Abstract
Extracellular vesicles (EVs) are small lipid-based membrane-bound vesicles secreted by most cells under both physiological and pathological conditions. A key function of EVs is to mediate cell–cell communication via transferring mRNAs, miRNAs and proteins from parent cells to recipient cells. These unique features of EVs have spurred a renewed interest in their utility for therapeutics. Given the growing evidence for EV-mediated renal diseases, strategies that could block the release or uptake of pathogenic EVs will be discussed in this review. Then, the therapeutic potential of EVs predominantly from stem cells in renal diseases will be outlined. Finally, we will focus on the specific application of EVs as a novel drug delivery system and highlight the challenges of EVs-based therapies for renal diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
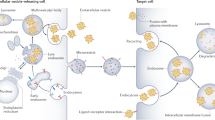
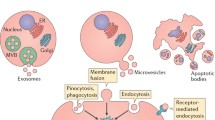
Extracellular vesicles (EVs) are nanoscale vesicles released by cells in physiological and pathological conditions. Depending on their size and biogenesis, EVs are classified into three major categories: exosomes, microvesicles and apoptotic bodies (van der Pol et al. 2012; Raposo and Stoorvogel 2013). Here, we focus on the first two classes of EVs. Exosomes, ranging from 30 to 150 nm in diameter, are formed by the fusion of intracellular multivesicular bodies with the plasma membrane (Colombo et al. 2014), whereas microvesicles, 50–1000 nm in size, are shed directly from the plasma membrane (Morel et al. 2011) (Fig. 34.1).
Biogenesis and characteristics of major classes of EVs. EVs can be classed as exosomes, microvesicles and apoptotic bodies based on their biogenesis and size. Exosomes are formed by the fusion of intracellular multivesicular bodies (MVBs) with the plasma membrane, whereas microvesicles are shed directly from the plasma membrane. EVs are taken up by cells by endocytosis, phagocytosis, pinocytosis or membrane fusion, and subsequently transfer cell membrane receptors or deliver effectors including mRNA, miRNA, DNA, lipid or protein into recipient cells. In addition, EVs could serve as a therapeutic target by inhibition of their production, release or cellular uptake
EVs were initially regarded as cell dust with no biological significance (Wolf 1967), but there is increasingly evidence for their important role in cell signalling and communication in normal and disease states (Karpman et al. 2017; Erdbrügger and Le 2016; Morrison et al. 2016; Zhang et al. 2016b; Camussi et al. 2010). In kidneys, EVs have been tightly linked to inflammation, fibrosis, thrombosis, adhesion, immune suppression, or growth and regeneration (Karpman et al. 2017; Erdbrügger and Le 2016; Morrison et al. 2016; Zhang et al. 2016b; Camussi et al. 2010). Therefore, EVs and their components could serve as the therapeutic targets, which can be inhibited to alleviate disease progression. Moreover, as EVs are suggested to participate in the tissue repair and immune modulation, they could be utilized directly as therapeutic agents in regenerative medicine and the treatment of autoimmune diseases. For example, EVs from mesenchymal stem cells protected against acute tubular injury and attenuated kidney inflammation (Bruno et al. 2009; Eirin et al. 2017; Rani et al. 2015).
Finally, given the natural role in transporting bioactive entities of EVs, they also have potential as drug carrier like a “Trojan horse” (van Dommelen et al. 2012; Fuhrmann et al. 2015). Recent studies indicate that EVs can function as efficient carriers of chemotherapeutic drugs (Tang et al. 2012; Yang et al. 2015), RNA drugs (Kamerkar et al. 2017; Alvarez-Erviti et al. 2011) and anti-inflammatory drugs (Sun et al. 2010; Zhuang et al. 2011). In this review, we will focus on recent developments in EV-based therapy as potential targets and as novel therapeutic agents, especially in the use of EVs as smart drug carriers.
