Abstract
Microfluidic paper-based analytical devices are an attractive tool for point of care diagnostics as they facilitate fast detection without the need for any sophisticated instrumentation and skilled professional. These devices are disposable, portable, and affordable; hence, they are utilized in almost all the diagnostic domains for carrying out the detection. There are various aspects associated with the paper-based devices, namely working principle, reaction mechanism, fabrication schemes (2D/3D), detection sensitivity, and readout mechanism. Over the period, continuous progress is envisioned in all these domains to enhance the sensitivity of the detection and several variants, namely miniaturization, the inclusion of nanoparticles, multi-functionalization, etc. are also explored to make the detection more efficient. This chapter provides a state of the art review of the various aspects of paper-based microfluidic devices, including their fabrication scheme, sensing methodology, and their several applications in DNA detection domain. Also, advantages, disadvantages, and future aspects of these devices are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
DNA being the most basic entity in the biological world, there exist several schemes for carrying out its detection. There can be enormous ways to detect the DNA, but the basic principle of detection remains the same. DNA analyte is taken close to the sensory surface, some biochemical change is observed near the surface, and a corresponding transduction signal is generated through this biochemical change, which helps in identifying the analyte characteristics. Hence there are several components related to the detection aspect, namely substrate, detection methodology, detection chemistry, etc. Substrate and detection chemistry are mostly responsible for sensory surface design, while the type of transduction signal helps decide the detection methodology. There exist a variety of substrates on which DNA detection can be carried out, and they are silicon (Bhatt et al. 2019), polymers (Bhatt et al. 2017), glass (Zou et al. 2013), nanoparticles (Zhao et al. 2003), paper (Kumar et al. 2018) and carbon nanotubes (Li and Lee 2015), etc. There are majorly four types of detection methodologies which are utilized for DNA detection, namely colorimetric detection (Kumar et al. 2018), fluorescence detection (Bhatt et al. 2017), electrochemical detection (Bhatt et al. 2019) and mass-based detection (Kumar et al. 2019). Sometimes colorimetric detection and fluorescence detection are considered under the same head, optical detection. The different type of detection methodologies requires a different set up in terms of requirement of detection electrodes, detection chemistry, or some combination of both. A variety of detection entities/structures are carved on to the different substrates to facilitate the various detection schemes like fluorescence-based, electrochemical-based, and mass-based. Interdigitated electrodes (Bhatt et al. 2019), crenelated electrodes (Nakano et al. 2011), cantilevers (Nakano et al. 2011), microarray dot electrodes (Yafouz et al. 2013), etc. are fabricated at various times to facilitate the detection.
Mostly detection schemes are based on either hybridization based chemistry or amplification-based chemistry. Hybridization-based chemistry requires adhesion of the sensing entity on the sensory surface, and in case of DNA detection, it can be carried out through various means, like physical adsorption, covalent immobilization and streptavidin-biotin immobilization (Kant et al. 2017). The amplification-based detection is mostly dependent on the amplification of the analyte to the detectable limit and its quantization in terms of a detectable signal. In this line, the polymerase chain reaction (PCR) is the most deployable technique for detection. PCR was discovered years back in 1893 by Mullis (Mullis et al. 1987), and since then, it has evolved in many directions to prove its utility in different aspects. Hence there are various combinations of substrates, electrode geometries, and detection schemes that can be clubbed together to achieve sensitive detection. Various methodologies possess several advantages and some disadvantages as well. Some schemes are very sensitive while some facilitate easier and affordable detection aspect. Out of various existing stated schemes, paper-based detection devices are gaining attention these days, due to their easy and fast detection, biodegradable and handy nature and easier and affordable availability.
Paper-based microfluidic devices, as the name suggests, utilize paper as the substrate, on which some sensory elements are coated. These sensory elements are responsible for showing some color change corresponding to the analyte. Hence mostly colorimetric detection technique is utilized for observing the detection event which has happened. This is the most basic detection device, which is used in day to day life, as it does not require any minimum set up and today, many handheld paper-based detection devices are available in the market. This chapter will deal in detail various modules related to paper-based devices, their evolution, their detection principle, fabrication schemes, and their applications.
