Abstract
Wastewater adversely affects humans and another animal including metal like Pb, As, Zn, Hg, and Cd in wastewater (domestic or industrial). These toxic metals affect human health and are a serious threat to the environment by the precipitation, adsorption, accumulation in the food chain and non-biodegradable nature, respectively. In the present study, treatment of industrial wastewater in terms of toxic Pb(II) removal was investigated by the using of copper oxide alginate (CuO/Alg) nanocomposite. The CuO/Alg nanocomposite was prepared by chemical reduction method in solution phase, and synthesized particles size were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), and Fourier transform infrared spectroscopy (FTIR). The wastewater sample collected from WWTP of the local electroplating industry is located in Okhla Industrial Area, New Delhi. A series of experimental approaches have been used to remove Pb2+ from industrial wastewater with CuO/Alg nanocomposite, which includes sorbent mass, competitive ion, contact time, and SEM. The SEM image of CuO/Alg nanocomposite showed that particles had a sheet-like shape and mean diameter of about 18.09 nm. The test was performed under the batch condition to determine the adsorption rate and uptake at equilibrium from single component solution. The maximum uptake value of Pb2+ in single component solution was 118.40 mg/g from wastewater. The CuO/Alg nanocomposite identified as the most promising sorbent with an effective potential of removal of Pb2+ from wastewater is due to their high metal uptake.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The contamination of water by heavy metal ions has become a serious environmental problem due to its toxicity and bioaccumulation tendencies. Across the world, surface and groundwater pollution by heavy metals is a major environmental issue [1, 2]. Heavy metal ions cause harmful effects on animals including humans and are toxic to living organisms through food chain transfer. Heavy metal ions can bind in a living organism to nucleic acids, proteins and small metabolites [3, 4]. These toxic heavy metal ions usually arise in mining industries, electroplating amenities, electric power industries, and other equipment manufacturing units and process waste streams from tanneries [1, 5, 6]. Many more environmental problems have been arising due to the release of toxic heavy metal ions in the natural environment and irrigation of agriculture area by the use of sewage water, these can be accumulated in the food chain due to their biodegradability and perseverance, thus may be a threat to human health [7,8,9]. In all heavy metals, Lead [Pb(II)] ion is a highly toxic substance, which can cause any adverse health effects including humans for exposure to organisms [10]. The level of exposure to Pb(II) ions in human can illation in lack of IQ level, learning inabilities, lack of attention shortfall, behavioral intricacies, undermined hearing and kidney failure [2, 11, 12]. Usually, children are more affected to lead ion toxicity than others as the lead ion concentration as per unit body weight (up to 40%) is much higher in children [13, 14].
Recently, the application of nanocomposite has become a fascinating area of research to remove pollutants from wastewater. The unique properties of nanocomposite are presenting distinct opportunities to remove toxic heavy metals in efficient and cost-effective approaches, and numerous nanocomposite has been used for this purpose [15,16,17]. Nanocomposite shows huge adsorption capacity mainly due to the more active sites for interaction with high surface area and metallic species [11, 12, 18, 19]. Metal oxide nanocomposites potentially provide more efficient and cost-effective wastewater treatment and processing techniques due to their size and removal efficiency [20, 21]. The present study aims to synthesis the CuO/Alg nanocomposite with the facile wet-chemical technique and to examine the removal efficiency of Pb(II) ions by the synthesized nanocomposite.
2 Materials and Methods
2.1 Chemicals Used
Copper nitrate trihydrate (Cu(NO3)2∙3H2O), sodium hydroxide (NaOH), and sodium alginate [(C6H8O6)n] pure were obtained from Sigma Aldrich Co Ltd. Double-distilled water was used for solution preparation. All the chemicals used in the study were analytical grade, and all the analysis was performed in triplicates.
2.2 Fabrication of CuO/Alg Nanocomposite
We have previously reported the synthesis of CuO/Alg nanocomposite [22] and briefly discussed here, as in this experimental part, pure copper oxide (CuO) has been synthesized by using copper nitrate trihydrate and sodium hydroxide as precursors. Two distinct solutions of copper nitrate trihydrate (1.0 M) and NaOH (8.0 M) were prepared in deionized water, respectively. Copper nitrate trihydrate (1.0 M) solution was stirred through dropwise (1 drop per 2 s) on a magnetic stirrer with sodium hydroxide (8.0 M) solution at 70 °C with 600 rpm till gel formation. The mixture was cooled at room temperature and separated by centrifuge at 800 rpm with deionized water for washing. The obtained powder was dried at 70–80 °C for 24 h and got a dry product of nanocomposite. For CuO/Alg nanocomposite, the addition of sodium alginate in copper nitrate trihydrate (1.0 M) solution was used.