2 Extracellular Vesicles as Potential Therapeutic Targets
Within the kidney, EVs can originate from blood cells, endothelial cells, podocytes or tubular epithelial cells (TECs), which have been strongly implicated in the pathogenesis of both acute kidney injury (AKI) and chronic kidney disease (CKD). Our group demonstrated that in the setting of proteinuric kidney disease, albumin triggered TECs to release exosomes packaged with CCL2 mRNA, which was delivered to macrophages and leads to interstitial inflammation (Lv et al. 2018a). Borges et al. identified that injured TECs released exosomes containing TGF-β mRNA to activate fibroblasts, contributing to the development of renal fibrosis in post-AKI kidneys (Borges et al. 2013). Moreover, microvesicle-mediated delivery of miR-21 among TECs could also drive the progressive renal fibrosis (Zhou et al. 2013a). Recent data found that transglutaminase-2, a matrix crosslinking enzyme for fibrotic remodelling, was secreted from TECs via exosomes (Furini et al. 2018). Thus, specifically inhibiting the biogenesis or uptake of these pathogenic EVs could be a potential therapeutic approach to alleviate disease progression (Fig. 34.1).
Various cellular components are known to be crucial for the biogenesis and release of EVs, and a number of possible therapeutic targets have been identified. For exosomes, ceramide is an important component in endosomal sorting and exosome biogenesis and its inhibition by GW4869 (neutral sphingomyelinase inhibitor) or amiloride (an antihypertensive agent) decreases exosome production (Trajkovic et al. 2008; Chalmin et al. 2010). GTPases Rab27b can regulate exosome release in some tumour cells, and this was demonstrated to be a therapeutic target (using RNAi) for reducing tumour progression (Peinado et al. 2012; Ostrowski et al. 2010; Bobrie et al. 2012). For microvesicles, the calpain inhibitor calpeptin or calpastatin can reduce the shedding of microvesicles (Zafrani et al. 2012; Yano et al. 1993), as well as blocking P2X receptors (Arvidsson et al. 2015). Furthermore, C1 inhibitor lessens the release of endothelial microvesicles, alleviating inflammatory diseases such as vasculitis (Mossberg et al. 2017). However, there are many limitations to target EV biogenesis and release because the precise mechanism remains elusive and is likely to vary among different cells.
In addition to reducing the level of EVs, inhibition of EV uptake into cells is also possible by certain substances and antibodies (Mulcahy et al. 2014). Blocking surface phosphatidylserine (which is important for cell adhesion) using Diannexin decreases the uptake of EVs derived from tumour cells (Lima et al. 2009; Al-Nedawi et al. 2009). Besides, an antibody to DEL1, annexin V, abciximab, chlorpromazine, cytochalasin D or cytochalasin B also have been demonstrated to block the uptake of EVs (Mulcahy et al. 2014; Dasgupta et al. 2012; Faille et al. 2012; Barrès et al. 2010), but it is difficult to translate these into therapeutic intervention due to the lack of specific mechanism regarding the key steps in EV trafficking and target definition.
3 Extracellular Vesicles as Therapeutic Agents
An increasing number of studies have demonstrated EVs, especially those derived from stem cells, and have innate therapeutic potential by virtue of their intrinsic cargoes, such as growth factors, soluble proteins and nucleic acids (Andaloussi et al. 2013). In kidney, mesenchymal stem cell-derived EVs of different origin also exhibit encouraging renoprotective efficacy, as shown in models of AKI, diabetic nephropathy, CKD and fibrosis. The application of these EVs in kidney diseases has been summarized in Table 34.1. For instance, Wang et al. showed that exosomes derived from bone marrow MSCs were able to transfer miR-let7c to damaged kidney cells and attenuate renal fibrosis in UUO mice (Wang et al. 2016). Kholia et al. reported that EVs derived from liver stem cells exhibited a regenerative, anti-inflammatory and anti-fibrotic role in aristolochic acid-induced kidney fibrosis (Kholia et al. 2018). In addition, EVs obtained from umbilical cord MSCs (Zhou et al. 2013b; Ju et al. 2015), Wharton’s jelly MSCs (Zou et al. 2014; Gu et al. 2016; Zhang et al. 2016a), adipose-derived MSCs (Eirin et al. 2017; Lin et al. 2016), kidney MSCs (Choi et al. 2014; Ranghino et al. 2017; Choi et al. 2015), as well as urine-derived MSCs (Jiang et al. 2016) also showed potential therapeutic benefits on kidney diseases.
