Abstract
Humoral immunity mediated by B cells plays an important role in combating different types of viral diseases. Two types of B cells (B-1 and B-2) originate in the primary lymphoid organ called bone marrow from hematopoietic stem cells (HSCs). Both B-1and B-2 cells develop separately through sequential steps (pro-B cells—pre-B cells—immature B cells) in bone marrow from distinct common lymphoid progenitors before their release as immature B cells into circulation. In the serous cavities, the immature B-1 cells differentiate into mature B-1 cells through transitional B cells. The immature B-2 cells differentiate into transitional B cells, which mature finally into marginal zone (MZ) and follicular (FO) B cells in the secondary lymphoid organs. The B-1 cells produce poly-specific natural antibodies (antibodies produced before infection), which provide first line of defense. The B-1 cells mainly defend mucosal and blood-borne pathogens in a T-cell independent manner. The MZ B cells produce immune response against blood-borne pathogens and undergo both T-independent and T-dependent activation. In addition, both B1 and MZ B cells behave like innate immune cells by expressing toll-like receptors (TLR) and produce immune response without or with their membrane-bound poly-reactive B-cell receptors (BCR). Finally, FO B cells are the conventional B cells of adaptive immunity and primarily responsible for T-dependent immune response by their membrane-bound mono-reactive BCR. The antigen-activated B-2 cells differentiate into antibodies secreting plasma cells and memory B cells. Along with natural antibodies, the non-neutralizing, neutralizing, and broadly neutralizing specific antibodies are important in combating various viral diseases. Additionally, B cells modulate T-cell functions by presenting antigens, providing co-stimulation, and secreting cytokines. This chapter describes different types of B cells, antibodies, and their role in combating viral diseases (HIV, influenza and hepatitis).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Serum therapy by Von Behring and Kitasato provided the first evidence that humoral response played an important role in the host resistance against infectious agents such as diphtheria and tetanus toxins. They explained that serum therapy gives disease resistance due to the presence of antibodies in the serum. Paul Ehrlich demonstrated the basis for serum therapy and established the field of humoral immunity. Today, the importance of antibodies as immunotherapeutic is well characterized and is used to treat a wide range of infectious diseases. Antibodies are main components in many effective vaccines and have been used to prevent many human diseases caused by viruses (Yamada 2011; Burton 2002).
The success of immune system in combating different types of viruses depends on both non-specific innate immune system and specific-adaptive immune system. Humoral immunity is a branch of adaptive immunity mediated by antibodies secreted from B cells, which plays an important role in combating viruses along with cell-mediated immunity (Bonilla and Oettgen 2010). Humoral immune response to antigens is categorized as primary and secondary response. In the primary response, T helper cells are of primary importance whereas in the secondary immune response, the specific-neutralizing antibodies play a primary role against reinfection and T cells are of secondary importance. The primary immune response can take 1–2 weeks and IgM is the predominant type of antibody elicited. The secondary immune response is more rapid than primary immune response and predominant type of antibody formed is IgG (Lefevre et al. 2009).
The T helper cells, typically follicular T helper cells (TFH cells), were activated by the same antigen and cooperate with antigen-activated mature B cells to differentiate into plasma cells and memory B cells. The activated B cells remain in the margins of T-cell zone and differentiate into short-lived plasma cells. The activated B cells which initiate germinal centers differentiate into long-lived plasma cells and memory B cells. The high-affinity and antigen-specific antibodies are secreted and maintained by both short-lived and long-lived plasma cells. The long-lived plasma cells migrate to survival niches in the bone marrow and persist for several years (Gourley et al. 2004). The protective natural antibodies are produced before the foreign antigen exposure or pathogens by B-1 cells and marginal zone B cells in a T-cell independent manner. About 80% of all circulating natural antibodies are IgM type and remaining are IgG and IgA (Palma et al. 2018). B cells provide several lines of protection against viruses. In the first line, natural antibodies provide defense against initial viral infections. The long-lived plasma cells generate high amounts of neutralizing IgG and provide second line of defense, and reactive long-lived memory B cells provide third line of defense against viruses (Dörner and Radbruch 2007).
