Abstract
Excessive use of conventional fossil fuels resulted in a hike in price, exhaustion, and change in climatic conditions. Therefore, a novel route to biofuel generation is another feasible option of sustainable process development. Continuous upgradation of technologies for biofuel generations from the first generation (1G) to the fourth generation (4G) gives new hopes to fulfill energy demands. Biofuel generation from multiple approaches such as physical, biological (includes microbial and enzymatic), chemical, and biochemical catalysis with nanotechnology from multiple feedstocks is the key to biofuel generation. Suitable conversion of cellulosic biorefinery and lignin biorefinery (via lignin valorization) is the key for complete utilization of lignocellulosic biomass. Utilization of the biological methods includes the use of microbial machinery from different domains which might open the door toward an environmentally benign process. Nanotechnology and its potential application with 1G to 4G have future promises for increasing yield and integration of technology. So, this book chapter covers a detailed process overview of biofuel generations and its challenges with the hope of overcoming it.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Increase in population and economic growth has increased the energy demand, and fossil fuels fulfill this energy demand as per the data revealed from the International Energy Agency (IEA) in 2011. As the consumption of fossil fuels is nonrenewable and emits greenhouse gases (GHG) which severely deplete environmental conditions (Buragohain et al. 2010), therefore, the notable increase in renewable energy sources such as solar, wind, and biomass gives new hope of a sustainable process. Biofuels are the renewable energy sources defined as the energy derived from biological carbon fixation, which plays a pivotal role in new energy sources. Therefore, the production of biofuel requires extensive research and development of technologies. These biomass require suitable conversion of biomass into biobutanol, bioethanol, biomethanol, bio-dimethyl ether, biomethane, Fischer-Tropsch (FT) fuel, biohydrogen, algae, and halophilic lipid-based biofuels.
Plant biomass is an abundant source of renewable energy which comprises of the various components such as carbon, hydrogen, oxygen, and traces of minerals. Availability of plant biomass is more reliable compared to another energy sources such as solar energy, wind energy, and hydropower, and it can easily grow in variable environmental conditions. Moreover, the low density of biomass over fossil fuel makes it more feasible for storing and transportation purposes. In developing countries, utilization of plant biomass for household purposes (burning of wood) underestimates its value as a potential biofuel. The burning of fossil fuels and plant biomass is the main contributor to the increase in CO2 level and has a direct impact on global warming. GHG emissions and global warming open up the door for the novel processes of biofuel generation.
Lignocellulosic biomass is made up of highly complex biopolymer of cellulose (40–60%), hemicellulose (10–40%), and lignin (15–30%) as major component which gives support to plant, and it is resistant to various microbial degradations as well as biochemical conversions (Himmel et al. 2007; DeMartini et al. 2013; Schutyser et al. 2018). Cellulose and hemicellulose mainly consist monomeric sugar unit, and after pre-treatment, lignocellulosic biomass sugar component via further saccharification is converted into suitable biofuel (Chen 2014). However, the lignin component is made up of the phenylpropane unit, which is more recalcitrant than the cellulose and hemicellulose. During kraft pulping process, lignin is utilized for the co-power generation, which can be separated, and suitably valorized into useful precursors for biofuel generation. However, the hydrogenation process is being used as a suitable method for converting lignin into a valuable product and converting raw material into biofuel (Chen 2014; Abdullah et al. 2017). Lignin valorization strategy utilizes the complete conversion of lignocellulosic biomass into biofuel generation.
Different methodologies are adapted from time to time for treating the plant biomass for the biorefinery route. This methodology has its advantages and limitations. Here, pulp and paper industries are the best examples of lignocellulosic biomass biorefinery, and they are emerging as fermentative production of bioethanol (Ragnar et al. 2000; Hahn-Hägerdal et al. 2006; Limayem and Ricke 2012). Various existing conventional techniques result in low yield of primary products obtained from the lignocellulosic biomass, but in case of lignin, it can be suitably valorized into valuable products (Davis et al. 2013). Further, the optimization process is done by understanding the process. Even biofuels productions have a direct impact on carbon sink; therefore, biofuels produced from oil-based fuels always have a better choice compared to others GHG emissions into the environment critics of biofuels, which ultimately depends on the route of production. Another selection of biofuel has the debate over food versus fuel; land used for cultivation for feedstock always competes with food demand and land uses for productions. Therefore, the governments of the countries take so many initiatives for the continuous growth of the biofuel industry, which includes providing fund for research and development along with mandating laws of Environmental Protection Agency (EPA) for blending of biofuel with conventional fuel. Renewable Fuel Standard (RFS) provides harness power of biofuels and infrastructure, and high productions of biofuels must be produced and widely available for customers with the competitive price of conventional biofuel (Richards 2013).
1.2 Classification of Biofuels
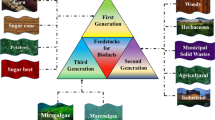
On the basis of origin of raw material biofuels are classified into first-generation (1G), second-generation (2G), third-generation (3G), and fourth-generation (4G) (Demirbas 2011). The first-generation biofuel, sugar, starch, vegetable oils, and fats are converted into bio-alcohol from (n = 1–4) fatty acid methyl esters (FAME). Second-generation biofuel depends on the carbon negative in terms of the carbon dioxide concentration in the environment, which majorly depends on the lignocellulosic biomass (plant material) (Gomez et al. 2008). The third and fourth generation of the biofuel utilize the algae and the blue-green algae (BGA) machinery for converting lipid into biofuel. In third generation, algae is directly used for the production of the biofuel, where in the fourth generation, metabolic engineered algae from the oxygenic photosynthetic microorganism create artificial carbon reservoir (Lü et al. 2011). Further, they are known as chemically synthesized biofuel and biologically synthesized biofuel. In chemical synthesis, biofuel is the generation with the help of a catalyst, and the action of various parameters such as pH, temperature, and pressure has played a significant role. Moreover, chemical methods, not benign environmental methods, in terms of the yield are preferable over biological methods. In the chemical process, the use of the catalyst is preferred over the various physical processes because of the more specific product formation and low cost of separation. Generally, physical processes yield more complex product formations, which are not easily separable and thus require a higher price for downstream processing. In biological methods, lignocellulosic biomass is microbially treated with bacteria, halophilic archaea, fungi, and algae. Moreover, other than these biological processes include various kinds of biocatalysts known as the enzymes used for the production of the biofuels’ generation. These enzymes are cellulases, xylanases, and lignolytic enzymes such as lignin peroxidase (LiP), manganese peroxidase (MnP), laccase, lytic polysaccharide monooxygenase (LPMO), multicopper oxidases, cellobiose dehydrogenase (CBDH), and lipases. Moreover, enzymatic use of the various processes of biofuel generations is the most environmentally friendly process which overcomes the numerous barriers of the chemical processes. Benefits of the enzymatic process yield specific product formation at optimum pH, temperature, and pressure. Therefore, enzyme from extremophile enzymes have added extra-advantages of various product formations at broad pH range and different temperature ranges from 4 to 80 °C, and they even act on higher pressure making them suitable for industrial process. To date, all the biological processes require a higher cost of production than the chemical process, and the cost-effectiveness of the biological process can only be reduced by the processes understanding of the microbial pathways, enzymatic reduction, and its substrate utilization strategy. However, new biological modified strains have shown various promises to overcome all barriers. In the last decade, there had been four industrial plants that started for the commercial production of the biofuel listed as “Project Liberty” by the joint venture of POET-DSM, 2G ethanol at Dupont, Abengoa Bioenergy Hybrid Kansas, and Crescentino by Beta Renewables in Europe (Valdivia et al. 2016).
Raw Materials
Availability of raw material is the key to success to achieve better economy and viability of the process. Biodiversity available on the earth is a good sink of energy as in the form of hydrocarbon and the production of hydrocarbon for biofuel generations are listed in the Table 1.1. As per requirement, feedstock can be classified on the basis of the source of origin. Feedstock can suitably be converted into biofuel and the abundance of this material in various forms are the source for different biofuel generations.
