Abstract
Insects have the ability to see ultraviolet (UV) radiation unlike vertebrates. Light sources that emit large amounts of UV radiation are often used to attract nocturnal insects. Devices that exploit this behavior, such as light traps are used for detecting pest outbreaks and forecasting. Some diurnal species have attraction to yellow color, and hence yellow pan traps are used for conducting surveys for pest outbreaks. Yellow illumination lamps have been used effectively to control the activity of nocturnal moths and thus reduce damage to fruits, vegetables, and flowers. Covering cultivation facilities with film that filters out near-UV radiation reduces the invasion of pests such as whiteflies and thrips into the facilities, thus reducing damage. Reflective material placed on cultivated land can control the approach of flying insects such as aphids. Hence, light trap has been found an essential part of Integrated Pest Management (IPM). According to the requirement, it can be set to either kill the pests or simply trap only. Trapped insects can also be used for study about the nature and their potential use in IPM. Light traps have been of significant importance in IPM and long-term planning.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The attraction of moths and other nocturnal insects to light is a well-known phenomenon and has been used for collecting nocturnal insects since the beginning of the eighteenth century. Nocturnal arthropods like many species of moths and beetles are easily attracted by artificial light sources (Nag and Nath 1991; Axmacher and Fiedler 2004). The first purpose-built devices termed light traps were used by the Romans in the first century AD (Morge 1973; Beavis 1995). Light-trapping refers to all methods of attracting and/or capturing nocturnal insects with lamps or artificial light sources that usually have a strong emission in the ultraviolet range of the spectrum, whether they are actually connected to a trap or just being operated in front of walls or other reflective surfaces.
Light sources vary greatly, ranging from gas lamps to mercury vapor lamps, black light, and fluorescent UV light tubes, and the collection of samples can either be manual or automatic (Brehm and Axmacher 2006). While the physiological background of the attraction to light is still under discussion (Hsiao 1972, 1973; Baker and Sadovy 1978), light traps have been widely used in nocturnal insect sampling for a long time (Ricklefs 1975; Morton et al. 1981; Thomas 1996; Holyoak et al. 1997; Axmacher et al. 2004a, b). Many light traps are often relatively expensive, but robust sampling devices can collect high numbers of specimens (Basset et al. 1997; Liu et al. 2007). Many of the insect species, mostly nocturnal are known positively phototropic and are attracted toward artificial light in large numbers. Gardeners may utilize this phenomenon to capture night-flying insects in a light trap. For most nocturnal insects, the attractive part of the light spectrum lies in the ultraviolet range, somewhere between 350 and 550 nm (Cleve 1954; Dufay 1964, 1965; Mikkola 1972; Hartstack 1979) though spectral sensitivity varies from species to species; in a number of nocturnal Lepidoptera taxa. Eguchi et al. (1982) reported peak sensitivities, especially around 440–480 and around 500–540 nm.
The use of light for sampling night-flying insects is a long-standing technique. Attracting nocturnal insects with ultraviolet light is now in general use and presents the most effective collecting method for nocturnal Lepidoptera, Trichoptera, and Ephemeroptera, Coleoptera, Hymenoptera, Diptera, Neuroptera, Orthoptera, and some other insect groups. Automatic light traps have also become standard equipment for insect pest control and pest management, as these devices are purely designed to kill or even destroy the insects attracted. There are several light-trap designs in common use.
There are two main approaches in the use of light traps. The qualitative approach aims at maximizing record and/or catch efficiency. For faunistic purposes, and for inventorying or assessing larger areas, it is usually preferable to use high-powered lights (e.g., 125 W lamps) and to choose sampling sites for maximum effect and across habitat types, such as ridge tops and forest edges. For ecological and habitat-related studies standardized comparisons and often target habitat- or niche-specific species, it is better to use low-powered lamps (e.g., 8 W fluorescent tubes) placed well inside the target habitats (Wirooks 2005).
There are basically two types of light-trapping devices: “light towers” or more basic devices such as white sheets spread behind light sources suitable for selective, manual collection, and automatic light traps. In the case of light towers, insects are collected in a jar equipped with a chemical to stun and kill the specimens after they land on the surface of the light tower. Alternative setups use a simple white sheet placed behind the light source. In automatic trap, insects are sampled after they are attracted toward transparent vanes, sliding down through a funnel where killing agents can be applied (Brehm and Axmacher 2006).
The capture rates of light traps are highly variable and affected by a wide array of factors relating to the trap design and environmental conditions. Sampling success is affected, for example, by the above-ground height of the light source and the type of trap illumination (Baker and Sadovy 1978; Bowden 1982) and attraction also varies between species (Bowden 1982). The timing of light traps should consider effects of background illumination by moonlight or anthropogenic light sources (Bowden and Church 1973; Morton et al. 1981; Bowden 1982; Nag and Nath 1991; Nowinszky 2004).
Light-trapping is most effective for sampling night-flying insects in the immediate vicinity up to 500 m from the light source. A light can work over greater distances up to 1 km or more when slightly elevated. The effectiveness depends on the direction of the wind as insects fly upwind, and on wind speed as many insects settle in strong wind. Flight activity is also dependent on temperature and humidity, and rain can stop or reduce it. As a result, care must be taken using light-trap catches for comparative purposes. For this, variables should be kept the same or near the same each time. This is termed standardization. Integrated pest management (IPM) uses a range of preventive measures to control pests. Frequent and reliable monitoring of pest populations is one of the most critical components of IPM program. One of such tools of IPM is the use of physical traps.
Insect traps are used to monitor and/or to directly reduce populations of insect pests. Visual lures use light, colors, and shapes to attract pests. Insect traps are sometimes used in pest management programs for mass trapping but are more often used to assess the seasonal and distributional patterns of pest populations. This information may then be used in other approaches in pest management. Therefore, trapping methods are one of the principal tools in insect pest management programs. The ability to attract specific insect species to the traps depends on the type of trapping equipment. Placement of traps is essential; they must be placed in such a manner that pests are attracted to them. Adequate number of traps is equally essential for effective monitoring.
2 Importance of Light Trap in Monitoring, Suppressing, and Species Diversity
Collections of a light trap provide significant clues to the diversity of insects active at night (Southwood and Henderson 2000), their respective affinity to different wavelengths of light and to understand and predict how populations function (Southwood and Henderson 2000).
Light traps are highly effective and can preserve specimens in relatively good state, important for sampling relatively frail specimens such as small moths. The main advantage of light-trapping is the large number of species recorded during a relatively short period. In Europe, for example, this can amount to 200 or more species of Lepidoptera in a single night under favorable conditions with the number of individuals running into the thousands. In the tropics, the total count of individuals and species can be even much higher, often exceeding the available capacity for recording or collecting. Furthermore, correlating the light-trap data with weather parameters predicts the period of maximum insect diversity and activity.
Light-trapping is most useful for inventory work to determine the geographical distributions of night-flying insects. This is because many species that are trapped at night are practically undetectable by other sampling methods. Light-trapping for native insects potentially reveals a rich diversity of insects. It provides information on biodiversity across seasons, landscapes, ecological areas, altitudes, and times of night (Patrick et al. 1992). Light does not attract insects, it confuses them and intercepts from their chosen flight path. Some insects fly repeatedly around the light, others simply settle at varying distances from the light and may fly off after varying times (Fry and Waring 1996).
