Abstract
Owing to their versatile properties, many biosurfactants are implicated in the cleanup of oil spills, heavy metals, and organopollutants. A number of biosurfactants have the potential to be used in the household detergent formulation as they are good stain remover and are quite compatible with enzymes and other additives used in detergents. This chapter presents an in-depth evaluation of the use of biosurfactants in bioremediation and in approaches for maintaining soil quality. However, many studies on the exogenous supplementation of biosurfactants in bioremediation showed the contradictory effect on biodegradation of pollutants. Hence, a thorough investigation of the efficacy and toxicity of biosurfactants is to be performed before implementing the biosurfactants in bioremediation. Biosurfactants can be a potential replacement to chemical surfactants only if they meet the large-scale production and cheap prices of synthetic surfactants. Use of inexpensive substrates, employing high yield strain, and developing cost-effective downstream processing are some of the approaches to reduce the cost of biosurfactants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biosurfactants
- Bioremediation
- Soil health
- Oil spill cleanup
- Rhamnolipids
- Sophorolipids
- Polyaromatic hydrocarbon
- Corexit 9500A
- Soil washing
15.1 Introduction
Surfactants are indispensable compounds in the modern world as cleansers, emulsifying agents, wetting agents, foaming agents, or dispersing agents. Because of their amphiphilic nature, surfactants have a tendency to adsorb at water-air, solid-water, and liquid-liquid interfaces and reduce the surface tension or interfacial tension. When surfactants cover the surface more closely, surface tension is reduced significantly. Another fundamental feature of surfactants is micellization or self-assembly. Surfactants alter the free energy, enthalpy, and entropy of the system (Mehta et al. 2010). The tendency of surfactants to adsorb at the water-air phase is determined by Gibbs free energy of adsorption, and Gibbs free energy of micellization represents the tendency of surfactants to form micelles at an appropriate concentration. Surface tension of the surfactant tail, the water-tail interface tension, as well the surfactant tail area contactable with the water molecules influence the Gibbs free energy of adsorption. During micellization, the partial molar volume of surfactants is changed depending on the average distance between the surfactants and the water molecules as well as between the surfactants molecules (Zdziennicka et al. 2018).
Biosurfactants are natural amphiphilic compounds produced by microbes. The hydrophobic moiety is usually fatty acid or fatty alcohol, and the hydrophilic moieties are sugars/carbohydrates or amino acids/peptides. Biosurfactants are classified into low molecular weight and high molecular weight biosurfactants. Low molecular weight biosurfactants effectively reduce the surface tension or interfacial tension and consist of glycolipids and lipopeptides. High molecular weight biosurfactants are usually extracellular amphiphilic polysaccharide or proteins such as lipopolysaccharides or lipopeptides and are good emulsifying agents (Banat et al. 2010, Roz and Rosenberg 2001). Glycolipids are produced by microbes such as Pseudomonas aeruginosa (rhamnolipids), Candida bombicola (sophorolipids), Rhodococcus erythropolis (trehalose lipids), and Pseudozyma antarctica (mannosylerythritol lipids). Some lipopeptide biosurfactant producers are Bacillus licheniformis (lichenysin), Pseudomonas fluorescens (viscosin), Serratia marcescens (serrawettin), and B. subtilis (surfactin). Some popular polymeric biosurfactants are emulsan (Acinetobacter calcoaceticus), alasan (Acinetobacter radioresistens), biodispersan (A. calcoaceticus), and liposan (C. lipolytica) (Sarubbo et al. 2015).
Biosurfactants have gained attention as an alternative to chemical surfactants due to the increasing awareness of environmental protection. Various attributes of biosurfactants are structural diversity, low toxicity, high biodegradability, and performance at extreme conditions. Compared to chemical surfactants, they are structurally diverse and are relatively high molecular weight compounds with more number of functional groups, which result in improved functionality and performances. Many biosurfactants outperform conventional surfactants in terms of surface activity and detergency. Apart from all these, biosurfactants are mild to humans, have relative low aquatic toxicity and derived completely from renewable sources. Hence, biosurfactants have huge applicability in many industries, such as personal care, domestic and industrial cleaning, agriculture, and enhanced oil recovery.
15.2 Physicochemical Attributes of Biosurfactants
Many biosurfactants have higher surface activity with low critical micelle concentration (CMC). Rhamnolipids exhibit better surface activity than sodium lauryl sulfate (SDS) due to their larger molecular area at the air-liquid interface. The CMC of rhamnolipid produced by Pseudomonas aeruginosa PA1 is 25.7 mg/L, while CMC of SDS is 2.6 g/L. In addition to this, rhamnolipids form a stable emulsion and were found to have potential in nano-/microsphere formulation of thermoplastic poly-methylmethacrylate (Mendes et al. 2015). The high surface activity of rhamnolipids is due to their high molecular weight and multiple oxygenated structures. They exhibit good frothability with more viscous and elastic froth phase and have the potential to be used as a replacement for conventional chemical frothers in the mineral processing industry (Khoshdast et al. 2012).
Biosurfactants are capable of emulsifying two immiscible liquid by reducing the interfacial tension. Biosurfactants such as rhamnolipids and surfactin are excellent emulsifier for a range of hydrocarbons such as aromatic compounds and vegetable oils, and their activity is comparable to SDS. However, the emulsifying activity of biosurfactant is pH dependent and greater emulsifying activity was observed at basic pH. This attribute is particularly advantageous for demulsification of strong emulsions formed by surfactin in the area of enhanced oil recovery (EOR). Surfactins demulsified by decreasing the pH are readily reusable and thus improving the economic viability of the process (Long et al. 2017, Lovaglio et al. 2011).
Biosurfactants effectively solubilize hydrophobic compounds in the aqueous system. Polyaromatic hydrocarbon (PAH) solubilization was found to follow a linear trend with the concentration of glycolipids biosurfactants above critical micelle concentration (CMC). This happens through micellar solubilization, where PAH disperse into the hydrophobic core of the micelles. Temperature, pH, ionic strength, and structural complexity of hydrophobic compounds are the various parameters affecting the solubility of hydrophobic compounds. Furthermore, the solubility of PAH can be greatly enhanced by mixing the biosurfactants such as rhamnolipids and sophorolipids (Li et al. 2015, Song et al. 2016).
Structural complexity and mosaic distribution of charge and polarity of biosurfactants molecules contribute to their superior performance and biological activity such as membrane binding. Biosurfactants bind weakly to protein and are less denaturing than chemical surfactants. Hence, biosurfactants are compatible with industrial enzymes and other additives in the different industrial formulations (Otzen 2017, Madsen et al. 2015).
Structural variation in the hydrophilic/hydrophobic moiety or degree of acetylation can alter the physicochemical properties of biosurfactants such as self-assembly and adsorption properties. Based on the hydrophobic moiety, sophorolipids are of two types- lactone and free acid forms (Fig. 15.1) (Penfold et al. 2011). In the presence of anionic surfactant sodium dodecyl benzene sulfonate [LAS], acidic [AS] and lactonic sophorolipids [LS] exhibit a complex and unusual phase behavior, depending on the concentration and composition (Table 15.1) (Penfold et al. 2011, 2012).
Structure of acidic sophorolipids (AS) and lactonic sophorolipids (LS). (Penfold et al. 2011)
15.3 Biosurfactants in Bioremediation
Bioremediation is the process to restore the contaminated site by biological means. This can be achieved by the addition of living organisms (bioaugmentation) or by addition of nutrients or microbial metabolites such as biosurfactants to stimulate the growth of indigenous population, which degrade the pollutants (biostimulation) (Das and Chandran 2011). Bioremediation can be used for the treatment of oil spills, metal contamination, and organic pollutants such as nitroaromatic compounds and halogenated biphenyls. Bioremediation has various advantages such as relatively low cost, less energy requirement, and efficiency of treatment. There are two methods of treatments – in situ and ex situ. Criteria for choosing the treatment methods are the degree of contamination, geographical area, environmental conditions, and feasibility (Barnes et al. 2002, Saxena et al. 2012). Biosorption is an emerging cost-effective cleanup technology for the removal of metals and organic pollutants, by using live or dead organisms, or their components. This involves various physicochemical phenomena like adsorption, absorption, ion exchange, surface complexation, and precipitation (Fomina and Gadd 2014).
Figure 15.2 shows the mechanisms of action of biosurfactants in natural and induced bioremediation of different pollutants (Lawniczak et al. 2013, Santos et al. 2016).