1.3 First-Generation Biorefinery
Concept of the biorefinery comes in the limelight for the researcher’s interest due to the limited resources of conventional energy sources and the rising price of the oil in the global market. 1G is produced from the food crops such as corn, sugar cane, rapeseed, cassava, etc. The first-generation biofuels are biodiesel, ethanol, and biogas, which are primarily producing so far. Biodiesel is the alternative biofuel to the diesel which is produced by the transesterification of the various vegetable oils and fats. However, small modification in the properties of biodiesel can make it a better substitute for the diesel. Whereas, bioethanol is the better substitute for the gasoline and is also known as the flexi fuel. Moreover, bioethanol is produced from various sources via fermentation of the sugar or starch material. Bioethanol is used as a feedstock for ethyl butyl ether (ETBE), which can easily blend with the gasoline. Another biofuel like biogas and biomethane is produced from the anaerobic digestion of the household and municipal waste. Biodiesel, bioethanol, and biogas can easily be produced from the food crop feedstock, but the increasing demand of the edible oils makes it difficult to use food crop for the biorefinery (Lee et al. 2014b). The big challenge of the production of the first-generation biofuel is the food to feedstock, high uses of the groundwater, fertilizer, and pesticide use on the crop.
1.3.1 Transesterification
The oil obtained from the various feedstock suitably transesterified into the fatty acid methyl esters (FAME) with the help of alcohol. The alcoholic group readily reacts with the triglyceride units in the presence of a suitable catalyst (homogenous or heterogeneous) with oil group and releases biodiesel and glycerol as high-value coproduct (Kulkarni et al. 2006; Meher et al. 2006). This mechanism can suitably be applied to all kinds of oil obtained from using algae, halophilic isolate, animal fat, and vegetable oil. The transesterification reaction is an equilibrium reaction in which continuous mixing is required for getting methyl esters (biodiesel). However, biodiesel obtained from transesterification reactions (given below) has similar characteristics as compared to diesel.
1.3.2 Ethanol Production
Ethanol is produced from sugar, starch, and cellulose-containing crops. Alcohol produced from the food crops and lignocellulosic biomass is known as the bioethanol, which can be easily produced by the biochemical process (Minteer 2016). Structure of starch consists of the polymeric unit of Dglucopyranose, which is first converted into glucose molecule with the action of enzymes “amylase.” Amylase produces the maltodextrin by liquefaction process. The process of saccharification of dextrin and oligosaccharides is hydrolyzed by the enzymes pullulanase and glucoamylase. The action of these enzyme converts the substrate into the smaller unit of glucose, maltose, and isomaltose. Then further, fermentation is carried by the yeast at 30 °C for ethanol production (Lee et al. 2014b).
1.3.3 Fermentation
The fermentation process converts organic compounds into simpler products with the action of the microbes and enzymes. Ethanol production from glucose utilizes fermentative consumption of pyruvate as an intermediate product (Ingram et al. 1987). The process can be classified as the aerobic and anaerobic among microbes depending up on oxygen requirement. Many microorganisms such as yeast (Saccharomyces cerevisiae), bacteria (Zymomonas mobilis), and halophilic bacteria (Halanaerobium saccharolyticum) convert butyrate and ethanol from glycerol and hydrogen from senegalensis (Kivistö et al. 2010). Most eukaryotes and prokaryotes follow Embden-Meyerhof-Parnas (EMP) also known as glycolysis pathway, and Zymomonas mobilis follows Entner-Doudorof (ED) pathway. Z. mobilis produces up to 97% of the theoretical yield of ethanol and also produces a 2.5-fold higher yield compared to S. cerevisiae (Weber et al. 2010). Moreover, Z. mobilis is suitably engineered for utilizing various types of sugar of lignocellulosic biomass such as glucose, mannose, and xylose for fermentation (Yanase et al. 2012). Another microorganism such as Clostridia can secrete hydrolytic complex to utilize both types of sugars (Lütke-Eversloh and Bahl 2011). However, these microorganisms are capable enough to ferment into C6 and C5 sugar or a mixture of both.
1.3.4 Anaerobic Fermentation
This process produces biogas under the absence of oxygen. However, anaerobic condition facilitates the growth of microorganism on organic biomass and converts it into methane (60–70%) and carbon dioxide (Naik et al. 2010). When we consider from the economic point, anaerobic digestion is an economically feasible process and also produces the biofertilizers for agricultural uses. The conventional process generally produces the gaseous fuel for the household purposes and electricity generation, but newer technology now focuses on the liquid fuel to overcome the separation process cost (Lee et al. 2014b). Utilizing simultaneous saccharification and fermentation (SSF) and anaerobic digestion in the case of birch wood by Saccharomyces cerevisiae efficiently converts sugar into ethanol, and stillage was subsequently utilized for the biomethane production (Kalyani et al. 2017).
1.3.5 Whole-Crop Utilization
In this concept, the whole crop utilization is applied for conversion of the whole plant into a useful product. Like Jatropha, oilseed contains 30–40% of oil. In the whole-crop utilization, different parts of the crop are treated separately. For example, oil produces the biodiesel, which can further be used for the conversion into oleochemicals. Remaining de-oiled cake is used for the gasification purposes, which can further convert into the syngas. Syngas finally is utilized for the production of various fuels.
1.4 Second-Generation Biofuels
Second-generation biofuels are produced from the nonfood crops such as agricultural residues (wheat straw, rice straw, and sugar cane bagasse, etc.) and organic waste from municipal solid waste. 2G biofuels have a key advantage over the first generation in terms of no competition with the food resources, their product formation with less emission of GHG, and less land requirement for the raw material. However, the mixed feedstock concept involved heterogeneous feed. When compared with 1G, the technological risk of operation is higher and also requires higher input cost for the process development. Process understanding in terms of the route selection requires higher technical knowledge and process modification (Margesin and Schinner 2001). Plant biomass consists of the plant cell, which comprised of the 75% of polysaccharide as a potential source of carbon (Pauly and Keegstra 2008). Lignocellulosic biomass is converted into the advanced biofuels through hydrolysis and fermentation (bioethanol and biobutanol) or gasification (FT biodiesel, dio-DME, and SNG). Raw materials for the advanced biofuels are rotational crops (poplar and Eucalyptus), Miscanthus, switchgrasses, and agricultural waste residues (Naik et al. 2010). Advanced bioethanol can be a suitable alternative of gasoline, and the sugars obtained from the hydrolysis are converted into Fischer-Tropsch diesel or biomass to liquids for the full substitution of the conventional diesel. In this, the lignocellulosic biomass with the help of suitable gasification is converted into syngas. Further, these syngas is utilized as the source of liquid hydrocarbon (kerosene, diesel, or bio-SNG), or this syngas can have transformed into the methane or dimethyl ether (DME) (Naik et al. 2010). 2G biofuels involve two main route strategies. First one is the thermochemical conversion route in which biomass conversion utilizes the thermal decay and chemical reformation in the presence of different concentrations of the oxygen. In the second one, biochemical conversion utilizes the conversion with the help of microbes using catalysts/biocatalysts. The major advantage of the thermochemical conversion over the biochemical is the conversion of the whole organic component into biofuel, but in the case of the biochemical conversion, it is only the polysaccharide that is converted into biofuel (Gomez et al. 2008).
1.4.1 Physical Process
1.4.1.1 Mechanical Extraction
In the mechanical extraction processes, crude oil from the oilseeds is recovered from the action of an external mechanical pressure generated by screw press. However, a small amount of the de-oiled cake (around 15–20% of oil) from the oilseed also comes out with the recovered oil. In some mechanical extraction processes, pre-pressing is followed by the solvent extraction for the high oil recovery around 30–40% (Stevens and Verhé 2004). On the other hand, in the full pressing system, high pressure of 9.5 MPa is applied to take out as possible oil content.
1.4.1.2 Briquetting
Various biomass resources obtained from agricultural, forest residues and other waste biomass come under second-generation fuel and are difficult to use as a direct biofuel due to uneven bulk. Therefore, this problem is overcome by the method of densification of the material to give near uniform compact shape. These densification methods are employed as pressing and maceration or combinations of both methods. In press, the density of the material increases by applying mechanical pressure in the early stage of compression. Further, an increase in the pressure has a negative impact on the density of material until it reaches the density of water, whereas in maceration, no such correlation of pressure exists, but chopping, grinding, and pulverization of the large material (like branches of the tree) are more effective methods. These methods are more effective compared to ultrafine grind material (Stevens and Verhé 2004; Osamu and Carl 1989).
