Abstract
Approximately 70% of patients receiving dialysis in Japan at the end of 2016 were over 65 years of age. Based on the survey the author and colleagues conducted, approximately 70% of Japanese elderly hemodialysis patients were in a state of frailty or prefrailty, and physical functions (muscle strength, walking speed, standing balance and flexibility) of elderly patients attending the dialysis clinic for outpatient care decreased to approximately 60–70% of those of healthy persons, respectively, and physical activity level (steps) was under 50%. This subsequent follow-up study revealed that a clear decline in physical functions and physical activity level could be a significant and independent risk factor that worsens prognosis. The authors also developed the questionnaire on perceived mobility difficulty to accurately grasp limitations in activities of daily living (ADL) and revealed that the ADL difficulty evaluation is a simple alternative method to assess the functional status and to predict subsequent prognosis in elderly hemodialysis patients. Moreover, the author and colleagues have introduced a disease management system consisting of periodic assessment and exercise therapy in a period of roughly 10 years, and clarified that the high attendance group (attended >75% of all available sessions in the management program) had significantly better survival and lower incidence of cardiovascular disease than the low attendance group. These findings suggest that periodic physical function assessment and encouragement for participation in physical activity should be part of disease management for frail hemodialysis patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Frailty
- Physical function
- Physical activity
- Activities of daily living
- Disease management
- Exercise therapy
- Rehabilitation
- Hemodialysis
- Chronic kidney disease
6.1 Introduction
With increasing rates of population aging and lifestyle diseases, the rate of occurrence of chronic kidney disease (CKD) requiring renal replacement therapy is increasing worldwide [1]. According to a study conducted by the Japanese Society for Dialysis Therapy (JSDT), there were approximately 330,000 patients, with an average age of 67.9 years, receiving dialysis in Japan at the end of 2016, and 70% of these patients were over 65 years of age [2]. Japan’s proportion of 65 years-and-over dialysis patients is nearly double that of the US and Europe, and it has been pointed out that the rate of annual increase of this segment of the population has also been extremely high over the past 20 years [3]. In this way, population aging is a striking feature in terms of the epidemiological background of Japan’s hemodialysis patients, and along with aging, more patients are showing physical frailty.
Physical frailty is the state of heightened vulnerability to external stresses, due to the weakening of various physical residual functions caused by aging [4, 5]. Not only does it make the body unable to maintain physiological systems and life functions in the event of incidents like infections or short-term hospitalizations, but it is also known for causing vulnerability to adverse outcomes including mortality, institutionalization, and falls [6,7,8]. Many factors are related and unified into a cycle of frailty associated with declining energetics and reserve. There is a broad international consensus that markers of frailty include age-associated weight (lean body mass) loss; chronic undernutrition; loss of muscle mass (sarcopenia); and declines in endurance, walking ability (walking speed), and physical activity (Fig. 6.1) [5]. In brief, frailty is independent of the illness conditions and disabilities a patient may have and may determine medical treatment progress after the illness [9]. However, frailty is essentially a reversible condition and may be reversed toward normal by appropriate intervention [9, 10]. Therefore, the diagnosis of physical frailty is of major significance [10].
6.2 The Prevalence of Physical Frailty in Hemodialysis Patients
Based on the concept and evaluation standards of physical frailty defined by Fried et al. [5], the author and colleagues evaluated 252 hemodialysis patients (average age 67 years) capable of independent mobility who were visiting hospitals for outpatient care and found that 19.4% of men and 23.3% of women, or roughly one-fifth of the patients, were in a state of frailty (Fig. 6.2). Considering that the frequency of frailty among the elderly aged 65–69 years without kidney disease living in the region is <1.9% [11], the prevalence of frailty among hemodialysis patients is approximately 10 times that among average seniors. Unfortunately, the rate of physical frailty is not the most important point. When McAdams-DeMarco et al. [12] studied the mortality rate of three groups of frail, intermediately frail, and non-frail hemodialysis patients over a period of 3 years, they reported that, although there was a significantly higher rate of mortality among the frail and intermediately frail groups compared to that in the non-frail group, there was no difference in the mortality rates of the frail and intermediately frail groups. This showed that it is not only necessary to pay attention to frail patient groups but to also identify and develop appropriate exercise-based approaches for those in a pre-frail condition [13].
6.3 Positioning Exercise Therapy for Hemodialysis Patients: Exercise Therapy for Disease Management
There are still no guidelines that have earned an international (broad) consensus regarding exercise therapies and exercise instructions for CKD patients, but the Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines that were published in 2005 have become a foundation for exercise guidance carried out as a part of lifestyle guidance [14]. The K/DOQI first indicated diabetes, hypertension, and dyslipidemia as basic risk factors for cardiovascular disease, noted the importance of appropriately managing anemia and metabolic abnormalities as the characteristic risk factors of kidney failure, and provided information to rectify lifestyle-related risks such as smoking and physical inactivity. Additionally, while it lists guidelines for exercise instructions for dialysis patients and recommends physical activity, it also specifies the consideration of the effects of complications and implementation based on an evaluation of physical functions. With regard to these physical function evaluations in particular, it states that they should be regularly conducted every 6 months, and that they should not only merely assess the level of physical functions, but be implemented as a part of disease management. There were no major developments regarding exercise instruction guidelines in the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline published in 2012 [15], but the guidelines published in 2016 by the European Renal Association and European Dialysis and Transplant Association (ERA-EDTA) included considerations focused on patients aged 65 years and over with CKD severity at Stage 3b (estimated glomerular filtration rate < 45 mL/min/1.73 m3) [16]. These guidelines contain many reviews about evaluation items for physical functions and exercise therapy effects and many points that should be referenced when implementing therapeutic exercise for Japanese dialysis patients, based on the fact that the average age of Japanese dialysis patients is over 65 years.