Natural antibodies are encoded by germ line variable genes and not shaped by post-recombination processes such as somatic hypermutation and class switching. Natural antibodies recruit antigens into the spleen to prevent infection of vital organs and also to induce early neutralizing antibodies without T-cell help. Natural antibodies activate complement to enhance B- and T-cell-specific immune responses. Therefore, natural antibodies are considered an important link between innate and adaptive immunity (Matter and Ochsenbein 2008). The natural antibodies are poly-reactive that they can bind to different structurally unrelated antigens and also auto-reactive that they can bind to self-antigens. Along with natural antibodies, poly-reactive antigen-binding B cells provide protection against multiple pathogens. In addition to poly-reactive antibodies, the specific neutralizing and cross-reactive antibodies also provide effective antiviral immune response (Warter et al. 2012).
The highly specific antibodies protect from viral infection by both neutralizing and Fc-mediated effector mechanisms. Neutralizing antibodies inhibit the viruses by binding through their antigen-binding sites or Fab region and also through their constant or Fc region. The non-neutralizing antibodies bind to many epitopes including neutralizing antibody targets and induce the destruction of microbe or infected host cells by innate immune system (Hua and Ackerman 2017). Recently, novel broadly neutralizing antibodies were characterized, which are able to neutralize diverse isolates of viruses (Sok and Burton 2018). This chapter describes about different types of B cells, antibodies, and their role in combating different types of viruses (HIV, influenza, and hepatitis).
2 B Cells
The B lymphocytes are subset of lymphocytes, which express diverse specific surface immunoglobulins and constitute 5–15% of total circulating lymphoid cells. The antigen-independent development of B cells starts from the fetal liver and bone marrow of an adult, where the hematopoietic stem cells differentiate into pro-B cells, which in turn differentiate pre-B cells to immature B cells. Along with the development of phenotypically distinct precursor cells, rearrangements of VDJ genes occur parallelly, which contribute to formation of diverse specific immature B cells with membrane-bound IgM. Immature B cells differentiate into mature B cells with membrane-bound IgM and IgD as its BCR in the secondary lymphoid organs. Two types of B lymphocytes (B-1 and B-2) have been identified. Mature B lymphocytes are activated by different types of antigens. There are three types of antigens activating the B lymphocytes. (1) T-independent antigen type 1, for example, LPS (lipopolysaccharide), which is polyclonal activator of both mature and immature B cells. LPS activates the B cells through TLR. (2) T-independent antigen type 2, for example, bacterial polysaccharides, which have repeated epitopes, require help from cytokines secreted by T helper cells but not their direct contact. (3) T-dependent antigens, for example, soluble protein antigens, require T helper cells’ direct contact and cytokines secreted by them to activate B lymphocytes (Maddaly et al. 2010).
The origin and development of B-1 and B-2 cells are described by two competing models. The selection model proposes that the response to a particular antigen decides the differentiation of B cell to become either B-1 or B-2. The other layered immune system hypothesis of Herzenberg proposes that they emerge from separate distinct progenitors. The B-1 cells present in serous cavities (peritoneal and pleural cavities) further differentiates into B-1a and B-1b. The B-2 cells in secondary lymphoid organs also differentiate into two types of subsets such as follicular (FO) and marginal zone (MZ) B cells after passing through T1, T2, and T3 transitional stages (Montecino-Rodriguez and Dorshkind 2012).
