. Bacteriorhodopsin functions as a rudimentary form of photosynthesis, associated with membrane-bound retinal pigment and proton pump. The color of the β-carotene, i.e., red/orange pigment, seeks the attention of dye industries especially food industry.
Lactose-Free Milk
Nearly 3/4 population of the world suffers from lactose intolerance. Resultantly, a major population is avoiding ingesting lactose-free dairy products thoroughly that has been prepared by use of the β-galactosidase isolated from Kluyveromyces lactis. The temperature of the dairy product is maintained within 5–25 °C for the stability and activity of the enzyme. However, such condition provides the suitable environment for the pathogens to grow and spoil the taste and flavor of the milk. This limitation can be overcome by the use of β-galactosidase isolated from a psychrophile. This enzyme would be active at low temperature and hydrolyze lactose throughout the entire process from production to shipment and storage which is significantly cost-effective and resulted into less chance of contamination.
Medical Applications
Anaerobic extremophiles produce antibiotics, antifungals, and antitumor molecules. Halobacteriaceae and Sulfolobus species generate antimicrobial peptides and diketopiperazines. The Diketopiperazines produced by Haloterrigena hispanica and Natronococcus occultus are used for treatment of pneumonia and cystic fibrosis.
Cyclodextrin Glycosyl Transferase (CGTase)
Cyclodextrin form the inclusion complexes along with different organic molecules which ultimately help in the ease of drug delivery system by improving the solubility of hydrophobic compounds. Thus, the application of cyclodextrin glycosyl transferase (CGTase) started at industrial level for the production of cyclodextrins.
11 Conclusions and Future Prospective
The knowledge of microbial diversity has been keep growing after the studies and thorough investigation of different extreme conditions such as high and low temeprature and anoxygenic environment. However, more extensive studies are required to investigate anaerobic diversity live in thermophilic, acidophilic, and halophilic environment, which can ultimately lead to the new species of bacteria and archaea.
Anaerobic bacteria and archaea have unique (unknown) physiological mechanism to counter the extreme environments by stabilizing their macromolecules (protein and lipid), in comparison to aerobic life. Extreme conditions are foremost requirement of the industrial biotechnology for the scale-up strategies of some products. More studies are required to understand molecular mechanism of anaerobes which will be later on exploited in industrial biotechnology for the human welfare. Especially, enzyme and lipid function of anaerobes at high temperature can ease the challenge of industrial biotechnology for large extent. However, cultural conditions required to scale up the production of the extremophiles are the major drawback at industrial level. Use of biotechnology and recombinant technology eases/address this limitation for the production of some extremozymes in large quantities up to some extent using other biological models such as E. coli and yeast. However, such limitations still persist for extremophiles. Therefore, utmost need is to develop new strategies to overcome such problems by involving anaerobic microorganism for the production of extremophile proteins at industrial level. This purpose can be only short out by collaboration between industries and research groups. Recent development/enhancement in the field of bioengineering and biotechnology paves the way to better understand the metabolism of anaerobic extremophilic microorganism for the purpose of its better exploitation at industrial level. Adaptation strategies used by these extremophiles involving unique enzyme system resulted into special metabolic products. In this context some enzymes like hydrolases are the best example with the ability to remain active in hyper-/hypothermic conditions and enzymes active in high alkaline or acidic environment isolated from hydrothermal and volcano vents. Tolerance to such extreme conditions makes these enzymes valuable for the scale up of industrially important products. The gene pool of such anoxic extremophiles needs to be studied thoroughly using metagenomic and genetic engineering. Such enzyme system can be used to express in other microorganisms to facilitate large-scale production of economically important chemicals and proteins. The demand of biotech industry-oriented product in current scenario can only be achieved by the better understanding of anaerobic extremophilic microorganisms using different techniques like metagenomics, metabolomics, and recombinant technology.