6.4 Physical Functions and Physical Activity Levels of Hemodialysis Patients
The abovementioned ERA-EDTA (2016) guidelines indicate that regular physical function evaluations should be carried out as a part of disease management [16]. For these evaluations, the guidelines recommend using simple evaluation indicators, that is, “field tests” that can be used in actual clinical settings, rather than test tools that use expensive special equipment. They specifically list the sit-to-stand test as an indicator of leg muscle strength, walking speed, and 6-min walk test [17,18,19,20,21,22,23]. As will be mentioned later, walking speed is a strong indicator that predicts the deterioration of physical functions as well as prognoses.
6.4.1 Status of Physical Functions and Physical Activity Levels
The radar chart in Fig. 6.3 shows the relative values of leg muscle strength (knee extensor strength), walking speed, standing balance (one-leg standing time), flexibility (range of joint motion), and physical activity levels of hemodialysis patients attending the dialysis clinic for outpatient care to which the author and colleagues belong, with the value of 100 being the average value for age- and sex-matched healthy individuals. The average age of the 252 hemodialysis patients participating in this study was 67.2 years, with an average dialysis history of 9.7 years; as such, they are approximately in line with the 2016 study results shown by the JSDT [2]. Therefore, it is thought that these results showed the characteristics of the physical functions and physical activity levels of dialysis patients in Japan. Supposing that the difference was equivalent to the degree of decline when compared to healthy persons, leg muscle strength, walking speed, standing balance, and range of joint motion decreased to approximately 60–70% of those of healthy persons, respectively, and physical activity level was under 50%. Furthermore, physical functions and physical activity levels decreased with age, but longer periods (years) of hemodialysis contributed greatly to the degree of decline in both functions and levels (Fig. 6.4) [24]. The results showed that when the period of dialysis was >15 years, there was a striking negative effect on these indicators.
Physical functions and physical activity levels in ambulatory patients on hemodialysis. Physical function (muscle strength, walking speed, balance, flexibility) and physical activity level (steps) for ambulatory hemodialysis patients are expressed as a percentage of the mean value for the normal subjects matched by age and sex. The mean age of hemodialysis patients enrolled in the study, who were able to ambulate without any assistance, was 67.2 years
6.4.2 Prognoses with Relation to Physical Functions and Physical Activity Levels
Figures 6.5, 6.6, 6.7, 6.8, 6.9, and 6.10 show the relationship between leg muscle strength, walking speed, and physical activity levels (the number of steps) of hemodialysis patients attending the dialysis clinic for outpatient care to which the author and colleagues belong and their prognoses (mortality rate and cardiovascular events). The average age for each was approximately 67 years; data that reflects the current aging of hemodialysis patients in Japan.
Lower extremity muscle strength and subsequent mortality in hemodialysis patients. Kaplan–Meier analysis of survival for 202 hemodialysis patients. Participants with knee extensor strength above the median value of 40% at baseline had significantly better survival than those with a lower value (P < 0.003 by log-rank test)
Maximum walking speed and subsequent cardio-cerebrovascular events in hemodialysis patients. Kaplan–Meier analysis of incidence rates of cardio-cerebrovascular events according to maximum waking speed quartiles for 188 hemodialysis patients. Participants with maximum walking speed above the value of 89 m/min for men, 85 m/min for women at baseline had a significantly lower cumulative incidence of cardio-cerebrovascular events compared to other patients during the follow-up period (P <0.001 by Log-rank test)
Physical activity (steps) and subsequent mortality in hemodialysis patients. Kaplan–Meier analysis of survival for 282 hemodialysis patients. Patients with physical activity above the 4000 steps per a non-dialysis day at baseline had significantly better survival than those with lower values (P < 0.001 by log-rank test)
The frequency distribution in Fig. 6.5 shows the results of muscle strength values taken when patients extended their leg at maximum strength in the extension direction from a 90-degree stance in a seated position, measured with a handheld dynamometer. The measurement unit is body weight ratio (%), to adjust the difference in physical constitution among patients [25, 26]. It has been shown in previous reports that a body weight ratio under 40% would not satisfy the muscle strength level necessary to walk without difficulty [27]. The results showed that when comparing dialysis patients with leg strength under this body weight ratio of 40% with others (those with a leg strength body weight ratio of 40% and over), there was a distinctly higher ensuing mortality rate for the former group [28] (Fig. 6.6). Further analysis using a Cox proportional hazards regression model clarified that patients with severely decreased leg strength (<40%) had a 2.7-fold higher risk of death than those with high leg muscle strength [28]. That is, a clear decline in leg muscle strength not only interferes with the activities of daily living (ADL), but it is also an independent risk factor that worsens prognoses.