2.1 Advantages
-
Easily repeatable and farmers can operate it, if learnt once.
-
Easily traps insect species.
-
Cost-effective in terms of labor and skills required. Cheaper and hence affordable.
-
Many types of insect specimens are undamaged and so are good for identification.
-
Specimens are easily contained for collection and identification.
-
Killing of insects favored instead of chasing and repelling.
-
Can be used at various places—agri and non-agri landscapes.
-
Do not possess danger and residue problem and gives immediate results.
-
Light traps are portable.
-
There is no problem of resistance.
-
Safe to man, animal, and natural enemies.
-
Favors sustainable agriculture.
-
Least or no chance of pollution.
-
Reward for effort is good—only a few hours give much data.
-
Components of light traps are easily obtained.
-
Additionally, light-trapping can be a particularly enjoyable social occasion with people sitting around a bright light awaiting the target species.
-
Simple light traps can be made at home using locally available resources.
2.2 Disadvantages
Insects see green, blue, and near ultraviolet (UV) light very well, but they see yellow and orange light poorly and cannot see red or infrared light. Different types of light sources produce light of different wavelengths (colors) and hence their effectiveness varies for catching insects. The disadvantages of light traps include limitation to nocturnal species, and difficulties in direct comparisons of quantitative data due to differences in light attraction between taxa (Basset 1988). Traps can be often heavy and inconvenient to carry in remote areas.
Light-trapping is still a selective method, and not all taxa of a given group (family, genus) are attracted to light with the same efficiency, and females of many species are less attracted than males or not at all. For ecological studies, it is sometimes seen as a drawback that light-trapping is an attraction method, and it is thus not possible to directly link the species recorded to their respective (larval) habitats.
-
Light-trapping is less productive when it is cold, wet, and windy.
-
It is less effective near bright artificial lights or when there is bright moonlight.
-
It is sometimes less effective when the traps are set too close to fresh water.
-
There are minor safety issues associated with this method.
-
Requires technical knowledge to operate.
-
Highly efficient traps are quite costly.
-
Not available in the rural parts, in most countries of the world.
-
Beneficial insects are also killed with harmful insects.
-
This method requires a source of electric power.
-
Light-trapping is not suitable in the following situations:
-
Windy or excessively wet conditions.
-
Too close to other sources of bright light.
-
Too close to large rivers and lakes because of the enormous numbers of freshwater insects encountered.
3 Role of Light Traps in IPM
Although different species respond uniquely to specific portions of the visible and non-visible spectrum (as perceived by humans), most traps or other devices relay on light to attract insects use fluorescent bulbs or bulbs that emit ultraviolet wavelengths (black lights). Light traps are used to monitor the activity of a range of insect species. Light traps are the only option for monitoring the activity of species for which there are no known (or commercially available) pheromones. Light traps are easy to construct and deploy and suitable for recognizable species that are not easily damaged in the trap (e.g., beetles).
Light traps are basically prepared on the principle that most of the night-flying insects are attracted toward the light source. Insects appear to be strongly attracted at light of 3650 A. These are designed in such a way that when the insects are attracted and come close to the light source fitted in the apparatus, they fall in the trap fitted. Light traps can be used in various fields of pest (pest) infestations. They are used in vegetable, agronomy fields, fruit orchards, agro products storage, research and sometimes in households, and so on.
Hundreds of species of moths, beetles, flies, and other insects, most of which are not pests, are attracted to artificial light. They may fly to lights throughout the night or only during certain hours. Key pests attracted to light include the European corn borer, codling moth, cabbage looper, cutworms and armyworms, diamondback moth, sod webworm moths, peach twig borer, leaf roller moths, potato leafhopper, other leafhoppers and plant hoppers, bark beetles, carpet beetles, adults of annual root grubs, bugs, house fly, stable fly, and mosquitoes. Lights and light traps are used with varying degrees of success in monitoring populations and in mass trapping. Light traps are used to monitor presence of insects and determine seasonal patterns of pest density. Light traps also provide useful information on the timing, relative abundance, or species composition of flights of European corn borer, white grubs, webworms, and other pests.
The UV light trap can be placed in food grain storage godowns to monitor storage insect pests. The light trap attracts stored product insects of paddy like lesser grain borer, Rhyzopertha dominica (Fabricius), Red flour beetle, Tribolium castaneum (Herbst), and saw-toothed grain beetle, Oryzaephilus surinamensis (Linnaeus) in large numbers. Psocids are of great nuisance in godowns are also attracted in large numbers. The trap is ideal for use in godowns meant for long-term storage of grains. Whenever infested stocks arrive in godowns and during post-fumigation periods to trap the resistant strains and leftover insects, traps can be used. In godowns of frequent transactions, the trap can be used for monitoring arthropod populations.
Light can be the most effective and safe tool used for the IPM purpose. These never deviate the principles of IPM. These traps favor in the distraction toward chemical pesticides. Worldwide pesticide problem was considered in light trap invention. Ill effects due to chemical pesticides are also avoided after the use of light traps. Pests can develop resistance against pesticides, but completely avoided in light trap. Light-trap application favors sustainable agriculture, since it doesn’t leave residual effects in the field. Light traps never harm the useful soil organisms. Species richness and abundance per night were positively correlated with temperature, and abundance was negatively correlated with air humidity (Fig. 8.1) (Dennis et al. 2014).
Effects of lamp type and weather parameters on moth species richness (a) and abundance. (b) The parameter estimates and 95% CI are derived from model averaging. All estimates are on a comparable scale due to standardization of the models. (Source: Dennis et al. 2014)
3.1 How It Has Been Improved?
The light trap has undergone lots of changes from its simple beginning as a kerosene lamp kept in front of a cloth sheet or water container to electrically operated ones to battery operated now. The ordinary light trap consists of an electric bulb emitting yellow light as attractant and a funnel to direct lured insects into a container containing water.
Light trap contains basically two parts in it. One is the light source to attract insects and other the killing device to kill insects. Often there is a container with water placed under light source. It is better to add soap in the water used for effective killing. Similarly, sticky plates can also be used instead of water container. In some cases, chemically treated nets can also be used to collect the trapped insects (Fig. 8.2).
Generalized light trap. (Source: www.uky.edu/Ag/Entomology/ythfacts/4h/unit2/hotm&ult.htm)
Vector-borne disease and insect surveillance throughout the world are most often conducted with Centers for Disease Control and Prevention (CDC) light traps. These traps were developed originally by Sudia and Chamberlain (1962) and provided a reliable method for monitoring disease vectors with minimal human exposure. Modifications have improved the CDC trap’s effectiveness (Stewart 1970; Johnston et al. 1973, Elston and Apperson 1977; Addison et al. 1979), but power consumption and bulkiness are a concern during field collections in rural areas and in developing countries. Burkett et al. (1998) first used light-emitting diodes (LEDs) to improve energy efficiency; however, the traps had several deficiencies, the bulbs were not easily interchangeable, the bandwidths were broad (±50 nm wide), the lighting array was limited to one color, and the reflected light diminished luminosity. To address these concerns in the new design, a newly designed LED light bulb was substituted for the standard incandescent bulb in current CDC traps (Cohnstaedt et al. 2008).