7.2 Evolution of Paper-Based Devices
The paper-based devices are the most deployable detection systems due to their easy and affordable availability and easy readout mechanisms. There exist several variants to paper based-microfluidic devices, namely, dipstick assays, lateral flow immunoassays (LFAs), and microfluidic paper-based analytical devices (μPADs). Dipsticks assays are the most preliminary and initial time paper-based devices that mainly referred to as urine test strips initially, which were developed by Jules Maunmene in 1850. Later it got transformed to pH test strips (Foster and Gruntfest 2009), which are widely accepted pH measurement platform even today. Further latex agglutination assay laid the basic foundation for LFAs (Wong and Tse 2008), and now it is widely used for the detection of various diseases. μPAD technology (Martinez et al. 2010) has been invented recently by inducing paper-based microfluidics to the paper-based devices, and it was also listed as one of the emerging technologies in 2009 by Technology Review magazine.
Paper-based devices mainly utilize hybridization/conjugation schemes on the sensory surface for carrying out the detection. Several conjugation schemes are developed for enhancing the detection sensitivity, which is discussed in detail in the forthcoming sections. As a substrate, a variety of paper materials, like filter paper and chromatography paper are used for devising paper-based devices, mostly in μPADs and dipstick assays. At a very basic regime, mainly for dipstick assays, the detection strips are simply fabricated by soaking the paper in a certain concentration of acid-alkali indicator mixture. For detection purpose, the strip is directly dipped into the analyte solution and color change that warrants the presence of the analyte is recorded. The other variety, namely LFAs, utilize a nitrocellulose membrane for making the devices. LFA test strip comprises of a sample pad, nitrocellulose membrane, conjugate pad, absorbent pad, and backing pad. The purpose of the absorbent pad is to provide a capillary-based driving force while the backing pad is to provide mechanical support to the device. Various capturing antibodies are deposited on the nitrocellulose membrane to form control and test lines capable of carrying out the detection. The techniques utilized for depositing these antibodies can be a hydrophobic force, a hydrogen bond, or electrostatic interaction. The sample pad is pretreated with buffer solution to help make analyte compatible with the other components of the device (Wong and Tse 2008). The analyte makes conjugate with particles already loaded in the conjugate pad and is further detected through sandwich or competitive mode of detection (Wong and Tse 2008). Figure 7.1 presents one such device based on LFA principle (Kumar et al. 2018), which is used for the detection of dengue NS1 antigen. The authors utilized gold decorated graphene oxide sheets on a tapered nitrocellulose membrane for achieving a detection limit of 4.9 ng/mL. 0.9° taper was used in the device for efficiency enhancement.

Reprinted with permission from Kumar et al. (2018) Copyright (2018) AIP
Lateral flow immunoassay device for the detection of dengue NS1. a Schematic (SP: Sample pad, CP: Conjugate pad, NC: Nitrocellulose membrane, T: Test line, C: Control Line, AP: Absorbent pad); b Detection scheme via nanoparticle aggregation; c Dimensional representation (in mm).
The most recent development, µPADs utilize capillary force in paper to drive the movement of analyte in the device. These devices can be 2D or 3D in nature. 2D µPADs comprises of formation of microchannels on the paper surface by patterning. Physisorption, mediator based deposition (some nanoparticles) or chemical coupling are used for immobilizing chemical/biological molecules on the paper surface. 3D µPADs are fabricated by stacking 2D µPAD layers one above another in a way to keep connection amongst each other (Martinez et al. 2008).
7.3 Principle of Detection/Reaction Mechanism
There are several reaction mechanisms that can be utilized in paper-based sensing devices (Hu et al. 2014). These are mostly detected through colorimetric detection schemes through chemical/biochemical reactions, as observed in pH test strips, biological reactions, and electrochemical reactions. In the case of chemical reactions, there are acid-alkali combinations which indicate the color change as in case of pH test strips. In the case of biological reactions, there come antibody-antigen chemistry, nucleic acid hybridization, and functional nucleic acid-based reactions. Antibody-antigen chemistry deals with detection via biological interaction of antigen with its respective antibody as coated on the test strip. This scheme is used in home pregnancy, tumor marker detection, and AIDS detection kits, etc. Nucleic acid hybridization is a more effective method for early detection of diseases, and here the detection is carried out through immobilizing a nucleic acid probe along with some kind of nanoparticles on the test strip and detecting the complementary target probe. Functional nucleic acids like aptamers, DNAzymes, and aptazymes can also be used as immobilization probe for detecting various ions, proteins, or some electrochemical molecules.