2.3 Characterization Techniques
An advanced X-ray diffractometer (Rigaku) was used for structural analysis of the synthesized product by using monochromatic Cu Kα radiation in the 2θ angular range of 20°–80°. The morphology of the samples was studied by using the transmission electron microscope (TEM) (JEOL, Tokyo, Japan) at 200 kV and scanning electron microscope (SEM) (JSM 6510LV JEOL, Tokyo, Japan). Fourier transform infrared (FTIR) (Bruker, Tensor 37) was used for functional group analysis.
3 Results and Discussion
3.1 X-Ray Diffraction (XRD) Analysis
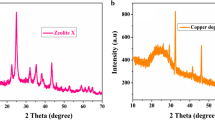
The highest peak in the result of XRD analysis corresponds to CuO/Alg (Fig. 1). The average particle size of synthesized sample can be determined through the results obtained from XRD analysis. In the peaks of the diffraction patterns, CuO/Alg showed a monoclinic structure, and Miller indices have been identified with JCPDF card no. 89-5895. The lattice framework parameters were determined from XRD data and the average crystallite sizes were calculated approximately 18.09 nm by the using of Debye–Scherrer formula. The latticework parameters are estimated from XRD data, a = 0.46 nm, b = 0.34 nm, and c = 0.50 nm.
where β is full-width half maxima of the peak, θ is angle, and λ is x-ray wavelength of XRD patterns.
3.2 Fourier Transform Infrared Spectroscopy (FTIR) Analysis
The FTIR spectra with the KBr pellet technique were examined in solid phase in the range of 400–4000 cm−1. CuO/Alg nanocomposite treated at 80 °C is shown in Fig. 2. The FTIR spectra exhibit three vibrations in the sample occurring at approximately 416, 498, and 599 cm−1, which can be associated with the vibrations of CuO, affirming the fabrication of pure CuO/Alg nanocomposite. The three absorption peaks at 599, 498, and 416 cm−1 are the characteristic peak of copper oxide. The absorption peaks of 599 and 498 cm−1 can be assigned to high-frequency Cu-O vibration stretching. The peak perceived at approximately at 3419 and 1020 cm−1 is observed due to the adsorption of water on the metal surface and corresponds to O–H stretching and deformation frequency, respectively. The peak at 1633 cm−1 is due to the C=O bond which may be the reason for the absorption of CO2 by the KBr because of KBr is highly CO2 adsorbent [7].
3.3 Transmission Electron Microscopy (TEM) Analysis
The surface morphology and size of the prepared CuO/Alg were examined by TEM as shown in Fig. 3. It can be clearly noticed that the synthesized nanocomposite contained significant nanosheets with size around 11 nm with some agglomeration. Finally, the particle sizes obtained from TEM are well measured by the XRD peak width.
3.4 Scanning Electron Microscope (SEM) Analysis
Based on SEM images, the nanocomposite is predominantly composed of CuO/Alg. In this study, the changes in surface morphology of the prepared CuO/Alg nanocomposite due to adsorption of Pb(II) ions was studied by the SEM images as shown in Fig. 4. The SEM image of nanocomposite reflects a homogeneous distribution of circular shaped nanoparticles with irregular distribution. The particles were distributed irregularly after adsorbing the lead ions.
3.5 Removal of Pb(II) from Wastewater
The removal of lead is performed by the batchwise at room temperature. For the effective removal of heavy metal ions such as Pb(II), the CuO/Alg nanocomposite was found fit in the presence of feasible ions because there is no requirement of adjusting pH and oxidation. Based on the result of SEM analysis, the particle size of the sample was found approximately 50–70 nm. The removal of Pb(II) with different CuO/Alg dosage (2–16 g/L) was studied. The optimum dose of the nanocomposite for solutions containing 16 g/L and lead removal efficiency was 114.2 mg/g with 120 min of contact time (Fig. 5). The maximum removal efficiency of lead was 118.4 mg/g at contact time 120 min, and maximum percentage removal was observed 92.2% with a CuO/Alg dosage of 8.0 g/L (Fig. 6).