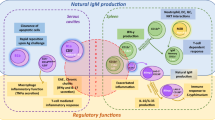
The B-1a cells produce poly-specific IgM natural antibodies in the serum of newborn or germ-free animals. The B-1b cells secrete antibodies after stimulation with thymus-independent antigens (Matter and Ochsenbein 2008). In addition to IgM, the B-1 cells also produce poly-reactive IgA antibodies for mucosal immunity (Suzuki et al. 2010). The MZ B cells express poly-reactive BCR and produce poly-specific IgM antibodies against blood-borne microorganisms. Both B-1 and MZ B cells express TLR and produce response to pathogen-associated or endogenous TLR ligands with or without recognition through BCR. Unlike B-1 cells, the MZ B cells generate response to T-dependent proteins and produce high-affinity isotype-switched antibodies. Although the FO B cells respond to T-independent antigens, they are primarily responsible for high-affinity IgG antibodies production against T-dependent antigens. The T-dependent antigens activate the FO B cells in the presence of T helper cells and their secreted cytokines. Antigen-activated FO B cells involves in the formation of germinal center (GC) reaction. The clonal expansion, isotype-switching, somatic hypermutation, and affinity maturation are characteristics of GC reaction. The activated FO B cells undergo proliferation and differentiation into antibody secreting long-lived plasma cells and memory B cells (Fig 6.1) (Hoffman et al. 2016). The external antigens are captured through BCR of B cells and they are degraded inside the B cells and the corresponding antigen fragments presented by B cells on MHC-II molecule to helper T cells are an important step in adaptive cellular immunity (Yuseff et al. 2013).
B cell surface not only consists of membrane-bound Ig, but also other complement component receptors and Fc receptors are expressed. In addition to that with the advancement of monoclonal antibody technology, many B-cell-specific surface molecules were identified. To bring common nomenclature, monoclonal antibodies were designated as clusters of differentiation (CD). The CD designation is embraced as a label for the target molecule rather than grouping of monoclonal antibodies with common reactivity. The target molecules or CD are involved in B-cell development, function and communication with extracellular environment. These CD molecules also provide cellular context which interprets the BCR signals; for example, in BCR signaling and B-cell development, CD79a (Igα) and CD79b (Igβ) are non-covalently attached with BCR and their cytoplasmic domains certainly contains conserved motifs for tyrosine phosphorylation and Src family kinase docking respectively (LeBien and Tedder 2008). In addition to production of antibodies, B cells also actively participate in cellular immune response mediated by T cells. The B cells directly modulate effector, memory, and regulatory T-cell functions via antigen-specific but antibody-independent mechanisms. B cells modulate T cells by antigen presentation, by providing co-stimulation, and by secreting cytokines. B cells are functionally subdivided into two types: (1) Be-1 cells and (2) Be-2 cells. In the presence of TH1-type cytokines, the Be-1 cells do not secrete significant amounts of IL-4, IL-13, or IL-2 but secrete IFN-γ and IL-12 including IL-10, TNF-α, and IL-6. In the presence of TH2-type cytokines, Be-2 cells do not secrete significant amounts of IFN-γ and IL-12 but secrete IL-4, IL-13, or IL-2 including IL-10, TNF-α, and IL-6. The regulatory B cells also called B-10 cells produce IL-10 cytokines that suppress the CD4+ T-cell responses. In addition to suppression, cytokines producing B cells enhance the T-cell-mediated immune responses (Lund and Randall 2010).
3 Antibodies
The B lymphocytes differentiate finally into plasma cells, which are the source for antibodies. The two competing natural selection and clonal selection theories for antibody formation were proposed by Jerne and Burnet, respectively, but finally, clonal selection theory was supported by experimental evidence (Burnet 1976).
Antibodies are chemically glycoprotein molecules. They are also called immunoglobulins and are present on the surface of B cells as BCR or free molecules in blood, plasma, and extracellular fluids. The fluids formerly were called humors and are part of humoral immune response. They have two principal functions in humoral defense. The first function is that it recognizes and binds to antigenic determinants or epitopes on the surface of foreign molecules, for example, envelope spikes on the virus surface. The second function is that it triggers elimination mechanisms, e.g., complement activation and phagocytosis by neutrophils and macrophages. The first function requires huge diversified specific antibodies and the second function requires common features in all different types of antibodies. The Y-shaped antibodies are made up of four proteins chains: the upper and lower parts of heavy and light chains are called variable and constant regions, respectively. The two heavy and two light chains are covalently linked by disulfide bonds at constant section. The two heavy chains again linked at their constant section contain hinge region, which provide flexibility to the antibodies (Fig. 6.2). The antibodies consist of three units, of which two units present at N-terminus of the chains are identical and responsible for antigen binding—the Fab (fragment antigen binding) arms. The variable domains of both heavy and light chains form the antigen-binding site or paratope. The variable domain again contains special hypervariable segments called complementary-determining regions, which are specific to antibodies. The third unit is called Fc (fragment crystalline) arm involved in effector functions (Hey 2015; Chan et al. 2009).