Along with colorimetric detection, there are also some modifications like fluorescence (Scida et al. 2013), chemiluminescence (Yu et al. 2011) and electrochemiluminescence detections which are currently being used in paper-based devices. In recent years, electrochemical detection is also being utilized through both redox and non-redox reactions (Han et al. 2013) for sensitivity enhancement of paper-based devices. Redox reactions are based on the electron transfer between the analyte molecules or particles while non-redox reactions deal with change in electrical properties of the analyte molecules like resistance, impedance, conductance or potential (Han et al. 2013).
Mostly μPADs report a color change which is observed through naked eyes, but this kind of measurement is not optimum, especially in clinical diagnostics, as a quantitative analysis is required to explain the sensitivity. The color change perception can also be different for different persons depending on the light availability as well. Hence some mode of quantification is required. Hence for application of these devices to the clinical trials, some camera or scanner is also utilized to express the exact color intensity, which can be further related to the concentration/amount of the analyte that can be detected. Zhu et al. have also explained the usage of mobile phone for detection signal quantification in point of care diagnostics (Zhu et al. 2013). Electrochemistry based techniques are further emerging well in terms of signal quantification. Also, the barcode detection scheme is also used for quantifying the analyte concentration (Cho and Paek 2001).
7.4 Fabrication Schemes of Microfluidic Paper-Based Devices
As stated in the earlier section, μPADs can be 2D or 3D in nature (Xia et al. 2016). Various techniques like plotting, photolithography, cutting, wax printing, screen-printing, plasma/inkjet etching are proposed for fabricating microchannels/geometries on the µPAD devices. Some of them are explained in detail here:
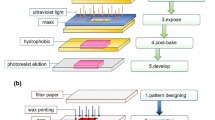
7.4.1 Wax Printing
Wax printing (Carrilho et al. 2009a) is the easiest, most economical, and eco-friendly method to achieve hydrophobic barriers in μPAD devices. There are different ways of wax printing, namely, painting through a wax pen, inkjet printing, and subsequent tracing by painting through wax pen or direct wax printing. The wax can be easily absorbed in the paper surface through heating. Figure 7.2a shows the basic schematic of the process. It is observed that test design can be printed and heated to obtain hydrophobic barriers. It can be observed from the schematic (Fig. 7.2d) that there is some deformation in the actual design after heating, the dashed lines present actual geometry while the carved geometry is a bit broader than the actual design. Hence while fabricating a μPAD device, this should be kept in mind while designing the mask.
Wax printing (Reprinted with permission from Carrilho et al. (2009a) Copyright (2009) American Chemical Society); a Schematic representation 1–3; b Test image digital design; c Printed design on Whatman no. 1 chromatography paper using solid ink printer; d Test design after heating. e Photolithography steps for fabricating μPAD devices (Reprinted with permission from Carrilho et al. (2009b) Copyright (2009) American Chemical Society)
7.4.2 Photolithography
Photolithography mainly deals with patterning paper sheets in a hydrophilic zone while they are surrounded by hydrophobic polymeric areas. Photolithography takes very less time for making microchannels on the surface in an inexpensive way by using photoresist (Carrilho et al. 2009b). Figure 7.2e shows the schematic of the fabrication steps which shows that hydrophilic zones can be created by coating a resist layer onto the paper surface both sides and subsequently exposing it from both sides through UV exposure and further developing and drying it.
7.4.3 Inkjet Printing
Inkjet printing is a new emerging cost-effective technique, which is a more precise printing technique to deliver biomolecules/indicator reagents into the microfluidic patterns for completing the sensing device. It utilizes direct design printing through digital inkjet printers to attain the device designs.
