Abstract
Today there is an enormous interest in developing safe, cost-effective and environmentally friendly technologies for nanoparticle synthesis. In the present study, extracellular synthesis of silver nanoparticles was carried by Trametes ljubarskyi KU382503.1, white rot fungus isolated from the decayed wood log. The reduction of the silver nanoparticles was monitored by UV–visible spectrophotometry, and the characterization of the AgNPs was carried out by FT-IR and transmission electron microscopy (TEM). The synthesized silver nanoparticles are very stable. Furthermore, the anticandidal activity of the AgNPs was assessed using agar well diffusion method. The biosynthesized AgNPs showed considerable activity against the Candida albicans. The present research opens a new path for the biological synthesis of AgNPs, and the process is easy and safe to scale up for biomedical and industrial applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Candida species belongs to the normal microbiota of an individual’s gastrointestinal tract, mucosal oral cavity, and vagina (Shao et al. 2007) , and this is responsible for many clinical manifestations from mucocutaneous overgrowth to bloodstream infections (Eggimann et al. 2003). These yeasts are forming symbiotic relationship in healthy humans and may cause systemic infection in immunocompromised state due to their great adaptability to different host niches (Sardi et al. 2013). Presently, a sudden increase in the number of yeasts that are impervious to antifungal drugs is recognized worldwide; therefore, the use of in vitro laboratory tests may fetch the doctor in selecting an appropriate therapy. Nowadays most of the commercially available effective antifungal agents are designed based upon polyenes (amphotericin B), triazoles (fluconazole, itraconazole, voriconazole, posaconazole) or echinocandins (caspofungin, micafungin and anidulafungin). In any case, administration of these antifungals is often assisted by various complications such as amphotericin B toxicity and adverse effects of some azoles including toxicity and drug interactions (Levin et al. 2007; Taxvig et al. 2007; Worth et al. 2008; Venkatakrishnan et al. 2000) and yeast resistance to antifungal therapy. Due to this, possibility for effective antifungal therapy must be found to avoid the mentioned adverse effects (White et al. 2002; Perea et al. 2001; Panáček et al. 2009).
Now, nanotechnology is emerging as expeditiously growing area with its applications in the field of science and technology for the purpose of manufacturing new material at the nanoscale level (Albrecht et al. 2006). Nanoparticle has multifunctional properties and received special attention due to their potential use in biomedical applications such as infection, prevention, and wound healing. Silver nanoparticles are known to show strong antimicrobial activity against bacteria (Morones et al. 2005; Panáček et al. 2006). Although the effects of silver nanoparticles against fungal pathogens have sustained only marginal attention from the researchers, AgNPs exhibited significant antifungal activity against clinical isolate strains of Candida spp. causing undesirably life-threatening fungal infections (Panáček et al. 2009). According to Kim et al. (2008), the AgNPs showed potent fungistatic activity against clinical isolates and ATCC strains of Trichophyton mentagrophytes and Candida spp. Consequently, AgNPs are proving to be an alternative for the development of new antifungal drugs; nevertheless, much effort is needed to understand the effects against other fungal pathogens (Qian et al. 2013) . The main drawback with the chemical and physical methods of silver nanoparticle formation is that they are extremely costly and also involve the use of toxic, hazardous chemicals and they contain potential environmental and biological stakes (Ingham et al. 2012). Fungi may be considered as good source for the synthesis of silver nanoparticles and ideal in the synthesis of metal nanoparticles, due to their capacity to accumulate large amount of enzymes. Comparatively with the other microbes, fungal mycelia mesh can withstand flow pressure and agitation and other conditions in the bioreactors compared to plant materials and bacteria (Gudikandula et al. 2015). White rot fungi are commonly used in bioremediation; it was reported that these fungi can also serve as a platform for the bioreduction of Ag nanoparticles (AgNPs) (Vigneshwaran et al. 2006).
In this study, the extracellular synthesis and characterization of AgNPs generated by the reduction of white rot fungi were reported. The biologically synthesized Trametes ljubarskyi were analysed and tested against Candida albicans . The results reported here cover the biological synthesis of AgNPs and their anticandidal activity.
All the chemicals were obtained from Hi-Media Pvt. Limited, Mumbai, India. Distilled water was used for the experiment. Candida albicans (ATCC 10231) was procured from the microbial-type culture collection centre Microbiologics, 200 Copper Avenue North, St. Cloud, MN 56303. This culture was grown on potato dextrose agar (PDA) medium at 25 °C for 3 days and maintained at 4 °C in a refrigerator. Trametes ljubarskyi KU382503.1 used in this study was isolated from Eturnagaram Forest, Warangal District, Telangana, India (18°20′20″N, 80°25′45″E). It was grown and maintained at 32 °C on malt extract agar medium (MEA: malt extract, 15 g; NH4Cl, 1 g; KH2PO4, 1 g; citric acid, 15N per 1000 mL of distilled water).