There are five classes or isotypes (IgG, IgM, IgA, IgD, and IgE), which are different in their C-terminus region of H chains termed γ, μ, α, δ, and ε, respectively. There are four subclasses of IgG (IgG1, IgG2, IgG3, and IgG4. The effector mechanisms are mainly dependent on the type of antibodies; for example, IgM and IgG 3 are complement activators, whereas IgG1 and IgGE activate macrophages and mast cells, respectively (Hoffman et al. 2016).
Antibodies act on viruses directly by neutralization or indirectly via interaction with complement or Fc receptors on innate immune cells (Fig. 6.3). The multiple effector functions of antibodies include (1) neutralization; (2) antibody-dependent cellular toxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP) of both viruses and infected cells through interaction with Fc receptors on innate immune cells; (3) complement-dependent cytotoxicity (CDC), where complement component C1q mediates formation of membrane attack complex on infected cells or opsonization-based phagocytosis mediated through complement receptors on innate immune effector cells; (4) antibodies bound to infected cells interact with dendritic cells to release type 1 interferons, which stimulate antiviral activity of NK cells and antiviral functions in infected cells, or antigen-presenting cells phagocytize the antibody–virus immune complex and present specific viral peptides to T cells for cellular immunity (Forthal 2014; Hua and Ackerman 2017).
Antiviral mechanism of antibodies. (a) Neutralization, (b) antibody-bound virus or infected cell interacts with Fc receptor on innate effector cell, (c) complement component-bound antibody initiates MAC formation on infected cell or interacts with complement-component receptor on innate effector cell, (d) infected cell bound by antibody interacts with dendritic cell to release type 1 interferon which in turn stimulates NK cells and antiviral proteins in infected cells, (e) antigen presentation of viral peptides to T cells. MAC membrane attack complex, APC antigen-presenting cell, MHC major histocompatibility complex, TCR T-cell receptor
Antibodies neutralize the pathogen by multiple mechanisms such as (1) pre-attachment neutralization, where antibodies directly bind with pathogen to agglutinate. For example, the neutralizing IgG antibodies aggregate the poliovirus to decrease their infectivity. (2) Interference with pathogen attachment—antibodies bind to pathogen ligands which are essential for pathogen attachment. For example, antibodies against HIV-1 gp120 interfere with their attachment to CD4+ T cells. (3) Post-attachment neutralization-inhibition of fusion or entry. For example, a monoclonal antibody 2F5 inhibits the fusion of viral and cellular membranes. (4) Inhibition of various steps in pathogen life cycle—once the pathogen internalized, in order to neutralize pathogen, antibodies must be internalized and interfere with the replication, genetic material expression, and release of pathogen. (5) Inhibition at later steps—antibodies inhibit the liberation of virus or budding (Forthal 2014).
4 Humoral Immune Response Against to Viruses
Viruses are intracellular pathogens and need host cells machinery for their survival. After synthesizing many viral copies, viruses burst out and reinfect healthy new host cells. These viruses directly kill the host cells and are called acutely cytopathic viruses (polioviruses, rabies, and small pox viruses). Some viruses do not kill the host cells and they are called non-cytopathic viruses (hepatitis B and C, herpes virus, herpes simplex virus 1 and 2, cytomegalovirus, and Epstein-Barr viruses). The role of immune system in antiviral defense is both protective and pathogenic, where it eliminates the viruses by effective immune response and in addition also causes more damage to host cells than viruses themselves. With regard to effective immunity, the viruses have developed an array of strategies to escape elimination by immune system through co-evolution (Dörner and Radbruch 2007).