In a climate of increased funding for vaccines, chemotherapy, and prevention of vector-borne diseases, fewer resources have been directed toward improving disease and vector surveillance. Recently developed light-emitting diode (LED) technology was applied to standard insect-vector traps to produce a more effective lighting system. This approach improved phlebotomine sand fly capture rates by 50%, and simultaneously reduced the energy consumption by 50–60%. The LEDs were incorporated into two lighting designs, (1) a LED combination bulb for current light traps and (2) a chip-based LED design for a modified Centers for Disease Control and Prevention (CDC) light trap (Cohnstaedt et al. 2008).
3.1.1 Current Research on Pest Control Using Light
The Ministry of Agriculture, Forestry and Fisheries of Japan has been implementing a research project called “Elucidation of biological mechanisms of photoresponse and development of advanced technologies utilizing light” since 2009 (Honda 2011). The National Agriculture and Food Research Organization (NARO), Japan is the core research institution, and more than 20 research groups are included. The main areas of research include basic research on reaction behavior, color perception, and polarized light perception with light of different wavelengths; the development of outbreak forecast technology using new light sources; and the development of pest control technology using new light sources.
In the first research area, electrophysiological techniques are used to comprehensively measure the sensitivity of different pests over a wide range of light wavelengths. In addition, the responses of stink bugs, thrips, whiteflies, planthoppers, leafhoppers, and other species to LEDs and other light sources are being studied to clarify the relationship between light wavelength and the behavioral ecology of pests. Workers have aimed at determining the wavelengths that are effective for attracting or repelling pests as well as those that affect behavioral activity and orientation to the light (Hironaka and Hariyama 2009; Cohnstaedt et al. 2008). The second research area includes development of LED light sources to replace incandescent lights for forecasting agricultural pest outbreaks. Find outdoor devices (light sources and traps) that can be used in locations with no electricity. Beneficial insects as parasitoid wasps are natural enemies of pests are also included to determine the light wavelengths that attract them effectively. Development of fundamental technologies for devices to survey and collect natural enemies is also a part of the investigation. Light traps that effectively attract biting midges which are vectors of viral diseases on livestock and to survey their outbreaks are being developed (Cohnstaedt et al. 2008).
In the third research area, behavioral effects like attraction or repulsion associated with different wavelengths of light are being analyzed for whiteflies, thrips, and leafminer flies, which damage vegetables grown in greenhouses; lepidopteran pests of vegetables and tea; Asian citrus psyllids for citrus fruit; fungus gnats for cultivated mushrooms; and green chafers for sugarcane. New pest control technologies for using LEDs are in progress in several countries. LED lighting devices that take up little space and have low energy consumption are desired. This will enable pest control in places where conventional light sources are impractical. Studies are also being carried out on wavelengths effective against pests. This research area aims to develop a new pest control technology, fully compatible with cultivation technology (Cohnstaedt et al. 2008).
3.2 How Does a Light Trap Work?
Insects are attracted to light trap because of the light in it. The mechanism in organisms that makes them react to light is called phototaxis. Phototaxis is an organism’s automatic movement toward or away from light. Using light traps is an effective way to control the number of insects and the potential infestation and damage they cause to humans.
The light trap traps insects in a field by attracting them towards the light. Coming nearer to the light, the insects get trapped and electrocuted through the screens, tapped with electricity. There are two types of phototaxis depending on the movement and reaction of the organism to the stimuli. It is called negative phototaxis when the organism moves away, while it is positive phototaxis when the organism moves toward the light. Cockroaches exhibit negative phototaxis and moths show a positive phototaxis. There is no definite answer to how organisms get in their system the mechanism of phototaxis. Some of the theories say that it is because the organisms think the light is the sun while some other theories say that some organisms use the moon as compass for traveling. When these organisms see a strong light in darkness, they think it is the moon.
3.3 Functioning of Light Trap
-
Once installed, insect light traps (ILTs) need little attention—just empty tray or replace the glue boards occasionally.
-
ILT units function well as long as the bulb is lit.
-
Traps with glue boards are better and more efficient than electrocutors.
-
ILTs can be placed over or near food and food contact surfaces.
-
Black light Blue (BLB) bulbs or combinations of BLB and black light (BL) bulbs are more effective than BL lamps alone.
-
Stunning circuitry improves the catch of glue board ILTs.
-
Ultraviolet emissions from ILTs can affect human health.
3.4 Where to Trap?
-
Trap as close as practical to the target insect community or host plants. This will ensure more target insects are exposed to the light and hence increase the success rate. If possible, set up the trap where it is sheltered from the wind. The best position for the operator is with their backs to the wind so they can see what comes into light as the insects fly upwind.
-
If the target is moths, do not trap close to freshwater rivers, ponds, or lakes on warm summer nights, because huge quantities of flies and caddis flies get attracted and interfere with the trapping of other groups.
-
To be effective, the light of the light trap must be brighter than the surrounding nightscape. Choose a site where the background is as dark as possible. Using a light trap in a lit-up suburb at night will be less effective, as is placing two light traps near each other (DOCCM-286730 Invertebrates: Light trapping v1.0).
3.5 When to Trap?
Each species has its own distinctive way; it responds to different colored lights and weather conditions. The best nights to trap are warm, humid with cloud cover and little wind. Different species are active at different times of the year and night: some are trapped at dusk, others during the night, and some others as daylight appears. Light-trapping from dusk till midnight is usually sufficient and cost-effective for inventory purposes. In general, avoid light-trapping during a full moon, although it can still work if the night is sufficiently hot and calm (DOCCM-286730 Invertebrates: Light trapping v1.0).
3.6 What Insects Come to Light?
Flies, moths, mayflies, and caddisflies are the most consistently trapped insects at night in almost all altitudes and locations (DOCCM-286730 Invertebrates: Light trapping v1.0).
The following insect orders regularly come to light:
Caddisflies (Trichoptera)—both marine and freshwater species |
|
Stoneflies (Plecoptera)—genera—Stenoperla and—Megaleptoperla |
|
Dobsonflies (Megaloptera) |
|
Mayflies (Ephemeroptera) |
|
Lacewings (Neuroptera) |
|
Wasps (Hymenoptera)—particularly Ichneumonidae |
|
Moths (Lepidoptera)—Geometridae, Noctuidae, Crambidae, Tortricidae, and Hepialidae |
|
Beetles (Coleoptera)—scarabs, click beetles, and longhorns |
|
Flies (Diptera)—many families |
|
Bugs (Hemiptera)—water boatmen and backswimmers |
|
Praying mantids (Mantoidea) |
|
4 Types
The monitoring of populations of both harmful insects and useful ones is a prerequisite for making decisions on the time and nature of pest management programs. There are many light traps based on design and the light source. Light sources range from simple oil lamps to different electric sources. Special light sources called black light have been developed and are efficient, because of the light they emit. They should be considered in situations when other light sources (house, street light) are competing with the traps.
Light traps, with or without ultraviolet light, attract certain insects. Light sources may include fluorescent lamps, mercury-vapor lamps, black lights, or light-emitting diodes (Fig. 8.3). Designs differ according to the behavior of the insects being studied. Light traps are widely used to survey nocturnal moths. Total species richness and abundance of trapped moths are influenced by several factors as night temperature, humidity, and lamp type. Grasshoppers and beetles are attracted to light at a long range but are repelled by it at short range. Farrow’s light trap has a large base so that it captures insects that may otherwise fly away from regular light traps. Light traps can attract flying and terrestrial insects and can be combined with other methods.