1.4.1.3 Distillation
In this process, raw materials are crushed followed by steam distillation. Further, collective vapors are condensed separately which then turn into liquid form. Advanced distillation process known as molecular distillation is applied where conventional methods cannot be applied for the productions of the fragrances (Stevens and Verhé 2004).
1.4.2 Thermochemical Conversion
Thermochemical conversion involves heating of biomass followed by chemical conversion into biofuel. This process includes combustion, gasification, liquefaction, and pyrolysis under oxygen-deficient or oxygen-controlled conditions and results in the generation of syngas majorly comprising of H2 and CO. Further, this syngas is processed into gaseous or liquid products (Lee et al. 2014b).
1.4.2.1 Combustion
In the combustion process, the chemical reactions usually take place in the air where fuel and air react with each other. Therefore, the products formed during this process are carbon dioxide and water, and it liberates energy in the form of heat (Lee et al. 2014b). Combustion is the simplest way to use biomass into energy with the low yield of products.
1.4.2.2 Gasification
Gasification is a type of pyrolysis carried out at a very high temperature and produces a mixture of gases known as producer gas (CO, H2, CH4, CO2, and N2). In the case of biomass, gasification producers are methanol, ammonia, and ethylene. Gasification utilizes conversion of the lignocellulosic biomass into the biofuel since the past 30 years. Gasification has many processes which simultaneously go on, such as drying, pyrolysis, and partial oxidation (Bridgwater and Boocock 2013). The syngas produced from the gasification process can be through catalytic and non-catalytic processes. In the non-catalytic process, higher temperature ranges 1000–1300 °C for the syngas production; in the catalytic process, it is operated at around 900 °C due to more advances in catalysis (Lee et al. 2014b). The key in gasification is controlling H2/CO ratio for getting the different product in higher concentration (Melin et al. 2015).
1.4.2.2.1 Biomethanol
The reaction of the biomass requires oxygen to produce producer gas, which is the mixture of various gases such as CO, CO2, H2, CH4, and N2 (known as syngas). These producer gases have the potential to produce stationary power generation. Gases are first cooled down to 300 °C. Further, gases are fed to shift reactor with the controlled condition of H2/CO ratio provided to 2.1 for the methanol synthesis. Remaining acid gases are then removed by scrubber or dissolved in solvent (Vamvuka et al. 2004). Further, high pressure of 6.1 MPa is used to compress purified syngas to increase methanol synthesis:
1.4.2.2.2 Methane
All conditions for the preparation of the syngas follow the same procedure. Here, the use of catalyst which catalyzes tar with methane decomposition requires higher H2/CO ratio of 3.1, which forms methane at 31 bar of pressure and temperature between 300–600°C, whereas the chemical reactions are:
1.4.2.2.3 Bioethanol Production
Biomass is majorly used for the power steam generation. Wherein, SO2 feed with the steam explosion around 205 °C for a short time of 5 min. Then, steam is recovered by forming the product. This product flash and vapor formed during flash is condensed to recover furfural. After this steam recovery, the liquid is recovering with the help of additional water use for the simultaneous fermentation. Saccharification to form the simplest sugar is done at low temperature up to 40 °C. Therefore, saccharification forms the simplest sugar of hexoses and pentoses, which further are converted into bioethanol via the fermentation process:
Combine production of the methanol and ethanol is achieved by using the following strategy. First, hydrolyzed biomass of cellulose and hemicellulose is used for ethanol production. The remaining part such as lignin with the remaining residue of the hydrolyzed biomass is used for the methanol production (Melin et al. 2015). In other strategies, biomass is hydrolyzed and coupled with hydrogenation with the use of a catalyst such as lignin with the remaining residue of the hydrolyzed biomass used for the methanol production (Melin et al. 2015). In other strategies, biomass is hydrolyzed and coupled with hydrogenation with the use of a catalyst such as Ru at 160 °C and 50 bar pressure. The major advantage of this method is the stability of sugar which produces alcohols:
1.4.3 Liquefaction
Biomass is subjected to the presence of alkali, glycerine, propanol, butanol, or direct liquefaction (Demirbas 2004). This technique product is water insoluble, with high viscosity, which requires solvents, carbon monoxide (reducing gases) or hydrogen, or catalyst to be present in biomass.
In thermochemical conversion, lignocellulosic biomass is directly converted into the liquid as heavy liquid oils.
1.4.4 Pyrolysis of Biomass
The basic thermochemical process converts biomass into hydrocarbon-rich gas mixture in the absence/controlled oxygen conditions (Demirbaş 1998). In this process, biomass is converted into the vital product known as bio-oil, and other liquid products like acetic acid, acetone, and methanol. Solid products like charcoal, and gaseous products like non-condensable gases are also formed on heating 477 °C in the presence of air. However, around 70% of bio-oil are produced on a mass basis, whereas this process economically viable does not require pre-treatment of the biomass (Alonso et al. 2010). Therefore, the corrosive nature of the bio-oil towards equipment, and poor thermal stability has to be overcome by upgrading oxygen content, whereas, hydrogenation leads alkali removal and oil require catalytic cracking (Demirbaş 1998). Pyrolysis of lignocellulosic biomass such as wood is a zonal process, in which thermal degradations of hemicellulose, cellulose, and lignin occur (Chum 1991). Moreover, pyrolysis comprises of five stages (Demirbaş 2000):
-
First stage: Removal of moisture with some volatile loss
-
Second stage: Degradation of hemicellulose and emissions of CO and CO2
-
Third stage: Exothermic reaction and emission of CH4, H2, C2H6
-
Fourth stage: External energy supply
-
Fifth stage: Completion of the process
1.4.4.1 Fast Pyrolysis
This process is performed in the higher temperature range of 577–977 °C with fast heating rate achieved in short residence time of 0.5–10 s. In fast pyrolysis, biomass is converted into vapors, aerosols, and char. With further cooling and condensation of vapor, the aerosol’s dark color liquid is formed, whereas in terms of yield, 60–75% bio-oil, 15–25% char, and 10–15% non-condensed gases are formed (Shafizadeh 1982).
1.4.4.2 Flash Pyrolysis
Flash pyrolysis occurs in higher temperature than fast pyrolysis of 777–1027 °C with the fast heating rate of more than 723 °C/s (<0.5 s short retention time) and very fine particle size of <0.2 mm. Bio-oil is formed from flash pyrolysis, is mixed with char, and makes a mixture of bioslurry. Further, this bioslurry is fed to the gasifier at 26 bar pressure with 654–954 °C to convert into syngas with the conversion efficiency of 70%. This bio-oil formed from flash pyrolysis is used for the engines and turbines for the power generation (Demirbas 2004).
Biomass-derived syngas is used for different biofuel productions contaminated with various particulates, tars, nitrogen, sulfur contaminants, and halides with trace elements. These contaminants are removed before entering into the catalytic reactor system (Sikarwar et al. 2017).
Production of the 2G biofuel not an issue for the feed to food compared with 1G biofuel, but fiber crops used as raw material, compete with the food crops. Moreover, the pesticides and fertilizer use also the primary concern for the production of the second-generation biofuel.
1.5 Third-Generation Biofuels
In the early stage of development, algae are used for the generation of the 3G biofuel. Algae include the microalgae, macroalgae from seaweed, and cyanobacteria, also known as blue-green algae (BGA). More than 800,000 algal species are exist on the earth, and algae can rapidly grow and harvested in a month, and minimal nutrient is required for their growth; even economic production in wastewater is an added advantage (Bowyer et al. 2018). The potential of oil production from algae is 100 times greater than in area basis than the soya bean and canola oils (Hu et al. 2008). For the better growth of the algae, the optimal temperature range is between 20 and 30 °C and slow growth observed is lower than 16 °C, whereas above 35 °C is deleterious for the algae growth (Menetrez 2012). Microalgae produce lipid through photosynthesis, and lipid content reaches around 20–80% of dry weight depending on the species. However, changes in the nutritional requirement can change the product yield of the lipid, protein, and carbohydrate (Shen et al. 2009). After that, lipid extracted by different extraction processes is used for the production of the biodiesel, and the leftovers, carbohydrate and starches (residual), are further processed and can be used for the production of ethanol (Shen et al. 2009; Coppola et al. 2009). Lipid extraction process includes mechanical and chemical extractions (Halim et al. 2012). Table 1.2 summarizes the various method of extraction.