Mechanisms of action of biosurfactants in natural and induced bioremediation of different pollutants. (Lawniczak et al. 2013)
15.3.1 Biosurfactants for Oil Spill Cleanup
Oil spills are a major cause of environmental pollution and have a drastic effect on marine and terrestrial ecosystem. Oil spills cause a havoc on local fauna and flora, ravage the farmland cultivation, and make the affected area unfit for habitation and human activities such as fishing and swimming. Aftermath effect of oil contamination can be seen by carcinogenic heavy metal and PAH accumulation in the food chain and decreased photosynthesis in the affected area. More than 2400 animals had been killed and 1000 plant species had been destroyed by an oil spill in Colombia in 2018 (Zachos 2018). People exposed to an oil spill can have acute and chronic health effects. Cough, headache, vomiting, diarrhea, and shortness of breath are some of the immediate health issues, and hematological, hepatic, pulmonary, and cardiac functions of people exposed to spill, especially cleanup workers, were aberrated years after the incidents (D’Andrea and Reddy 2018). According to US Clean Water Act and Oil Pollution Act of 1990, bioremediation agents or chemical agents such as dispersants, sinking agents, miscellaneous oil spill control agent, and burning agents can be added to combat oil spills (National Oil and Hazardous Substances Pollution Contingency Plan).
Crude oil consists of alkanes, cycloalkanes, aromatics, and a small fraction of asphaltenes. During degradation, alkanes are readily degraded by microbes while aromatics remain recalcitrant. After an oil spill, microbial community acts synergistically to degrade the crude oil. Analysis of microbial community in deepwater horizon oil spill revealed the presence of alkane-degrading Marinobacter and polyaromatic hydrocarbon-degrading Alpha- and Gammaproteobacteria (Dombrowski et al. 2016).
Dispersants are emulsifying, dispersing, or solubilizing agents and contain three constituents – surfactants, solvents, and additives. Addition of dispersants is among the primary response, added to mitigate the surface and subsurface oil slick after the oil spill. Surfactants in dispersants reduce the interfacial tension between water and oil, thus enhance the dissolution of oil into the water. Ideally, dispersants should increase the bioavailability of crude oil and improve the biodegradation rate. However, the use of dispersants for treatment of oil spill is still controversial, considering their detrimental effects on the marine ecosystem. The toxicity of dispersant along with crude oil is more pronounced than crude alone and can slow down the biodegradation by altering the ingenious microbial community. Furthermore, simulation study showed that dispersant can alter the microbial community and negatively affect biodegradation rate (Kleindienst et al. 2015). Dispersants are highly toxic to marine life, can bioaccumulate and increase the PAH uptake by fish during oil spill (Ramachandran et al. 2004).
Synthetic dispersants have been used to combat the oil spills. Approximately two million gallons of Corexit 9500A was used to disperse the deepwater horizon oil spill. Formulation of a safer dispersant can address the environmental concern raised by synthetic surfactants (Athas et al. 2014).
Corexit 9500A is highly toxic to marine organisms such as zooplankton and octocorals. Studies showed that Corexit 9500A can have a devastating effect on the coral reef as it increases the mortality of coral larvae and could change the marine biodiversity and dynamics of the marine food chain (Almeda et al. 2014, Frometa et al. 2017, Goodbody-Gringley et al. 2013). One of the active ingredients in Corexit 9500A is surfactant dioctyl sodium sulfosuccinate (DOSS), which cause the pulmonary and dermatological adverse effect in the oil spill cleanup workers (Anderson et al. 2011). Environmental samples collected from deepwater horizon oil spill still contain DOSS 6 years after the spillage (White et al. 2014). Hence, continuous monitoring of dispersant and damage assessment posttreatment is needed to evaluate the use of dispersant as a measure to mitigate the future oil spills (Passow et al. 2017). Many of the components used in dispersant formulation are also used in the household detergent formulation and can make some household detergent more toxic than dispersant such as Corexit 9500A (Word et al. 2015).
In this scenario, biosurfactants are a potential alternative to synthetic dispersants as they are quite efficacious in action and totally environmentally safe. Biosurfactant from Candida bombicola was found to be promising as a dispersant due to its excellent dispersant activity and stability at different temperatures and pH and in presence of salt (Freitas et al. 2016). It was shown that the efficiency of rhamnolipid to disperse the crude oil was decreased after settling. Hence, the addition of additives is necessary to increase the stability of the emulsion. An environmentally benign silica nanoparticle modified with rhamnolipid resulted in a stable oil-in-water emulsion and worked well as a dispersant for crude oil in seawater system (Holakoo 2011, Pi et al. 2015). BioSURF, a rhamnolipid-based commercial dispersant formulation from Bionetix® International, is designed to combat oil slick and oil spills on rocks, beaches, and soil surfaces. Hydrocarbon degradation rate is also enhanced, as the BioSURF is fortified with micronutrients (Amanda 2018).
Lipopolysaccharide produced by Acinetobacter calcoaceticus disrupted the oil slick on the water surface, improved the dissolution of hydrocarbon to form a stable emulsion, and enhanced the natural biodegradation than the chemical dispersants. Microbial adhesion to hydrocarbon was also improved in the presence of lipopolysaccharide (Crescenzi et al. 2002). A biological oil spill dispersing agent containing biosurfactants such as sophorolipids, rhamnolipids, trehalose lipids, lipoprotein, and other auxiliary agents was found promising when applied onsite during oil leakage or oil pollution (Zheng 2012). Even a mixture of biosurfactants-chemical dispersant can exhibit higher efficiency in oil removal and can reduce the impact of secondary pollution caused by chemical surfactants. A lipopeptide-sodium dihexyl sulfosuccinate formulation based on hydrophilic-lipophilic deviation concept showed better oil dispersion and improved solubilization of crude oil in the water column (Rongsayamanont et al. 2017). Modified sophorolipid derivatives developed by SyntheZyme are very effective in oil dispersion and emulsification and can be used as a dispersant (biobased surfactants).
Gas hydrate, an ice-like structure formed as a result of the reaction between natural gas and water, is an undesirable event in natural gas pipelines. Hydrate formation can lead to the shutdown of onshore and offshore operations. Rhamnolipid was quite as effective as antiagglomerant at low concentration and forms a less stable emulsion, which is advantageous for phase separation and product recovery (York and Firoozabadi 2008).
Biosurfactant is a potential replacement to chemical surfactants as it can reduce the oil viscosity, disperse the hydrocarbon, stabilize the oil emulsion, and help in the deposition of paraffin/asphalt (De Cássia et al. 2014). Advantages of using biosurfactant are their superior performance even at very low concentration when compared to chemical surfactants. Mono-rhamnolipid was highly effective at a sub-CMC level for solubilization of hydrocarbon and can be employed in surfactant-enhanced aquifer remediation (Zhong et al. 2016).
The downside of biosurfactants-enhanced bioremediation is that native microbes start utilizing biosurfactants before utilizing the contaminants. When rhamnolipids were supplemented to accelerate the degradation of pesticides in soil slurry system, biodegradation of pesticides was suppressed as the microbial inoculum, Streptomyces species, started utilizing the rhamnolipids (Mata-Sandoval et al. 2001).
Apart from biosurfactant-mediated bioremediation, soil washing or in situ flushing with biosurfactants is also feasible. In situ flushing with surfactants is used to treat soil and groundwater contaminated with dense nonaqueous phase liquid (DNAPL), which can retain in the polluted site for many years, if untreated. Surfactants reduce the interfacial tension between water and NAPL and increase the solubility and mobility of the pollutants. Hence, the contaminant can be recovered from the polluted site at an accelerated rate (Strbak 2000). Many technologies have been developed to automate the delivery of biosurfactants to treat the contaminated site. DO-IT (dissolved oxygen in situ) treatment developed by the ETEC LLC is used to inject biosurfactants “petrosolv” for the recovery of the contaminant from the groundwater [Advanced Bioremediation Solution ETEC].
To study the effect of biosurfactants on indigenous microbes in presence of crude oil spill, Saborimanesh and Mulligan (2015) measured the cell surface hydrophobicity of bacterial communities in presence of hydrocarbon, sophorolipids, and hydrocarbon and sophorolipids combination. Microbes are hydrophobic in presence of hydrocarbon, which is due to their tendency to interact with hydrophobic substrates. In presence of sophorolipids, cells are hydrophilic and have a limited bioavailability to utilize sophorolipids. Hydrophobicity was significantly decreased in the cell-sophorolipid-hydrocarbon system, because sophorolipids increased the bioavailability of hydrocarbons to microbes through micellar dispersion of hydrocarbons. This study showed that indigenous microbes play a significant role in hydrocarbon degradation by changing the microbial dynamics and cell surface hydrophobicity via cell surface modification (Saborimanesh and Mulligan, 2015).
A combination of food grade amphiphiles such as lecithin and tween 80 can result in smaller and stable emulsion of crude oil than Corexit 9500A and can be an effective dispersant for crude oil (Athas et al. 2014). Similar way, a more potent dispersant can be developed by blending the biosurfactants with less toxic amphiphiles. An optimized formulation of glycolipids biosurfactants such as rhamnolipids and glycolipids, sorbitol-based nonionic surfactants, and solvent ethylene glycol butyl ether exhibited high dispersion effectiveness for crude oil. The formulation also exhibited low dispersant-to-oil ratio and could diminish the environmental impact of dispersant by reducing the amount of dispersant to be added to the oil spill. In addition to this, the above formulation retained high dispersion activity at various environmental factors such as low temperature, high salinity, and high pH, and was having low aquatic toxicity as well (Song et al. 2013).