Insect vision and lighting spectra. The upper graph represents a schematic of the conserved insect photoreceptors and their maximum sensitivities in the ultraviolet, blue, and green spectra. The lower graph indicates the spectra emitted from ultraviolet light-emitting diode and incandescent lights. The overlap of the insect vision and the lighting system yields the light visible to insect eyes. Note that 94% of the incandescent bulb energy is expended in the infrared. (Source: Cohnstaedt et al. 2008)
For field work, the choice of lamp type is often determined by the actual field conditions than purely by scientific considerations. If there is access to the electricity network or to a portable generator, mercury vapor lamps, black-light lamps or blended (mixed light) lamps are the best choice because their emittance in the UV range is higher than standard household light bulbs (tungsten bulbs). If weight and size are an issue in field situations without a mains power supply, fluorescent tubes are a perfect alternative which can run from rechargeable 12 V batteries.
4.1 Mercury Vapor and Other Lamps
High pressure mercury vapor lamps come in several sizes of which the 80 and 125 W versions are most used by entomologists. A larger 250 W version (which is no longer manufactured) is even more effective but also more tiring for human eyes. All of those lamps require separate electronic ballast (choke) inserted between the lamp and the power outlet. There are also 80 W versions which can be run without ballast. The so-called black-light bulbs (125 W) produce almost no visible light; for the human eye, they seem dark blue (Fig. 8.4a, b). For many groups, the 160 W blended (mixed light) lamps are less effective than 125 W mercury vapor lamps but require no external ballast. There is also a 160 W black-light bulb, which does not need ballast.
4.2 Fluorescent UV Light Tubes
The low pressure fluorescent tubes or neon tubes generally produce a bluish light and are available in a range of sizes in different lengths: 6 W (22.5 cm), 8 W (30 cm), 15 W (45 cm), 20 W (60 cm). Two special types emitting UV light are commonly used for light-trapping: the so-called “super actinic” tubes producing pale blue light and “black-light” tubes are comparable to the black-light bulbs and are virtually invisible from a distance. While fluorescent tubes can be operated with a voltage converter from a generator or mains power supply, in the field, they are best directly run from 12 V rechargeable batteries. A number of studies have compared the relative performance of different lamp types and attraction to different insect orders (Williams 1951; Bretherton 1954; Williams et al. 1955; Lam and Stewart 1969; Mikkola 1972; Taylor and Brown 1972; Taylor and French 1974; Blomberg et al. 1978; Walker and Galbreath 1979; Leinonen et al. 1998).
Insects are easily attracted to Bluish-purple (UV) radiation, because these rays are visible to insects. Yellow pan traps with yellow sticky plates are used to catch daylight insects. Lamps with yellow illumination are also used to control pest deeds during night. Thus, the extent of damage to flowers, vegetables, and fruits can be reduced. The population of hovering bugs can be limited by covering the cultivated land using reflective materials (Shimoda and Honda 2013).
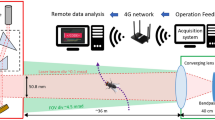
An autonomous monitoring system using black lights (Ultra Violet) and LED lights is ensured with a low cost image sensor to capture the images of trapped pests. A solar panel of 10 W is used to charge the battery to power the LED and the UV lights (Fig. 8.5a, b). The power generated through solar panel is stored in a battery for operating the system during the night. A solar charge controller guarantees effective charging of the battery and enhances the life of the battery. The captured images are sent to a remote control station. The information thus acquired is used for the estimation of pest density in farms. The experiment is carried out by placing the designed trap in three different fields.
4.3 Black-Light Traps
A black light, also referred to as a UV-A light, Wood’s lamp, or simply ultraviolet light, is a lamp that emits long-wave (UV-A) ultraviolet light and not much visible light. It may be available as fluorescent black-light tubes, incandescent bulbs, mercury vapor black-light lamps, and LED black-light bulbs (Fig. 8.6).
More species were captured when sampling on warm nights than when sampling, during the same number of nights, with no specific temperature restrictions (Fig. 8.7). At low sample sizes (<10 nights), slightly more species were captured when sampling was restricted to the warmest summer nights, but at larger sample sizes (>10 nights), slightly more species were captured when sampling during the warmest nights. With the latter, “WARMEST MONTHLY” strategy, after 30 nights, we captured 60% of all species when using the trap with 250 W mercury lamp and 55% when using the trap with the 40 W ultraviolet fluorescent tube (Dennis et al. 2014).
Sample-based rarefaction curves with 95% confidence intervals generated from data obtained using four sampling strategies and the traps with a 40 W black-light bulb (a) and a 250 W mercury lamp (b). (Source: Dennis et al. 2014)
4.4 Solar Light Trap
It is a small scale photovoltaic system, called solar power insect trap. By absorbing sun radiant light, the solar module generates electrical energy, stores it in the batteries during the daylight, then discharges the electricity to supply the pest spectrum light at night (Fig. 8.8a, b). The solar panel absorbs the sunlight available during the daytime and is stored in the battery.
Later during the night, the UV LED uses this energy to emit light which attracts insects. The light emitted by the UV LED attracts most of the nocturnal insects at a particular wavelength. Automatic dusk-to-dawn system is installed which helps to automatically switch on the device during night and switch-off during day (Fig. 8.9a, b). The basin contains liquid medium to trap the insects that are attracted by the UV LED light.
The development of solar light trap and successful demonstration in different crop areas by the farmers in four districts of West Bengal resulted as an alternative to pesticides. Solar light trap is considered important for eco-friendly nature and low cost to both the farmers and agricultural experts. The solar light trap model will be effective against insect pests in cultivated areas in the near future. Government and non-government organizations may utilize this as an IPM tool (Bera 2015).
Solar energy-based insect traps use ultraviolet light-emitting diode tubes to lure pests and 12 volt battery as power supply to light-emitting diode tube. The battery charging system derives electrical energy from 20 watts of solar cell for use at night. This proposed solar-based insect trap has an automatic control system to lure insect pests when there is no sunlight, and the system will stop when the sun shines. The results of the system installation test showed that this trap could lure several types of insect pests (Nichanant and Chonmapat 2015).
Thailand farmers face problems of pests that harm crops and result in substantial losses. So, it is essential for farmers to use pesticides to prevent crop damage. A study revealed that the ultraviolet light diode tube could be used to lure “Coconut Hispine Beetle” (Plesispa reichei Chapuis), a pest of coconut. A solar energy-based insect pests trap was developed using ultraviolet light-emitting diode tube to lure the insect pests and 12 volt battery as power supply to light-emitting diode tube. The battery charging system derives electrical energy from 20 watts of solar cell for use at night (Nichanant et al. 2015).
Light-trapping is an ideal method for surveying nocturnal moths. The influence of weather, time of year, and light source on nightly catches of macro moths in light traps was studied. The workers compared four strategies for sampling by estimating observed species richness using rarefaction. The authors operated two traps with different light sources for 225 consecutive nights from mid-March to October in eastern Germany in 2011. Species richness and abundance per night were mainly related to night temperature, humidity, and lamp type. By monitoring the peak activity of moths during warm nights, an understanding on the bioecology of species could be ascertained (Dennis et al. 2014).