The distribution in Fig. 6.7 shows the results of measuring the walking speed (maximum walking speed) when patients were asked to walk as quickly as possible, without running, along a 10-m corridor [29]. When reviewing later cardiovascular events by the four quartiles of those walking speed results (Q1–Q4), as shown in Fig. 6.8, it was observed that the group of patients with the slower walking speeds (Q1 and Q2) had distinctly higher rates of cardiovascular events than the other patient groups (Q3 and Q4). That is, patients who had a maximum walking speed above the value of 89 m/min for men and 85 m/min for women at baseline had a significantly lower cumulative incidence of cardio-cerebrovascular events compared to other patients during the follow-up period. Further analysis using a Cox proportional hazards regression model showed that the hazard ratio for clinical events per 10 m/min increase in maximum walking speed was 0.77 [29]. Based on our subsequent analysis, if the lowest values for Q3 walking speed (men: 89 m/min, women: 85 m/min) are exchanged for a comfortable walking speed, they align with those used for diagnostic criteria for frailty (1.0 m/s) or sarcopenia (0.8 m/s) [19, 30,31,32], which shows that walking speed can be an important marker predicting frailty diagnoses and prognoses of hemodialysis patients.
The distribution in Fig. 6.9 shows the results of patients walking steps measured on the 4 days that patients were not receiving dialysis. Surprisingly, the greatest number of patients (mode) were in the group that walked an average of under 1000 steps per day, despite these being non-dialysis days that were not limited by the time constraints of dialysis treatment. Furthermore, when reviewing the relationship between this physical activity level (number of steps) and prognoses, there was a clear and significantly greater ensuing mortality rate for patients with an average of <4000 steps per day on non-dialysis days than those who had a greater average number of steps (Fig. 6.10) [33]. Viewing the relationship between prognoses and changes in physical activity levels over time, it was clear that there was a major difference in ensuing mortality rates when comparing those whose activity levels decreased by 30% and those whose activity levels increased by 30%, relative to each patient’s activity level (number of steps) from 1 year prior [34]. Furthermore, when reviewing the relationship between the increase or decrease in physical activity levels (number of steps) (positive or negative, with the boundary of 0) and mortality risk (hazard ratio), it was shown that mortality risks increased sharply when the number of steps declined (Fig. 6.11) [34]. In this way, because physical activity levels are strongly influenced by physical functions such as leg muscle strength and walking speed, these could be useful disease management indicators that keenly reflect physical conditions and subsequent outcomes [35, 36].
Change in physical activity (steps) and mortality hazard ratio in hemodialysis patients. The cubic spline survival analyses exhibit the association between change in physical activity and mortality, after adjusted by age, sex, time on hemodialysis, body mass index, primary kidney disease, comorbidity index, serum albumin, and baseline physical activity
6.5 Activities of Daily Living of Hemodialysis Patients
As with the aforementioned physical activity levels, an accurate grasp of the ADL is important for surmising a target person’s condition, as well as their physical and even mental aspects [19, 20, 37]. If a physical function impairment should occur, such as a bone fracture due to a fall, or a cerebral stroke, a person can quickly change from being independent in their ADL to being dependent in their ADL (Fig. 6.12) Thus, it is easy to grasp the reasons or causal relationships for a decline in ADL [38, 39]. However, for patients with CKD requiring dialysis treatment, it is often difficult to grasp the causal relationship merely based on their having a chronic illness. In particular, ADL limitations have already begun to occur before the initiation of dialysis in CKD patients [40]. For the above acute diseases, the decline in physical activity level or ADL is the result of the occurrence of a physical dysfunction, but for chronic illnesses, it is sometimes the case that a decline in physical activity level or ADL leads to further decline in physical functions. That is, for hemodialysis patients, the decline of physical functions, physical activity level, and ADL may all influence one another and create a vicious cycle that has adverse effects on quality of life (QOL) and prognoses (Fig. 6.13) [26, 41]. Therefore, it is necessary to regularly conduct ADL evaluations in addition to physical functions and physical activity levels previously mentioned in Sect. 6.4 [42].
6.5.1 ADL Dependency Evaluations
Figure 6.14 shows the relationship between mortality risk and ADL dependency scores using 5 basic ADLs and 8 instrumental ADLs, for a total of 13 ADLs (functional status score), with a study target of 7000 dialysis patients from Japan (n = 1700) and overseas [43]. In this large-scale study, scores were determined by assigning 1 point for every ADL that a patient can conduct independently (dependent score: 0), with scores of <8 out of 13 determined to be indicators of a severe level of ADL decline. As shown in Fig. 6.14, patients with a score of <8 have a higher mortality risk than those with higher scores, and this effect increases with age: for patients over 65 years of age, those with scores <8 have nearly three times the mortality risk of those with a perfect score of 13. Therefore, maintaining ADL independence for senior dialysis patients is an extremely important therapeutic strategy for keeping their prognoses from worsening [20].