References
Akin DE, Borneman WS, Windham WR (1988) Rumen fungi: morphological types from Georgia cattle and the attack on forage cell walls. BioSystem 21:385–339
Akin DE, Borneman WS, Lyon CE (1990) Degradation of leaf blades and stems by monocentric and polycentric isolates of ruminal fungi. Anim Feed Sci Technol 31:205–221
Alain K, Marteinsson VT, Miroshnichenko ML, Bonch-Osmolovskaya EA, Prieur JL (2002) Birrien, Marinitoga piezophila sp. nov., a rod-shaped, thermo-piezophilic bacterium isolated under high hydrostatic pressure from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 52:1331–1339
Andreesen JR, Bahl H, Gottschalk G (1989) Introduction to the physiology and biochemistry of the genus clostridium. Biotechnology handbooks. In: Nigel PM, Clarke DJ (eds) Clostridia. Plenum Press, New York/London
Antranikian G (2009) Extremophiles and biotechnology. In: Encyclopedia of Life Sciences (ELS). Wiley, Chichester. https://doi.org/10.1002/9780470015902.a0000391.pub2
Barnard D, Casanueva A, Tuffin M, Cowan D (2010) Extremophiles in biofuel synthesis. Environ Technol 31:8–9
Bauchop T (1981) The anaerobic fungi in rumen fibre digestion. Agri Environ 6:339–348
Benjelloun H, Rabe Ravelona M, Lebeault JM (2007) Characterization of growth and metabolism of commercial strains of propionic acid bacteria by pressure measurement. Eng Life Sci 7:143–148
Broeker J, Mechelke M, Baudrexl M, Mennerich D, Hornburg D et al (2018) The hemicellulose-degrading enzyme system of the thermophilic bacterium Clostridium stercorarium: comparative characterisation and addition of new hemicellulolytic glycoside hydrolases. Biotechnol Biofuels 11:229. https://doi.org/10.1186/s13068-018-1228-3
Brul S, Stumm CK (1994) Symbionts and organelles in ancrobic protozoa and fungi. Trends Ecol Evol 9:319–324
Bryant MP (1972) Commentary on the Hungate technique for culture of anaerobic bacteria. American J Clinical Nutr 25:1324–1328
Buchanan RE, Gibbons NE (1974) Bergey’s manual of determinative bacteriology. The Williams and Wilkins Co., Baltimore
Clarke A, Morris GJ, Fonseca F, Murray BJ, Acton E, Price HC (2013) A low temperature limit for life on earth. PLoS One 8:e66207
Coker JA (2016) Extremophiles and biotechnology: current uses and prospects [version 1; referees: 2 approved]. F1000Res 5:(F1000 Faculty Rev):396. https://doi.org/10.12688/f1000research.7432.1
Conrad R (1999) Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol Ecol 28:193–202
Cousin FJ, Jouan-Lanhouet S, Théret N (2016) The probiotic Propionibacterium freudenreichii as a new adjuvant for TRAIL-based therapy in colorectal cancer. Oncotarget 7:7161–7178
Dalmaso GZL, Ferreira D, Vermelho AB (2015) Marine extremophiles: a source of hydrolases for biotechnological applications. Mar Drug 13:1925–1965
Daly DFM, McSweeney PLH, Sheehan JJ (2010) Split defect and secondary fermentation in Swiss-type cheeses: a review. Dairy Sci Technol 90:3–26
Des Marais DJ (2000) Evolution: when did photosynthesis emerge on Earth? Science 289:1703–1705
Dolly M, Spitia S, Silva E, Schwarz WH (2000) Isolation of mesophilic solvent producing strains from Colombian sources: physiological characterization, solvent production and polysaccharide hydrolysis. J Biotechnol 79:117–126
Engle M, Mandelco L, Wiegel J (1994) Clostridium thermoalcaliphilum sp. nov., an anaerobic and thermotolerant facultative alkaliphile. Int J Syst Bacteriol 44:111–118