Viruses are chemically nucleoprotein particles with a size of ~20–200 nm. Due to their small size, viruses move freely in the lymphatic system and interact with B cells in secondary lymphoid organs. The highly repetitive structures of viral particles allow the cross-linking of BCRs, which is an initial step in the activation of B cells. Further, the repetitive viral structures also bind with natural antibodies, fix complement, and also carry TLR ligands (DNA or RNA) for TLR 7/8 or 9 to activate B cells directly (Zabel et al. 2013).
4.1 Human Immunodeficiency Virus
AIDS (acquired immune deficiency syndrome) is caused by HIV (human immunodeficiency virus). HIV is genetically highly diverse virus and can be found in two types: HIV-1 and HIV-2. HIV-1 is the most common and falls into three groups: M, N, and O. Group M is the most common and divided into subtypes or clades (A–D, F–H, J, and K), of which B is predominant subtype in the Western world and C in India, China, and Africa. The CD4+ T cells, macrophages, and different subsets of dendritic cells are the major targets of HIV-1 infection. HIV-1 belongs to Retroviridae family and lentivirus genus. HIV-1 is an enveloped virus containing two positive sense RNA strands. The enveloped glycoproteins of HIV-1 present as trimers of gp120/gp41 heterodimers on the surface of virus (Phogat et al. 2007).
After few days of following HIV-1 postinfection, anti-gp41 antibodies and anti-gp120 antibodies are produced, but these antibodies are unable to neutralize the infecting virus strain or autologous virus. The autologous neutralizing antibodies are produced several months postinfection, which are not able to neutralize the heterologous virus. The cross-reacting antibodies, which can neutralize many heterologous viruses, develop 2–4 years after seroconversion, but in some patients develop earlier. Some HIV patients like “Elite neutralizers” develop highly cross-reacting antibodies or broadly neutralizing antibodies (bNAbs) 20-months postinfection. Diversity of HIV strains and molecular peculiarities of HIV envelope protein are some obstacles in recognition and neutralization of HIV strains. However, some patients produce bNAbs that neutralize many HIV strains. The discovery and well characterization of bNAbs has given new hope in the treatment of HIV, so that vaccines should be prepared that induce broadly neutralizing antibodies and antibodies with particular functional activity. Not only systemic immune response but also mucosal immune response is produced against HIV virus. Even though, very little is known about mucosal immune response, some studies have shown that both seronegative and positive patients develop HIV-specific mucosal IgA, which inhibits HIV transcytosis and replication in epithelial cells (Mouquet 2014; Baum 2010).
Non-neutralizing antibody plays an important role in the prevention and control of HIV in humans. In addition, limited protection was observed with RV144 vaccine in the absence of neutralizing antibodies, which indicates the role of non-neutralizing functions by antibodies (Zolla-Pazner 2016; Mayr et al. 2017). Some studies reported that they found anti-HIV antibodies are part of innate immune system. They defend HIV by binding to the HIV protein Tat. Natural antibodies for host receptor CCR5 prevent HIV infection effectively at major sites of virus entry such as mucosal tissues (Ward 2001; Lopalco 2010).
4.2 Influenza Virus
Influenza or flu viruses cause respiratory diseases and continuously threaten human health. Genome high mutational rates of influenza viruses cause emergences of new strains by genetic drifts. Influenza affects 5–30% of global population and causes hospitalization and death of many people. They are enveloped viruses and contain single-stranded, segmented, and negative sense 7–8 RNA strands. Influenza viruses are categorized into four types: A, B, C, and D. Influenza A virus is again classified based on the antigenic properties of two surface viral glycol proteins such as hemagglutinin (HA) and neuraminidase (NA) with 18 (H1-H18) and 11 (N1-N11) antigenic subtypes, respectively. Influenza A (H1N1 and H3N2) virus causes seasonal influenza epidemics in humans along with other members of influenza viruses such as influenza B virus (Sautto et al. 2018).