Mortality risk (per year) by age and ADL (functional status score). The functional status (FS) score has a total of 13 ADL items consisting of 5 basic ADLs (eating, getting dressed, bathing, using the toilet, transferring from bed to chair) and 8 instrumental ADLs (using the telephone, getting places beyond walking distance, grocery shopping, preparing meals, doing housework or handyman work, doing laundry, taking medications, managing money)
6.5.2 ADL Difficulty Evaluations
Generally, when evaluating ADLs in clinical setting, each assessment is rated on a two-point scale (independent and dependent) (Fig. 6.12). In the previous section (see Sect. 6.4.1), hemodialysis patients were evaluated in two groups, independent and dependent. However, most of clinically stable patients who receive outpatient dialysis services can perform basic ADL and instrument ADL tasks without assistance [44, 45]. On the other hand, even though these outpatient patients are able to travel and move independently, few are actually able to accomplish these tasks “comfortably” and many have a sense of hardship. Thus, it is often impossible to grasp ADL issues using the above-mentioned two-valued standard (Fig. 6.12). We developed the questionnaire on perceived mobility difficulty using a 5-point scale based on difficulty (1, not possible; 2, severe difficulty; 3, moderate difficulty; 4, mild difficulty; and 5, ease) for hemodialysis patients [45] (Fig. 6.15). This questionnaire comprises 12 items divided into the following three categories, which were obtained by a factor analysis: “basic ADL,” “ambulation,” and “walking up or down stairs. With a target group of 216 hemodialysis patients (average age of 67 years), Fig. 6.16a shows the frequency distribution results on the scale of dependency using the Functional Independence Measure, and Fig. 6.16b shows the result distribution using the difficulty scale we developed, for the same group of patients. As these results show, the results of the dependency evaluation indicated that nearly all subjects had independent mobility, and thus there was a concentration of perfect scores. Meanwhile, when the evaluation scale is based on difficulty, there was a wide variety of scores. Furthermore, Fig. 6.17 shows the distribution (percentage) of scores 1–5 for each of the 12 items [45]. Since the study subjects were patients capable of visiting the clinic, roughly <10% were a score of impossible (dependent). On the other hand, if we consider those who had difficulty (3 or below) even while being capable (independent) as having limited ADL, nearly 50% of patients were ADL-limited with regard to “walking 600 m,” which would influence their activities in the periphery of their home, such as shopping. When sorted by level of difficulty based on the percentage of the ADL limitation, the highest item was “climbing stairs (to the third floor)” at 70%, followed by “walking one kilometer” at 60% [45].
The questionnaire on perceived mobility difficulty. This questionnaire on perceived mobility difficulty for hemodialysis patients comprises 12 items divided into the following three categories, which were obtained by a factor analysis: “basic ADL,” “ambulation,” and “walking up or down stairs.” ADL activities of daily living
Additionally, as a follow-up survey, we reviewed the prognoses according to the tertiles of the ADL difficulty scores that we had developed above and found that the group with the greatest level of difficulty had a mortality risk nearly four times that of the group with the least difficulty. In this way, ADL difficulty evaluation is a simple alternative method to assess the functional status and to predict subsequent prognoses in hemodialysis patients [19].
6.6 The Practice of Exercise Therapy as Disease Management
6.6.1 Protocol of Exercise Therapy (Flow Chart)
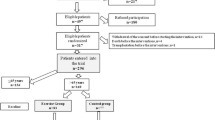
Figure 6.18 shows a therapeutic exercise flowchart developed by the authors and colleagues. The criteria (patient conditions) for implementation of this protocol include: those who have not been hospitalized in the past 3 months, those who are undergoing stable dialysis treatment, and those recognized as clinically stable by their attending physician. In practice, this exercise flowchart begins with classifying patients who should do exercise therapy based on periodic assessment data for their physical functions, physical activity levels, and ADLs. In this first classification (as discussed in Sects. 6.4 and 6.5), patients are sorted by those whose indicators are not declining: leg muscle (knee extensor) strength and walking speed for physical function, number of steps for physical activity level, and difficulty in ADLs. The cutoff points for this categorization are as follows: 40% of body weight for leg strength [28]; maximum walking speeds of 90 m/min (1.5 m/s) [29] or comfortable walking speeds of 60 m/min (1.0 m/s) [32], or 48 m/min [31] for walking speeds; 4000 steps on non-dialysis day [33]; and no increase (no adverse change) in the ADL difficulty level. Other physical performance tests and cutoff points are listed in Table 6.1 [30, 36, 46,47,48,49].
As a result of the above categorization, those not determined to have a decline in any of the above items (indicators) are instructed to continue their current self-management without adding any specific exercise therapy. However, those who have a decline in any of the items are asked to undergo an exploration of the causes after a detailed examination of the changes in their symptoms, complications, and treatment content, in addition to periodic diagnostic assessments by their doctor. When causes are determined, medical treatment is justifiably given priority, but in many other cases, there are no clear reasons, with many seeing a gradual decline after a short-term hospitalization (up to 1 week) or activity limitations due to shunt issues or infections. In these cases, patients are asked to gradually progress in exercise therapies while monitoring in detail any changes in symptoms.
The most important points, as mentioned in a statement in the K/DOQI Clinical Practice Guidelines (2005) [14], are to periodically conduct physical function, activity level, and ADL assessments every 6 months, as well as showing the results of these periodic assessments to each patient individually, having the patient understand their own situation, and allowing patients to manage their own progression [50]. This process then leads to improved adherence to the exercise therapy program.