7.4.4 Laser Treatment
Laser treatment uses polymerization of photopolymers to guide the flow of fluids in the device. This process is mostly a one-step process for cutting the paper according to the designed pattern (Nie et al. 2013) through laser, hence is a quick process for cutting a variety of paper-based devices.
7.4.5 Plasma Treatment
In this technique, the paper is initially hydrophobized via octadecyl trichlorosilane (OTS) salinization, and further, the silanized paper is plasma treated via a mask with a channel network. The plasma exposed portions turn hydrophilic (Yan et al. 2015) to make distinct hydrophobic/hydrophilic areas.
7.4.6 Wet Etching
The fabrication of hydrophobic filter paper-based μPADs through selective wet etching comprises of two steps, first is hydrophobic patterning of hydrophilic filter paper through a patterning agent, trimethoxyoctadecylsilane and the second step is the alignment of paper mask penetrated with glycerol contained NaOH solution onto the hydrophobic filter paper to allow selective etching of silanized filter paper using an etching agent (Cai et al. 2014).
The mentioned techniques are mostly capable of fabricating 2D μPAD devices. Further, 3D μPAD devices are mostly fabricated by stacking the 2D μPAD devices in some manner to retain the fluidic connections. The stacking can be carried out by some adhesive joints or some other joining techniques. As 3D devices have higher utility concerning the sample handling and performance; more than one sample can be detected simultaneously through 3D devices, but these devices cost higher as compared to the 2D devices. The research is being carried out to enhance the simplicity and cost-effectiveness of the 3D devices. Philips et al. proposed a simple methodology for assembling the 3D μPAD devices, by using spray adhesive to permanently join the 2D films and removing time-taking alignment and assembly task (Lewis et al. 2012). Some authors have also used a toner as the thermal adhesive to reduce the equipment requirement (Schilling et al. 2013). The researchers have also utilized craft-cutting and lamination scheme (Cassano and Fan 2013) and also simple folding of fabricated 2D μPAD devices to attain 3D structures (Liu et al. 2012) to make these devices more robust and cost-effective. Table 7.1 presents the application of various fabrication schemes for μPAD devices and the corresponding sensitivity of the reported devices.
Substrates like filter paper, nitrocellulose membrane, and glass fiber membrane, etc. are used for making μPAD devices. These are used for detection of various analytes such as bovine serum albumin, glucose, uric acid, H2O2, and also ions through a variety of detection modes. Different combinations have reported the different level of sensitivity to make them usable in various modalities.
7.5 Applications of μPADs in DNA Sensing
The μPADs technology is growing very fast and is being used in various modalities for carrying out the detection. Although conventional devices do not have that level of sensitivity, the signal enhancement techniques are continuously being incorporated in the detection devices. The enhancement is mostly enzyme-based or metal ion based. μPAD technology is being used for detecting a variety of analytes, namely, bacteria, cells, ions, proteins, DNA, enzymes, antigens, etc. As DNA is the most basic entity for point-of-care diagnostics, this section further discusses the application of μPAD technology for DNA detection. Both kinds of assays, sandwich and competitive can be used for detection of proteins and nucleic acids.
Scida et al. utilized hybridization-based detection of DNA on an origami-based PAD. A competitive assay was designed where analyte, single-stranded DNA (ssDNA) and quencher labeled ssDNA competed to hybridize to a fluorescence-tagged ssDNA. The fluorescence growth was recorded to confirm the detection (Scida et al. 2013). Lu et al. proposed a 3D folded paper device for sensitive DNA detection (Lu et al. 2012). A folded electrochemical DNA detection device was fabricated, which comprised of gold nanoparticles/graphene modified screen-printed electrodes. Complementary DNA-thionine tagged dsDNA-nanoporous gold bioconjugate served as an efficient amplification label for detection of DNA. Along with the extracted DNA, the device was further tested for its performance for human serum assay and is found to perform well with that also. Liu et al. proposed an aptamer-based μPAD device for electrochemical detection of adenosine (Liu et al. 2012). The device is printed on a single piece of paper and folded to 3D configuration and laminated in a plastic casing. An aptamer immobilized on microbeads is utilized to bind to analyte target and further releasing the glucose oxidase-labeled DNA. The connected capacitor shows an increase in instantaneous current to express higher sensitivity.