Euzeby J (2013) List of new names and new combinations previously effectively, but not 789 validly. Int J Syst Evol Microbiol 63:2365–2367
Finegold SM, Song Y, Liu C (2002) Taxonomy – general comments and update on taxonomy of clostridia and anaerobic cocci. AnaerobeAnaerobe 8:283–285
Foster JW (1949) Chemical activities of the fungi. Academic Press, New York
Haddad M, Vali H, Paquette J, Guiot SR (2014) The role of Carboxydothermus hydrogenoformans in the conversion of calcium phosphate from amorphous to crystalline state. PLoS One 9(2):e89480
Hayakawa K, Mizutani J, Wada K, Masai T, Yoshihara I, Mitsuoka T (1990) Effects of soybean oligosaccharides on human faecal flora. Microb Ecol Health Dis. http://www.microbecolhealthdis.net/index.php/mehd/article/view/7555/8897
Hillaire MC, Jouany JP (1989) Effects of rumen anaerobic fungi on the digestion of wheat straw and the end products of microbial metabolism studies in a semi continuous in vitro system. In: Nolan JV, Leng RA, Demeyer DI (eds) The roles of protozoa and fungi in ruminant digestion. Penambul Books, Armidale, pp 269–272
Hungate RE (1969) A roll tube method for the cultivation of strict anaerobes. Methods in Microbiol 3B:117–132
Joblin KN, Naylor GE (1989) Fermentation of woods by rumen anaerobic fungi. FEMS Microbiol Lett 65:119–122
Joblin K, Naylor G, Odongo N, Garcia M, Viljoen G (2010) Ruminal fungi for increasing forage intake and animal productivity. In: Odongo NE, Garcia M, Viljoen GJ (eds) Sustainable improvement of animal production and health. Food and Agriculture Organization of the United Nations, Rome, pp 129–136
Jolivet EL, Haridon S, Corre E, Forterre P, Prieur D (2003) Thermococcus gammatolerans sp. nov., a hyperthermophilic archaeon from a deep-sea hydrothermal vent that resists ionizing radiation. Int J Syst Evol Microbiol 53:847–851
Jun D, Huang V, Beatty JT (2017) Heterologous production of the photosynthetic reaction center and light harvesting 1 complexes of the thermophile Thermochromatium tepidum in the mesophile Rhodobacter sphaeroides and thermal stability of a hybrid Core complex. Appl Environ Microbiol 83(20):e01481–e01417
Keller MW, Lipscomb GL, Nguyen DM, Crowley AT, Schut GJ et al (2017) Ethanol production by the hyperthermophilic archaeon Pyrococcus furiosus by expression of bacterial bifunctional alcohol dehydrogenases. J Microbial Biotechnol 10(6):1535–1545
Kononen E, Valsanen ML, Finegold SM, Heine R, Jousimies-Somer H (1996) Cellular fatty acid analysis and enzyme profiles of Porphyromonas catoniae – a frequent colonizer of the oral cavity in children. Anaerobe 2(5):329–335
Kunkel A, Vorholt JA, Thauer RK, Hedderich R (1998) An Escherichia coli hydrogenase-3-type hydrogenase in methanogenic archaea. Eur J Biochem 252:467–476
Lee SS, Ha JK, Cheng K-J (2000a) Relative contributions of bacteria, protozoa, and fungi to in vitro degradation of orchard grass cell walls and their interactions. Appl Environ Microbiol 66:3807–3813
Lee SS, Ha JK, Cheng KJ (2000b) Influence of an anaerobic fungal culture administration on in vivo ruminal fermentation and nutrient digestion. Anim Feed Sci Technol 88:201–217
Leedle JAZ, Hespell RB (1980) Differential carbohydrate media and anaerobic replica plating techniques in delineating carbohydrate utilizing subgroups in rumen bacterial populations. Appl Environ Microbiol 39:709–719