Because of their superior performance at various physicochemical conditions, biosurfactants can be incorporated into high pressure-hot water washing that is used to remove oil spills from shorelines and hard surfaces. Biosurfactants from Pseudomonas aeruginosa could disperse oil 2–3 times greater than water alone when applied to oil-contaminated Alaskans gravel samples (Harvey et al. 1990).
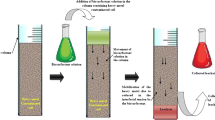
Bioaugmentation with biosurfactant-producing microbes can be used to address the challenges with biosurfactant-enhanced bioremediation. Systematic environmental molecular bioremediation technology, an approach that combines bioaugmentation and biostimulation with biosurfactants, was quite effective for ex situ treatment of oil-contaminated soil (Lin et al. 2010). Remarkable degradation of total petroleum hydrocarbon and polyaromatic hydrocarbon in multi-contaminated soil was achieved by phytoremediation supplemented with rhamnolipids (Liduino et al. 2018). Ex situ bioremediation of crude oil-contaminated soil with biosurfactants based biostimulation is shown in Figs. 15.3 and 15.4.
Systematic environmental molecular bioremediation technology, a highly effective ex situ bioremediation strategy reported by Lin et al. (2010). Incorporation of bioaugmentation and biostimulation shortens the treatment time and improves the biodegradation efficiency of land farming
Ex situ bioremediation of crude oil-contaminated soil using multiple approach. Here, the contamination level and treatment efficiency were monitored by measuring total recoverable petroleum hydrocarbons (TRPH). (Kuyukina et al. 2010)
Polycyclic aromatic hydrocarbons and polychlorinated biphenyl are the major organic pollutants in soil, particularly in the land exposed to emission sources such as industrial plants, oil refineries, agricultural farms, and landfills. Combustion can also be another major cause of this pollution. A recent study showed that the concentration of carcinogenic benzopyrene in agricultural and park soils of some urban areas of Havana exceeded the regulatory guidance value. Hence, periodic monitoring of organic pollutants in this kind of soil is necessary as people have direct contact with such soil (Pacheco et al. 2018).
Soil contamination with petroleum compound changes the microbial dynamics of soil to an extent that microbial community is fit to degrade the contaminant present. Many of these microbes can produce biosurfactants, which aid to emulsify the contaminant present in the soil. Some of the biosurfactant-producing genera found in the petroleum-contaminated soil are Rhodotorula, Candida, Yarrowia, Geotrichum, Galactomyces, and Cystobasidium (Yalçın et al. 2018). Apart from microbial composition, crude oil spillage alters the physicochemical properties of soil such as soil moisture content and soil permeability. Prolonged exposure of soil to petroleum hydrocarbons alter the soil wettability and induce the water repellency. In addition to this, soil water capillary water height rise and soil saturated hydraulic conductivity also decreased. Water repellency affects plant growth, changes the ecological balance, and makes the soil more prone to erosion (Roy 1999, Wei and Li 2018). Treatment with biosurfactants dispels the oil from soil particles, reduces the wettability of soil, and converts them from oil wet to water wet (Ukwungwu et al. 2017).
15.3.2 Biosurfactants for Bioremediation of Heavy Metals and Organopollutants
Pesticides and heavy metals are a big threat to the soil ecosystem. Soil washing, soil vapor extraction, thermal desorption, phytoremediation and solidification/stabilization are used to treat the pesticides and heavy metals contaminated soil. Conventional soil washing uses a combination of synthetic compounds such as SDS and EDTA. A combination of microbial-derived compounds such as rhamnolipids and citric acid effectively removed the organochlorine pesticides and heavy metals such as lindane and cadmium from contaminated soil by increasing their solubilization and desorption. These combinations are quite environmentally friendly, restore the soil ecological balance, and can cut the remediation cost by using a combination rather than individual compounds (Wan et al. 2015).
Rhamnolipids can be used as a soil bioremediation agent for the removal of heavy metals such as cadmium, nickel, lead, and zinc (Wang and Mulligan 2004, Herman et al. 1995). Sorption and desorption kinetic models revealed that rhamnolipids are compatible with various soil materials. Anionic rhamnolipids form an ionic bond with cationic cadmium ion and helps to leach out the heavy metals from the soil. Reduction of interfacial tension by rhamnolipids solubilize the cadmium ion, thus get detached from soil particles [Asci et al. 2008]. Compared to sophorolipids and lipopeptides, rhamnolipids facilitate the leaching of metals such as molybdenum, nickel, and vanadium from hazardous spent hydrosulphurozate catalyst generated by petroleum refineries (Alsaqer et al. 2018).
Rhamnolipid biosurfactant blend, JBR-425, effectively removed aged metals Zn, Cu, Pb, and Cd from the field soil deposited with metal, when compared to cationic synthetic surfactant, 1-dodecyl pyridinium chloride, and nonionic synthetic surfactant, oleyl dimethyl benzyl ammonium chloride. Remediation procedure can release metals from soil and enhance the bioavailability, which can lead to ecotoxicity of soil biota. However, treatment with JBR-425 resulted in reduced metal accumulation and increased the growth rate of two earthworm species, Eisenia fetida and Lumbricus terrestris (Slizovskiy et al. 2011).
Mobilization of heavy metals such as arsenic, copper, lead, and zinc was greatly enhanced in the presence of rhamnolipid biosurfactants. It was shown that heavy metals are incorporated into biosurfactants micelles and metal bridging might play a role in that. Hence, soil flushing with rhamnolipids can be a feasible technology to remove arsenic and other heavy metals from mine tailings (Wang and Mulligan 2009a, Wang and Mulligan 2009b).
Efficient surface-active and detergent activity of surfactin was found to be useful in the cleanup of radioactive cesium and other contaminants. Kaneka Corp., a Japanese chemical company had successfully carried out a radioactive decontamination of areas affected by the Fukushima No. 1 nuclear power plant using their biosurfactants, Kaneka Surfactin, which is composed of surfactin. Radioactive decontamination of the road using surfactin is shown in Fig. 15.5 (Sajna et al. 2015).
Biosurfactants are good additives for the phytoremediation of soil contamination. Addition of biosurfactants promotes the microbial colony formation at root surfaces and stimulates the rhizodegradation of pollutants. The plants were healthier, when compared to the addition of chemical surfactants (Al mansoori et al. 2015). Apart from rhizodegradation, rhizospheric microbes are involved in biotransformation and volatilization of organic and inorganic pollutants and biomethylation of heavy metals, which make the contaminants less toxic and more water-soluble. Since many rhizobacteria are biosurfactant producers, amendment of contaminated soil with biosurfactant-producing rhizobacteria, prior to phytoremediation, can be a promising strategy (Lal et al. 2018).
Bioaugmentation with biosurfactant-producing microbe is an effective strategy to treat persistent organic pollutants. Endosulfan, a restricted organochlorine pesticide, still used in developing countries comes under persistent organic pollutant and has a debilitating effect on humans. Biosurfactant-producing Bordetella petrii species could degrade α and β isomers of endosulfan up to 82%. Bioremediation of endosulfan-contaminated soil can be achieved by bioaugmentation with biosurfactant-producing microbes (Odukkathil and Vasudevan 2015, Odukkathil and Vasudevan 2016).
15.4 Role of Biosurfactants in Soil Health
Anthropogenic activities affect soil health and damage the ecological equilibrium supported by soil. There are physical, chemical, and biological indicators used to assess soil health [Table 15.2] (Cardoso et al. 2013). It is important to check the soil quality as soil health is the primary requirement for agriculture and environmental sustainability. Extensive farming, deforestation, and industrial effluent disposal severely affect soil health.
Surfactants are ubiquitous compounds present in the industrial formulation. Chemical surfactants used in household detergent and agricultural formulation often end up dispersed into soil and cause a negative impact on soil biota and deter soil quality. Replacing chemical surfactants with natural surfactants such as biosurfactants could be best solution to reduce the damage caused by the environmentally harmful chemicals.
15.4.1 Biosurfactants in Wastewater Treatment
Biosurfactants showed a great potential for the formulation of eco-friendly adsorbents used in wastewater treatment. A lignocellulosic biocomposite modified with natural lipopeptide biosurfactant obtained from corn steep liquor exhibited improved dye elimination and sulfate removal, when used for the treatment of winery wastewater (Perez-Ameneiro et al. 2015). The outcome can be hopefully extrapolated for the microbial-derived biosurfactants, considering the emulsification and bioadsorption properties of biosurfactants.