Wei et al. proposed a DNA hydrogel mediated μPAD device for the detection of multiple target samples (Wei et al. 2015). The device was also capable of providing a signal-off readout in the case when the target is present. It is done by making hydrogel flow in the channel and further stopping the flow in the channel. When the target is present, no hydrogel formation is reported, and a smooth sample flow was recorded to express signal-on readout. Multiple targets like Pb2+, cocaine, and adenosine is simultaneously detected by the fabricated device. Cunningham et al. fabricated a paper-based electrochemical device for detecting DNA and thrombin by target induced conformational switching (Cunningham et al. 2014). The SlipChip concept was used for fabricating e-sensor. For ssDNA detection, stem-loop functionalized on 5’ end with thiol group was used as an electrode-bound receptor. 3’ end was containing methylene blue to act as an electroactive reporter. While for thrombin detection, thrombin aptamer was immobilized on the sensory gold surface, which allows redox reporter (methylene blue) to approach the working electrode. Gong et al. proposed a lab-on-paper device for Hepatitis B virus DNA detection (Gong et al. 2015). The device was designed in a way to be used for three different operations, namely pre-concentration, separation, and detection.
Davaji and Lee proposed first of its kind paper-based micro-calorimetric biochemical detection scheme for glucose and DNA (Davaji and Lee 2014), to accommodate the variability of temperature through various reaction for sensitive detection. The device presented detection through colorimetric scheme and Fig. 7.3 shows the schematic of the detection device along with the equivalent model of the reaction scheme. The device has shown temperature changes in biotin and streptavidin reaction.

Reprinted with permission from Davaji and Lee (2014) Copyright (2014) Elsevier
a Schematic of the fabricated paper-based device; b Top and cross-sectional view of the reaction site (along with equivalent model); c Fabricated device; d Knife plotter cut paper strips as a reaction substrate and a microfluidic channel.
Table 7.2 further lists various fabrication schemes, substrate selection, assay type, and limit of detection for the discussed μPAD devices for DNA detection.
7.6 Conclusions
As it is ubiquitously accepted that the paper is the most affordable entity, and this has appealed most researchers to make it usable for mankind through variable research schemes. Lab-on-paper is the most basic devices, which are readily available in the market for point-of-care diagnostics of many diseases. These devices are affordable and mostly provide reliable results for preliminary detection. Keeping the popularity of these paper-based devices in mind, this chapter discusses various aspects of μPAD devices. The chapter at first discusses the evolution of paper-based devices and the way they have moved through all these years. The various reaction schemes, fabrication methods, and detection methodologies are then discussed concerning the mostly μPAD devices. DNA being the most basic entity for detection is of preliminary importance for detection domain. Hence, the chapter further organized to discuss the application of μPADs for detection of DNA and related entities. The chapter further shows a comparison of various fabrication schemes to develop μPAD and their corresponding application for detecting various entities. As can be observed through table also, mostly colorimetric type of detection is used in μPAD devices but with time, various modifications like fluorescence, chemiluminescence, etc. are also being these days. Electrochemical μPAD devices are the most recent evolution in μPAD technology.
There are also some disadvantages associated with the paper-based devices, like dependence of external environmental conditions, humidity, availability of surrounding lights, and mostly dependent on the analyte concentration. These parameters may result in the false readout, as the perception of color varies for person to person depending on the surrounding light and also a lower concentration analyte can lead to negative results. Hence these devices are being refined more and more to make the detection easier and reliable.