The removal of Pb(II) ions is also shown in Fig. 4b which was increased gradually with increasing contact time and CuO/Alg dose. The short stability time was reported by other researchers for adsorption of heavy metals ions on nanocomposite [6, 8, 23]. Similar behaviors have been reported by many authors [10, 12, 19] for the uptake of heavy metal ions from aqueous solution by different metals’ adsorbent. At lower contact time and dose, the percentage removal of Pb(II) was low for CuO/Alg nanocomposite because protons in large quantities compete with positive metal ions for the adsorption sites. Therefore, it obstructs the surface of metal ions to reach the functional group. Consequently, the percentage of lead ion [Pb(II)] removal may decrease at low doses of nanocomposites [9, 11, 15]. This is in contrast to other traditional porous adsorptions, which have to be absorbed through other spreading stages [4]. This result is promising because contact time plays a major role in economic viability for waste treatment plants .
4 Conclusion
It was concluded from the present study that the CuO/Alg nanocomposite was synthesized by wet-chemical reduction method in solution phase. The XRD spectrum of CuO/Alg exhibits that the particle size was approximately 18.09 nm which well agreed with the XRD data. The study of surface morphology of the nanocomposite was done in the context of SEM, TEM, and FTIR spectroscopy techniques. The CuO/Alg nanocomposite was successfully experimented to remove Pb(II) from industrial wastewater. The removal process was conducted in the acidic and basic environment with a contact time of 10, 30, 60, 90, 120, 150, 180, 210, and 240 min. The obtained data indicated that the removal efficiency of Pb(II) ion was higher and nanocomposite was found to be dependent on experimental conditions. From the reported observation, CuO/Alg has no commercial value and is a good, inexpensive source of the readily available biomaterial. It can be concluded that this can be positively used as a cost-effective and environment-friendly nanocomposite for the removal of Pb(II) ions from wastewater.
References
Farghali AA, Bahgat M, Enaiet Allah A, Khedr MH (2013) Adsorption of Pb(II) ions from aqueous solutions using copper oxide nanostructures. Beni-Suef Univ J Basic Appl Sci 2:61–71. https://doi.org/10.1016/j.bjbas.2013.01.001
Heidari A, Younesi H, Mehraban Z (2009) Removal of Ni(II), Cd(II), and Pb(II) from a ternary aqueous solution by amino functionalized mesoporous and nano mesoporous silica. Chem Eng J 153:70–79. https://doi.org/10.1016/j.cej.2009.06.016
Liu X, Hu Q, Fang Z, Zhang X, Zhang B (2009) Magnetic chitosan nanocomposites: a useful recyclable tool for heavy metal ion removal. Langmuir 25:3–8. https://doi.org/10.1021/la802754t
Hussain M (2014) Synthesis, characterization and applications of metal oxide nanostructures. Linköping University Electronic Press, Department of Science and Technology, Linköping University
Yin P, Xu Q, Qu R, Zhao G, Sun Y (2010) Adsorption of transition metal ions from aqueous solutions onto a novel silica gel matrix inorganic–organic composite material. J Hazard Mater 173:710–716. https://doi.org/10.1016/j.jhazmat.2009.08.143
Jiang M, Wang Q, Jin X, Chen Z (2009) Removal of Pb(II) from aqueous solution using modified and unmodified kaolinite clay. J Hazard Mater 170:332–339. https://doi.org/10.1016/j.jhazmat.2009.04.092
Munagapati VS, Yarramuthi V, Nadavala SK, Alla SR, Abburi K (2010) Biosorption of Cu(II), Cd(II) and Pb(II) by Acacia leucocephala bark powder: kinetics, equilibrium and thermodynamics. Chem Eng J 157:357–365. https://doi.org/10.1016/j.cej.2009.11.015
Uheida A, Iglesias M, Fontàs C, Hidalgo M, Salvadó V, Zhang Y, Muhammed M (2006) Sorption of palladium(II), rhodium(III), and platinum(IV) on Fe3O4 nanoparticles. J Colloid Interface Sci 301:402–408. https://doi.org/10.1016/j.jcis.2006.05.015