Mechanistically, the protective effect of MSC-EVs on kidney diseases depends on their transfer of genetic material including mRNA and miRNA (Rani et al. 2015; Grange et al. 2017; Nargesi et al. 2017). This was confirmed in many studies when degradation of the RNAs in MSC-EVs using RNase could abolish aforementioned therapeutic benefits (Bruno et al. 2009; Choi et al. 2015; Zou et al. 2016), suggesting RNA-dependent biological effect. EVs derived from the Drosha-knockdown MSCs also showed global downregulation of miRNAs, resulting in ineffective renal repair of glycerol-induced AKI (Collino et al. 2015). Gene ontology analysis further showed that those genes shuttled by MSC-EVs were involved in healing pathways associated with renal regeneration (Collino et al. 2015). Moreover, EVs can also deliver proteins from MSCs to injured kidney cells. Proteins related to cell proliferation, adhesion, migration and morphogenesis have been identified in the vesicles by extensive proteomic analysis (Eirin et al. 2017; Shen et al. 2016; Jiang et al. 2016; Kim et al. 2012). In this regard, an elegant study showed that adipose-derived MSC-EVs attenuated renal inflammation in a porcine model of coexisting metabolic syndrome and renal artery stenosis by their cargo of IL-10 (Eirin et al. 2017).
In addition to MSC-EVs, other sources of cell-derived EVs, such as endothelial colony-forming cells (ECFCs), endothelial progenitor cells (EPCs) and hypoxic TECs, have shown significant beneficial effects as well (Table 34.1). In models of ischemic AKI, both ECFC-derived exosomes and EPC-derived EVs ameliorated renal injury via transfer of miRNAs (Viñas et al. 2016; Cantaluppi et al. 2012). In anti-Thy1.1-induced model of glomerulonephritis, EPC-derived EVs alleviated mesangial cell activation, leukocyte infiltration and apoptosis, which was related to its content of mRNAs coding for anti-apoptotic factors and the complement inhibitors (Cantaluppi et al. 2014). Interestingly, Dominguez et al. found that EVs derived from hypoxic TECs significantly improved renal tubular damage, fibrosis and microvascular pruning in established renal IRI (Dominguez et al. 2017). However, paradoxically, EVs from injured TECs also contribute to the progression of interstitial inflammation and fibrosis (Lv et al. 2018a; Borges et al. 2013; Zhou et al. 2013a; Furini et al. 2018), and the dual role of TEC-derived EVs needs to be further clarified.
4 Extracellular Vesicles as Smart Drug Carriers
Currently, the most preferred drug delivery systems are nanoparticle platforms based on liposomes, albumin, polymeric micelles and nanosized polymer-drug conjugates, which effectively improve the pharmacokinetics and biodistribution of drugs (Kamaly et al. 2016). However, their immunogenicity, stability and toxicity still remain elusive. In this case, EV-based drug delivery—with many of advantages, such as high permeability, less immunogenicity and non-cytotoxicity—appears to be a superior choice, overcoming the limitations observed with nanoparticles (Ha et al. 2016; Lv et al. 2018b). So far, EVs have been eloquently demonstrated to be as therapeutic nanocarriers for delivering a variety of cargos, including siRNAs, miRNAs, proteins and drugs (van Dommelen et al. 2012; Fuhrmann et al. 2015). But the application of EVs in kidney diseases has just begun its journey.
4.1 Cargo-Loading Techniques
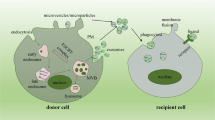
In order to employ EV-based drug delivery, it is essential to consider the methods of cargo loading and their suitability under different circumstances. In brief, cargo encapsulation can be performed exogenously or endogenously (van Dommelen et al. 2012; Fuhrmann et al. 2015; Batrakova and Kim 2015) (Fig. 34.2). For exogenously loading, the cargos were packaged into pre-assembled EVs ex vitro. A number of methods, including electroporation, sonication, direct transfection and simple incubation, are valid strategies for drug incorporation in this regard (Syn et al. 2017). For example, simple incubation is a versatile and feasible approach employed in many cases, through which several small lipophilic molecules, such as curcumin (antioxidant agents) (Sun et al. 2010; Zhuang et al. 2011), doxorubicin (Rani et al. 2015; Tian et al. 2014) and paclitaxel (Yang et al. 2015) (anti-cancer agents), are passively loaded into exosomes, but the loading capacity is low. Besides, potential limitations of electroporation may include size-dependent loading efficiency, denaturation and degradation of organic molecules and colloidal stability of the exosomal preparation (Syn et al. 2017).