The results of a study at Zonal Agricultural Research Station (ZARS), Mohitnagar, and farmers’ demonstration in different crops in four districts revealed that the newly developed solar light-trap model is effective for monitoring insect pests. It is the most effective IPM tool which provide better safeguard to the nature in comparison to the other methods of pest control (Bera 2015).
5 Impact of Light on Insects
5.1 Attraction
Lighting attracts a large number of a wide range of invertebrates but moths are best known for this behavior (Frank 1988). Other groups drawn to artificial lights include beetles, lacewings, aphids, caddisflies, crane flies, midges, hoverflies, true flies, scorpionflies, damselflies and dragonflies, termites, butterflies, diurnal jumping spiders, ant-lions, bush crickets, and wasps (Eisenbeis and Hassel 2000; Feltwell 2010; Nakamura and Yamashita 1997). UV, green, and blue lights have short wavelengths, and high frequencies are best discriminated by most insects and are highly attractive. Consequently, lights that emit little or no UV such as low pressure sodium lamps are less attractive to insects (Bruce-White and Shardlow 2011).
The distance that invertebrates are attracted to light varies greatly depending on environmental factors and the species. Moths are known to fly to light from distances varying from 3 to 130 m, but greater distances up to 500 m have been recorded (Frank 2006). Attraction to artificial light has been utilized by entomologists for centuries as a way to survey and monitor moth and other invertebrate populations. Many trappers will not run traps in the same area on consecutive nights, in case the artificial light affects moth breeding and feeding behavior (Bruce-White and Shardlow 2011). From light traps one can generate data for long-term studies and analysis. One can detect trends or predict populations.
Insects can die or become injured when they collide with a hot lamp or become disorientated and exhausted making them susceptible to predation. In some instances, huge numbers of dead moths have been observed around outdoor lighting. Certain species of fast-flying bats such as the Noctule (Nyctalus noctula) and Common pipistrelle (Pipistrellus pipistrellus) (Blake et al. 1994; Rydell 1992) feed on large numbers of insects attracted to lights. It has also been found that mercury vapor street lights increase bat predation on moths, because the lights interfere with the ability of moths to detect the ultrasonic sound bursts used by bats to locate prey (Bruce-White and Shardlow 2011).
Moths and other invertebrates attracted to night lighting often rest on surfaces close to the light source during the day. Many invertebrate species use camouflage, but they may end up resting on an unsuitable background making them clearly visible to predators. Birds soon learn to hunt for invertebrates resting on surfaces close to artificial light, and amphibians and reptiles in some tropical areas perch and wait to prey on invertebrates attracted to light. Even common toads (Bufo bufo) in Britain prey on flying insects attracted to street lamps at night. Nocturnal mammals such as hedgehogs and shrews also eat invertebrates attracted to artificial lights. The Bridge orbweaver (Larinioides sclopetarius) has an inborn preference to build webs near artificial light. This behavior might originate from the spider favoring hunting grounds near to water bodies reflecting moonlight. This spider and several others are able to take advantage of the attraction of flying insects to artificial lighting (Bruce-White and Shardlow 2011).
Increased predation is not the only effect. Lighting can affect moths in many ways; it can disturb their flight, navigation, vision, migration, dispersal, egg-laying, mating, feeding, and camouflage. The combination of such wide-ranging effects has considerable impact. Two-thirds of the 337 moth species with sufficient monitoring data to determine the population trend in Britain have decreased over the last 35 years. Light pollution has been identified as a possible contributing factor. It is difficult to separate the impacts of artificial lighting from other impacts of urban development such as habitat loss and air pollution (Bruce-White and Shardlow 2011).
An investigation was conducted by Sharma et al. (2017) on occurrence, distribution, and population dynamics of 51 phototactic harmful insect species in chickpea ecosystem at Jabalpur, India. This will serve as baseline data, useful at present and in the future for surveillance and monitoring of insects for forecasting and also in the use of light trap as IPM component against pests of chickpea and other crops.
Light sources attract nocturnal insects. Some sources attract more insects than others. Donners et al. (2018) developed a model to quantify the attractiveness of light sources based on the spectral output. This model is fitted using data from field experiments comparing large number of different light sources. The model was validated using two additional datasets, one for all insects and one excluding numerous Diptera.
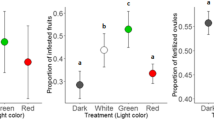
Nirmal et al. (2017) evaluated different light traps with different colored lights, this revealed that tungsten filament light was found to be most effective followed by yellow, blue, and green lights. Red light was the least effective based on number of insects caught in the light trap.
5.1.1 Attraction to Polarized Light
Polarized light pollution sources are also attractive to many invertebrates. Polarized light pollution is the process whereby light undergoes linear polarization by reflecting off smooth surfaces or by scattering in the atmosphere or under water. Artificial lights are not necessarily part of this form of light pollution, but artificial lighting can make the situation worse (Bruce-White and Shardlow 2011).
5.1.2 Attraction to Reflected Light
Light reflected off colored artificial surfaces has the potential to impact on invertebrate populations. Select colors are attractive to pollinating insects as they are intensely connected with flower colors. Yellow objects are attractive to diurnal pollinators, while white or pale grey objects attract more insects at dusk (Long et al. 2010). For example, pollen beetles are attracted to yellow color and are likely to be localized. Large objects may attract many insects from a considerable distance; if combined with an increased risk of fatality, it could have significant ecological impacts (Bruce-White and Shardlow 2011). In particular, pale grey wind turbines attract insects at dusk. This in consequence attracts increased numbers of predators, leading to increased fatality of bats and birds (Young et al. 2003; Long et al. 2010).
6 Activity Levels
Artificial light at night confuses invertebrates and alters night activity. Artificial light can also alter the rate of development in invertebrates. A high general level of illumination at night affects artificial lighting and results in night-flying insects to cease flying and settle. This prevents insects from performing normal nightly activities such as feeding and breeding. It has been found that Corn earworm moth (Helicoverpa zea Boddie) activity in a bioclimatic chamber was suppressed by light intensities as low as 0.1 lux (less than a fifth of full moonlight) and that field oviposition rates were significantly lessened in full moon (Nemec 1971).
A study looking at Speckled wood butterfly larvae (Parar geaegeria Linnaeus) revealed that a higher growth rate related to longer photoperiods (by artificial light) led to significantly higher predation on the butterfly larvae from a parasitoid (Gotthard 2000). It revealed that artificial light influence growth rate and predator–prey balance, benefiting a few species while possibly negatively affecting others. Several daylight invertebrates as butterflies have larvae that feed at night, a behavior that helps avoid predation. Nocturnal British butterfly caterpillars include Wall brown (Lasiommata megera Linnaeus) and Grayling (Hipparchia Semele Linnaeus) are selected as prime species in the UK Biodiversity Action Plan (UKBAP). Other night-feeding species include Scotch argus (Erebia aethiops Esper), Meadow brown (Maniol ajurtina Linnaeus), Marbled white (Melanargia galathea Linnaeus), and Ringlet (Aphantopus hyperantus Linnaeus) (Lewington 2003). It is possible that artificial lighting affects behavior of larvae, either make them more vulnerable to predation or inhibit feeding.
6.1 Monitoring
Light-trapping is suitable for continuous monitoring of invertebrates species. The method must be simple, repeatable, fully documented, and standardized. The trapping must be done at the appropriate time of year at appropriate place and time.