References
Bhatt G, Kant R, Mishra K et al (2017) Impact of surface roughness on dielectrophoretically assisted concentration of microorganisms over PCB based platforms. Biomed Microdevices 19:28. https://doi.org/10.1007/s10544-017-0172-5
Bhatt G, Mishra K, Ramanathan G, Bhattacharya S (2019) Dielectrophoresis assisted impedance spectroscopy for detection of gold-conjugated amplified DNA samples. Sensors Actuators, B Chem 288:442–453. https://doi.org/10.1016/j.snb.2019.02.081
Cai L, Xu C, Lin SH et al (2014) A simple paper-based sensor fabricated by selective wet etching of silanized filter paper using a paper mask. Biomicrofluidics. https://doi.org/10.1063/1.4898096
Carrilho E, Martinez AW, Whitesides GM (2009a) Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal Chem 81:7091–7095. https://doi.org/10.1021/ac901071p
Carrilho E, Phillips ST, Vella SJ et al (2009b) Paper microzone plates. Anal Chem 81:5990–5998. https://doi.org/10.1021/ac900847g
Cassano CL, Fan ZH (2013) Laminated paper-based analytical devices (LPAD): fabrication, characterization, and assays. Microfluid Nanofluidics 15:173–181. https://doi.org/10.1007/s10404-013-1140-x
Cho JH, Paek SH (2001) Semiquantitative, bar code version of immunochromatographic assay system for human serum albumin as model analyte. Biotechnol Bioeng 75:725–732. https://doi.org/10.1002/bit.10094
Cunningham JC, Brenes NJ, Crooks RM (2014) Paper electrochemical device for detection of DNA and thrombin by target-induced conformational switching. Anal Chem 86:6166–6170. https://doi.org/10.1021/ac501438y
Davaji B, Lee CH (2014) A paper-based calorimetric microfluidics platform for bio-chemical sensing. Biosens Bioelectron 59:120–126. https://doi.org/10.1016/j.bios.2014.03.022
Foster LS, Gruntfest IJ (2009) Demonstration experiments using universal indicators. J Chem Educ. https://doi.org/10.1021/ed014p274
Gong MM, Nosrati R, San Gabriel MC et al (2015) Direct DNA analysis with paper-based ion concentration polarization. J Am Chem Soc 137:13913–13919. https://doi.org/10.1021/jacs.5b08523
Han KN, Li CA, Seong GH (2013) Microfluidic chips for immunoassays. Annu Rev Anal Chem 6:119–141. https://doi.org/10.1146/annurev-anchem-062012-092616
Hu J, Wang SQ, Wang L et al (2014) Advances in paper-based point-of-care diagnostics. Biosens Bioelectron 54:585–597. https://doi.org/10.1016/j.bios.2013.10.075
Kant R, Bhatt G, Sundriyal P, Bhattacharya S (2017) Relevance of adhesion in fabrication of microarrays in clinical diagnostics. In: Mittal KL, Etzler FM (eds) Adhesion in pharmaceutical, biomedical and dental fields. Scrivener Publishing LLC, USA, pp 257–298
Kumar A, Singh P, Awasthi M, Bhattacharya S (2019) α-Fe2O3 loaded rGO nanosheets based fast response/recovery CO gas sensor at room temperature. Appl Surf Sci 465:56–66. https://doi.org/10.1016/j.apsusc.2018.09.123
Kumar S, Bhushan P, Krishna V, Bhattacharya S (2018) Tapered lateral flow immunoassay based point-of-care diagnostic device for ultrasensitive colorimetric detection of dengue NS1. Biomicrofluidics 10(1063/1):5035113
Lewis GG, Ditucci MJ, Baker MS, Phillips ST (2012) High throughput method for prototyping three-dimensional, paper-based microfluidic devices. Lab Chip 12:2630–2633. https://doi.org/10.1039/c2lc40331e
Li J, Lee EC (2015) Carbon nanotube/polymer composite electrodes for flexible, attachable electrochemical DNA sensors. Biosens Bioelectron 71:414–419. https://doi.org/10.1016/j.bios.2015.04.045
Li X, Tian J, Garnier G, Shen W (2010) Fabrication of paper-based microfluidic sensors by printing. Colloids Surf B Biointerfaces 76:564–570. https://doi.org/10.1016/j.colsurfb.2009.12.023
Liu H, Xiang Y, Lu Y, Crooks RM (2012) Aptamer-based origami paper analytical device for electrochemical detection of adenosine. Angew Chem Int Edit 51:6925–6928. https://doi.org/10.1002/anie.201202929