Flow of the production of EV-based drug formulations. EV-based drug delivery requires the correct choice of source cell type for the specific application and should ideally be patient-derived to avoid triggering immune response. The therapeutic cargo can include different types of siRNA, miRNA, proteins or small-molecule compound such as curcumin or chemotherapeutics. Drug loading can be carried out either endogenously or exogenously. Endogenous loading is achieved by loading source cell with a therapeutic agent or transfecting source cell with drug-encoding gene which is then released in EVs upon collection. Exogenous loading allows the isolation of EVs before their loading with therapeutic cargo with the help of electroporation or by simple co-incubation. Importantly, the generation process should meet the quality requirements
For endogenously loading, the drug-loaded EVs are isolated from the modified parent cells through genetic engineering or medication with cytotoxic drugs. This method is convenient and requires very few manipulation steps. It is reported that paclitaxel is incorporated by MSCs and released in exosomes (Pascucci et al. 2014), as well as other anti-cancer agents: etoposide, carboplatin, irinotecan, epirubicin and mitoxantrone (Lv et al. 2012), which are loaded in exosomes with strong anti-proliferative activity. Moreover, recent studies demonstrated that the therapeutic protein and its genetic material could be loaded into EVs when parental cells were transfected with drug-encoding gene (Zeelenberg et al. 2008; Lee et al. 2015; Yim et al. 2016), but that might confer risks of genotoxicity and adverse host immune response. Of note, each cargo-loading strategy has its advantages and limitations depending on the type of therapeutic cargo and site of the disease, and thus further nuanced understanding is needed to select the optimal approach for mass production.
4.2 EVs as Delivery Vehicles for Nucleic Acids
It is known that EVs naturally carry nucleic acids, making them stable in the circulation and protecting from degradation. Given this, EVs may offer unique advantages for genetic therapy, and key studies using EVs as carriers for genetic materials are highlighted below. The first report on EV-mediated transfer of exogenous nucleic acids was published in 2010, when it was shown that THP-1 cells, which were transfected with a miR-150 mimic, secreted miR-150-enriched EVs and that could be functionally delivered to recipient cells (Zhang et al. 2010). Subsequent study conducted by Akao et al. found that THP-1 monocytes transfected with miR-143 mimic ex vivo secreted miR-143-containing EVs in nude mice after intravenous injection (Akao et al. 2011). Furthermore, when injected intravenously into UUO mice, engineered MSCs that overexpressed miR-let7c attenuated renal fibrosis via secreting miR-let7c-loaded exosomes (Wang et al. 2016). All these studies have eloquently corroborated such modes of miRNA transfer.
Small interference RNA (siRNA) is used to disrupt genes of interest and has great potential for the treatment of a range of diseases. Several studies have been conducted to test the usefulness of EVs as delivery vehicle for siRNA, and the first study conducted by Alvarez-Erviti et al. found that by expressing a neuron-targeting protein on the surface of exosomes, they could specifically deliver siRNA to the brain and resulted in a specific gene knockdown (Alvarez-Erviti et al. 2011). Importantly, the treatment displayed minimal toxicity and immune stimulation, even following repeated administration, suggesting EVs are suitable to deliver vectors in RNA interference therapy. This notion has been further confirmed by Wahlgren et al. that the gene MAPK1 was selectively silenced in monocytes and lymphocytes by using siRNA-loaded exosomes derived from human plasma (Wahlgren et al. 2012). More recently, an elegant research employed fibroblast-like mesenchymal cell-derived exosomes to deliver siRNA or short hairpin RNA specific to oncogenic KRAS, achieving enhanced therapeutic efficacy in suppressing tumour growth and improving the overall survival (Kamerkar et al. 2017). Notably, the therapeutic effects of engineered exosomes were greater than siRNA-loaded liposomes (Kamerkar et al. 2017). Beyond miRNA and siRNA delivery, EVs were also exploited to encapsulate adeno-associated viruses (AAVs), which were substantially more efficient than free AAVs for the delivery of genetic cargo into recipient cells (Maguire et al. 2012). Collectively, these studies emphasize the potential of using EVs for the therapeutic delivery of nucleic acids.