1.5.1 Open Pond
A natural water reservoirs like lake and lagoon and artificial reservoirs system like ponds, shallow ponds, and tanks are the open pond system. Advantage of the open system is that it is easy to construct and operate, but poor utilization of the light, evaporation loss, loss of the CO2 to the environment, and chances of contamination are the significant key challenges of the open pond system. This system can be suitably installed with the wastewater treatment plant (Beal et al. 2012). But the major drawbacks of this system lacks control of various parameters.
1.5.2 Photobioreactor (PBRs)
The system is closed, therefore it provides a better internally maintained control system for the growth of pure algal culture as they maintain various parameters of inadequate amount such as CO2, water, temperature, light intensity, mixing density, and pH of the system (Lee 1999; Mussgnug et al. 2007). In PBRs, algae are cultured in glass or plastic tube, where they are grown in light of the particular wavelength which supports the optimal growth of the algae. Approximately, 7.6–10 cm light penetrates inside the PBRs due to the turbidity of the algal growth into PBRs (Lee 1999; Mussgnug et al. 2007; Müller et al. 1993; Rapala and Sivonen 1998). For the easy operations of the PBRs, various conditions optimized like volume size to the area for the optimum light penetration, spatial distribution of light, CO2 transfer rate, and monitoring of the growth inside the reactor (Menetrez 2012). Their installation and operational costs are higher, but product yield in terms of oil is obtained around 40–55% (Hu et al. 2008; Chisti 2007). Due to the less expensive operation of the open system, it can be suitably hybrid with the closed type of PBRs for optimizing cost and product yield (Menetrez 2012).
Algae are an excellent source to produce various biofuels such as biodiesel, ethanol, and petroleum. From various reports, productivity from the algae is 602 times higher than various crops like corn and switchgrasses (Menetrez 2012). The significant advantage of algae is higher productivity, and small land for the cultivation is required. Transesterification of oil recovered from algae produces biodiesel chemically and biologically. Moreover, biological production utilizes a lipase enzyme which converts lipids into biodiesel at room temperature. This operation at room temperature cuts the energy requirement for biodiesel production (Fig. 1.1).
1.6 Fourth-Generation Biofuel
The use of the new synthetic biological tools has emerged as the fourth-generation biofuel. In this generation, the breakthrough has made promises to photobiological biofuels and electro biofuels (Aro 2016). Synthetic biology of the algae and cyanobacteria involves various designs of the new promising strains by using recombinant deoxyribonucleic acid (DNA) technologies. This technology uses the synthesis of the new strain by using new devices and the integration of the new biological parts or redesigning of the biological system for the biofuel generation.
The fourth-generation biofuels are inexhaustible, cheap, and readily available resources compared in (Table 1.3) with other generation of biofuel. During photosynthesis process, water molecule splits into hydrogen and oxygen by the action of solar energy with the interaction of photosystems I and II which is the key for the biofuel production at a large scale (Inganäs and Sundström 2016). Moreover, artificial photolysis is emerging as the new door for the biofuel generation (Inganäs and Sundström 2016). Even CO2 water can also use as an alternative strategy for biofuel production, which utilizes carbon-based solar fuel.
1.6.1 Direct Process for Solar Fuel
This technology utilizes the combination of sensitizers and catalyst for performing individual reactions and generating approximately 30% of the solar fuel from water, but no complete device to date is made for direct fuel productions (Inganäs and Sundström 2016). In this process, oxidation of the water and reduction of the substrate are principle mechanisms. Further, proton from substrate utilized for the hydrogen fuel generation. The catalyst used is generally made up of abundant metals like cobalt, iron, and manganese, etc. In another way, an artificial photosynthesis follows the molecular mechanism; it involves the enzymes which are catalytically active used as biocatalyst like hydrogenase enzyme, which is used for hydrogen production by dark fermentation (Tamagnini et al. 2007). In direct process, with the help of the nanotechnologies, hydrogen production follows the non-molecular mechanism light-driven catalysis on the surface. This surface area can be metal, semiconductors, or fabrication of nanostructured carbon-based material. Limitations of the non-molecular mechanism are expensive, and rare metals are used as the catalyst. Involvement of molecular and non-molecular mechanism involved makes the process complex thus less understood. Limitations of the non-molecular mechanism are the use of rare metals as the catalyst which makes it expensive.
1.7 Microbial Conversion
Production of the biofuel is still a challenge because the production cost is more than the raw material. In bioprocessing, microbial conversion step converts lignocellulosic biomass into biofuel by microbial action (no action of enzyme). This method has gained more interest from researchers over the first-generation biofuel production in terms of the cost (Lynd et al. 2005). The new trend of bioprocess states that the potential of ethanol production through microbial route or its combinations mainly utilizes the cellulose machinery with other components (hemicellulose and lignin) of the pre-treated biomass with the high yield of the ethanol. Various aerobic fungi can degrade pre-treated feedstocks directly into the CO2, and only a minor amount of ethanol is produced. However, the electrons yielded are transferred to the respiratory chain for oxidative phosphorylation, and the metabolic pathway does not utilize the substrate level phosphorylation for ethanol production. The rule of thumb of alcohol production from sugar is that 2 moles of alcohol is produced from each mole of hexose sugar. Yeast like Saccharomyces cerevisiae uses the glycolytic pathway to form alcohol. All the industrial processes are operated at higher pH and temperature range, which suitably use consortia of potent fungal strain like Sarocladium strictum with thermophiles like Halomonas for the improved degradation of wheat straw (Cortes-Tolalpa et al. 2018) (Table 1.4).
1.8 Enzymatic Conversion to Biofuel
Biofuel production from lignocellulosic biomass has given the new option to fulfill global energy demand but has a greater challenge of global warming. Production of biological-derived energy known as “bioenergy” is a major concern with the biocatalysis (utilizes the various enzymes to catalyze biomass conversion) (Rubin 2008). The complex structure of the lignocellulosic biomass first gets hydrolyzed into its simplest fractions with various hydrolyzing enzyme cocktails. However, the use of enzyme cocktail proved the maximum conversion into industrial product formation from lignocellulosic biomass conversion. Enzyme consortium is another way used to deconstruct recalcitrant substrate into a useful product (Lopes et al. 2018). This enzyme consortium conversion requires detailed studies on various enzymes and their action to work synergistically. To date, Sprizyme® from Novozyme, a commercial enzymatic cocktail is available in three versions (in the market) for the complete conversion of sugar. Moreover, Cellic®CTec3 (2017) from Novozyme is using cellulose conversion into ethanol. Enzyme cocktails enable the enzyme to hydrolyze cell wall structure due to their specific action. Moreover, the product of one enzyme acts as the substrate of another enzyme and also gives an option to the individual enzyme to act on an available substrate. During enzyme-substrate reaction, enzyme inhibitors are also produced, which can inhibit the hydrolysis process. Therefore, a complete enzyme mechanism can be studied to increase lignocellulosic biomass conversion. Wherein, each substrate in cellulosic biomass has its characteristic and feature which require the distinct condition to convert it into its simplest form.
1.8.1 Cellulases
There are a group of three enzymes which act synergistically on cellulose and completely convert it into glucose. These enzymes are endoglucanase, exoglucanase, and β-glucosidase.
Endoglucanase: This enzyme randomly hydrolyzes β 1-4 glucosidase linkages at the amorphous region and forms 2 Carbon units of cellobiose from cellulose chain.
Exoglucanase: Other names are cellobiohydrolase, avicelase, and exocellulases, which cleaves at exterior ends of the crystalline part of cellulose and produces cellobiose as a product.