6.6.2 The Therapeutic Exercise Program in Practice
As shown in the therapeutic exercise flowchart of Fig. 6.18, exercise training program is prescribed for those patients who are determined as having a functional decline if it is necessary and safe for them to engage in exercises. Moreover, exercise training prescriptions should be individualized to the patient’s physical function with an emphasis placed on regular engagement and evaluation of progress. There are primarily two options: unsupervised exercise (instructing the patient to do the exercises at home on non-dialysis days) or supervised exercise (conducting the exercises under the supervision of medical staff such as a physical therapist). The authors take full advantage of the physical conditions of having patients come for hemodialysis outpatient treatment three times per week for implementation of therapeutic exercise. In principle, individual therapeutic exercise sessions are performed under the supervision of a physical therapist by using short periods of 10 or 15 min before the start of the patients’ hemodialysis treatments at dialysis day. Earlier, many studies reported that the adherence to exercise, that is, the rate of continuation of exercise implementation, is poor under unsupervised condition than under supervision [51, 52]. It has been also suggested that the amount of exercise plays a significant role in attaining health-related benefits, namely, using the time before treatment on dialysis day means that they would have a minimum frequency of three times per week for conducting therapeutic exercises. This makes it possible to attain the target level of physical function relatively early, as well as the levels of everyday physical activity and ADLs. Figure 6.19 shows a scene in which an outpatient is performing individualized exercise programs before the start of hemodialysis treatment on a dialysis day.
Exercise recommendations for patients with end-stage renal disease are suggested based on the FITT principle: frequency, intensity, time, and type [50, 53, 54]. To summarize, patients should exercise 2–3 times per week at the beginning of the training; the frequency can then be increased to 3–5 times per week. Intensity should be tailored according to patient tolerance to exercise and should be gradually increased. The duration of exercise should be progressively increased depending on the physiological and physical condition. The types of exercises should include aerobic, resistance, flexibility, and balance exercises.
However, frail elderly hemodialysis patients encounter patient-related and structural barriers in exercising, including time restriction associated with continuous dialysis treatment, limited physical capacity, low adherence to conventional exercise programs, and lack of social interaction and support, and challenges in the physical environment [50, 55, 56]. In recent years, therapeutic exercise has been developed within hemodialysis treatment times (after treatment begins), and its safety and results have been verified [51, 57]. This can be a useful option for patients who do not have time before the start of hemodialysis treatment, or in cases where vital signs such as blood pressure are not stable (Fig. 6.20). Moreover, recent studies reported that intradialytic electrical muscle stimulation can improve muscle strength, exercise capacity, and quality of life (QOL) in hemodialysis patients [58,59,60]. Electrical muscle stimulation is a new method for exercise therapy that can be used by patients while lying down; this method has no time restriction, requires no volitional effort, and places no hemodynamic stress on the patient (Fig. 6.21).
6.6.3 Long-Term Effect of Introducing a Disease Management System
The K/DOQI Clinical Practice Guidelines (2005) [14] include a statement that periodic physical function assessment and encouragement for participation in physical activity should be part of disease management for dialysis patients, but there have been very few reports verifying the outcomes of introducing these systems in practice. The author and colleagues introduced the flowchart shown in Fig. 6.18 over a period of roughly 10 years and verified the prognoses in hemodialysis patients who have been in this system for 3 years retrospectively [61]. Those results showed that high attendance (>75% attended) group had significantly lower mortality rates and rates of cardiovascular events compared to low attendance (≤75% attended) group (Fig. 6.22). In this way, it was shown that a system of periodically conducting physical function evaluations as a part of disease management protocols and intervening individually based on that data brings about effective outcomes in terms of long-term prognoses.
The prognoses after introducing a disease management system (see Fig. 6.19) for hemodialysis patients. Kaplan–Meier analysis of survival (a) and cardiovascular events (b) in 266 patients undergoing hemodialysis. The high attendance group (attended >75% of all available sessions in the management program) had significantly better survival and lower incidence of cardiovascular disease than the low attendance group (≤75% attendance)
6.7 Future Topics
Over the course of the past 10 years, many systematic reviews have been published that focus on CKD patients who have started dialysis treatment [57, 62,63,64,65]. However, they all have a relatively young average age of <60 years, and in which there have been very few trials actually evaluating the impact of exercise on frailty regarding elder frail patients with end-stage renal disease treated with hemodialysis [66, 67]. Furthermore, there have been few reports using prospective, randomized, and controlled trials or exercise therapy. The EXerCise Introduction to Enhance performance in dialysis patient trial (EXCITE) demonstrated the effectiveness of a 6-month personalized, home-based walking exercise program to improve walking capacity and muscle strength compared to “usual care” [64, 68]. However, this multicenter randomized, controlled trial in the dialysis population revealed that during the 6 months of training, 31% of participants withdrew from the exercise group compared with 15% from the placebo, and 47% of those who completed the study were designated as having low adherence to the protocol (<60% of sessions). Therefore, further study is needed to develop an effective strategy for promoting adherence rates to exercise therapy in frail elderly hemodialysis population, namely, there are many issues that remain unclear for senior dialysis patients.
In recent years, the age of patients starting hemodialysis treatment is gradually increasing. The content of this chapter (exercise therapy as disease management) can be summarized as frailty prevention, but developing frailty is not only attributable to physical functions but is also related to nutritional conditions, mental functions (cognitive functions), psychosocial aspects, and environmental factors. It is anticipated that these proportions of patients will increase as the population aging progresses. In summary, dialysis patients are in certain need of comprehensive care “renal rehabilitation.”