Dissolved air flotation (DAF) is a method of wastewater treatment to remove oily waste effluent. The efficiency of the process can be enhanced by the use of biosurfactants. Both laboratory and in silica analysis revealed that there was a substantial increase in efficiency of DAF to remove oil when biosurfactants were used as the collector (Rocha e Silva et al. 2018; Silva et al. 2018). Addition of biosurfactants can enhance microbial enzymatic activity and growth rate in the soil, and enhance the degradation of organic waste. Gong et al. (2017) reported that growth rate of earthworm, Eisenia fetida and the efficiency of vermicompost were improved on the addition of rhamnolipids (Gong et al. 2017). Biosurfactants can enhance bioenergy recovery from organic waste. Rhamnolipids and surfactin were proved to have a significant effect on hydrogen production from waste-activated sludge and organic fraction of municipal solid waste, respectively (Sharma and Melkania 2017). During the anaerobic digestion of waste-activated sludge, rhamnolipids increased the rate of acidogenesis and decreased the rate of methanogenesis, which resulted in the subsequent improved production of hydrogen within a short fermentation time (Zhou et al. 2017) [Fig. 15.6].
Effect of rhamnolipids (RL) on hydrogen production from waste-activated sludge. Apart from their effect on volatile fatty acid production and conversion efficiency of methanogenesis, rhamnolipids also influence electron-proton transfer and internal resistance decrease in microbial electrolysis cell. (Zhou et al. 2017, Adapted from Bensaid et al. 2015)
15.4.2 Biosurfactants in Detergent Industry
Graywater resulting from household activities contains a large number of surfactants from detergents and hygienic products. Methyl ester sulfonate, olefin sulfonates, alkyl benzene sulfonates, alkyl ether sulfates, isotridecanol ethoxylates, benzalkonium chloride, n-hexadecyl trimethyl, and ammonium chloride are the common surfactants found in the graywater. In developing countries, graywater is usually drained into the soil. Many soil properties such as soil salinity and soil pH are elevated, which leads to deterioration of soil composition and permeability. Hence, graywater should be properly disposed, and surfactants’ concentration in graywater should be kept minimum (Mohamed et al. 2018). To reduce the adverse effect of chemical surfactants on soil health, environmentally safe detergents containing biobased surfactants should be put to use.
Biosurfactants can be a potential replacement for chemical surfactants in detergent if they can meet large-scale production and cheap prices of synthetic surfactants. Biosurfactants can efficiently clean the stain out of soiled clothes by dispersing and solubilizing organic compounds and are compatible with enzymes used in detergents. Furthermore, they have antimicrobial and biofilm-disrupting properties (Otzen 2017). A number of dishwashing, hard surface cleaning, and laundry detergents containing sophorolipids as one of the ingredients are out in the market. Develter and Lauryssen (2010) reported the usefulness of sophorolipid in hard surface cleaning and automatic dishwashing rinse aid formulation, owing to their outstanding surface activity and low foaming properties.
A detergent formulation containing rhamnolipids as surfactant, sodium tripolyphosphate as a builder and sodium sulfate as filler had exhibited high stain removal efficiency that is comparable to commercial detergents (Bafghi and Fazaelipoor 2012). Studies showed that addition of lipopeptide biosurfactants from Bacillus subtilis SPB1 to commercial detergents enhanced the stain removal and wash quality (Bouassida et al. 2018). Applicability of mannosylerythritol lipids in laundry detergent formulation was explored by fabric wash analysis using biosurfactants derived from Pseudozyma sp. NII 08165. Pseudozyma biosurfactants exhibit good washing performance and are stable at high temperature and alkaline pH (Sajna et al. 2013).
Sophorolipids can be good laundry detergent additives as they possess good wetting property, emulsification index, antimicrobial activity, and clean fabric strain effectively (Joshi-Navare et al. 2013). When sophorolipids, rhamnolipids, and accell biosurfactants derived from the undisclosed yeast strain were tested to see their efficiency in removing the beef stain from cloth, in combination with either bacterial or yeast lipase enzyme, sophorolipids along with bacterial enzyme gave a satisfactory performance (Parry et al. 2012). Detergent containing a cocktail of both biosurfactants and chemical surfactants utilizing the synergistic action of sophorolipids, rhamnolipids, cellobiose lipids was shown to have enhanced oily soil detergency (Hall et al. 1995). However, a formulation containing both glycolipid biosurfactants and non-glycolipid biosurfactants in micellar phase showed an improved detergency and had been found to be suitable for all cleaning purpose, ranging from laundry detergent to hard surface cleaning. It has been noted that employing micellar phase sophorolipids is more suitable for hard surface cleaning as it possesses an efficient foam breaking activity since over-foaming of the hard surface cleaner is a disadvantage as it requires a lot of rinsing to remove the foams. Addition of micellar non-glycolipid surfactants along with sophorolipids helps to give suitable foaming properties to the hard surface cleaning formulation, as non-glycolipid biosurfactants help in the initial foaming and sophorolipids subsequently curb the foaming (Develter and Fleurackers 2010). The low foaming property of sophorolipids and their high-temperature stability can be exploited for jet washing, a washing method which uses water pressure to remove dirt from the object, and are widely used in dishwashing machine and high-tech washing machines. A mixture of lactone and acidic forms of sophorolipids exhibits better washing performance than conventionally used nonionic surfactants (Furuta et al. 2004). A formulation containing sophorolipids, cellobiose lipids, and a number of bacterial biosurfactants exhibits good flushing performance, dispersibility, foaming power, and dermatological compatibility and is found to be suitable for manual dishwashing applications (Hees and Fabry 1997).
15.4.3 Biosurfactants in Agriculture
Owing to their nontoxic and biodegradable nature, biosurfactants in agriculture can be used to achieve a sustainable environment. There are a number of applications of biosurfactants in crop protection. According to US EPA’s fact sheet about rhamnolipids, rhamnolipid is an effective biofungicide against plant pathogens such as Pythium and Phytophthora species and can be used in agricultural, horticultural, and turf settings (Rhamnolipid Biosurfactant (110029) Fact Sheet). Besides being adjuvants for boosting the performance of pesticide, sophorolipids can also be used as a wetting agent, emulsifier, dispersant, and defoamer for the preparation of pesticides (Giessler-Blank et al. 2016). Sophorolipids derivatives can be effective biopesticide as their application could control plant pathogens. Among differently modified sophorolipids derivatives tested on plant pathogens, which include hydrogenated sophorolipids, sophorolipids ester derivatives, sophorolipids amide derivatives, sophorolipids biogenic amide derivatives; amide derivative shows highest antibacterial activity and sophorolipids biogenic amide derivatives show highest antifungal activity (Schofield et al. 2012).
Cellobiose lipids are more potent fungicidal agent than sophorolipids. When the antibiotic activity of both sophorolipids and cellobiose lipids were compared against Filobasidiella neoformans and Candida tropicalis, minimum inhibitory concentration (MIC) value of cellobiose lipids is much less than that of sophorolipids. Cellobiose lipids exhibit higher antifungal activity at acidic pH, while the advantage of using sophorolipids as antifungal agents is their high solubility (Kulakovskaya et al. 2014). Mode of action of cellobiose lipids in antifungal activity involves membrane permeability of target organism due to amphiphilicity of cellobiose lipids followed by membrane leakage of ATP and potassium ions (Trilisenko et al. 2012). Surfactin act as elicitor on wheat plant against Zymoseptoria tritici infection by stimulating both salicylic acid- and jasmonic acid-dependent signaling pathways and provided 70% protection against Septoria tritici blotch (STB) disease, caused by Z tritici (Le Mire et al. 2018). Biosurfactants overproducing producing Bacillus subtilis exhibit plant promoting trait as well (Paraszkiewicz et al. 2017). Mannosylerythritol lipids significantly reduced the infection of powdery mildew in wheat leaf (Yoshida et al. 2015).
15.5 Efficacy and Toxicity Studies of Biosurfactants
It is very necessary that efficacy and toxicity of biosurfactants should be evaluated before proceeding for their potential applications in bioremediation. Biosurfactants are relatively low toxic compounds compared to chemical surfactants. If the biosurfactant is intended for dispersant application, toxicity studies should be determined for marine test species. Toxicity was determined by LC 50 value (Median Lethal concentration), which is performed for dispersant alone and dispersant and oil mixture. LC 50 is a method that evaluates the rate of population mortality and is the concentration of a compound at which 50% population is killed in a given period of time. Marine test species such as Phaeodactylum tricornutum, Oncorhynchus mykiss, Daphnia magna, and Selenastrum capricornutum Printz are usually used for studying aquatic toxicity. However, indicator organisms vary according to the spillage site. Phytotoxicity is studied by germination index (GI), which measures the relative vegetable seed germination and relative root elongation in presence of biosurfactants (De Cássia et al. 2014). In vitro analysis of cytotoxicity and endocrine disruption ability must also be studied. Rufino et al. (2014) demonstrated the low toxicity of biosurfactants from Candida lipolytica against seeds of Brassica oleracea, Solanum gilo, and Lactuca sativa L. and the micro-crustacean Artemia salina. When acute and chronic toxicities of three synthetic surfactants (PES-61, Corexit 9500, Triton X-100) and three microbiologically produced surfactants (BioEM-Glycolipid surfactant produced by Pseudomonas aeruginosa, emulsan, PES-51- mixture of d-limonene and a bacterial fermentation by-products) were determined and compared, biosurfactants exhibited intermediate toxicity to marine species, Mysidopsis baha and Menidia beryllina than synthetic surfactants (Edward et al. 2003). Before introducing the dispersant to oil spill site, a detailed field test is required to assess the effectiveness of biosurfactants to disperse at various environmental parameter such as temperature, pH and salinity. The effect of crude oil properties such as viscosity on dispersant activity should also be thoroughly studied.