Lu J, Ge S, Ge L et al (2012) Electrochimica acta electrochemical DNA sensor based on three-dimensional folding paper device for specific and sensitive point-of-care testing. Electrochim Acta 80:334–341. https://doi.org/10.1016/j.electacta.2012.07.024
Martinez AW, Phillips ST, Whitesides GM (2008) Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc Natl Acad Sci 105:19606–19611. https://doi.org/10.1073/pnas.0810903105
Martinez AW, Phillips ST, Whitesides GM, Carrilho E (2010) Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem 82:3–10. https://doi.org/10.1021/ac9013989
Mullis KB, Erlich HA, Arnheim N et al (1987) Process for amplifying, detecting, and/or cloning nucleic acid
Nakano M, Suehiro J, Konishi K et al (2011) Development of rapid oral bacteria detection apparatus based on dielectrophoretic impedance measurement method. IET Nanobiotechnol 5:25–31. https://doi.org/10.1049/iet-nbt.2010.0011
Nie J, Liang Y, Zhang Y et al (2013) One-step patterning of hollow microstructures in paper by laser cutting to create microfluidic analytical devices. Analyst 138:671–676. https://doi.org/10.1039/c2an36219h
Schilling KM, Jauregui D, Martinez AW (2013) Paper and toner three-dimensional fluidic devices: programming fluid flow to improve point-of-care diagnostics. Lab Chip 13:628–631. https://doi.org/10.1039/c2lc40984d
Scida K, Li B, Ellington AD, Crooks RM (2013) DNA detection using origami paper analytical devices. Anal Chem 85:9713–9720. https://doi.org/10.1021/ac402118a
Wei X, Tian T, Jia S et al (2015) Target-responsive DNA hydrogel mediated stop-flow microfluidic paper-based analytic device for rapid, portable and visual detection of multiple targets. Anal Chem 87:4275–4282. https://doi.org/10.1021/acs.analchem.5b00532
Wong RC, Tse HY (2008) Quantitative, false positive, and false negative issues for lateral flow immunoassays as exemplified by onsite drug screens. In: Lateral Flow Immunoassay. Humana Press, pp 1–19
Xia Y, Si J, Li Z (2016) Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: a review. Biosens Bioelectron 77:774–789. https://doi.org/10.1016/j.bios.2015.10.032
Yafouz B, di Kadri NA, Ibrahim F (2013) Microarray dot electrodes utilizing dielectrophoresis for cell characterization. Sensors (Basel) 13:9029–9046. https://doi.org/10.3390/s130709029
Yan C, Yu S, Jiang Y et al (2015) Fabrication of paper-based microfluidic devices by plasma treatment and its application in glucose determination. Acta Chim Sin 72:1099. https://doi.org/10.6023/a14060496
Yu J, Ge L, Huang J et al (2011) Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab Chip 11:1286–1291. https://doi.org/10.1039/c0lc00524j
Zhang Y, Zhou C, Nie J et al (2014) Equipment-free quantitative measurement for microfluidic paper-based analytical devices fabricated using the principles of movable-type printing. Anal Chem 86:2005–2012. https://doi.org/10.1021/ac403026c
Zhao X, Tapec-Dytioco R, Tan W (2003) Ultrasensitive DNA detection using highly fluorescent bioconjugated nanoparticles. J Am Chem Soc 125:11474–11475. https://doi.org/10.1021/ja0358854
Zhu H, Isikman SO, Mudanyali O et al (2013) Optical imaging techniques for point-of-care diagnostics. Lab Chip 13:51–67
Zou L, Li Y, Cao S, Ye B (2013) Gold nanoparticles/polyaniline Langmuir-Blodgett Film modified glassy carbon electrode as voltammetric sensor for detection of epinephrine and uric acid. Talanta 117:333–337. https://doi.org/10.1016/j.talanta.2013.09.035
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bhatt, G., Bhattacharya, S. (2019). Paper-Based Microfluidic Devices for the Detection of DNA. In: Bhattacharya, S., Kumar, S., Agarwal, A. (eds) Paper Microfluidics. Advanced Functional Materials and Sensors. Springer, Singapore. https://doi.org/10.1007/978-981-15-0489-1_7
Download citation
DOI: https://doi.org/10.1007/978-981-15-0489-1_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0488-4
Online ISBN: 978-981-15-0489-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)