4.3 EVs as Delivery Vehicles for Proteins
In addition to delivering nucleic acids, EVs are also used to deliver large molecules such as proteins. Haney and colleagues found that exosomes loaded with the antioxidant protein catalase were successfully delivered across the blood–brain barrier (BBB) and provided significant neuroprotective effects in a model of Parkinson’s disease (Haney et al. 2015). In this study, catalase was incorporated into pre-assembled exosomes ex vivo using different methods, and identified sonication and extrusion approaches achieved better loading efficiency, sustained release and protein preservation (Haney et al. 2015). Similar results were reported by Yuan et al., showing that macrophage-derived exosomes efficiently crossed the BBB and delivered a cargo protein to the brain, further indicating the potency of EVs as nanocarriers for brain delivery of therapeutic proteins (Yuan et al. 2017). The cargo protein in the study was also loaded in an exogenous way by mixing with exosomes; in addition, the therapeutic protein can be packaged into EVs by transfecting parental cells as well. For example, HEK-293T cells transfected with suicide gene secreted EVs enriched in suicide mRNA and protein, which were subsequently used to treat schwannoma tumours in an orthotopic mouse model, leading to reduced tumour growth (Mizrak et al. 2013). Overall, these studies suggest that EVs can serve as novel nanocarriers to effectively deliver therapeutic proteins.
4.4 EVs as Delivery Vehicles for Drugs
EVs have been utilized as delivery vehicles for therapeutic drugs in extensive research (Tang et al. 2012; Yang et al. 2015; Sun et al. 2010; Zhuang et al. 2011). Early studies from the Zhang group (Sun et al. 2010; Zhuang et al. 2011) demonstrated an anti-inflammatory small-molecule compound curcumin could be incorporated into exosomes by mixing curcumin with murine tumour cell line (EL-4) or microglia cell (JSI124)-derived exosomes, and found that exosomal curcumin exhibited enhanced anti-inflammatory activity in LPS-induced septic shock mouse model. Interestingly, exosomal packaging leads to an increase in the solubility, stability and bioavailability of curcumin (Sun et al. 2010), suggesting EVs are capable to modify the bioavailability of the native drug. For another natural phytochemical compound celastrol, exosome-mediated delivery also improved drug biodistribution and subsequently enhanced its anti-tumour efficacy (Aqil et al. 2016). This study further highlighted the benefits of EVs in enhancing the properties of drugs, such as solubility, stability and bioavailability.
Besides, the deployment of EVs encapsulating chemotherapeutics such as paclitaxel and doxorubicin has yielded promising results, representing encouraging anti-cancer efficacy with minimal cytotoxicity towards non-cancerous cells (Tang et al. 2012; Yang et al. 2015; Syn et al. 2017; Tian et al. 2014; Pascucci et al. 2014; Jang et al. 2013; Saari et al. 2015; Toffoli et al. 2015; Srivastava et al. 2016; Martins-Marques et al. 2016). For example, anti-cancer drug-loaded exosomes or exosome-like vesicles were shown to traffic to tumour tissue and reduce tumour growth in mice without overt adverse effects (Tian et al. 2014; Jang et al. 2013). Importantly, exosomes had superior therapeutic effects when compared to liposomes (Jang et al. 2013). Moreover, the administration of doxorubicin loaded in exosomes resulted in significantly less drug accumulation in non-target organs and prevented the onset of off-target cardiotoxicity compared with mice treated with unmodified doxorubicin (Saari et al. 2015; Toffoli et al. 2015; Srivastava et al. 2016; Martins-Marques et al. 2016). Thus, the advantages of exosomes packaging may improve the safety profile of cytotoxic agents and present further opportunities to address cancer therapy.
5 Benefits and Challenges of Extracellular Vesicle Therapy
Unarguably, the field of EV-based therapeutics holds significant promise to enable targeted drug delivery with superior efficiency (Table 34.2). Compared with existing liposomes or polymeric nanoparticles, the outstanding advantage of EV-based therapy is their natural lipid and surface protein composition, which enable them to evade phagocytosis, extend blood half-life and reduce long-term safety issues. Moreover, the small size of EVs facilitates their extravasation, translocation through physical barriers and passage through extracellular matrix (van Dommelen et al. 2012; van den Boorn et al. 2011). Several studies have demonstrated that EVs successfully cross the BBB and deliver cargos into the brain, but whether EVs are able to pass through the glomerular filtration barrier remains unclear. In addition, EVs encapsulation also makes the new drug candidates such as proteins and nucleic acids more stable and targetable to treatment site (Zhu et al. 2012; Bruno et al. 2013).