Two important hypotheses such as original antigenic sin (OAS) and immune imprinting or antigenic seniority explain how humoral immunity gets affected by influenza viruses. Both are dependent on humoral immune memory response rather than on de novo humoral immune response to drifted or altered influenza viruses (Guthmiller and Wilson 2018). The original antigenic sin concept refers that the first exposure of influenza variant in early life dictates lifelong immunity to all variants of influenza viruses in subsequent encounters. The immune memory produced by the first influenza variant influences the immune response to subsequently expose influenza distinct variants, but how this sequential exposure shapes the immune response remains obscure. The antigenic seniority concept better explains the hierarchical nature of immune response to previously exposed variants of influenza virus. According to the antigenic seniority concept, the first exposed influenza variant in childhood takes the senior antigenic position in immune repertoire and subsequent exposed strains take the junior positions. The immune response to the first exposure is larger than the responses to subsequent exposures. Understanding of how previous exposure shapes the antibody responses to vaccination and infection is critical for the development of universal influenza vaccine (Henry et al. 2018).
Neutralizing antibodies produced against HA head region of influenza are strain-specific and bind to highly variable regions. Antibodies neutralize more strains of influenza and are called broadly neutralizing or cross-reactive antibodies. The broadly neutralizing antibodies produced against influenza virus mainly target the conserved regions of HA stem domain. The existence of non-neutralizing antibodies also clears the influenza virus infection by exploiting non-neutralizing effector mechanisms (Sicca et al. 2018).
The natural antibodies against influenza virus are produced by B-1 cells and therefore reported to suggest that innate and acquired humoral immunity comes from separate effector arms of immune system (Baumgarth et al. 1999).
4.3 Hepatitis C Virus
Hepatitis C (HCV) is non-cytopathic virus which infects millions of people around the world, and it is transmitted mainly by unsafe injections and transfusions. During acute infections few people (20%) only clear the virus spontaneously and remaining 70–80% people suffer from chronic infection. The acute hepatitis C infections are asymptomatic. The chronic viral hepatitis by HCV leads to cirrhosis, hepatocellular carcinoma, and end-stage liver disease (Webster et al. 2015).
Hepatitis C virus is small enveloped virus and has positive single-stranded RNA genome encoding a single polyprotein which undergoes posttranslational modification by cellular and viral-encoded proteases into structural (core proteins and envelope proteins) and nonstructural proteins. The HCV RNA genome interacts with core proteins to form the nucleocapsid that is surrounded by a lipid membrane, called the viral envelope, in which envelope glycoproteins are anchored (Irshad et al. 2008).
The clearance of HCV is associated with induction of cellular immune response. In addition, evidence is accumulating that neutralizing antibodies also contribute to HCV clearance. Acutely HCV-infected individuals produce antibodies against epitopes present on the structural and nonstructural proteins of HCV virus. A small fraction of antibodies called neutralizing antibodies are able to inhibit virus binding, entry, and uncoating. The glycoproteins E1 and E2 are major targets for the neutralizing antibodies. Neutralizing antibodies are generated against HVR1 region of envelope glycoprotein E2. The generation of neutralizing antibodies in the early phase of infection correlates with the resolution of HCV infection in some acutely infected people. In contrast, chronic infected people showed delayed induction of neutralizing antibodies (Lapa et al. 2019).
The HVR1 antibodies are type-specific but some evidence shows that anti-HVR1 antibodies are cross-reactive. Some neutralizing antibodies against E1 and E2 envelope proteins show cross-neutralizing potential (Drummer 2014). However, there is strong evidence on the production of broadly neutralizing antibodies against HCV infections (Kinchen et al. 2018). The HCV virus also has immunologic regions for virus escape or non-neutralizing antibodies in the envelope glycoproteins (Fuerst et al. 2018). Similar to HIV virus, the humoral immune response also evolves with time in HCV. The evolution of Ab specificity (non-neutralizing—autologous neutralizing—heterologous neutralizing—broadly neutralizing) during the course of infection produces increased breadth in the neutralizing activity (Murira et al. 2016). There are also reports on natural antibodies which recognize the linear epitopes on hepatitis C envelope glycoprotein E2 to confer additional neutralization (Tarr et al. 2012).