6.2 Suppression of Nocturnal Insects
Fruit-piercing moths such as Eudocimatyr annus Guenee and Oraesiae marginata Fabricius damage fruits. Fruit damage can be prevented by running yellow fluorescent lamps in the orchard at night (Nomura 1967; Nomura et al. 1965). This method is based on the fact that when moths encounter light above certain brightness at night, by which their compound eyes are light-adapted as in the day (Meyer-Rochow 1974; Walcott 1969). The light adaptation suppresses nocturnal activities as flying, sucking fruit juice, and mating. This technique of suppressing behavior using yellow fluorescent light is also used to prevent damage to chrysanthemums and carnations by the cotton bollworm Helicoverpa armigera Hubner (Yase et al. 1997), damage to green perilla (major Asiatic leafy vegetable) by the common cutworm Spodoptera litura (Fabricius), and damage to cabbage by the webworm, Hellula undalis Fabricius (Yase et al. 2004). Recently, green fluorescent lamps have also been developed for the control of nocturnal moths. These lamps suppress the behavioral activity of select moth species similar to yellow fluorescent lamps (Yamada et al. 2006; Kono and Yase 1996; Yase et al. 1997). Because LED lighting is becoming considerably cheaper, yellow-emitting LEDs have been recently applied to suppress population of nocturnal moths (Hirama et al. 2007; Yabu 1999; Yoon et al. 2012). LEDs produce highly monochromatic lights (i.e., with a narrow range of wavelength) across the spectrum from UV to red. This optical characteristic of LEDs is an advantage as it affects normal pest behavior and practical application is possible.
Study by Sermsri and Torasa (2015) suggested use of ultraviolet light diode tube to lure the Coconut Hispine Beetle, a pest of coconut. A solar energy-based trap was developed by using ultraviolet light and 12 volt battery as power supply to light-emitting diode tube. The battery charging derives electrical energy from 20 watts solar cell at night. The results of a study by Bera (2015) at Zonal Adaptive Research Centre, West Bengal, India and farmer demonstration in different crops in four districts revealed that the newly developed solar light trap model is effective for monitoring insect pests. Mafia et al. (2018) developed an efficient light trap model which allowed the capture of 3.6 times as many as insects as the conventional model with a 61% gain in control efficiency. The use of this new model represents another integrated management alternative for lepidopteran pests of eucalyptus plantations and other cultured plants in Brazil.
6.3 Control of Pest Infestation
The use of UV-absorbing plastic films that block near-UV light radiation (300–400 nm) in polyhouse cultivation has been effective for preventing a variety of pests from entering polyhouses (Nakagaki et al. 1982, 1984; Raviv and Antignus 2004). Insect eyes are highly sensitive to near-UV light radiation, and vision in the UV range is important for orientation in several species (Prokopy and Owens 1983). A polyhouse covered with UV absorbing film appears dark for insects. For insects, given access to a space with UV radiation and a space from which near-UV radiation was removed several species avoid the latter. Studies have shown a reduced incidence of insects such as aphids, whiteflies, and thrips in facilities covered with UV absorbing film (Costa et al. 2002; Nguyen et al. 2009; Nonaka and Nagai 1985; Ohta and Kitamura 2006). In addition to vinyl chloride films, products made of highly durable polyolefin films have recently been developed to block near-UV radiation and to prevent infestation inside greenhouses. Honey bees play crucial role in pollination, also become inactive in facilities covered with near-UV ray absorbing film. So care is required when pollinators are used in polyhouses (Shimoda and Honda 2013).
6.4 Inhibition of Flight by Reflective Mulching Films
The use of mulching sheets that reflect light in open cultivated fields is proven to deflect alate aphids (e.g., soybean field covered with a silver mulch sheet; Kimura 1982). Spreading reflective sheets over the ground surface has also been reported to prevent the invasion or outbreak of thrips and whiteflies (Nagatsuka 2000; Simmons et al. 2010; Tsuchiya et al. 1995). The principle by which reflecting light prevents the invasion of pests is not fully understood. Some free-flying insects exhibit the dorsal light reaction, as reported earlier (Goodman 1965; Jander 1963; Neville 1960). They stabilize their horizontal orientation by the light shining on their dorsal side, as happens with light from the sun when flying. But insects are unable to continue normal flight when the light comes from the ground. By covering the ground with highly reflective mulching sheets, the light reflection from below disturbs the normal flight.
References
Addison LD, Watson BJ, Webber LA (1979) Apparatus for the use of CO2 gas with a CDC light trap. Mosq News 39:803–804
Axmacher JC, Fiedler K (2004) Manual versus automatic moth sampling at equal light sources: a comparison of catches from Mt. Kilimanjaro. J Lepidopterists’ Soc 58:196–202
Axmacher JC, Holtmann G, Scheuermann L, Brehm G, Müller- Hohenstein K, Fiedler K (2004a) Diversity of geometrid moths (Lepidoptera: Geometridae) along an Afrotropical elevational rainforest transect. Divers Distrib 10:293–302
Axmacher JC, Tünte H, Schrumpf M (2004b) Diverging diversity patterns of vascular plants and geometrid moths during forest regeneration on Mt Kilimanjaro, Tanzania. J Biogeogr 31:895–904
Baker RR, Sadovy Y (1978) The distance and nature of the light-trap response of moths. Nature 276:818–821
Basset Y (1988) A composite interception trap for sampling arthropods in tree canopies. Aust J Entomol 27:213–219
Basset Y, Springate ND, Aberlenc HP, Delvare G (1997) A review of methods for sampling arthropods in tree canopies. Canopy Arthropods 35:27–52
Beavis IC (1995) The first light trap, 1st century AD. Entomol Rec J Var 197:155
Bera KP (2015) Development of a new solar light trap model and its utilisation as IPM tool in agriculture. JETIR 2(3):549–554
Blake D, Hutson AM, Racey PA, Rydell J, Speakman JR (1994) Use of lamplit roads by foraging bats in southern England. J Zool (Lond) 234:453–462
Blomberg O, Itmies J, Kuusela K (1978) The influence of weather factors on insect catches in traps equipped with different lamps in northern Finland. Annales Entomologici Fennici 44:56–62
Bowden J (1982) An analysis of factors affecting catches of insects in light traps. Bull Entomol Res 72:535–556
Bowden J, Church BM (1973) The influence of moonlight on catches of insects in light-traps in Africa. Part II. The effect of moon phase on light trap catches. Bull Entomol Res 63:129–142
Brehm G, Axmacher JC (2006) A comparison of manual and automatic moth sampling methods (Lepidoptera: Arctiidae, Geometridae) in a rain forest in Costa Rica. Environ Entomol 35:757–764