β-glucosidase: This enzyme cleaves 2C chain molecule (cellobiose) into glucose.
These all three enzymes are collectively known as cellulytic enzyme, majorly produced from Trichoderma reesei. Various thermophilic potent strains such as Thermophilic clostridia and Thermoanerobacterium are also reported in producing cellulases (Lamed and Zeikus 1980).
1.8.2 Xylanases
Xylan sugar is an important component of hemicellulose which is made up of 5C chain unit (pentosans). Xylanases are the enzymes, which are used to degrade xylan unit linked with the lignin and cellulose component. Removing the xylan unit from lignocellulosic biomass gives easy accessibility to other hydrolyzing enzymes or chemicals for the increasing saccharification of cellulose. Various microbial species like Trichoderma reesei and Humicola insolens are the potent strain which degrades xylan at higher temperatures of 40–60 °C (Binod et al. 2018).
1.8.3 Lignolytic Enzymes
These are lignin-degrading enzymes which utilize lignin phenolic and non-phenolic group as substrates comprising of lignin peroxidase (LiP), manganese peroxidase (MnP), laccases, versatile peroxidase (VP) and dye-oxidizing enzymes (DyP A and DyP B). The action mechanism of LiP acts on the surface of the phenolic and non-phenolic aromatic component, and MnP acts on phenolic substrates by the action of Mn3+, and VP has characterstics of both LiP and MnP (Schoemaker and Piontek 1996). Lignin-degrading enzymes remove lignin in the pre-treatment step of lignocellulosic biomass and expose site for easy degradation of cellulose.
1.8.4 Cellobiose Dehydrogenase (CBDH)
This enzyme is the oxidative enzyme used to oxidize various sugars such as lactose, glucose, and cellobiose. Wherein, the substrate is oxidized by the action of CBDH and reduction of flavin into flavin adenine dinucleotide (Knöös et al. 2014). In another way, CBDH activates cellulases by product inhibition of cellobiose. Moreover, another enzyme of this class is lytic polysaccharide monooxygenase copper (LPMO) enzyme, and it needs an external electron donor for its activity to cleave C1, C4, or both glycosidic bonds of cellulose. LPMO acts on first C-H bond of cellulose followed by the oxygen-dependent chain for the degradation into the product (Eibinger et al. 2014).
1.9 Effect of Surfactant on Enzymatic Hydrolysis
Surfactant positively enhances enzymatic hydrolysis and reduces hydrolysis time and dosage of enzymes (Helle et al. 1993). Surfactant hydrophobically interacts with the lignin part of lignocellulosic biomass and doubles the yield by increasing surface modifications or disruption; even surfactant acts as enzyme stabilizer (Kim et al. 1982). However, nonionic surfactant used in enzymatic hydrolysis such as Tween (80, 20) and polyethylene glycol (PEG) has more effect than the ionic surfactant as it increases higher surface for hydrolysis (Binod et al. 2018) (Table 1.5).
1.10 Biofuel from Nanotechnology
Nanotechnological advancement for improving bioprocess requires nontoxic NPs for microbial growth, which is economically produced and environment friendly. However, nanomaterial provides high surface area, high catalytic activity, stability, storage, durability, and higher potentials of 3R principle (recovery, reusability, and recycling). Previous reports showed the successful implementation of nanotechnology with all types of biofuel-producing processes such as transesterification, anaerobic digestion, gasification, FAME, and renewable hydrocarbons shows efficient and economic feasible only at lab scale (Zhang et al. 2010). Various nanocatalysts such as titanium dioxide, magnesium oxide calcium oxide, and strontium oxide are developed with high catalytic performance. Nanotechnology is also applied with the enzyme (biocatalyst) immobilization in nanoencapsulation, entrapment with silaffin, and adsorption for providing higher surface area, higher stability, higher enzyme loading and also gives the chance of reusability. Nanoparticles are used for the extraction of lipid from algae without harming cells. Nanotechnology can suitably bring breakthrough in advanced fermentation, pyrolysis, jet fuels, catalytic conversion gasification, biofuel cell, carbon capture storage, and nano-based precision forming technology (Nizami and Rehan 2018). Nanoparticles recently gained lots of interest due to their feasibility to enhance effect metabolic engineering by improving performance or product yield. Biohydrogen produced by the microbes shows enhanced production in anaerobic conditions using NPs. Whereas, these NPs improve reaction kinetics with increased transfer of electrons. Though several NPs have been reported to enhance dark fermentation for hydrogen production with incorporation of gold NPs, which shows stimulatory effect on substrate utilization by 56% and increases yield by 46%, this enhanced surface area to volume ratio provides better accessibility of binding site with bacteria as well as enzyme (Zhang and Shen 2007; Sekoai et al. 2019). Various mesoporous NPs have been developed to overcome the barrier of higher dosing of NPs for improving the production of biofuel. When NPs supplemented (zero-valent metals, metal oxide, and carbon-based NPs) with the anaerobic fermenting bacteria which further increase the hydrolysis process of organic material by increasing substrate utilization, lipase catalyzing biodiesel production by transesterification reaction produces biodiesel; this enzyme suitably nano-immobilized, produces biodiesel, and increases the reusability of the enzyme. Therefore, the use of nanotechnology also improves bioethanol production by immobilizing cellulases using manganese oxide dependent (NPs), it increases hydrolysis and catalytic efficiency of cellulase. Immobilization of enzyme by NPs acts as enzyme protector from the intermediate inhibitor. Nevertheless, NPs modified enzyme can sustain in extreme environmental conditions and supports higher product formation with reusability. Since nanotechnological implementation is still under laboratory scale, it requires higher knowledge and techno-economic assessments for process modification before its commercialization.
1.11 Lignin Strategy to Biofuel
1.11.1 Lignin Structure
Lignin matrix consists of heterogeneous structure of aromatic alcohol such as p-coumaryl, coniferyl, and sinapyl alcohols. The aromatic monolignols from lignin form guaiacol (G) from coniferyl alcohol, p-hydroxy phenyl (H) from coumaryl alcohol, and syringyl from sinapyl alcohol (S) gives lignin with distinctive characteristics from each other. Variation in monolignol compositions forms distinctive properties of wood. Where G/S-Units ratio are highly present in softwood lignin, and a variable composition ratio of G/S/H is present in hardwood lignin (Laskar et al. 2013; Lee et al. 2014a). Aromatic structure of lignin transferred to hydrocarbon (HC) fuels via chemical treatment produces products having similar properties like gasoline and diesel (Ragauskas et al. 2006).
1.11.2 Lignin Valorization
In the cellulosic biorefinery, the cellulosic part, which is consist of 45–60%, is utilized for the ethanol production; the rest of the ~20% part of lignin leftover is the key motivation for the lignin valorization into biofuel and generation of value-added by-product. This strategy involves depolymerization of lignin by using hydrogen as the reducing agent to form aromatic product with low oxygen content with higher stability. Due to the presence of different lignin monomers, degree of interaction varies to form useful product (Ragauskas et al. 2014). This barrier of interaction overcome by different pre-treatment strategies of lignocellulosic biomass. In case of hydrogenation operated at mild conditions with the help of a suitable catalyst, which ensures the desired product formation in complex reaction. In an in situ catalytic characterization, hydrogenation process yields guaiacol conversion with cyclohexanol selectivity of 99% and ~94% in 7 h, 220 °C, and initial pressure of 2 MPa with the suitable ration of water/methanol/lignin as feedstock (20:5:0.5) (Yu et al. 2013). Presently, in highly selective hydrogenation at mild conditions, step-by-step precipitation is done with the help of catalyst (Ni/SiO2). This highly selective hydrogenation process is operated in 7h, 120 °C, and 2 MPa for complete conversion into guaiacol (Shu et al. 2016). Moreover, in wet impregnation technique of hydrogenation palladium is suitably doped with alumina as a catalyst, where 3 weight % of catalyst converts 4-ethylphenyl(cyclohexanol) in aqueous phase reaction operated for 6h, 60 °C (Yi et al. 2016). In lignin valorization other than the chemical catalyst, biological catalyst like lignolytic enzyme is also utilized for future trend of biofuel generation. The value added HC fuels produced from lignin are able to converted into C7-C18 hydrocarbon, which have suitable applications in jet fuel (Wang et al. 2015). This lignin to biofuel strategy involves degradation of lignin in such a way that O2/C and H/C ratios match to conventional fuel. In case of hydrodeoxygenation (HDO) technique, lignin monomers increase the ratio of H/C while decreasing their O/C ratio. This process includes cleavage of CO bond in monomeric lignin unit and oxygen removal achieved by high-pressure hydrogen, which forms water vapor with the decrease in O/C ratio. Further more, double bond of aromatic carbon is saturated with the help of hydrogen using catalyst (palladium and active carbon) (Ge et al. 2017).