Baffled flask test (BFT) is a standard test to study the efficacy of dispersant for the possible use in the oil spill. BFT should be performed with different variables such as temperature, oil type, mixing speed, and oil viscosity. BFT is superior and reproducible, when compared to swirl flask test, and is expected to be an official US Environmental Protection Agency’s (EPA) test soon. However, detailed field trial is inevitable as laboratory-scale test does not necessarily reflect the dispersant ability in vivo (Venosa and Holder 2015). Dispersion effectiveness of Corexit 9500 on 23 crude oil measured by BFT varied from 3.4% to 93% and is higher for lighter, less viscous oil relative to heavier, more viscous oil. Furthermore, BFT revealed that dispersion effectiveness is a function of oil viscosity and gave good indication of dispersibility of oil with different variables such as mixing speed, oil type, temperature, etc. (Holder et al. 2015).
Lawniczak et al. (2013) designed a guideline for successful biosurfactant-mediated bioremediation (Fig. 15.7). Under the guideline, biocompatibility between biosurfactants, pollutants, native microbes, and plants should be taken into consideration at first. Influence of native microbes on the degradation of biosurfactants should be studied in detail. An optimal concentration of biosurfactants at which an efficient biodegradation happen should be found out.
Critical steps in biosurfactant-mediated bioremediation. (Lawniczak et al. 2013)
It is also imperative to perform a field study to check whether the developed technology is feasible.
15.6 Cost-Effective Production of Biosurfactants
Replacement of chemical compounds with biobased counterpart is a growing trend due to increased environmental awareness. One of the remarkable features of biosurfactant is biodegradability and environmental safety. Ideally, production of biosurfactants should be a green process from the environmental point of view, due to the utilization of renewable feedstock and no generation of hazardous by-products. However, cradle-to-grave analysis of acidic sophorolipids production by a knock-out yeast for a handwash formulation revealed that use of vegetable oil and glucose as a substrate for the production of biosurfactants can contribute to much more environmental damage when compared to petroleum-derived surfactants. Hence, bioprocess development with second-generation biomass and efficient production and purification should be encouraged to reduce environmental damage (Baccile et al. 2017).
Waste cooking oil can be a cheap substrate for the production of biosurfactants. This can be a safe and environmentally sustainable solution for the disposal of waste cooking oil compared to use of waste cooking oil as an animal feed or disposal in industrial effluent which can clog the sewer in cold weather. Cultivation of Pseudomonas SWP-4 in medium containing waste cooking oil as sole carbon source resulted in the production of rhamnolipids at a yield of 1.9 g/L (Lan et al. 2015).
Agro-industrial waste is the abundant low-cost carbon source. Use of agro-industrial waste as a raw material for the production of value-added compounds can reduce the production cost and minimize environmental pollution (Sadh et al. 2018). Rane et al. (2017) used cheap agro-industrial waste such as molasses, orange peels extract, bagasse extract, banana peels extract and potato peels extract as a substrate for the production of biosurfactants from Bacillus subtilis isolate. The use of waste material as a substrate and fermentation under aseptic conditions can significantly reduce the production cost for the large-scale production of biosurfactants (Vipulanandan and Mohanty 2004). Various animal fats and tallow can be a potential substrate for biosurfactant production. Cationic biosurfactants produced by Alcaligenes aquatilis sp. from chicken tallow effectively remove chromium from contaminated soils (Magthalin et al. 2016). Yarrowia lipolytica, a biosurfactant producer, was reported to grow well in tallow derivative containing media and resulted in the production of single cell protein, microbial lipids, and lipase (Papanikolaou et al. 2007). Brewery waste is a good carbon source for the production of biosurfactants from Bacillus subtilis (Moshtagh et al. 2018).
Bacillus species is a potential candidate to use starchy agro-industrial waste as substrate as they are abundant in amylase enzyme. A bioprocess was developed for the simultaneous production of keratinase, amylase, and biosurfactants from a medium containing feather meal, potato peel and rapeseed cake as a carbon substrate (Bhange et al. 2016).
Downstream processing is the most expensive step of a bioprocess and account for 60-80 % of the production cost of biosurfactants. Conventional biosurfactants recovery methods such as solvent extraction have various disadvantages. In situ foam fractionation and ultrafiltration are the best choices for cost-effective continuous removal of biosurfactants. Continuous removal can result in improved yield and fermentation efficiency as product inhibition is lessened (Najmi et al. 2018).
Since high concentration of biosurfactants is usually found in the foam fraction of the fermentation broth, adsorption of biosurfactants from foam fraction is a cost-effective purification method. An integrated process foam adsorption with foam flow-through back which recirculate cell-containing collapsed foam into the bioreactor can be used for simultaneous production and recovery of rhamnolipids at high yield and purity (Anic et al. 2018). High viscosity, low dissolved oxygen, and product inhibition are the major drawbacks with large-scale production of sophorolipids. A semicontinuous sophorolipid fermentation using a novel bioreactor with dual ventilation pipes and dual sieve-plates coupled with a novel two-stage separation system resulted in a yield of 477 g/l with an improved productivity from 0.5 g g-1 (in the batch fermentation) to 0.6 g g-1 (Zhang et al. 2018).
Various recombinant organisms have been developed for the heterologous production of biosurfactants that address the challenges of biosurfactant production by natural host microbes. Pathogenicity of host-microbe and production of biosurfactants as a mixture of congeners or isomers, which aggravate the purification process and dependence on carbon sources such as vegetable oil and glucose that contribute to negative environmental impact, are the major challenges with biosurfactant production by host microbes. Development of custom-made biosurfactants and production of biosurfactants under extreme conditions can also be achieved by genetic engineering. Pseudomonas stutzeri Rhl was constructed for the heterologous production of rhamnolipid under anaerobic conditions (Zhao et al. 2015). A recombinant Bacillus subtilis was used for the production of custom-made biosurfactants called FA-Glu with applicability as a dispersant to clean up oil spills and produce biosurfactants better in medium containing glycerol than glucose (Colona et al. 2011).
Genetic-engineered Pseudomonas putida has been developed that utilized sustainable carbon sources such as crude glycerol and second-generation xylose and produced a set of predesigned rhamnolipid congener composition. Biosurfactants are usually synthesized as a set of congener with variation, in either the hydrophobic chain or hydrophobic moiety. The type of congener predominant in the biosurfactants determines their surface activity and other physicochemical properties. In this case, mono rhamnolipids have good foaming action, while di rhamnolipids are good emulsifiers (Tiso et al. 2017). A recombinant Starmerella bombicola with cytochrome P450 cyp1 gene of Ustilago maydis produced sophorolipids with a palmitic acid acyl chain, instead of oleic acid acyl chain (Geys et al. 2018). Hence, custom-made biosurfactants can be synthesized by playing around with biosynthetic pathways.
15.7 Conclusion
Biodegradation of pollutants is mainly achieved by the activity of indigenous microbes in the environment. It has been noted that surface-active compounds produced by microbes play an important role in the uptake of hydrophobic pollutants. Exogenous supplementation of biosurfactants improves the bioavailability of pollutants and thus accelerates the bioremediation. Considering the adverse effects of chemical surfactants, biosurfactants is the best choice to address environmental pollution. The book chapter primarily reviews the use of various biosurfactants to treat various environment contamination and improve soil sustainability. A major limitation with biosurfactant-assisted bioremediation is that biosurfactants are easily degraded than the pollutants and most microbes consume biosurfactants before utilizing the pollutants, eventually suppressing the rate of degradation. Besides the negative effects of biosurfactants addition on bioremediation, lack of consistency during scale-up experiments still questions whether biosurfactant-mediated bioremediation is a feasible technology. Hence, biosurfactant toxicity and biodegradability and substrate specificity and efficacy are the major factors to be considered for implementing biosurfactant-mediated bioremediation. Still, trials with the incorporation of biosurfactants with soil washing, in situ soil flushing, and phytoremediation were found promising. Several studies have shown that biosurfactants can be good cleaning agents owing to their amphiphilic nature and excellent performance at extreme conditions, which enable them to meet the diverse demands of the detergent industry. Using biosurfactants in detergents and cleaning agents can pave the way for environmental sustainability and preserving the human health. Being a high-value product, biosurfactants have a long way to go to meet the application in bioremediation. Use of high-titer biosurfactant-producing strains, inexpensive substrates, and a cost-effective downstream processing can make the bioprocess look appealing for remediation and sustainable technologies.