Lencina AM, Ding Z, Schurig-Briccio LA, Gennis RB (2012) Characterization of the Type III sulfide:quinone oxidoreductase from Caldivirga maquilingensis and its membrane binding. Biochim Biophys Acta 1827(3):266–275
Liu P, Lu Y (2018) Concerted metabolic shifts give new insights into the syntrophic mechanism between propionate-fermenting Pelotomaculum thermopropionicum and hydrogenotrophic Methanocella conradii. Front Microbiol 9:1551. https://doi.org/10.3389/fmicb.2018.01551
Lowe SE, Theodorou MK, Trinci APJ, Hespell RB (1985) Growth of anaerobic rumen fungi on defined and semidefined media lacking rumen fluid. J Gen Microbiol 131:2225–2229
Martin WF, Sousa FL (2016) Early microbial evolution: the age of anaerobes. Cold Spring Harb Perspect Boil 8(2):a018127. https://doi.org/10.1101/cshperspect.a018127
McFarland LV (1995) Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am J Infect Control 23:295–305
Mellaert LV, Barbe S, Anne J (2008) Clostridium spores as anti-tumour agents. Trends Microbiol 14:190–196
Miller TL, Wolin MJ (1974) A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol 27:985–987
Minton NP, Clarke DJ (1989) In: Saha BC, Lamed R, Zeikus JG (eds) Clostridial enzymes. Plenum Press, New York
Mock J, Wang S, Huang H, Kahnt J, Thauer RK (2014) Evidence for a hexaheteromeric methylenetetrahydrofolate reductase in Moorella thermoacetica. J Bacterial 196(18):3303–3314
Muller M, Mentel M, van Hellemond JJ, Henze K, Woehle C et al (2012) Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol Mol Biol Rev 76:444–495
Oro J, Miller SL, Lazcano A (1990) The origin and early evolution of life on Earth. Ann Rev Earth Planet Sci 18(1):317–356
Orpin CG (1975) Studies on the rumen flagellate Neocallimastix frontalis. J Gen Microbiol 91:249–262
Prajakta BM, Suvarna PP, Singh RP, Rai AR (2019) Potential biocontrol and superlative plant growth promoting activity of indigenous Bacillus mojavensis PB-35(R11) of soybean (Glycine max) rhizosphere. SN Appl Sci 1:1143. https://doi.org/10.1007/s42452-019-1149-1
Prokofeva MI, Kublanov IV, Nercessian O, Tourova TP, Kolganova TV et al (2005) Cultivated anaerobic acidophilic/acidotolerant thermophiles from terrestrial and deep-sea hydrothermal habitats. Extremophiles 9:437–448
Schiraldi C, Marulli F, Martino IDLA, Rosa MD (1999) A microfiltration bioreactor to achieve high cell density in Sulfolobus solfataricus fermentation. Extremophiles 3:199–204
Schiraldi C, Giuliano M, De Rosa M (2002) Perspectives on biotechnological applications of archaea. Arch Microbiol 1(2):75–86
Segawa T, Miyamoto K, Ushida K, Agata K, Okada N et al (2003) Seasonal change in bacterial flora and biomass in mountain snow from the Tateyama mountains, Japan, analyzed by 16S rRNA gene sequencing and real-time PCR. Appl Environ Microbiol 71:123–130
Shao X, Zhou J, Olson DG, Lynd LR (2016) A markerless gene deletion and integration system for Thermoanaerobacter ethanolicus. Biotechnol Biofuels 9:100. https://doi.org/10.1186/s13068-016-0514-1
Singh RP, Singh RN, Manchanda G, Tiwari PK, Srivastava AK et al (2014) Biofuels: a way ahead. Ind J Appl Res 4(1):3–6
Singh RP, Manchanda G, Li ZF, Rai AR (2017) Insight of proteomics and genomics in environmental bioremediation. In: Bhakta JN (ed) Handbook of research on inventive bioremediation techniques. IGI Global, Hershey. https://doi.org/10.4018/978-1-5225-2325-3