6.8 Conclusion
Based on the survey the author and colleagues conducted previously, approximately 70% of Japanese elderly patients with hemodialysis are in a state of frailty or prefrailty, and physical functions and physical activity level of Japanese elderly patients attending the dialysis clinic for outpatient care decrease to approximately 50–70% of those of healthy persons. Moreover, a clear decline in physical functions and physical activity level is a significant and independent risk factor that worsens prognosis. The authors and colleagues developed the questionnaire on perceived mobility difficulty to accurately grasp limitations in ADL and revealed that the ADL difficulty evaluation is a simple alternative method to assess the functional status and to predict subsequent prognosis in elderly hemodialysis patients. The authors also have introduced a disease management system consisting of periodic assessment and exercise therapy and clarified that the high attendance group in the management program had significantly better survival and lower incidence of cardiovascular disease than the low attendance group. Therefore, periodic physical function assessment and encouragement for participation in physical activity should be part of disease management for frail hemodialysis patients.
References
Hamer RA, El Nahas AM. The burden of chronic kidney disease. BMJ. 2006;332(7541):563–4. https://doi.org/10.1136/bmj.332.7541.563.
Masakane I, Taniguchi M, Nakai S, Tsuchida K, Wada A, Ogata S, et al. Annual dialysis data report 2016, JSDT renal data registry. Renal Replace Ther. 2018;4(1):45. https://doi.org/10.1186/s41100-018-0183-6.
Kimata N, Tsuchiya K, Akiba T, Nitta K. Differences in the characteristics of dialysis patients in Japan compared with those in other countries. Blood Purif. 2015;40(4):275–9. https://doi.org/10.1159/000441573.
Bortz WM 2nd. A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57(5):M283–8.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56.
Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63.
Hamerman D. Toward an understanding of frailty. Ann Intern Med. 1999;130(11):945–50. https://doi.org/10.7326/0003-4819-130-11-199906010-00022.
Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. https://doi.org/10.1111/j.1532-5415.2006.00745.x.
Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–7. https://doi.org/10.1681/asn.2007020221.
Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, de Boer IH, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–21. https://doi.org/10.1053/j.ajkd.2012.05.017.
Kojima G, Iliffe S, Taniguchi Y, Shimada H, Rakugi H, Walters K. Prevalence of frailty in Japan: a systematic review and meta-analysis. J Epidemiol. 2017;27(8):347–53. https://doi.org/10.1016/j.je.2016.09.008.
McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. https://doi.org/10.1111/jgs.12266.
Johansen KL, Dalrymple LS, Delgado C, Chertow GM, Segal MR, Chiang J, et al. Factors associated with frailty and its trajectory among patients on hemodialysis. Clin J Am Soc Nephrol. 2017;12(7):1100–8. https://doi.org/10.2215/cjn.12131116.
National Kidney Foundation. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153.
KDIGO CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:1–150.
Farrington K, Covic A, Aucella F, Clyne N, de Vos L, Findlay A, et al. Clinical practice guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR <45 mL/min/1.73 m2). Nephrol Dial Transplant. 2016;31(suppl 2):ii1–ii66. https://doi.org/10.1093/ndt/gfw356.
Hsieh RL, Huang HY, Chen SC, Lin WH, Wu CW, Chang CH, et al. Changes in physical functional performance and quality of life in hemodialysis patients in Taiwan: a preliminary study. J Nephrol. 2010;23(1):41–8.
Kutner NG, Zhang R, Huang Y, Painter P. Gait speed and mortality, hospitalization, and functional status change among hemodialysis patients: a US renal data system special study. Am J Kidney Dis. 2015;66(2):297–304. https://doi.org/10.1053/j.ajkd.2015.01.024.
Painter P, Marcus RL. Assessing physical function and physical activity in patients with CKD. Clin J Am Soc Nephrol. 2013;8(5):861–72. https://doi.org/10.2215/cjn.06590712.
Painter P, Roshanravan B. The association of physical activity and physical function with clinical outcomes in adults with chronic kidney disease. Curr Opin Nephrol Hypertens. 2013;22(6):615–23. https://doi.org/10.1097/MNH.0b013e328365b43a.
Saito GK, Jassal SV. The ‘Sit-to-Scale’ score--a pilot study to develop an easily applied score to follow functional status in elderly dialysis patients. Nephrol Dial Transplant. 2007;22(11):3318–21. https://doi.org/10.1093/ndt/gfm454.
Segura-Orti E, Martinez-Olmos FJ. Test-retest reliability and minimal detectable change scores for sit-to-stand-to-sit tests, the six-minute walk test, the one-leg heel-rise test, and handgrip strength in people undergoing hemodialysis. Phys Ther. 2011;91(8):1244–52. https://doi.org/10.2522/ptj.20100141.
Torino C, Manfredini F, Bolignano D, Aucella F, Baggetta R, Barilla A, et al. Physical performance and clinical outcomes in dialysis patients: a secondary analysis of the EXCITE trial. Kidney Blood Press Res. 2014;39(2–3):205–11. https://doi.org/10.1159/000355798.
Saitoh M, Matsunaga A, Yokoyama M, Fukuda M, Yoshida A, Masuda T. Effects of long-term hemodialysis therapy on physical function in patietns with chronic renal failure. J Jpn Soc Dial Ther. 2007;40(2):147–53.
Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78(1):26–32.
Kutsuna T, Matsunaga A, Matsumoto T, Ishii A, Yamamoto K, Hotta K, et al. Physical activity is necessary to prevent deterioration of the walking ability of patients undergoing maintenance hemodialysis. Ther Apher Dial. 2010;14(2):193–200. https://doi.org/10.1111/j.1744-9987.2009.00750.x.