References
Açıkel YS (2011) Use of biosurfactants in the removal of heavy metal ions from soils. In: Khan M, Zaidi A, Goel R, Musarrat J (eds) Biomanagement of metal-contaminated soils. Environmental pollution, vol 20. Springer, Dordrecht, pp 183–223
Advanced Bioremediation Solution ETEC. https://www.etecllc.com/services. Accessed 7 Aug 2018
Al mansoori AF, Hasan HA, Idris M et al (2015) Potential application of a biosurfactant in phytoremediation technology for treatment of gasoline-contaminated soil. Ecol Eng 84:113–120
Almeda R, Hyatt C, Buskey EJ (2014) Toxicity of dispersant Corexit 9500A and crude oil to marine microzooplankton. Ecotoxicol Environ Saf 106:76–85. https://doi.org/10.1016/j.ecoenv.2014.04.028
Alsaqer S, Marafi M, Banat IM et al (2018) Biosurfactant-facilitated leaching of metals from spent hydrodesulphurization catalyst. J Appl Microbiol 125:1358–1369
Amanda S (2018) Bionetix Biosurf uses nature to clean oil spills on its own surf and turf. https://www.pollutionequipmentnews.com/bionetix-biosurf-uses-nature-to-clean-oil-spills-on-its-own-surf-and-turf. Accessed 7 Aug 2018
Anderson SE, Franko J, Lukomska E et al (2011) Potential immunotoxicological health effects following exposure to Corexit 9500A during cleanup of the Deepwater horizon oil spill. J Toxicol Environ Health A 74:1419–1430. https://doi.org/10.1080/15287394.2011.606797
Anic I, Apolonia I, Franco P et al (2018) Production of rhamnolipids by integrated foam adsorption in a bioreactor system. AMB Express 8:122. https://doi.org/10.1186/s13568-018-0651-y
Asci Y, Nurbas M, Acikel YS (2008) A comparative study for the sorption of Cd(II) by soils with different clay contents and mineralogy and the recovery of Cd(II) using rhamnolipid biosurfactant. J Hazard Mater 154:663–673
Aşçi Y, Nurbas M, Acikel YS (2008) Removal of zinc ions from a soil component Na-feldspar by a rhamnolipid biosurfactant. Desalination 223:361–365
Athas JC, Jun K, McCafferty C et al (2014) An effective dispersant for oil spills based on food-grade amphiphiles. Langmuir 30:9285–9294. https://doi.org/10.1021/la502312n
Baccile N, Babonneau F, Banat IM et al (2017) Development of a cradle-to-grave approach for acetylated acidic Sophorolipid biosurfactants. ACS Sustain Chem Eng 5:1186–1198
Bafghi MK, Fazaelipoor HM (2012) Application of Rhamnolipid in the formulation of a detergent. J Surfact Deterg 15:679–684
Banat IM, Franzetti A, Gandolfi I et al (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87:427–444
Barnes D, Laderrach S, Showers C (2002) Treatment of Petroleum Contaminated Soils in Cold, Wet, Remote Regions, Report No. 9E92G49, US Forest Service, Technology and Development Program, Missoula, MT. https://www.fs.fed.us/t-d/pubs/pdfpubs/pdf02712801/pdf02712801_300dpi.pdf. Accessed 2 Aug 2018
Bensaid S, Ruggeri B, Saracco G (2015) Development of a photosynthetic microbial electrochemical cell (PMEC) reactor coupled with dark fermentation of organic wastes: medium term perspectives. Energies 8:399–429. https://doi.org/10.3390/en8010399
Bhange K, Chaturvedi V, Bhatt R (2016) Simultaneous production of detergent stable keratinolytic protease, amylase and biosurfactant by Bacillus subtilis PF1 using agro industrial waste. Biotechnol Rep 10:94–104
Bio-based surfactants., http://www.allied-c-s.co.jp/pdf/report12.pdf. Accessed 7 Aug 2018
Bouassida M, Fourati N, Ghazala I et al (2018) Potential application of Bacillus subtilis SPB1 biosurfactants in laundry detergent formulations: compatibility study with detergent ingredients and washing performance. Eng Life Sci 18:70–77. https://doi.org/10.1002/elsc.201700152
Cardoso EJBN, Vasconcellos RLF, Bini D et al (2013) Soil health: looking for suitable indicators, what should be considered to assess the effects of use and management on soil health? Sci Agric 70:274–289
Chrzanowski Ł, Wick LY, Meulenkamp R et al (2009) Rhamnolipid biosurfactants decrease the toxicity of chlorinated phenols to Pseudomonas putida DOT-T1E. Lett Appl Microbiol 48:756–762
Colona WJ, Marti ME, Pynn M et al (2011) Integrations of biosurfactant production into advanced biorefineries. Conference: 2011 AIChE annual meeting. Doihttps://aiche.confex.com/aiche/2011/webprogram/Paper236248.html. Accessed 12 Sept 2018
Crescenzi F, Buffagni M, Angelil D et al (2002) A new biosurfactant for use in the cleanup of oil spills on sea water environment. In: Brebbia CA (ed) Oil and Hydrocarbon Spills III. WIT Press, Southampton. https://doi.org/10.2495/OIL020231
D’Andrea MA, Reddy GK (2018) The development of Long-term adverse health effects in oil spill cleanup workers of the deepwater horizon offshore drilling rig disaster. Front Public Health 6:117. https://doi.org/10.3389/fpubh.2018.00117
Das N, Chandran P (2011, 2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int. https://doi.org/10.4061/2011/941810
De Cássia FSS, Almeida DG, Rufino RD et al (2014) Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int J Mol Sci 15:12523–12542
Develter D, Fleurackers S (2010) Sophorolipids and rhamnolipids. In: Johansson I, Kjellin URM (eds) Surfactants from renewable resources. Wiley, Chichester
Develter DW, Lauryssen LM (2010) Properties and industrial applications of sophorolipids. Eur J Lipid Sci Technol 112:628–638. https://doi.org/10.1002/ejlt.200900153
Dombrowski N, Donaho JA, Gutierrez T et al (2016) Reconstructing metabolic pathways of hydrocarbon-degrading bacteria from the Deepwater horizon oil spill. Nat Microbiol 17:16057. https://doi.org/10.1038/nmicrobiol.2016.57
Edward KR, Lepo JE, Lewis MA (2003) Toxicity comparison of biosurfactants and synthetic surfactants used in oil spill remediation to two estuarine species. Mar Pollut Bull 46:1309–1316
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14
Freitas BG, Brito JGM, Brasileiro PPF et al (2016) Formulation of a commercial biosurfactant for application as a dispersant of petroleum and by-products spilled in oceans. Front Microbiol 7:1646. https://doi.org/10.3389/fmicb.2016.01646
Frometa J, DeLorenzo ME, Pisarski EC et al (2017) Toxicity of oil and dispersant on the Deepwater gorgonian octocoral Swiftia exserta, with implications for the effects of the Deepwater horizon oil spill. Mar Pollut Bull 122:91–99. https://doi.org/10.1016/j.marpolbul.2017.06.009
Furuta T, Igarashi K, Hirata Y (2004) Low-foaming detergent compositions. US patent US20040171512A1
Geys R, Graevel MD, Lodens S et al (2018) Increasing uniformity of biosurfactant production in Starmerella bombicola via the expression of chimeric cytochrome P450s. Colloids Interf 42. https://doi.org/10.3390/colloids2040042
Giessler-Blank S, Schilling M, Thum O et al (2016) Use of sophorolipids and derivatives thereof in combination with pesticides as adjuvant/additive for plant protection and the industrial non-crop field. US patent US9351485B2
Gong X, Wei L, Yu X et al (2017) Effects of Rhamnolipid and microbial inoculants on the vermicomposting of green waste with Eisenia fetida. PLoS One 12:e0170820. https://doi.org/10.1371/journal.pone.0170820
Goodbody-Gringley G, Wetzel DL, Gillon D et al (2013) Toxicity of Deepwater Horizon source oil and the chemical dispersant, Corexit® 9500, to coral larvae. PLoS One 8:e45574. https://doi.org/10.1371/journal.pone.0045574
Hall PJ, Haverkamp J, Kralingen CV et al (1995) Synergistic dual-surfactant detergent composition containing sophorolipid. US patent US5417879A
Harvey S, Elashvili I, Valdes JJ et al (1990) Enhanced removal of Exxon Valdez spilled oil from Alaskan gravel by a microbial surfactant. Nat Biotechnol 8:228–230
Hees U, Fabry B (1997) Use of mixture of glyco-lipid and surfactant in hand dishwashing detergent. German patent DE19600743A1
Herman DC, Artiola JF, Miller RM (1995) Removal of cadmium, Lead, and zinc from soil by a Rhamnolipid biosurfactant. Environ Sci Technol 29:2280–2285