However, before EV-based therapy can be translated to the clinic, several hurdles need to be overcome (Table 34.2). First, many properties and mechanisms about EV biology such as the biochemical composition of EV currently remain elusive, and the production or uptake mechanism yet poorly described. Even though from the same cell types, EVs may have contradictory effects as a consequence of differences in cell culture conditions and differences in the purification protocols used or due to a lack of robust extracellular vesicle characterization (Andaloussi et al. 2013; Zhu et al. 2012; Bruno et al. 2013). In addition, a major bottleneck in the translation of EV-based therapy into clinic is the lack of good manufacturing practice (GMP) standards. To develop clinical-grade EVs, sterile generation, high scale and efficient production of sufficient amounts of EVs with therapeutic payloads for clinical testing are required. Very recently, Mendt and colleagues have illustrated the process and feasibility of generating GMP-grade exosomes (Mendt et al. 2018). Finally, regarding the particularity of kidney, the glomerular filtration barrier is the primary obstacle that excludes EVs from accessing podocytes or tubular cells. The level of EVs accumulation in the kidney is highly restricted based on the injury degree of the glomerulus; thus, effective engineering of the size, shape and surface charge will conduce to EVs passing through renal barriers and their advancement to the clinic.
6 Conclusions
EVs are important conveyers of information between cells and have been strongly implicated in numerous biological and pathological processes. Targeting EVs directly to inhibit their pathogenic effects or exploiting their innate potential for renal regenerative medicine is promising therapeutic strategy. Moreover, although EV-based therapy has just begun its journey, they provide an enormous promise and a fresh therapeutic area for delivery of different drugs such as small-molecule compounds, particularly therapeutic nucleic acid delivery.
References
Akao Y, Iio A, Itoh T, Noguchi S, Itoh Y, Ohtsuki Y et al (2011) Microvesicle-mediated RNA molecule delivery system using monocytes/macrophages. Mol Ther 19:395–399
Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J (2009) Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A 106:3794–3799
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345
Andaloussi SEL, Mäger I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12:347–357
Aqil F, Kausar H, Agrawal AK, Jeyabalan J, Kyakulaga AH, Munagala R, Munagala R et al (2016) Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp Mol Pathol 101:12–21
Arvidsson I, Ståhl AL, Hedström MM, Kristoffersson AC, Rylander C, Westman JS et al (2015) Shiga toxin-induced complement-mediated hemolysis and release of complement-coated red blood cell-derived microvesicles in hemolytic uremic syndrome. J Immunol 194:2309–2318
Barrès C, Blanc L, Bette-Bobillo P, André S, Mamoun R, Gabius HJ et al (2010) Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood 115:696–705
Batrakova EV, Kim MS (2015) Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release 219:396–405
Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC et al (2012) Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 72:4920–4930
Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA et al (2013) TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 24:385–392
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F et al (2009) Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20:1053–1067
Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G (2013) Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev 22:758–771
Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L (2010) Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78:838–848
Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC et al (2012) Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 82:412–427
Cantaluppi V, Medica D, Mannari C, Stiaccini G, Figliolini F, Dellepiane S et al (2014) Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol Dial Transplant 30:410–422
Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP et al (2010) Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 120:457–471
Choi HY, Moon SJ, Ratliff BB, Ahn SH, Jung A, Lee M et al (2014) Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS ONE 9:e87853
Choi HY, Lee HG, Kim BS, Ahn SH, Jung A, Lee M et al (2015) Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem Cell Res Ther 6:18
Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P et al (2015) AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying MicroRNAs. J Am Soc Nephrol 26:2349–2360
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289
Dasgupta SK, Le A, Chavakis T, Rumbaut RE, Thiagarajan P (2012) Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation 125:1664–1672
Dominguez JH, Liu Y, Gao H, Dominguez JM 2nd, Xie D, Kelly KJ (2017) Renal tubular cell-derived extracellular vesicles accelerate the recovery of established renal ischemia reperfusion injury. J Am Soc Nephrol 28:3533–3544
Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ et al (2017) Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int 92:114–124
Erdbrügger U, Le TH (2016) Extracellular vesicles in renal diseases: more than novel biomarkers? J Am Soc Nephrol 27:12–26