2 Synthesis of Silver Nanoparticles from White Rot Fungi
The strain Trametes ljubarskyi KU382503.1 (Fig. 23.1a, b) was cultured in a 250 mL Erlenmeyer flask containing 100 mL MEA broth at 30 °C and 150 revolutions per minute (rpm) for 5 days. After incubation, biomass was separated by double filtration and then properly washed with sterile distilled water to remove traces of medium components. The obtained culture filtrate was challenged with 1 mM silver nitrate in equal volume and incubated at 30 °C under shaking conditions at 150 rpm. The control (without addition of AgNO3) was also kept simultaneously under the same conditions.
3 Characterization of Silver Nanoparticles
3.1 UV–Vis Absorption
Silver nanoparticle synthesis was confirmed by sampling the reaction mixture at regular intervals, and the absorption maxima was scanned by UV–Vis spectra at the wavelength of 350–470 nm (ELICO SL-159 Spectrophotometer).
3.2 TEM
Transmission electron microscopy (TEM) was obtained using JEOL (JEM-1010) instrument with an accelerating voltage of 80 kV. AgNPs were loaded on carbon-coated copper grids, and solvent was allowed to evaporate under the infrared light for 30 min. The particle size and surface morphology of nanoparticles were evaluated using ImageJ 1.45s software.
3.3 FT-IR
Fourier transform infrared spectroscopy (FT-IR) of silver nanoparticles was carried out by using Digital Excalibur 3000 series, Japan, in diffuse reflectance mode (DRS-800) in the range of 400–4000 cm−1 at a resolution of 4 cm−1. FT-IR reveals the biomolecules responsible for the bioreduction of silver ions and stabilization of silver nanoparticles in the solution.
3.4 Anticandidal Activity
Anticandidal activity was performed using agar well diffusion method. Approximately 20 mL of potato dextrose agar medium was poured in the sterilized petri plates and allowed to solidify. The test fungus Candida albicans was suspended in a saline solution (0.85%) NaCl and adjusted to a turbidity of 0.5 McFarland standards (108 CFU/mL). One millilitre of the test culture was speeded over the PDA plates using sterilized spreader. Agar wells of 8 mm diameter were prepared with the help of sterilized stainless steel borer. The wells were loaded with different concentrations of AgNP solution (40, 60 and 80 μL). The plates were kept for incubation at 32 °C for 48 h; later the diameter of zone of inhibition was measured in millimetres. Silver nitrate solution and culture filtrate extract were used as negative controls.
4 Biogenic Silver Nanoparticles and Anticandidal Activity
4.1 Biosynthesis of Silver Nanoparticles
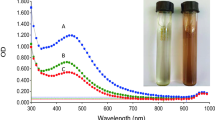
The nanoparticle synthesis reaction was started after the culture filtrate extract was introduced right into 1 mM liquid (AgNO3) solution. After 12 h of incubation in shaking condition, the reaction blend was turned into dark brown colour solution (Fig. 23.2). The colour modification was taking place due to the fact that the energetic metabolites present in the culture filtrate essence reduce the silver metal ions right into silver nanoparticles. The intensity of the colour change was increased in direct proportion to the incubation period of nanoparticle synthesis. It could be due to the excitation of surface area plasmon vibration (SPR) and also reduction of AgNO3 (Mulvaney 1996).
4.2 Characterization of Silver Nanoparticles
UV–Vis absorption spectra is the most prominent method to observe the forming and stabilization of silver nanoparticles in the aqueous solution, as the spectral response of silver nanoparticles is mainly based on the diameter. The plasmon peak resonance moves to longer wavelengths and broadens as the diameter increases (Ninganagouda et al. 2014). So the aqueous bioreduction of silver nanoparticles could be easily observed by UV–Vis, which is sensitive to several factors such as particles shape, particle–particle interaction with the medium and size (Khan et al. 2013). The synthesized sample reaction mixture exhibits the strong peak at 420 nm (Fig. 23.3) at the end of 12 h and remained closed at 418 nm. This reveals that the reaction was very rapid, and the particles are well distributed in the solution. Trametes ljubarskyi has never been reported to biosynthesize AgNPs.