Holakoo L (2011) On the capability of rhamnolipids for oil spill control of surface water. Masters thesis, Concordia University, https://spectrum.library.concordia.ca/1745/. Accessed 7 Aug 2018
Holder EL, Conmy RN, Venosa AD (2015) Comparative laboratory-scale testing of dispersant effectiveness of 23 crude oils using four different testing protocols. J Environ Protect 6:628–639. https://doi.org/10.4236/jep.2015.66057
Joshi-Navare K, Khanvilkar P, Prabhune A (2013) Jatropha oil derived Sophorolipids: production and characterization as laundry detergent additive. Biochem Res Int 2013:169797. https://doi.org/10.1155/2013/169797
Khoshdast H, Abbasi H, Sam A et al (2012) Frothability and surface behavior of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa MA01. Biochem Eng J 60:127–134
Kleindienst S, Seidel M, Ziervogel K et al (2015) Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. PNAS 112:14900–14905
Kulakovskaya E, Baskunov B, Zvonarev A (2014) The antibiotic and membrane-damaging activities of cellobiose lipids and sophorose lipids. J Oleo Sci 63:701–707
Kuyukina MS, Ivshina IB, Ritchkova MI et al (2010) Bioremediation of crude oil-contaminated soil using slurry-phase biological treatment and land farming techniques. Soil Sediment Contam 12:85–99
Lai CC, Huang YC, Wei YH et al (2009) Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J Hazard Mater 167:609–614
Lal S, Ratna S, Said OB et al (2018) Biosurfactant and exopolysaccharide-assisted rhizobacterial technique for the remediation of heavy metal contaminated soil: an advancement in metal phytoremediation technology. Environ Technol Innov 10:243–263. https://doi.org/10.1016/j.eti.2018.02.011
Lan G, Fan Q, Liu Y et al (2015) Rhamnolipid production from waste cooking oil using Pseudomonas SWP-4. Biochem Eng J 101:44–54
Lawniczak L, Marecik R, Chrzanowski L (2013) Contributions of biosurfactants to natural or induced bioremediation. Appl Microbiol Biotechnol 97:2327–2339
Le Mire G, Siah A, Brisset MN et al (2018) Surfactin protects wheat against Zymoseptoria tritici and activates both salicylic acid- and Jasmonic acid dependent defense responses. Agriculture 8:11–23. https://doi.org/10.3390/agriculture8010011
Li S, Pi Y, Bao M et al (2015) Effect of rhamnolipid biosurfactant on solubilization of polycyclic aromatic hydrocarbons. Mar Pollut Bull 101:219–225
Liduino VS, Servulo EFC, Oliveira FJS (2018) Biosurfactant-assisted phytoremediation of multi-contaminated industrial soil using sunflower (Helianthus annuus L.). J Environ Sci Health A Tox Hazard Subst Environ Eng 53:609–616. https://doi.org/10.1080/10934529.2018.1429726
Lin TC, Pan PT, Cheng SS (2010) Ex situ bioremediation of oil-contaminated soil. J Hazard Mater 176:27–34. https://doi.org/10.1016/j.jhazmat.2009.10.080
Long X, He N, He Y et al (2017) Biosurfactant surfactin with pH-regulated emulsification activity for efficient oil separation when used as emulsifier. Bioresour Technol 241:200–206
Lovaglio RB, Jose Santos FJ, Jafelicci M Jr et al (2011) Rhamnolipid emulsifying activity and emulsion stability: pH rules. Colloids Surf B Biointerfaces 85:301–305
Madsen JK, Pihl R, Moller AH et al (2015) The anionic biosurfactant rhamnolipid does not denature industrial enzymes. Front Microbiol 6:292. https://doi.org/10.3389/fmicb.2015.00292
Magthalin CJ, Varadharajan A, Swarnalatha S et al (2016) Utilization of chicken tallow for the production of cationic biosurfactant and thereof for decontamination of Cr(III) containing soil. Procedia Environ Sci 35:895–913
Mata-Sandoval JC, Karns J, Torrents A (2001) Influence of rhamnolipids and triton X-100 on the biodegradation of three pesticides in aqueous phase and soil slurries. J Agric Food Chem 49:3296–3303
Mehta SK, Sharma S, Mehta N et al (2010) Biomimetic Amphiphiles: properties and potential use. In: Sen R (ed) Biosurfactants, advances in experimental medicine and biology, vol 672. Springer, New York, pp 102–120
Mendes A, Filgueiras L, Pinto J et al (2015) Physicochemical properties of Rhamnolipid biosurfactant from Pseudomonas aeruginosa PA1 to applications in microemulsions. J Biomater Nanobiotechnol 6:64–79. https://doi.org/10.4236/jbnb.2015.61007
Mohamed RM, Al-Gheethi AA, Noramira J et al (2018) Effect of detergents from laundry greywater on soil properties: a preliminary study. Appl Water Sci 8:16–24. https://doi.org/10.1007/s13201-018-0664-3
Moshtagh B, Hawboldt K, Zhang B (2018) Optimization of biosurfactant production by Bacillus Subtilis N3-1P using the brewery waste as the carbon source. Environ Technol. https://doi.org/10.1080/09593330.2018.1473502
Najmi Z, Ebrahimipour G, Franzetti A et al (2018) In situ downstream strategies for cost-effective biosurfactant recovery. Biotechnol Appl Biochem 65:523–532
National Oil and Hazardous Substances Pollution Contingency Plan., https://www.api.org/~/media/Files/Certification/ICP/ICP-Certification-Programs/1169_2017_GovRefDocs/1169_USA_40-CFR-300_Eff-04-2017.pdf. Accessed 4 Aug 2018
Odukkathil G, Vasudevan N (2015) Biodegradation of endosulfan isomers and its metabolite endosulfate by two biosurfactant producing bacterial strains of Bordetella petrii. J Environ Sci Health B 50:81–89
Odukkathil G, Vasudevan N (2016) Residues of endosulfan in surface and subsurface agricultural soil and its bioremediation. J Environ Manag 165:72–80
Otzen DE (2017) Biosurfactants and surfactants interacting with membranes and proteins: same but different? Biochim Biophys Acta Biomembr 1859:639–649
Pacheco S, Hilber I, Faure R et al (2018) Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in urban and semi-urban soils of Havana, Cuba. J Soil Sediment. https://doi.org/10.1007/s11368-018-2137-6
Papanikolaou S, Chevalot I, Galiotou-Panayotou M et al (2007) Industrial derivative of tallow: a promising renewable substrate for microbial lipid, single-cell protein and lipase production by Yarrowia lipolytica. Electron J Biotechnol 10. https://doi.org/10.2225/vol10-issue3-fulltext-8
Paraszkiewicz K, Bernat P, Siewiera P et al (2017) Agricultural potential of rhizospheric Bacillus subtilis strains exhibiting varied efficiency of surfactin production. Sci Hort 225:802–809
Parry AJ, Parry NJ, Peilow AC et al (2012) Detergent compositions comprising biosurfactant and enzyme. WO patent WO2012010405A1
Passow U, Sweet J, Quigg A (2017) How the dispersant Corexit impacts the formation of sinking marine oil snow. Mar Pollut Bull 125:139–145
Penfold J, Chen M, Thomas RK et al (2011) Solution self-assembly of the sophorolipid biosurfactant and its mixture with anionic surfactant sodium dodecyl benzene sulfonate. Langmuir 27:8867–8877
Penfold J, Thomas RK, Shenc HH et al (2012) Adsorption and self-assembly of biosurfactants studied by neutron reflectivity and small angle neutron scattering: glycolipids, lipopeptides and proteins. Soft Matter 8:578–591
Perez-Ameneiro M, Vecino X, Cruz JM et al (2015) Wastewater treatment enhancement by applying a lipopeptide biosurfactant to a lignocellulosic biocomposite. Carbohyd Polym 131:186–196
Pi G, Mao L, Bao M et al (2015) Preparation of oil-in-seawater emulsions based on environmentally benign nanoparticles and biosurfactant for oil spill remediation. ACS Sustain Chem Eng 3:2686–2693
Ramachandran SD, Hodson PV, Khan CW et al (2004) Oil dispersant increases PAH uptake by fish exposed to crude oil. Ecotoxicol Environ Saf 59:300–308
Rane AN, Baikar VV, Kumar RV et al (2017) Agro-industrial wastes for production of biosurfactant by Bacillus subtilis ANR 88 and its application in synthesis of silver and gold nanoparticles. Front Microbiol 8:492. https://doi.org/10.3389/fmicb.2017.00492
Rhamnolipid biosurfactant (110029) Fact Sheet. https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-110029_01-May-04.pdf. Accessed 4 Sept 2018
Rocha e Silva FCP, Rocha e Silva NMP, Luna JM et al (2018) Dissolved air flotation combined to biosurfactants: a clean and efficient alternative to treat industrial oily water. Rev Environ Sci Biotechnol 17:591–602