1.12 Sustainability Criteria
1.12.1 Food and Feedstock
Crop food used for the biofuel generation in 1G affects the food requirement in developing countries; even increasing the price of agricultural product, cereal, and sugar is the key public agenda. Therefore, biofuel expansion requires both increased agricultural productivity for food and feedstock for animal and biomass material for biofuel. This would lead conversion of forest land, and further, this would affect the increase in GHG and a potential risk to biodiversity. Moreover, 3G biofuel gives hope for the high product yield, and land requirement, but the downstream cost is higher to be reduced.
1.12.2 Water Requirement
Even 2G biofuel requires nonfood crop, but it competes with land for cultivation of lignocellulosic biomass such as Jatropha, which also requires other resources like water uses (400–700 times higher than other energy sources) and 90% more than the production of feedstocks (Mandil and Shihab-Eldin 2010).
1.12.3 Emissions
As expected biofuel reduces GHG emissions by 60–94% compared to the fossil fuels, but when we compare the scenario with climatic change and lifecycle assessment, biofuel is worse than gasoline (Highina et al. 2014; Holma et al. 2013).
1.12.4 Biodiversity
Biofuel production and conversion of land affect the environment, sustainability, and biodiversity. Conversion of land and forest may affect a wide range of flora and fauna of the ecosystem. No methods are available, which can tell about the direct impact on biodiversity and its damage (Araújo et al. 2017). Therefore, biodiversity is considered foremost, integrated with the biofuel generation planning and agricultural farming (Morgera et al. 2009).
1.12.5 Policies
Policies and its implementation for biofuel generation play a pivotal role in the development of sustainable development, reducing barriers, greater economics, better implementation of technologies, and funding agencies. However, many countries like Brazil, the USA, China, and India have their own regulation policies for the development of biofuel and blending with the flexi fuel. These policies suitably help for reducing subsidies to conventional fuel and mandate blending of biofuel to curb out CO2 emissions (Table 1.6).
1.13 Conclusions
In this book chapter, biofuel production disscussed on different process methodologies, uses of different feedstocks, its key challenges. The production of biofuel plays a pivotal role in the current scenario. In case, 1G technology impacts on food prices, and competition with the food crop is the key challenge. Wherein, the concept of 2G comes into the biofuel production, which is economically better than 1G, but again its competition with the land uses and other resources makes it difficult. Further, concept of 3G uses of algae and BGA strains, which require low area and no competition with food feedstock and high product yield opens a new door, but its downstream processing cost is still a challenge. This 3G technology is still in the developmental phase for increasing higher product yield. The genetic manipulation and engineered strain reduces its downstream cost and yield known as 4G of biofuel. Apart from 1G to 4G new advancement in nanotechnology, has potential with technological advancement increases the effectiveness of processes. Generation of biofuel from the chemical process is commercially viable but still have challenge to environment. Therefore, biologically and biochemically preccesses are promising and environmentally friendly. Furthermore, several attempts are made for making the enzymatic process economically viable, and still industrial important enzymes are required. Biofuel production requires several issues for careful analysis such as; feedstock selection, process design, reduction of cost, cultivation practices, land uses and its practices, GHG, cocktail preparation, effect on soil, and mineral, etc. Shortly, the processes compacted and designed by removing pre-treatment strategy. Lignin valorization strategy is also gaining interest to convert lignin as biofuel in recent trends. The suitable blending of biofuel with existing fuel is the alternate best option. However, large-scale production is the ultimate requirement shortly to fulfill our energy need. Nevertheless, there is still a need for technological understanding with advancement and integration of the concept of chemistry, bioprocess, and chemical engineering in biofuel generation.
1.14 Summary
This book chapter gives the idea about how biofuels are formed from various feedstocks using multiple technology strategies. Biofuel production requires various chemical, biological, biochemical processes, and nanotechnologies. Moreover, biofuel formed from these technology showed similar characteristic properties as fuel from fossil fuel would be blended suitably to open the doors of sustainable process development. In the future complete development of biofuel technology will reduce dependence on fossil fuels.
Abbreviations
- 1G:
-
First generation
- 2G:
-
Second generation
- 3G:
-
Third generation
- 4G:
-
Fourth generation
- BGA:
-
Blue-green algae
- C:
-
Carbon
- CBDH:
-
Cellobiose dehydrogenase
- Centimeter:
-
cm
- CH4:
-
Methane
- CNG:
-
Compressed natural gases
- CO:
-
Carbon monoxide
- CO2:
-
Carbon dioxide
- DME:
-
Dimethyl ether
- DNA:
-
Deoxyribonucleic acid
- ED:
-
Entner-Doudorof
- EMP:
-
Embden-Meyerhof-Parnas
- ETBE:
-
Ethyl butyl ether
- FAME:
-
Fatty acid methyl esters
- FT:
-
Fischer-Tropsch
- GHG:
-
Greenhouse gases
- H2:
-
Hydrogen
- HC:
-
Hydrocarbons
- HDO:
-
Hydrodeoxygenation
- Lignin peroxidase:
-
LiP
- LPG:
-
Liquefied petroleum gas
- LPMO:
-
Lytic polysaccharide monoxygenase
- Manganese peroxidase:
-
MnP
- Mn:
-
Manganese
- MPa:
-
Mega Pascal
- N2:
-
Nitrogen
- NOx:
-
Nitrogen oxide
- NPs:
-
Nanoparticles
- PBR:
-
Photobioreactor
- PEG:
-
Polyethylene glycol
- PM:
-
Particulate matter
- SNG:
-
Syngas
References
Abdullah B, Muhammad SAFAS, Mahmood NAN (2017) Production of biofuel via hydrogenation of lignin from biomass. In: New advances in hydrogenation processes-fundamentals and applications. InTech, London
Alonso DM, Bond JQ, Dumesic JA (2010) Catalytic conversion of biomass to biofuels. Green Chem 12:1493–1513
Araújo K, Mahajan D, Kerr R, Silva MD (2017) Global biofuels at the crossroads: an overview of technical, policy, and investment complexities in the sustainability of biofuel development. Agriculture 7:32
Aro E-M (2016) From first generation biofuels to advanced solar biofuels. Ambio 45(Suppl 1):S24–S31
Beal CM, Hebner RE, Webber ME, Ruoff RS, Seibert AF, King CW (2012) Comprehensive evaluation of algal biofuel production: experimental and target results. Energies 5:1943–1981
Beopoulos A, Cescut J, Haddouche R, Uribelarrea J-L, Molina-Jouve C, Nicaud J-M (2009) Yarrowia lipolytica as a model for bio-oil production. Prog Lipid Res 48:375–387
Binod P, Gnansounou E, Sindhu R, Pandey A (2018) Enzymes for second generation biofuels: recent developments and future perspectives. Bioresour Technol Rep 5:317–325
Bowyer J, Howe J, Levins RA, Groot H, Fernholz K, Pepke E, Henderson C (2018) Third generation biofuels implications for wood-derived fuels. Available online: http://www.dovetailinc.org/report_pdfs/2018/dovetail3gbiofuel0218.pdf. Accessed on 28 Feb 2019