Bretherton RF (1954) Moth traps and their lamps: an attempt at comparative analysis. Entomol Gaz 5:145–154
Bruce-White C, Shardlow M (2011) A review of the impact of artificial light on invertebrates. Buglife—The Invertebrate Conservation Trust Peterborough P 33 www.buglife.org.uk/News/newsarchive/News+Archive+2011/Save+bugs+from+light+pollution
Burkett DA, Butler JF, Kline DL (1998) Field evaluation of colored light-emitting diodes as attractants for woodland mosquitoes and other diptera in north Central Florida. J Am Mosq Control Assoc 14(2):186–195
Cleve K (1954) Einfluss der Wellenl.nge des Lichtes auf den Lichtfang der Schmetterlinge. In Titschak E (ed) Deutscher Entomologentag in Hamburg 30 Juli bis 3. August 1953. Jena (Fischer), pp 107–113
Cohnstaedt LEE, Gillen JI, Munstermann LE (2008) Light-emitting diode technology improves insect trapping. J Am Mosq Control Assoc 24(2):331
Costa HS, Robb KL, Wilen CA (2002) Field trials measuring the effects of ultraviolet-absorbing greenhouse plastic films on insect populations. J Econ Entomol 95(1):113–120
Dennis J, Franzén M, Ranius T (2014) Surveying moths using light traps: effects of weather and time of year. PLoS One 9(3):e92453
DOCCM-286730 Invertebrates: Light trapping v1.0 (2016) Inventory and monitoring toolbox: invertebrates. Department of Conservation Te Papa Atawhai
Donners M, van Grunsven RH, Groenendijk D, van Langevelde F, Bikker JW, Longcore T, Veenendaal E (2018) Colors of attraction: modeling insect flight to light behavior. J Exp Zool A Ecol Integr Physiol 329(8–9):434–440
Dufay C (1964) Contribution a l’Étude du phototropisme des Lépidoptères noctuidae. Masson. Annales des Sciences Naturelles - Zoologie et Biologie Animale Paris 12e série 6:281–406
Dufay C (1965) étude du phototropisme des Lépidoptères Noctuidae. Applications aux chasses à la lumière. Alexanor 4(81–88):131–136
Eguchi E, Watanabe K, Hariyama T, Yamamoto K (1982) A comparison of electrophysiologically determined spectral responses in 35 species of Lepidoptera. J Insect Physiol 28(8):675–682
Eisenbeis G, Hassel F (2000) Zur Anziehung nachtaktiver Insekten durch Straßenlaternen. Natur und Landschaft 75(4):145–156
Elston R, Apperson C (1977) A light-activated on-off switch for the CDC light trap. J Med Entomol 14(2):254–255
Feltwell J (2010) Types of invertebrates attracted to artificial lighting. Personal Communication
Frank KD (1988) Impact of outdoor lighting on moths: an assessment. J Lepid Soc 42(2):63–93
Frank KD (2006) Effects of artificial night light on moths. In: Rich C, Longcore T (eds) Ecological consequences of artificial night lighting. Island Press, Washington, DC, pp 345–364
Fry R, Waring P (1996) A guide to moth traps and their use. Amat Entomol 24:1–60
Goodman LJ (1965) The role of certain optomotor reactions in regulating stability in the rolling plane during flight in the desert locust, Schistocerca gregaria. J Exp Biol 42(3):385–407
Gotthard K (2000) Increased risk of predation as a cost of high growth rate: an experimental test in a butterfly. J Anim Ecol 69(5):896–902
Hartstack AW (1979) Light sources, trap design and other factors affecting moth catch. In: Rabb RL, Kennedy GG (eds) Movement of highly mobile insects: concepts and methodology in research. North Carolina State University, Raleigh, pp 232–241
Hirama J, Seki K, Hosodani N, Matsui Y (2007) Development of a physical control device for insect pests using a yellow LED light source: results of behavioral observations of the Noctuidae family. J Sci High Technol Agric (Japan) 19:34–40
Hironaka M, Hariyama T (2009) Insect orientation to natural and artificial light. Jpn J Appl Entomol Zool 53:135–145
Holyoak M, Jarosik V, Novak I (1997) Weather-induced changes in moth activity bias measurement of long-term population dynamics from light trap samples. Entomol Exp Appl 83:329–335
Honda K (2011) Reactions to light in insects and practical applications. J Appl Biomech 35:233–236
Hsiao HS (1972) Attraction of moths to light and to infrared radiation. San Francisco Press, San Francisco, p 89
Hsiao HS (1973) Flight paths of night-flying moths to light. J Insect Physiol 19:1971–1976
Jander R (1963) Insect orientation. Annu Rev Entomol 8:95–114
Johnston J, Weaver J, Sudia W (1973) Flashlight batteries as a power source for CDC miniature light traps. Mosq News 33:190–194
Kimura Y (1982) Control of aphid infestation by mulching with silver-colored polyethylene films. Plant Prot 36:469–473
Kono S, Yase J (1996) Characteristic of physical control and using technology. Utilization of color sense of insects. Plant Prot 50:30–33
Lam JJ, Stewart PA (1969) Modified traps using blacklight lamps to capture nocturnal tobacco insects. J Econ Entomol 62:1378–1381
Leinonen R, Soderman G, Itamies J, Rytkonen S, Rutanen I (1998) Intercalibration of different light-traps and bulbs used in moth monitoring in northern Europe. Entomol Fenn 9(1):37–51
Lewington R (2003) Pocket guide to the butterflies of Great Britain and Ireland. British Wildlife Publishing, Gillingham
Liu Y, Axmacher JC, Li L, Wang C, Yu Z (2007) Ground beetle (Coleoptera: Carabidae) inventories: a comparison of light and pitfall trapping. Bull Entomol Res 97(6):577–583
Long CV, Flint JA, Lepper PA (2010) Insect attraction to wind turbines: does colour play a role? Eur J Wildl Res 57(2):323–331
Mafia RG, Loureiro EB, Silva JB, Simões JAC, Zarpelon TG, Junior NB, Damacena MB (2018) A new light trap model as an alternative for controlling pests in Eucalyptus plantations. Neotrop Entomol 47(2):326–328
Meyer-Rochow VB (1974) Fine structural changes in dark-light adaptation in relation to unit studies of an insect compound eye with a crustacean-like rhabdom. J Insect Physiol 20(3):573–589
Mikkola K (1972) Behavioural and electrophysiological responses of night-flying insects, especially Lepidoptera, to near-ultraviolet and visible light. In: Annales zoologici fennici. Societas Biologica Fennica Vanamo, Helsinki, pp 225–254
Morge G (1973) Entomology in the western world in antiquity and in medieval times. In: Smith RF, Mittler TE, Smith CN (eds) History of entomology. Annual Reviews Inc, Palo Alto, CA, pp 37–80
Morton R, Tuart LD, Wardhaugh KG (1981) The analysis and standardisation of light-trap catches of Heliothis armiger (Hübner) and H. punctiger Wallengren (Lepidoptera: Noctuidae). Bull Entomol Res 71(2):207–225
Nag A, Nath P (1991) Effect of moon light and lunar periodicity on the light trap catches of cutworm Agrotis ipsilon (Hufn.) moths. J Appl Entomol 111:358–360
Nagatsuka H (2000) Effects of reflective sheet for whiteflies and thrips. Plant Prot 54:359–362
Nakagaki S, Sekiguchi K, Onuma K (1982) The growth of vegetable crops and establishment of insect and mite pests in a plastic greenhouse treated to exclude near UV radiation.(2) establishment of insect and mite pests. Bull Ibaraki-Ken Hortic Exp Sta 10:39–47