Rongsayamanont W, Soonglerdsongpha S, Khondee N et al (2017) Formulation of crude oil spill dispersants based on the HLD concept and using a lipopeptide biosurfactant. J Hazard Mater 334:168–177
Roy JL (1999) Soil water repellency at old crude oil spill sites. Dissertation, University of Alberta
Roz EZ, Rosenberg E (2001) Natural roles of biosurfactants. Environ Microbiol 3:229–236
Rufino RD, de Luna JM, Takaki GMC et al (2014) Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron J Biotechnol 17:34–38
Saborimanesh N, Mulligan CN (2015) Effect of Sophorolipid biosurfactant on oil biodegradation by the natural oil-degrading Bacteria on the weathered biodiesel, diesel and light crude oil. J Bioremed Biodegr 6:314. https://doi.org/10.4172/2155-6199.1000314
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5:1. https://doi.org/10.1186/s40643-017-0187-z
Sajna KV, Sukumaran RK, Jayamurthy H et al (2013) Studies on biosurfactants from Pseudozyma sp. NII 08165 and their potential application as laundry detergent additives. Biochem Eng J 78:85–92
Sajna KV, Höfer R, Sukumaran RK et al (2015) White biotechnology in biosurfactants. In: Pandey A, Höfer R, Taherzadeh M, Nampoothiri KM, Larroche C (eds) Industrial biorefineries and White biotechnology. Elsevier, Amsterdam, pp 499–521
Santos DK, Rufino RD, Luna JM et al (2016) Biosurfactants: multifunctional biomolecules of the 21st century. Int J Mol Sci 17(3):401. https://doi.org/10.3390/ijms17030401
Sarubbo LA, Rocha RB Jr, Luna JM et al (2015) Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem Ecol 31:707–723
Saxena K, Aseri GK, Gupta AD et al (2012) Bioremediation of Xenobiotics. In: Mohee R, Mudhoo A (eds) Bioremediation and sustainability: research and applications. Wiley, Hoboken, pp 367–398
Schofield MH, Thavasi TR, Gross RA (2012) Modified sophorolipids for the inhibition of plant pathogens. WO patent WO2013052615A1
Sharma P, Melkania U (2017) Biosurfactant-enhanced hydrogen production from organic fraction of municipal solid waste using co-culture of E. coli and Enterobacter aerogenes. Bioresour Technol 243:566–572
Silva EJ, Almeida DG, Luna JM et al (2018) Use of bacterial biosurfactants as natural collectors in the dissolved air flotation process for the treatment of oily industrial effluent. Bioprocess Biosyst Eng 41:1599–1610
Slizovskiy IB, Kelsey JW, Hatzinger PB (2011) Surfactant-facilitated remediation of metal-contaminated soils: efficacy and toxicological consequences to earthworms. Environ Toxicol Chem 30:112–123. https://doi.org/10.1002/etc.357
Song D, Liang S, Zhang Q et al (2013) Development of high efficient and low toxic oil spill dispersants based on sorbitol Derivants nonionic surfactants and glycolipid biosurfactants. J Environ Protect 4:16–22. https://doi.org/10.4236/jep.2013.41B004
Song D, Liang S, Yan L et al (2016) Solubilization of polycyclic aromatic hydrocarbons by single and binary mixed Rhamnolipid-Sophorolipid biosurfactants. J Environ Qual 45:1405–1412. https://doi.org/10.2134/jeq2015.08.0443
Strbak L (2000) In Situ Flushing with surfactants and Cosolvents. National Network of environmental studies fellowship report for US Environmental Protection Agency. Office of Solid Waste and Emergency Response Technology Innovation Office, Washington, DC
Tiso T, Zauter R, Tulke H et al (2017) Designer rhamnolipids by reduction of congener diversity: production and characterization. Microb Cell Factories 16:225. https://doi.org/10.1186/s12934-017-0838-y
Trilisenko LV, Kulakovskaya EV, Kulakovskaya TV et al (2012) The antifungal effect of cellobiose lipid on the cells of Saccharomyces cerevisiae depends on carbon source. Springerplus 1:18–26. https://doi.org/10.1186/2193-1801-1-18
Ukwungwu SV, Abbas AJ, Nasr GG et al (2017) Wettability effects on Bandera gray sandstone using biosurfactants. J Eng Technol 6. http://www.joetsite.com/wp-content/uploads/2017/07/Vol.-62-47-2017.pdf
Venosa AD, Holder E (2015) Laboratory-Scale Testing of Dispersant Effectiveness of 20 Oils Using the Baffled Flask Test. http://oilspilltaskforce.org/wp-content/uploads/2015/08/Venosa-and-Holder-baffled-flask.pdf. Accessed 11 Sept 2018
Vipulanandan C, Mohanty KK (2004) Biosurfactant produced from used vegetable oil for removal of metals from wastewaters and soils. EPA project report R828598C787
Wan J, Meng D, Long T et al (2015) Simultaneous removal of Lindane, Lead and cadmium from soils by Rhamnolipids combined with citric acid. PLoS One 10:e0129978. https://doi.org/10.1371/journal.pone.0129978
Wang S, Mulligan C (2004) Rhamnolipid foam enhanced remediation of cadmium and nickel contaminated soil. Water Air Soil Pollut 157:315–330
Wang S, Mulligan CN (2009a) Arsenic mobilization from mine tailings in the presence of a biosurfactant. Appl Geochem 24:928–935
Wang S, Mulligan CN (2009b) Rhamnolipid biosurfactant-enhanced soil flushing for the removal of arsenic and heavy metals from mine tailings. Process Biochem 44:296–301
Wei Y, Li G (2018) Effect of oil Pollution on water characteristics of loessial soil. IOP Conference Series: Earth and Environmental Science, 170 032154. https://doi.org/10.1088/1755-1315/170/3/032154
White HK, Lyons SL, Harrison SJ et al (2014) Long-term persistence of dispersants following the Deepwater horizon oil spill. Environ Sci Technol Lett 1:295–299
Word JQ, Clark JR, Word LS (2015) Comparison of the acute toxicity of Corexit 9500 and household cleaning products. Hum Ecol Risk Assess 21:707–725
Yalçın HT, Ergin-Tepebaşı G, Uyar E (2018) Isolation and molecular characterization of biosurfactant producing yeasts from the soil samples contaminated with petroleum derivatives. J Basic Microbiol 58:782–792. https://doi.org/10.1002/jobm.201800126
York JD, Firoozabadi A (2008) Comparing effectiveness of Rhamnolipid biosurfactant with a quaternary ammonium salt surfactant for hydrate anti-agglomeration. J Phys Chem B 112:845–851
Yoshida S, Koitabashi M, Nakamura J et al (2015) Effects of biosurfactants, mannosylerythritol lipids, on the hydrophobicity of solid surfaces and infection behaviours of plant pathogenic fungi. J Appl Microbiol 119:215–224
Zachos E (2018) 2,400 Animals Die in Oil Spill in Colombia, https://news.nationalgeographic.com/2018/03/oil-spill-colombia-animals-killed-spd/. Accessed 3 Aug 2018
Zdziennicka A, Krawczyk J, Szymczyk K et al (2018) Macroscopic and microscopic properties of some surfactants and biosurfactants. Int J Mol Sci 19. https://doi.org/10.3390/ijms19071934
Zhang Y, Jia D, Sun W et al (2018) Semicontinuous sophorolipid fermentation using a novel bioreactor with dual ventilation pipes and dual sieve-plates coupled with a novel separation system. Microb Biotechnol 11:455–464
Zhao F, Shi R, Zhao J et al (2015) Heterologous production of Pseudomonas aeruginosa rhamnolipid under anaerobic conditions for microbial enhanced oil recovery. J Appl Microbiol 118:379–389
Zheng H (2012) Biological oil spilling dispersing agent and preparation method thereof. Chinese patent CN102335493A, 1 Feb 2012
Zhong H, Zhang H, Lui Z et al (2016) Sub-CMC solubilization of dodecane by rhamnolipid in saturated porous media. Sci Rep 6:33266. https://doi.org/10.1038/srep33266
Zhou A, Zhang J, Cai W et al (2017) Comparison of chemosynthetic and biological surfactants on accelerating hydrogen production from waste activated sludge in a short-cut fermentation-bioelectrochemical system. Int J Hydrog Energy 42:9044–9050
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sajna, K.V., Gottumukkala, L.D. (2019). Biosurfactants in Bioremediation and Soil Health. In: Kumar, A., Sharma, S. (eds) Microbes and Enzymes in Soil Health and Bioremediation. Microorganisms for Sustainability, vol 16. Springer, Singapore. https://doi.org/10.1007/978-981-13-9117-0_15
Download citation
DOI: https://doi.org/10.1007/978-981-13-9117-0_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9116-3
Online ISBN: 978-981-13-9117-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)