Bridgwater A, Boocock D (2013) Developments in thermochemical biomass conversion: volume 1. Springer, Dordrecht
Buragohain B, Mahanta P, Moholkar VS (2010) Biomass gasification for decentralized power generation: the Indian perspective. Renew Sust Energ Rev 14:73–92
Chen H (2014) Chemical composition and structure of natural lignocellulose. In: Biotechnology of lignocellulose. Springer, Dordrecht/Heidelberg
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chum HL (1991) Polymers from biobased materials. Noyes Data Corp, Park Ridge
Chung D, Cha M, Guss AM, Westpheling J (2014) Direct conversion of plant biomass to ethanol by engineered Caldicellulosiruptor bescii. Proc Natl Acad Sci 111(24):8931–8936
Coppola F, Simonciniand E, Pulselli R (2009) Bioethanol potentials from marine residual biomass: an energy evaluation. Energy Environ 122:379–387
Cortes-Tolalpa L, Norder J, van Elsas JD, Falcao Salles J (2018) Halotolerant microbial consortia able to degrade highly recalcitrant plant biomass substrate. Appl Microbiol Biotechnol 102:2913–2927
Davies FK, Work VH, Beliaev AS, Posewitz MC (2014) Engineering limonene and bisabolene production in wild type and a glycogen-deficient mutant of Synechococcus sp. PCC 7002. Front Bioeng Biotechnol 2:21
Davis R, Tao L, Tan E, Biddy M, Beckham G, Scarlata C, Jacobson J, Cafferty K, Ross J, Lukas J (2013) Process design and economics for the conversion of lignocellulosic biomass to hydrocarbons: dilute-acid and enzymatic deconstruction of biomass to sugars and biological conversion of sugars to hydrocarbons. National Renewable Energy Lab.(NREL), Golden
Deeba F, Pruthi V, Negi YS (2016) Converting paper mill sludge into neutral lipids by oleaginous yeast Cryptococcus vishniaccii for biodiesel production. Bioresour Technol 213:96–102
Demartini JD, Pattathil S, Miller JS, Li H, Hahn MG, Wyman CE (2013) Investigating plant cell wall components that affect biomass recalcitrance in poplar and switchgrass. Energy Environ Sci 6:898–909
Demirbaş A (1998) Yields of oil products from thermochemical biomass conversion processes. Energy Convers Manag 39:685–690
Demirbaş A (2000) Mechanisms of liquefaction and pyrolysis reactions of biomass. Energy Convers Manag 41:633–646
Demirbas A (2004) Combustion characteristics of different biomass fuels. Prog Energy Combust Sci 30:219–230
Demirbas A (2011) Competitive liquid biofuels from biomass. Appl Energy 88:17–28
Eibinger M, Ganner T, Bubner P, Rosker S, Kracher D, Haltrich D, Ludwig R, Plank H, Nidetzky B (2014) Cellulose surface degradation by a lytic polysaccharide monooxygenase and its effect on cellulase hydrolytic efficiency. J Biol Chem 289(52):35929–35938. jbc. M114. 602227
Ge Y, Dababneh F, Li L (2017) Economic evaluation of lignocellulosic biofuel manufacturing considering integrated lignin waste conversion to hydrocarbon fuels. Procedia Manuf 10:112–122
Gomez LD, Steele-King CG, Mcqueen-Mason SJ (2008) Sustainable liquid biofuels from biomass: the writing’s on the walls. New Phytol 178:473–485
Hahn-Hägerdal B, Galbe M, Gorwa-Grauslund MF, Lidén G, Zacchi G (2006) Bio-ethanol – the fuel of tomorrow from the residues of today. Trends Biotechnol 24:549–556
Halim R, Gladman B, Danquah MK, Webley PA (2011) Oil extraction from microalgae for biodiesel production. Bioresour Technol 102:178–185
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30:709–732
Harun R, Singh M, Forde GM, Danquah MK (2010) Bioprocess engineering of microalgae to produce a variety of consumer products. Renew Sust Energ Rev 14:1037–1047
Helle SS, Duff SJ, Cooper DG (1993) Effect of surfactants on cellulose hydrolysis. Biotechnol Bioeng 42:611–617
Highina B, Bugaje I, Umar B (2014) A review on second generation biofuel: a comparison of its carbon footprints. Eur J Eng Technol 2(2):1–7
Himmel ME, Ding S-Y, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315:804–807
Hirokawa Y, Maki Y, Tatsuke T, Hanai T (2016) Cyanobacterial production of 1, 3-propanediol directly from carbon dioxide using a synthetic metabolic pathway. Metab Eng 34:97–103
Holma A, Koponen K, Antikainen R, Lardon L, Leskinen P, Roux P (2013) Current limits of life cycle assessment framework in evaluating environmental sustainability – case of two evolving biofuel technologies. J Clean Prod 54:215–228
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huang J, Chen D, Wei Y, Wang Q, Li Z, Chen Y, Huang R (2014) Direct ethanol production from lignocellulosic sugars and sugarcane bagasse by a recombinant Trichoderma reesei strain HJ48. Sci World J 2014:1–8
Inganäs O, Sundström V (2016) Solar energy for electricity and fuels. Ambio 45:15–23
Ingram L, Conway T, Clark D, Sewell G, Preston J (1987) Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol 53:2420–2425
Kalyani DC, Zamanzadeh M, Müller G, Horn SJ (2017) Biofuel production from birch wood by combining high solid loading simultaneous saccharification and fermentation and anaerobic digestion. Appl Energy 193:210–219
Kanda H, Li P, Yoshimura T, Okada S (2013) Wet extraction of hydrocarbons from Botryococcus braunii by dimethyl ether as compared with dry extraction by hexane. Fuel 105:535–539
Kim M, Lee S, Ryu DD, Reese E (1982) Surface deactivation of cellulase and its prevention. Enzym Microb Technol 4:99–103
Kivistö A, Santala V, Karp M (2010) Hydrogen production from glycerol using halophilic fermentative bacteria. Bioresour Technol 101:8671–8677
Knöös P, Schulz C, Piculell L, Ludwig R, Gorton L, Wahlgren M (2014) Quantifying the release of lactose from polymer matrix tablets with an amperometric biosensor utilizing cellobiose dehydrogenase. Int J Pharm 468:121–132
Kremer TA, Lasarre B, Posto AL, Mckinlay JB (2015) N2 gas is an effective fertilizer for bioethanol production by Zymomonas mobilis. Proc Natl Acad Sci 112:2222–2226
Kulkarni MG, Gopinath R, Meher LC, Dalai AK (2006) Solid acid catalyzed biodiesel production by simultaneous esterification and transesterification. Green Chem 8:1056–1062
Kumar R, Kumar P (2017) Future microbial applications for bioenergy production: a perspective. Front Microbiol 8:450
Lamed R, Zeikus J (1980) Glucose fermentation pathway of Thermoanaerobium brockii. J Bacteriol 141:1251–1257
Laskar DD, Yang B, Wang H, Lee J (2013) Pathways for biomass-derived lignin to hydrocarbon fuels. Biofuels Bioprod Biorefin 7:602–626
Lee C-G (1999) Calculation of light penetration depth in photobioreactors. Biotechnol Bioprocess Eng 4:78–81
Lee H, Hamid S, Zain S (2014a) Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. Sci World J 2014:1–20
Lee S, Speight JG, Loyalka SK (2014b) Handbook of alternative fuel technologies. CRC Press, Boca Raton
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energy Combust Sci 38:449–467
Lin PP, Mi L, Morioka AH, Yoshino KM, Konishi S, Xu SC, Papanek BA, Riley LA, Guss AM, Liao JC (2015) Consolidated bioprocessing of cellulose to isobutanol using Clostridium thermocellum. Metab Eng 31:44–52
Lopes A, Ferreira Filho EX, Moreira L (2018) An update on enzymatic cocktails for lignocellulose breakdown. J Appl Microbiol 125:632–645
Lü J, Sheahan C, Fu P (2011) Metabolic engineering of algae for fourth generation biofuels production. Energy Environ Sci 4:2451–2466
Lütke-Eversloh T, Bahl H (2011) Metabolic engineering of Clostridium acetobutylicum: recent advances to improve butanol production. Curr Opin Biotechnol 22:634–647