4.2.1 FT-IR
FT-IR spectrum of synthesized SNPs was carried out to understand the possible biomolecules responsible for the capping and also stabilization of nanoparticles. For this, the sample was analysed in the scan array from 3500 to 500 cm−1 of close to IR spectra by FT-IR. The peaks at 3396 cm−1 and also 1638 cm−1 were assigned for O–H bond of phenols and also N–H bond of key amines, specifically (Fig. 23.4). This recommends that the hydroxyl groups of phenols as well as amide groups of proteins forming a layer of the nanoparticles act as capping agents to prevent agglomeration and provide stability to the reaction medium. Similar type of results was found in Syzygium alternifolium stem bark extract-mediated synthesis of silver nanoparticles (Yugandhar et al. 2015).
4.2.2 TEM
A transmission electron microscopy (TEM) picture of the AgNPs is shown in Fig. 23.5. The shapes of AgNPs were either round or near to spherical. A total amount of 120 particles were counted to determine their dimensions. The results showed that the particles size varied from 1 to 25 nm. The particle dimension histogram of AgNPs is shown in Fig. 23.6.
The results of UV–visible absorption spectroscopy as well as TEM recommended that the AgNPs were polydispersed spherical or near to spherical shapes varying from 1 to 25 nm and also were exceptionally similar to the reports of Qian et al. (2013) as well as Verma et al. (2010). We used the extracellular filtrate to synthesize AgNPs, and the procedure was devoid of any type of toxic chemicals or solvents. This would not only result in “natural” AgNPs but also simplify the downstream handling. Provided these benefits, the Trametes ljubarskyi KU382503.1 represents a promising prospect for large-scale production of AgNPs.
4.3 Anticandidal Activity
The AgNPs of Trametes ljubarskyi KU382503.1 at 80 μL/well showed good anticandidal activity ranging from 10 to 20 mm. From the results, it was clearly known that the anticandidal activity was directly proportional to the concentration of AgNPs. A negative control, i.e. fungal culture filtrates, did not show any activity against tested strain. Fluconazole used as standard against fungi showed the inhibition zones of 30 mm (Fig. 23.7). This is the first report on biosynthesis of silver nanoparticles from Trametes ljubarskyi and their anticandidal activity. Nanoscale silver has been shown to be among the potential antifungal agents, and also specifically biologically synthesized silver nanoparticles could be a lot more reliable in the medicinal applications as a result of their varied surface coatings. Hwang et al. (2012) established the mechanism of action of AgNPs on C. albicans. It was documented that the cells revealed to AgNPs showed increased reactive oxygen species and also hydroxyl radical production leading to the apoptotic features such as phosphatidylserine externalization, DNA and nuclear fragmentation, activation of metacaspases and mitochondrial disorder.
San Chan and Don (2013), in their study, synthesized silver nanoparticles from white rot fungi Schizophyllum commune and reported that AgNPs have great promise as antimicrobial agent against S. aureus, S. epidermidis, E. coli and C. albicans. Very few are reported on the silver nanoparticle synthesis by white rot fungi, but many reports are available on plant- and chemical-mediated synthesis of silver nanoparticles and their activity against Candida sp. In Kim et al.’s (2008) [14] study, nano-Ag was synthesized chemically, and its antifungal effects on clinical isolates and ATCC strains of Trichophyton mentagrophytes and Candida species were investigated, and they revealed that silver nanoparticles may have considerable antifungal activity.
5 Conclusion
In the present study, the focus was on biological synthesis of silver nanoparticles using culture filtrate of Trametes ljubarskyi. The physical property of synthesized nanoparticle was characterized using relevant techniques. The synthesized nanoparticles are active against Candida albicans . This is the first report on the anticandidal activity of AgNPs synthesized using white rot fungi. The synthesized nanoparticles have the potential to be exploited in the preparation of antifungal drugs, and there is a wide scope for detailed investigation in the future for the application of AgNPs in the field of medicine for controlling the pathogen. Based on these results, it is concluded that Trametes ljubarskyi is an efficient and effective source for the synthesis of silver nanoparticles and good source against Candida albicans.