Yamamoto S, Matsunaga A, Kamiya K, Miida K, Ebina Y, Hotta K, et al. Walking speed in patients with first acute myocardial infarction who participated in a supervised cardiac rehabilitation program after coronary intervention. Int Heart J. 2012;53(6):347–52.
Matsuzawa R, Matsunaga A, Wang G, Yamamoto S, Kutsuna T, Ishii A, et al. Relationship between lower extremity muscle strength and all-cause mortality in Japanese patients undergoing dialysis. Phys Ther. 2014;94(7):947–56. https://doi.org/10.2522/ptj.20130270.
Abe Y, Matsunaga A, Matsuzawa R, Yoneki K, Harada M, Watanabe T, et al. Evaluating the association between walking speed and reduced cardio-cerebrovascular events in hemodialysis patients: a 7-year cohort study. Renal Replace Ther. 2016;2(1):54. https://doi.org/10.1186/s41100-016-0063-x.
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. https://doi.org/10.1016/j.jamda.2013.11.025.
Ostir GV, Kuo YF, Berges IM, Markides KS, Ottenbacher KJ. Measures of lower body function and risk of mortality over 7 years of follow-up. Am J Epidemiol. 2007;166(5):599–605. https://doi.org/10.1093/aje/kwm121.
Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51(3):314–22.
Matsuzawa R, Roshanravan B, Shimoda T, Mamorita N, Yoneki K, Harada M, et al. Physical activity dose for hemodialysis patients: where to begin? Results from a prospective cohort study. J Ren Nutr. 2018;28(1):45–53. https://doi.org/10.1053/j.jrn.2017.07.004.
Shimoda T, Matsuzawa R, Yoneki K, Harada M, Watanabe T, Matsumoto M, et al. Changes in physical activity and risk of all-cause mortality in patients on maintence hemodialysis: a retrospective cohort study. BMC Nephrol. 2017;18(1):154. https://doi.org/10.1186/s12882-017-0569-7.
Matsuzawa R, Matsunaga A, Wang G, Kutsuna T, Ishii A, Abe Y, et al. Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(12):2010–6. https://doi.org/10.2215/cjn.03660412.
Matsuzawa R, Roshanravan B. Management of physical frailty in patients requiring hemodialysis therapy. Contrib Nephrol. 2018;196:101–9. https://doi.org/10.1159/000485707.
Lo D, Chiu E, Jassal SV. A prospective pilot study to measure changes in functional status associated with hospitalization in elderly dialysis-dependent patients. Am J Kidney Dis. 2008;52(5):956–61. https://doi.org/10.1053/j.ajkd.2008.04.010.
Koyama T, Sako Y, Konta M, Domen K. Poststroke discharge destination: functional independence and sociodemographic factors in urban Japan. J Stroke Cerebrovasc Dis. 2011;20(3):202–7. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.11.020.
Ghosal MK, Burman P, Singh V, Das S, Paul N, Ray BK, et al. Correlates of functional outcome among stroke survivors in a developing country--a prospective community-based study from India. J Stroke Cerebrovasc Dis. 2014;23(10):2614–21. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.06.001.
Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–47. https://doi.org/10.1056/NEJMoa0904655.
Arai Y, Kanda E, Kikuchi H, Yamamura C, Hirasawa S, Aki S, et al. Decreased mobility after starting dialysis is an independent risk factor for short-term mortality after initiation of dialysis. Nephrology (Carlton). 2014;19(4):227–33. https://doi.org/10.1111/nep.12202.
Shimoda T, Matsuzawa R, Yoneki K, Harada M, Watanabe T, Yoshida A, et al. Combined contribution of reduced functional mobility, muscle weakness, and low serum albumin in prediction of all-cause mortality in hemodialysis patients: a retrospective cohort study. J Ren Nutr. 2018;28(5):302–8. https://doi.org/10.1053/j.jrn.2017.12.012.
Jassal SV, Karaboyas A, Comment LA, Bieber BA, Morgenstern H, Sen A, et al. Functional dependence and mortality in the international dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2016;67(2):283–92. https://doi.org/10.1053/j.ajkd.2015.09.024.
Kutsuna T, Matsunaga A, Takagi Y, Motohashi S, Yamamoto K, Matsumoto T, et al. Development of a novel questionnaire evaluating disability in activities of daily living in the upper extremities of patients undergoing maintenance hemodialysis. Ther Apher Dial. 2011;15(2):185–94. https://doi.org/10.1111/j.1744-9987.2010.00905.x.
Watanabe T, Kutsuna T, Yoneki K, Harada M, Shimoda T, Matsunaga Y, et al. Determinants of difficulty in activities of daily living in ambulatory patients undergoing hemodialysis. Renal Replace Ther. 2018;4(1):8. https://doi.org/10.1186/s41100-018-0146-y.
Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, et al. Added value of physical performance measures in predicting adverse health-related events: results from the health, aging and body composition study. J Am Geriatr Soc. 2009;57(2):251–9. https://doi.org/10.1111/j.1532-5415.2008.02126.x.
Roshanravan B, Robinson-Cohen C, Patel KV, Ayers E, Littman AJ, de Boer IH, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24(5):822–30. https://doi.org/10.1681/asn.2012070702.
Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandinelli S, et al. Use of the short physical performance battery score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64(2):223–9. https://doi.org/10.1093/gerona/gln022.
Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, et al. Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66(1):89–96. https://doi.org/10.1093/gerona/glq167.
Roshanravan B, Gamboa J, Wilund K. Exercise and CKD: skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. Am J Kidney Dis. 2017;69(6):837–52. https://doi.org/10.1053/j.ajkd.2017.01.051.
Konstantinidou E, Koukouvou G, Kouidi E, Deligiannis A, Tourkantonis A. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med. 2002;34(1):40–5.
Howden EJ, Coombes JS, Strand H, Douglas B, Campbell KL, Isbel NM. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis. 2015;65(4):583–91. https://doi.org/10.1053/j.ajkd.2014.09.017.
Koufaki P, Greenwood S, Painter P, Mercer T. The BASES expert statement on exercise therapy for people with chronic kidney disease. J Sports Sci. 2015;33(18):1902–7. https://doi.org/10.1080/02640414.2015.1017733.
Smart NA, Williams AD, Levinger I, Selig S, Howden E, Coombes JS, et al. Exercise & Sports Science Australia (ESSA) position statement on exercise and chronic kidney disease. J Sci Med Sport. 2013;16(5):406–11. https://doi.org/10.1016/j.jsams.2013.01.005.
Johansen KL. Resistance exercise in the hemodialysis population - who should do the heavy lifting? Am J Nephrol. 2016;44(1):29–31. https://doi.org/10.1159/000446866.
Painter P. Physical functioning in end-stage renal disease patients: update 2005. Hemodial Int. 2005;9(3):218–35. https://doi.org/10.1111/j.1492-7535.2005.01136.x.
Sheng K, Zhang P, Chen L, Cheng J, Wu C, Chen J. Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am J Nephrol. 2014;40(5):478–90. https://doi.org/10.1159/000368722.
Bruggemann AK, Mello CL, Dal Pont T, Hizume Kunzler D, Martins DF, Bobinski F, et al. Effects of neuromuscular electrical stimulation during hemodialysis on peripheral muscle strength and exercise capacity: a randomized clinical trial. Arch Phys Med Rehabil. 2017;98(5):822–831.e821. https://doi.org/10.1016/j.apmr.2016.12.009.
Miura M, Hirayama A, Oowada S, Nishida A, Saito C, Yamagata K, et al. Effects of electrical stimulation on muscle power and biochemical markers during hemodialysis in elderly patients: a pilot randomized clinical trial. Renal Rep Ther. 2018;4(1):33. https://doi.org/10.1186/s41100-018-0174-7.
Schardong J, Dipp T, Bozzeto CB, da Silva MG, Baldissera GL, Ribeiro RC, et al. Effects of intradialytic neuromuscular electrical stimulation on strength and muscle architecture in patients with chronic kidney failure: randomized clinical trial. Artif Organs. 2017;41(11):1049–58. https://doi.org/10.1111/aor.12886.
Yamamoto S, Matsuzawa R, Abe Y, Hoshi K, Yoneki K, Harada M, et al. Utility of regular management of physical activity and physical function in hemodialysis patients. Kidney Blood Press Res. 2018;43(5):1505–15. https://doi.org/10.1159/000494016.
Cheema BS, Singh MA. Exercise training in patients receiving maintenance hemodialysis: a systematic review of clinical trials. Am J Nephrol. 2005;25(4):352–64. https://doi.org/10.1159/000087184.
Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64(3):383–93. https://doi.org/10.1053/j.ajkd.2014.03.020.
Manfredini F, Mallamaci F, D’Arrigo G, Baggetta R, Bolignano D, Torino C, et al. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol. 2017;28(4):1259–68. https://doi.org/10.1681/asn.2016030378.
Smart N, Steele M. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology (Carlton). 2011;16(7):626–32. https://doi.org/10.1111/j.1440-1797.2011.01471.x.
Matsufuji S, Shoji T, Yano Y, Tsujimoto Y, Kishimoto H, Tabata T, et al. Effect of chair stand exercise on activity of daily living: a randomized controlled trial in hemodialysis patients. J Ren Nutr. 2015;25(1):17–24. https://doi.org/10.1053/j.jrn.2014.06.010.
Matsuzawa R, Hoshi K, Yoneki K, Harada M, Watanabe T, Shimoda T, et al. Exercise training in elderly people undergoing hemodialysis: a systematic review and meta-analysis. Kidney Int Rep. 2017;2(6):1096–110. https://doi.org/10.1016/j.ekir.2017.06.008.
Baggetta R, D’Arrigo G, Torino C, ElHafeez SA, Manfredini F, Mallamaci F, et al. Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: a secondary analysis of the EXCITE trial. BMC Geriatr. 2018;18(1):248. https://doi.org/10.1186/s12877-018-0938-5.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Matsunaga, A. (2020). Exercise Interventions in Dialysis Patients. In: Kato, A., Kanda, E., Kanno, Y. (eds) Recent Advances of Sarcopenia and Frailty in CKD. Springer, Singapore. https://doi.org/10.1007/978-981-15-2365-6_6
Download citation
DOI: https://doi.org/10.1007/978-981-15-2365-6_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2364-9
Online ISBN: 978-981-15-2365-6
eBook Packages: MedicineMedicine (R0)