Sparrow FKJR (1960) Aquatic phycomycetes, 2nd edn. University of Michigan Press, Ann Arbor
Subhashini DV, Singh RP, Manchanda G (2017) OMICS approaches: tools to unravel microbial systems. Directorate of Knowledge Management in Agriculture, Indian Council of Agricultural Research. ISBN:9788171641703. https://books.google.co.in/books?id=vSaLtAEACAAJ
Thauer RK (1998) Biochemistry of methanogenesis: a tribute to Marjory Stephenson. Microbiol-UK 144(9):2377–2406
Theodorou MK, Gill MK, King-Spooner C, Beever DE (1990) Enumeration of anaerobic chytridiomycetes as thallus forming units: a novel method for the quantification of fibrolytic fungal populations from the digestive tract ecosystem. Appl Environ MicrobioI 56:1073–1078
Theodorou MK, Brookman J, Trinci APJ (2005) Anaerobic fungi. In: Makkar HPS, McSweeney CS (eds) Methods in gut microbial ecology for ruminants. Springer, Dordrecht, pp 55–66
Thiel A, Michoud G, Moalic Y, Flament D, Jebbar M (2014) Genetic manipulations of the hyperthermophilic piezophilic archaeon Thermococcus barophilus. Appl Environ Microbiol 80(7):2299–2306
Thierry A, Maillard MB (2002) Production of cheese flavour compounds derived from amino acid catabolism by Propionibacterium freudenreichii. Lait 82:17–32
Thierry A, Maillard MB, Richoux R, Kerjean JR, Lortal S (2005) Propionibacterium freudenreichii strains quantitatively affect production of volatile compounds in Swiss cheese. Lait 85:57–74
Trinci APJ, Davies DR, Gull K, Lawrence MI, Nielsen BB et al (1994) Anaerobic fungi in herbivorous animals. Mycol Res 98:129–152
Tsai KP, Calza RE (1993) Optimization of protein and cellulase secretion in Neocallimastix frontalis EB188. Appl Microbiol Biotechnol 39:477–482
Wilson CA, Wood TM (1992) The anaerobic fungus Neocallimastix frontalis: isolation and properties of a cellulosome-type enzyme fraction with the capacity to solubilize hydrogen-bond-ordered cellulose. Appl Microbiol Biotechnol 37:125–129
Yarlett N, Orpin CG, Munn EA, Yarlett NC, Greenwood CA (1986) Hydrogenosomes in the rumen fungus Neocallimastix patriciarum. Biochem J 236:729–739
Youssef NH, Couger MB, Struchtemeyer CG, Liggenstoffer AS, Prade RA et al (2013) The genome of the anaerobic fungus Orpinomyces sp. strain C1A reveals the unique evolutionary history of a remarkable plant biomass degrader. Appl Environ Microbiol 79:4620–4634
Zeikus JG, Henning DL (1975) Methanobacterium arbophilicum sp. nov. an obligate anaerobe isolated from wet wood of living trees. Anto van Leeu J Microbiol Serol 41:543–552
Zhang J, Wang E, Singh R, Guo C, Shang Y et al (2019) Grape berry surface bacterial microbiome: impact from the varieties and clones in the same vineyard from central China. J Appl Microbiol 126:204–214. https://doi.org/10.1111/jam.14124
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Srivastava, A.K., Saroj, A., Nayak, A., Nishad, I., Gautam, K. (2020). Microbial Life in Stress of Oxygen Concentration: Physiochemical Properties and Applications. In: Singh, R., Manchanda, G., Maurya, I., Wei, Y. (eds) Microbial Versatility in Varied Environments. Springer, Singapore. https://doi.org/10.1007/978-981-15-3028-9_11
Download citation
DOI: https://doi.org/10.1007/978-981-15-3028-9_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3027-2
Online ISBN: 978-981-15-3028-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)