References
Albrecht MA, Evans CW, Raston CL (2006) Green chemistry and the health implications of nanoparticles. Green Chem 8:417–432
Eggimann P, Garbino J, Pittet D (2003) Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis 23:685–702
Gudikandula K, Metuku RP, Pabba SK, Laxmi SV, Nidadavolu HB, Burra S, Alha SC (2015) Fungus-mediated synthesis of silver nanoparticles and their activity against gram positive and gram negative bacteria in combination with antibiotics. Walailak J Sci Technol 12:613–622
Hwang IS, Lee J, Hwang JH, Kim KJ, Lee DG (2012) Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J 279:1327–1338
Ingham CJ, Boonstra S, Levels S, De Lange M, Meis JF, Schneeberger PM (2012) Rapid susceptibility testing and microcolony analysis of Candida spp. cultured and imaged on porous aluminum oxide. PLoS One 7:e33818
Khan M, Khan M, Adil SF, Tahir MN, Tremel W, Alkhathlan HZ, Al-Warthan A, Siddiqui MR (2013) Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int J Nanomedicine 2:1507–1506
Kim KJ, Sung WS, Moon SK, Choi JS, Kim JG, Lee DG (2008) Antifungal effect of silver nanoparticles on dermatophytes. J Microbiol Biotechnol 18:1482–1484
Levin MD, den Hollander JG, Van Der Holt B, Rijnders BJ, Van Vliet M, Sonneveld P, Van Schaik RH (2007) Hepatotoxicity of oral and intravenous voriconazole in relation to cytochrome P450 polymorphisms. J Antimicrob Chemother 60(5):1104–1107
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346
Mulvaney P (1996) Surface plasmon spectroscopy of nanosized metal particles. Langmuir 12:788–800
Ninganagouda S, Rathod V, Singh D (2014) Characterization and biosynthesis of Silver nanoparticles using a fungus Aspergillus niger. Int Lett Nat Sci 10:49–57
Panáček A, Kolář M, Večeřová R, Prucek R, Soukupová J, Kryštof V, Hamal P, Zbořil R, Kvítek L (2009) Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 30:6333–6340
Panáček A, Kvítek L, Prucek R, Kolar M, Vecerova R, Pizurova N, Sharma VK, Nevečná TJ, Zboril R (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 110:16248–16253
Perea S, López-Ribot JL, Kirkpatrick WR, McAtee RK, Santillán RA, Martı́nez M, Calabrese D, Sanglard D, Patterson TF (2001) Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45:2676–2684
Qian Y, Yu H, He D, Yang H, Wang W, Wan X, Wang L (2013) Biosynthesis of silver nanoparticles by the endophytic fungus Epicoccum nigrum and their activity against pathogenic fungi. Bioprocess Biosyst Eng 36:1613–1619
San Chan Y, Don MM (2013) Optimization of process variables for the synthesis of silver nanoparticles by Pycnoporus sanguineus using statistical experimental design. J Korean Soc Appl Biol Chem 56:11–20
Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Giannini MM (2013) Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 62:10–24
Shao LC, Sheng CQ, Zhang WN (2007) Recent advances in the study of antifungal lead compounds with new chemical scaffolds. Yao Xue Xue Bao 42:1129–1136. (Article in Chinese)
Taxvig C, Hass U, Axelstad M, Dalgaard M, Boberg J, Andeasen HR, Vinggaard AM (2007) Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol Sci 100:464–473
Venkatakrishnan K, von Moltke LL, Greenblatt DJ (2000) Effects of the antifungal agents on oxidative drug metabolism. Clin Pharmacokinet 38:111–180
Verma VC, Kharwar RN, Gange AC (2010) Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 5:33–40
Vigneshwaran N, Kathe AA, Varadarajan PV, Nachane RP, Balasubramanya RH (2006) Biomimetics of silver nanoparticles by white rot fungus, Phanerochaete chrysosporium. Colloids Surf B Biointerfaces 53:55–59
White TC, Holleman S, Dy F, Mirels LF, Stevens DA (2002) Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother 46:1704–1713
Worth LJ, Blyth CC, Booth DL, Kong DC, Marriott D, Cassumbhoy M, Ray J, Slavin MA, Wilkes JR (2008) Optimizing antifungal drug dosing and monitoring to avoid toxicity and improve outcomes in patients with haematological disorders. Intern Med J 38:521–537
Yugandhar P, Haribabu R, Savithramma N (2015) Synthesis, characterization and antimicrobial properties of green-synthesised silver nanoparticles from stem bark extract of Syzygium alternifolium (Wt.) Walp. 3 Biotech 5:1031–1039
Acknowledgement
The authors are thankful to Ministry of Environment and Forest, New Delhi, for providing financial assistance.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gudikandula, K., Jaffar, S., Charya, M.A.S. (2020). Biogenic Silver Nanoparticles from Trametes ljubarskyi (White Rot Fungus): Efficient and Effective Anticandidal Activity. In: Khasim, S.M., Long, C., Thammasiri, K., Lutken, H. (eds) Medicinal Plants: Biodiversity, Sustainable Utilization and Conservation. Springer, Singapore. https://doi.org/10.1007/978-981-15-1636-8_23
Download citation
DOI: https://doi.org/10.1007/978-981-15-1636-8_23
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1635-1
Online ISBN: 978-981-15-1636-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)