5 Future Perspectives and Conclusions
B cells are the main players of humoral immunity against viruses. B cells also act as antigen-presenting cells, contributing to cell-mediated immunity. Both B-1 and B-2 cells secrete natural and highly specific antibodies for the prevention and control of viral infections, respectively. B-2 cells capture the viral antigens through BCR and differentiate into memory B cells and plasma cells, which secrete different types of specific adaptive antibodies. Recently, many studies reported the generation of broadly neutralizing antibodies, which can neutralize different strains of viruses. Understanding the production of antibodies and their antiviral mechanisms will provide new ways to develop novel, efficient prophylactic and therapeutic vaccines.
References
Baum LL (2010) Role of humoral immunity in host defense against HIV. Curr HIV/AIDS Rep 7(1):11–18
Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA (1999) Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A 96(5):2250–2255
Bonilla FA, Oettgen HC (2010) Adaptive immunity. J Allergy Clin Immunol 125(2 Suppl 2):S33–S40
Burnet FM (1976) A modification of Jerne’s theory of antibody production using the concept of clonal selection. CA Cancer J Clin 26(2):119–121
Burton DR (2002) Antibodies, viruses and vaccines. Nat Rev Immunol 2(9):706–713
Chan CE, Chan AH, Hanson BJ, Ooi EE (2009) The use of antibodies in the treatment of infectious diseases. Singap Med J 50(7):663–672; quiz 673
Dörner T, Radbruch A (2007) Antibodies and B cell memory in viral immunity. Immunity 27(3):384–392
Drummer HE (2014) Challenges to the development of vaccines to hepatitis C virus that elicit neutralizing antibodies. Front Microbiol 5:329
Forthal DN (2014) Functions of antibodies. Microbiol Spectr 2(4):1–17
Fuerst TR, Pierce BG, Keck ZY, Foung SKH (2018) Designing a B cell-based vaccine against a highly variable hepatitis C virus. Front Microbiol 8:2692
Gourley TS, Wherry EJ, Masopust D, Ahmed R (2004) Generation and maintenance of immunological memory. Semin Immunol 16(5):323–333
Guthmiller JJ, Wilson PC (2018) Harnessing immune history to combat influenza viruses. Curr Opin Immunol 53:187–195
Henry C, Palm AE, Krammer F, Wilson PC (2018) From original antigenic sin to the universal influenza virus vaccine. Trends Immunol 39(1):70–79
Hey A (2015) History and practice: antibodies in infectious diseases. Microbiol Spectr 3(2):AID-0026-2014
Hoffman W, Lakkis FG, Chalasani G (2016) B cells, antibodies, and more. Clin J Am Soc Nephrol 11(1):137–154
Hua CK, Ackerman ME (2017) Increasing the clinical potential and applications of anti-HIV antibodies. Front Immunol 8:1655
Irshad M, Khushboo I, Singh S, Singh S (2008) Hepatitis C virus (HCV): a review of immunological aspects. Int Rev Immunol 27(6):497–517
Kinchen VJ, Zahid MN, Flyak AI, Soliman MG, Learn GH, Wang S, Davidson E, Doranz BJ, Ray SC, Cox AL, Crowe JE Jr, Bjorkman PJ, Shaw GM, Bailey JR (2018) Broadly neutralizing antibody mediated clearance of human Hepatitis C virus infection. Cell Host Microbe 24(5):717–730.e5
Lapa D, Garbuglia AR, Capobianchi MR, Del Porto P, Hepatitis C (2019) Virus genetic variability, human immune response, and genome polymorphisms: which is the interplay? Cells 8(4):E305
LeBien TW, Tedder TF (2008) B lymphocytes: how they develop and function. Blood 112(5):1570–1580
Lefevre EA, Carr BV, Prentice H, Charleston B (2009) A quantitative assessment of primary and secondary immune responses in cattle using a B cell ELISPOT assay. Vet Res 40(1):3
Lopalco L (2010) Natural anti-CCR5 antibodies in HIV-infection and exposure. J Transl Med 9(Suppl 1):S4