Nakagaki S, Amagai H, Onuma K (1984) The growth of vegetable crops and establishment of insect and mite pests in a plastic greenhouse treated to exclude near UV radiation. (4) establishment of insect pest on tomatoes. Bull Ibaraki Hortic Exp Sta 12:89–94
Nakamura T, Yamashita S (1997) Phototactic behavior of nocturnal and diurnal spiders: negative and positive phototaxis. Zool Sci 14(2):199–204
Nemec SJ (1971) Effects of lunar phases on light-trap collections and populations of bollworm moths. J Econ Entomol 64:860–864
Neville AC (1960) Aspects of flight mechanics in anisoptera dragonflies. J Exp Biol 37(3):631–656
Nguyen THN, Borgemeister C, Max J, Poehling HM (2009) Manipulation of ultraviolet light affects immigration behavior of Ceratothripoides claratris (Thysanoptera: Thripidae). J Econ Entomol 102(4):1559–1566
Nichanant S, Chonmapat T (2015) Solar energy-based insect Pest trap. Soc Behav Sci 197:2548–2553
Nirmal A, Sidar YK, Gajbhiye R, Anil K, Ganguli JL (2017) A review on evaluation of light trap against different colored electric bulbs for trapping phototrophic insects. Bull Environ Pharmacol Life Sci 6(1):209–211
Nomura K (1967) Studies on orchard illumination against fruit piercing moths. III. Inhibition of moths’ flying to orchard by illumination. Jpn J Appl Entomol Zool 11:21–28
Nomura K, Oya S, Watanabe I, Kawamura H (1965) Studies on orchard illumination against fruit-piercing moths. I. Analysis of illumination effects, and influence of light elements on moths’ activities. Jpn J Appl Entomol Zool 9:179–186
Nonaka K, Nagai K (1985) Pest management using ultraviolet absorbing films. Agric Hortic 60:323–326
Nowinszky L (2004) Nocturnal illumination and night flying insects. J Appl Ecol Environ Res 2(1):17–52
Ohta I, Kitamura T (2006) Insect pest control by ultraviolet-absorbing plastic films for greenhouse crops. Crop Prod Plast Film 232:3–8
Patrick BH, Lyford B, Ward J, Barratt BIP (1992) Lepidoptera and other insects of the Rastus Burn Basin, the Remarkables, Otago. J R Soc New Zealand 22(4):265–278
Prokopy RJ, Owens ED (1983) Visual detection of plants by herbivorous insects. Annu Rev Entomol 28:337–364
Raviv M, Antignus Y (2004) UV radiation effects on pathogens and insect pests of greenhouse-grown crops. Photochem Photobiol 79:219–226
Ricklefs RE (1975) Seasonal occurrence of night-flying insects on Barro Colorado Island, Panama Canal Zone. J N Y Entomol Soc 83:19–32
Rydell J (1992) Exploitation of insects around streetlamps by bats in Sweden. Funct Ecol 6:744–750
Sermsri N, Torasa (2015) Solar energy based insect pest trap. Procedia Soc Behav Sci 197:2548–2553
Sharma AK, Mandloi R, Pachori R (2017) Study on biodiversity of phototactic harmful insect fauna collected in light trap in chickpea (Cicer arietinum Linn.) ecosystem. Int J Agric Sci 9(12):4037–4041
Shimoda M, Honda KI (2013) Insect reactions to light and its applications to pest management. Appl Entomol Zool 48(4):413–421
Simmons AM, Kousik CS, Levi A (2010) Combining reflective mulch and host plant resistance for sweetpotato whitefly (Hemiptera: Aleyrodidae) management in watermelon. Crop Prot 29(8):898–902
Southwood R, Henderson PA (2000) Ecological methods. Wiley-Blackwell, Hoboken
Stewart WWA (1970) A modified CDC light trap. Mosq News 30:188–189
Sudia WD, Chamberlain RW (1962) Battery-operated light trap, an improved model. Mosq News 22:126–129
Taylor LR, Brown ES (1972) Effects of light-trap design and illumination on samples of moths in the Kenya highlands. Bull Entomol Res 62:91–112
Taylor LR, French RA (1974) Effects of light-trap design and illumination on samples of moths in English woodland. Bull Entomol Res 63:583–594
Thomas AW (1996) Light-trap catches of moths within and above the canopy of a northeastern forest. J Lepid Soc 50:21–45
Tsuchiya M, Masui S, Kuboyama N (1995) Reduction of population density of yellow tea thrips (Scirtothrips dorsalis Hood) on mandarin orange (Citrus unshiu Marc.) trees by application of white solution with/without reflective-sheet mulching. Japanese Journal of. Appl Entomol Zool 39:305–312
Walcott B (1969) Movement of retinula cells in insect eyes on light adaptation. Nature 223:971–972
Walker AK, Galbreath RA (1979) Collecting insects at lights: a test of four types of lamp. N Z Entomol 7:83–85
Williams CB (1951) Comparing the efficiency of insect traps. Bull Entomol Res 42:513–517
Williams CB, French RA, Hosnisic MM (1955) A second experiment on testing the relative efficiency of insect traps. Bull Entomol Res 46:193–204
Wirooks L (2005) Die.kologische Aussagekraft des Lichtfangs. Eine Studie zur Habitatbindung und kleiner.einigen Verteilung von Nachtfaltern und ihren Raupen. Havixbeck-Hohenholte Verlag Wolf & Kreuels, p 320
Yabu T (1999) Control of insect pests by using illuminator of ultra-high luminance light emitting diode (LED). Effect of the illumination on the flight and mating behavior of Helicoverpa armigera. Plant Prot 53:209–211
Yamada M, Uchida T, KUramitsu O, Kosaka S, Nishimura T, Arikawa K (2006) Insect control lighting for reduced and insecticide-free agriculture. Matsushita-Denko-Giho 54(1):30–35
Yase J, Yamanaka M, Fujii H, Kosaka S (1997) Control of tobacco budworm, Helicoverpa armigera (Hubner), beet armyworm, Spodoptera exigua (Hubner), common cutworm, Spodoptera litura (Fabricius), feeding on carnation, roses and chrysanthemum by overnight illumination with yellow fluoresent lamps. Bull Natl Agric Res Cent West Reg 93:10–14. (in Japanese)
Yase J, Nagaoka O, Futai K, Izumida T, Kosaka S (2004) Control of cabbage webworm, Hellula undalis Fabricius (Lepidoptera: Pyralidae) using yellow fluorescent lamps. Jpn J Appl Entomol Zool 46:29–37
Yoon J, Nomura M, Ishikura S (2012) Analysis of the flight activity of the cotton bollworm Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) under yellow LED lighting. Jpn J Appl Entomol Zool 56(3):103–110
Young Jr DP, Erickson WP, Strickland MD, Good RE, Sernka KJ (2003) Comparison of Avian responses to UV-light-reflective paint on wind turbines: subcontract report, July 1999–December 2000 (no. NREL/SR-500-32840). National Renewable Energy Lab., Golden
Acknowledgement
The authors are grateful to the authority of the Department of Food and Public Distribution and ICAR-Indian Institute of Vegetable Research for their help and encouragement.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kammar, V., Rani, A.T., Kumar, K.P., Chakravarthy, A.K. (2020). Light Trap: A Dynamic Tool for Data Analysis, Documenting, and Monitoring Insect Populations and Diversity. In: Chakravarthy, A. (eds) Innovative Pest Management Approaches for the 21st Century. Springer, Singapore. https://doi.org/10.1007/978-981-15-0794-6_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-0794-6_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0793-9
Online ISBN: 978-981-15-0794-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)