Faille D, El-Assaad F, Mitchell AJ, Alessi MC, Chimini G, Fusai T et al (2012) Endocytosis and intracellular processing of platelet microparticles by brain endothelial cells. J Cell Mol Med 16:1731–1738
Fuhrmann G, Herrmann IK, Stevens MM (2015) Cell-derived vesicles for drug therapy and diagnostics: opportunities and challenges. Nano Today 10:397–409
Furini G, Schroeder N, Huang L, Boocock D, Scarpellini A, Coveney C et al (2018) Proteomic profiling reveals the transglutaminase-2 externalization pathway in kidneys after unilateral ureteric obstruction. J Am Soc Nephrol 29:880–905
Grange C, Iampietro C, Bussolati B (2017) Stem cell extracellular vesicles and kidney injury. Stem Cell Investig 4:90
Gu D, Zou X, Ju G, Zhang G, Bao E, Zhu Y (2016) Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int 2016:2093940
Ha D, Yang N, Nadithe V (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B 6:287–296
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z et al (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207:18–30
Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J et al (2013) Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 7:7698–7710
Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC et al (2016) Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther 7:24
Ju GQ, Cheng J, Zhong L, Wu S, Zou XY, Zhang GY et al (2015) Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS ONE 10:e0121534
Kamaly N, He JC, Ausiello DA, Farokhzad OC (2016) Nanomedicines for renal disease: current status and future applications. Nat Rev Nephrol 12:738–753
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA et al (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546:498–503
Karpman D, Ståhl AL, Arvidsson I (2017) Extracellular vesicles in renal disease. Nat Rev Nephrol 13:545–562
Kholia S, Herrera Sanchez MB, Cedrino M, Papadimitriou E, Tapparo M, Deregibus MC et al (2018) Human liver stem cell-derived extracellular vesicles prevent aristolochic acid-induced kidney fibrosis. Front Immunol 9:1639
Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW et al (2012) Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res 11:839–849
Lee J, Kim J, Jeong M, Lee H, Goh U, Kim H et al (2015) Liposome-based engineering of cells to package hydrophobic compounds in membrane vesicles for tumor penetration. Nano Lett 15:2938–2944
Lima LG, Chammas R, Monteiro RQ, Moreira ME, Barcinski MA (2009) Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett 283:168–175
Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT et al (2016) Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol 216:173–185
Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL et al (2012) Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 287:15874–15885
Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL et al (2018a) Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol 29:919–935
Lv LL, Wu WJ, Feng Y, Li ZL, Tang TT, Liu BC (2018b) Therapeutic application of extracellular vesicles in kidney disease: promises and challenges. J Cell Mol Med 22:728–737
Maguire CA, Balaj L, Sivaraman S, Crommentuijn MH, Ericsson M, Mincheva-Nilsson L et al (2012) Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther 20:960–971
Martins-Marques T, Pinho MJ, Zuzarte M, Oliveira C, Pereira P, Sluijter JP et al (2016) Presence of Cx43 in extracellular vesicles reduces the cardiotoxicity of the anti-tumour therapeutic approach with doxorubicin. J Extracell Vesicles 5:32538
Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M et al (2018) Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 3:99263
Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP et al (2013) Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther 21:101–108
Morel O, Jesel L, Freyssinet JM, Toti F (2011) Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol 31:15–26
Morrison EE, Bailey MA, Dear JW (2016) Renal extracellular vesicles: from physiology to clinical application. J Physiol 594:5735–5748
Mossberg M, Ståhl AL, Kahn R, Kristoffersson AC, Tati R, Heijl C et al (2017) C1-inhibitor decreases the release of vasculitis-like chemotactic endothelial microvesicles. J Am Soc Nephrol 28:2472–2481
Mulcahy LA, Pink RC, Carter DR (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3:24641
Nargesi AA, Lerman LO, Eirin A (2017) Mesenchymal stem cell-derived extracellular vesicles for renal repair. Curr Gene Ther 17:29–42
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A et al (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12:19–30
Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E et al (2014) Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release 192:262–270
Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18:883–891
Ranghino A, Bruno S, Bussolati B, Moggio A, Dimuccio V, Tapparo M et al (2017) The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res Ther 8:24
Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther 23:812–823
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383
Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M (2015) Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J Control Release 220:727–737
Shen B, Liu J, Zhang F, Wang Y, Qin Y, Zhou Z et al (2016) CCR68 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int 2016:1240301