Lynd LR, van Zyl WH, Mcbride JE, Laser M (2005) Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 16:577–583
Mandil C, Shihab-Eldin A (2010) Assessment of biofuels potential and limitations. Geopolit Energy 32:6–11
Margesin R, Schinner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Meher L, Sagar DV, Naik S (2006) Technical aspects of biodiesel production by transesterification – a review. Renew Sust Energ Rev 10:248–268
Melin K, Kohl T, Koskinen J, Hurme M (2015) Performance of biofuel processes utilising separate lignin and carbohydrate processing. Bioresour Technol 192:397–409
Menetrez MY (2012) An overview of algae biofuel production and potential environmental impact. Environ Sci Technol 46:7073–7085
Minteer S (2016) Alcoholic fuels. CRC Press, Boca Raton
Morgera E, Kulovesi K, Gobena A (2009) Case studies on bioenergy policy and law: options for sustainability. In: FAO legislative study. FAO, Rome
Mubarak M, Shaija A, Suchithra T (2015) A review on the extraction of lipid from microalgae for biodiesel production. Algal Res 7:117–123
Müller C, Reuter W, Wehrmeyer W, Dau H, Senger H (1993) Adaptation of the photosynthetic apparatus of Anacystis nidulans to irradiance and CO2-concentration. Bot Acta 106:480–487
Mussgnug JH, Thomas-Hall S, Rupprecht J, Foo A, Klassen V, Mcdowall A, Schenk PM, Kruse O, Hankamer B (2007) Engineering photosynthetic light capture: impacts on improved solar energy to biomass conversion. Plant Biotechnol J 5:802–814
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sust Energ Rev 14:578–597
Nielsen DR, Leonard E, Yoon S-H, Tseng H-C, Yuan C, Prather KLJ (2009) Engineering alternative butanol production platforms in heterologous bacteria. Metab Eng 11:262–273
Nizami A-S, Rehan M (2018) Towards nanotechnology-based biofuel industry. Biofuel Res J 5:798–799
Nylund N-O, Aakko-Saksa P, Sipilä K (2008) Status and outlook for biofuels, other alternative fuels and new vehicles. VTT, Espoo
Osamu K, Carl H (1989) Biomass handbook. Gordon Breach Science Publisher, New York
Pauly M, Keegstra K (2008) Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J 54:559–568
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Hallett JP, Leak DJ, Liotta CL (2006) The path forward for biofuels and biomaterials. Science 311:484–489
Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, Davison BH, Dixon RA, Gilna P, Keller M (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344:1246843
Ragnar M, Henriksson G, Lindström ME, Wimby M, Blechschmidt J, Heinemann S (2000) Pulp. In: Ullmann’s encyclopedia of industrial chemistry. WileyVCH, Weinheim, pp 1–92
Rapala J, Sivonen K (1998) Assessment of environmental conditions that favor hepatotoxic and neurotoxic Anabaena spp. strains cultured under light limitation at different temperatures. Microb Ecol 36:181–192
Richards E (2013) Careers in biofuels. US Bureau of Labor Statistics
Romero-García J, Martínez-Patiño C, Ruiz E, Romero I, Castro E (2016) Ethanol production from olive stone hydrolysates by xylose fermenting microorganisms. Bioethanol 2:51–65
Rubin EM (2008) Genomics of cellulosic biofuels. Nature 454:841
Schoemaker HE, Piontek K (1996) On the interaction of lignin peroxidase with lignin. Pure Appl Chem 68:2089–2096
Schutyser W, Renders T, van den Bosch S, Koelewijn SF, Beckham GT, Sels BF (2018) Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem Soc Rev 47:852–908
Sekoai PT, Ouma CNM, du Preez SP, Modisha P, Engelbrecht N, Bessarabov DG, Ghimire A (2019) Application of nanoparticles in biofuels: an overview. Fuel 237:380–397
Shafizadeh F (1982) Introduction to pyrolysis of biomass. J Anal Appl Pyrolysis 3:283–305
Shen Y, Pei Z, Yuan W, Mao E (2009) Effect of nitrogen and extraction method on algae lipid yield. Int J Agric Biol Eng 2:51–57
Shen CR, Lan EI, Dekishima Y, Baez A, Cho KM, Liao JC (2011) High titer anaerobic 1-butanol synthesis in Escherichia coli enabled by driving forces. Appl Environ Microbiol 77(9):2905–2915
Shu R, Zhang Q, Xu Y, Long J, Ma L, Wang T, Chen P, Wu Q (2016) Hydrogenation of lignin-derived phenolic compounds over step by step precipitated Ni/SiO 2. RSC Adv 6:5214–5222
Sikarwar VS, Zhao M, Fennell PS, Shah N, Anthony EJ (2017) Progress in biofuel production from gasification. Prog Energy Combust Sci 61:189–248
Speight JG (2011) The biofuels handbook. Royal Society of Chemistry, Cambridge
Stevens C, Verhé R (2004) Renewable bioresources: scope and modification for non-food applications. Wiley, Chichester
Tamagnini P, Leitão E, Oliveira P, Ferreira D, Pinto F, Harris DJ, Heidorn T, Lindblad P (2007) Cyanobacterial hydrogenases: diversity, regulation and applications. FEMS Microbiol Rev 31:692–720
Valdivia M, Galan JL, Laffarga J, Ramos JL (2016) Biofuels 2020: biorefineries based on lignocellulosic materials. Microb Biotechnol 9:585–594
Vamvuka D, Arvanitidis C, Zachariadis D (2004) Flue gas desulfurization at high temperatures: a review. Environ Eng Sci 21:525–548
Wang H, Ruan H, Pei H, Wang H, Chen X, Tucker MP, Cort JR, Yang B (2015) Biomass-derived lignin to jet fuel range hydrocarbons via aqueous phase hydrodeoxygenation. Green Chem 17:5131–5135
Weber C, Farwick A, Benisch F, Brat D, Dietz H, Subtil T, Boles E (2010) Trends and challenges in the microbial production of lignocellulosic bioalcohol fuels. Appl Microbiol Biotechnol 87:1303–1315
Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, Horning T, Tsuruta H, Melis DJ, Owens A (2012) Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci 109:E111–E118
Yanase H, Miyawaki H, Sakurai M, Kawakami A, Matsumoto M, Haga K, Kojima M, Okamoto K (2012) Ethanol production from wood hydrolysate using genetically engineered Zymomonas mobilis. Appl Microbiol Biotechnol 94:1667–1678
Yang S, Mohagheghi A, Franden MA, Chou Y-C, Chen X, Dowe N, Himmel ME, Zhang M (2016) Metabolic engineering of Zymomonas mobilis for 2, 3-butanediol production from lignocellulosic biomass sugars. Biotechnol Biofuels 9:189
Yi J, Luo Y, He T, Jiang Z, Li J, Hu C (2016) High efficient hydrogenation of lignin-derived monophenols to cyclohexanols over Pd/γ-Al2O3 under mild conditions. Catalysts 6:12
Yu Y-X, Ying X, Wang T-J, Zhang Q, Zhang X-H, Zhang X (2013) In-situ hydrogenation of lignin depolymerization model compounds to cyclohexanol. J Fuel Chem Technol 41:443–447
Yu A-Q, Juwono NKP, Foo JL, Leong SSJ, Chang MW (2016) Metabolic engineering of Saccharomyces cerevisiae for the overproduction of short branched-chain fatty acids. Metab Eng 34:36–43
Zhang Y, Shen J (2007) Enhancement effect of gold nanoparticles on biohydrogen production from artificial wastewater. Int J Hydrog Energy 32:17–23
Zhang XL, Yan S, Tyagi RD, Surampalli RY, Zhang TC (2010) Application of nanotechnology and nanomaterials for bioenergy and biofuel production. In: In Bioenergy and biofuel from biowastes and biomass. American Society of Civil Engineers (ASCE), pp 478–496. https://doi.org/10.1061/9780784410899.ch21
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chauhan, A.K. (2020). Biofuel: Types and Process Overview. In: Srivastava, M., Srivastava, N., Mishra, P., Gupta, V. (eds) Nanomaterials in Biofuels Research. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-13-9333-4_1
Download citation
DOI: https://doi.org/10.1007/978-981-13-9333-4_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9332-7
Online ISBN: 978-981-13-9333-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)