Lund FE, Randall TD (2010) Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol 10(4):236–247
Maddaly R, Pai G, Balaji S, Sivaramakrishnan P, Srinivasan L, Sunder SS, Paul SF (2010) Receptors and signaling mechanisms for B-lymphocyte activation, proliferation and differentiation--insights from both in vivo and in vitro approaches. FEBS Lett 584(24):4883–4894
Matter MS, Ochsenbein AF (2008) Natural antibodies target virus-antibody complexes to organized lymphoid tissue. Autoimmun Rev 7(6):480–486
Mayr LM, Su B, Moog C (2017) Non-neutralizing antibodies directed against HIV and their functions. Front Immunol 8:1590
Montecino-Rodriguez E, Dorshkind K (2012) B-1 B cell development in the fetus and adult. Immunity 36(1):13–21
Mouquet H (2014) Antibody B cell responses in HIV-1 infection. Trends Immunol 35(11):549–561
Murira A, Lapierre P, Lamarre A (2016) Evolution of the humoral response during HCV infection: theories on the origin of broadly neutralizing antibodies and implications for vaccine design. Adv Immunol 129:55–107
Palma J, Tokarz-Deptuła B, Deptuła J, Deptuła W (2018) Natural antibodies – facts known and unknown. Cent Eur J Immunol 43(4):466–475
Phogat S, Wyatt RT, Karlsson Hedestam GB (2007) Inhibition of HIV-1 entry by antibodies: potential viral and cellular targets. J Intern Med 262(1):26–43
Sautto GA, Kirchenbaum GA, Ross TM (2018) Towards a universal influenza vaccine: different approaches for one goal. Virol J 15(1):17
Sicca F, Neppelenbroek S, Huckriede A (2018) Effector mechanisms of influenza-specific antibodies: neutralization and beyond. Expert Rev Vaccines 17(9):785–795
Sok D, Burton DR (2018) Recent progress in broadly neutralizing antibodies to HIV. Nat Immunol 19(11):1179–1188
Suzuki K, Maruya M, Kawamoto S, Fagarasan S (2010) Roles of B-1 and B-2 cells in innate and acquired IgA-mediated immunity. Immunol Rev 237(1):180–190
Tarr AW, Urbanowicz RA, Jayaraj D, Brown RJ, McKeating JA, Irving WL, Ball JK (2012) Naturally occurring antibodies that recognize linear epitopes in the amino terminus of the hepatitis C virus E2 protein confer noninterfering, additive neutralization. J Virol 86(5):2739–2749
Ward S (2001) Natural anti-HIV antibodies. Trends Immunol 22(10):544
Warter L, Appanna R, Fink K (2012) Human poly- and cross-reactive anti-viral antibodies and their impact on protection and pathology. Immunol Res 53(1–3):148–161
Webster DP, Klenerman P, Dusheiko GM (2015) Hepatitis C. Lancet 385(9973):1124–1135
Yamada T (2011) Therapeutic monoclonal antibodies. Keio J Med 60(2):37–46
Yuseff MI, Pierobon P, Reversat A, Lennon-Duménil AM (2013) How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol 13(7):475–486
Zabel F, Kündig TM, Bachmann MF (2013) Virus-induced humoral immunity: on how B cell responses are initiated. Curr Opin Virol 3(3):357–362
Zolla-Pazner S (2016) Non-neutralizing antibody functions for protection and control HIV in humans and SIV and SHIV in non-human primates. AIDS 30(16):2551–2553
Acknowledgements
The authors are grateful to YVU Kadapa and Krishna University, Machilipatnam, for the support extended.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mallaiah, D., Bramhachari, P.V. (2020). B Cells and Their Role in Combating Viral Diseases. In: Bramhachari, P. (eds) Dynamics of Immune Activation in Viral Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-15-1045-8_6
Download citation
DOI: https://doi.org/10.1007/978-981-15-1045-8_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1044-1
Online ISBN: 978-981-15-1045-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)