Srivastava A, Amreddy N, Babu A, Panneerselvam J, Mehta M, Muralidharan R et al (2016) Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci Rep 6:38541
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C et al (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606–1614
Syn NL, Wang L, Chow EK, Lim CT, Goh BC (2017) Exosomes in cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol 35:665–676
Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J et al (2012) Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun 3:1282
Tang TT, Lv LL, Lan HY, Liu BC (2019) Extracellular vesicles: opportunities and challenges for the treatment of renal diseases. Front Physiol 10:226
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ et al (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35:2383–2390
Toffoli G, Hadla M, Corona G, Caligiuri I, Palazzolo S, Semeraro S et al (2015) Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine 10:2963–2971
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F et al (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247
van den Boorn JG, Schlee M, Coch C, Hartmann G (2011) SiRNA delivery with exosome nanoparticles. Nat Biotechnol 29:325–326
van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64:676–705
van Dommelen SM, Vader P, Lakhal S, Kooijmans SA, van Solinge WW, Wood MJ et al (2012) Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. J Control Release 161:635–644
Viñas JL, Burger D, Zimpelmann J, Haneef R, Knoll W, Campbell P et al (2016) Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int 90:1238–1250
Wahlgren J, Karlson TDL, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P et al (2012) Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res 40:e130
Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C et al (2016) Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther 24:1290–1301
Wolf P (1967) The nature and significance of platelet products in human plasma. Br J Haematol 13:269–288
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R et al (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 32:2003–2014
Yano Y, Shiba E, Kambayashi J, Sakon M, Kawasaki T, Fujitani K et al (1993) The effects of calpeptin (a calpain specific inhibitor) on agonist induced microparticle formation from the platelet plasma membrane. Thromb Res 71:385–396
Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H et al (2016) Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun 7:12277
Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E et al (2017) Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142:1–12
Zafrani L, Gerotziafas G, Byrnes C, Hu X, Perez J, Lévi C et al (2012) Calpastatin controls polymicrobial sepsis by limiting procoagulant microparticle release. Am J Respir Crit Care Med 185:744–755
Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A et al (2008) Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res 68:1228–1235
Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z et al (2010) Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 39:133–144
Zhang G, Zou X, Huang Y, Wang F, Miao S, Liu G et al (2016a) Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing Nrf2/ARE activation in rats. Kidney Blood Press Res 41:119–128
Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z (2016b) Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 311:F844–F851
Zhou Y, Xiong M, Fang L, Jiang L, Wen P, Dai C et al (2013a) miR-21-containing microvesicles from injured tubular epithelial cells promote tubular phenotype transition by targeting PTEN protein. Am J Pathol 183:1183–1196
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y et al (2013b) Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 4:34
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y et al (2012) Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 315:28–37
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC et al (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19:1769–1779
Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G et al (2014) Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther 5:40
Zou X, Gu D, Xing X, Cheng Z, Gong D, Zhang G et al (2016) Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res 8:4289–4299
Acknowledgements
This chapter was modified from a paper reported by our group in Front Physiol (Tang et al. 2019).
This study was supported by grants from the National Key Research and Development Program of China (2018YFC1314004), the National Natural Science Foundation of China (No.81720108007, 81670696, 81470922 and 31671194), the Clinical Research Center of Jiangsu Province (No. BL2014080) and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (No. KYCX18_0171).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tang, TT., Liu, BC. (2019). Extracellular Vesicles: Opportunities and Challenges for the Treatment of Renal Fibrosis. In: Liu, BC., Lan, HY., Lv, LL. (eds) Renal Fibrosis: Mechanisms and Therapies. Advances in Experimental Medicine and Biology, vol 1165. Springer, Singapore. https://doi.org/10.1007/978-981-13-8871-2_34
Download citation
DOI: https://doi.org/10.1007/978-981-13-8871-2_34
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8870-5
Online ISBN: 978-981-13-8871-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)