Ruparelia JP, Duttagupta SP, Chatterjee AK, Mukherji S (2008) Potential of carbon nanomaterials for removal of heavy metals from water. Desalination 232:145–156. https://doi.org/10.1016/j.desal.2007.08.023
Mohapatra M, Anand S (2007) Studies on sorption of Cd(II) on Tata chromite mine overburden. J Hazard Mater 148:553–559. https://doi.org/10.1016/j.jhazmat.2007.03.008
Gupta VK, Agarwal S, Saleh TA (2011) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185:17–23. https://doi.org/10.1016/j.jhazmat.2010.08.053
Gebru KA, Das C (2017) Removal of Pb (II) and Cu (II) ions from wastewater using composite electrospun cellulose acetate/titanium oxide (TiO 2) adsorbent. J Water Process Eng 16:1–13. https://doi.org/10.1016/j.jwpe.2016.11.008
Hassan KH, Mahdi ER (2016) Synthesis and characterization of copper, iron oxide nanoparticles used to remove lead from aquous solution. 04: 730–738
Moezzi A, Soltanali S, Torabian A, Hassani A (2017) Removal of lead from aquatic solution using synthesized iron nanoparticles. Int J Nanosci Nanotechnol 13:83–90
Hu J, Chen G, Lo IMC (2006) Selective removal of heavy metals from industrial wastewater using maghemite nanoparticle: performance and mechanisms. J Environ Eng 132:709–715. https://doi.org/10.1061/(ASCE)0733-9372(2006)132:7(709)
Afkhami A, Conway BE (2002) Investigation of Removal of Cr(VI), Mo(VI), W(VI), V(IV), and V(V) Oxy-ions from industrial waste-waters by adsorption and electrosorption at high-area carbon cloth. J Colloid Interface Sci 251:248–255. https://doi.org/10.1006/jcis.2001.8157
Yang M, He J, Hu X, Yan C, Cheng Z (2011) CuO nanostructures as quartz crystal microbalance sensing layers for detection of trace hydrogen cyanide gas. Environ Sci Technol 45:6088–6094. https://doi.org/10.1021/es201121w
Türker AR (2007) New sorbents for solid-phase extraction for metal enrichment. Clean–Soil, Air, Water 35: 548–557. https://doi.org/10.1002/clen.200700130
Ghorpade A, Ahammed MM (2017) Water treatment sludge for removal of heavy metals from electroplating wastewater. Environ Eng Res 23:92–98. https://doi.org/10.4491/eer.2017.065
Engates KE, Shipley HJ (2011) Adsorption of Pb, Cd, Cu, Zn, and Ni to titanium dioxide nanoparticles: effect of particle size, solid concentration, and exhaustion. Environ Sci Pollut Res 18:386–395. https://doi.org/10.1007/s11356-010-0382-3
Al-Saad KA, Amr MA, Hadi DT, Arar RS, Al-Sulaiti MM, Abdulmalik TA, Alsahamary NM, Kwak JC (2012) Iron oxide nanoparticles: applicability for heavy metal removal from contaminated water. Arab J Nucl Sci Appl 45:335–346
Siddiqui VU, Khan I, Ansari A, Siddiqui WA, Akram K (2018) Advances in polymer sciences and technology. Springer Singapore, Singapore
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40. https://doi.org/10.1016/j.susmat.2016.06.002
Acknowledgements
The author, Afzal Ansari gratefully acknowledges for the financial assistance in terms of “Non-NET fellowship” by University Grant Commission (UGC), New Delhi. Further, the authors are also grateful to the Department of Applied Sciences, Faculty of Engineering and Technology, Jamia Millia Islamia, New Delhi, for providing the experimental facility.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Ansari, A., Siddiqui, V.U., Akram, M.K., Siddiqi, W.A., Sajid, S. (2020). Removal of Pb(II) from Industrial Wastewater Using of CuO/Alg Nanocomposite. In: Ahmed, S., Abbas, S., Zia, H. (eds) Smart Cities—Opportunities and Challenges. Lecture Notes in Civil Engineering, vol 58. Springer, Singapore. https://doi.org/10.1007/978-981-15-2545-2_16
Download citation
DOI: https://doi.org/10.1007/978-981-15-2545-2_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2544-5
Online ISBN: 978-981-15-2545-2
eBook Packages: EngineeringEngineering (R0)