Abstract
Arsenic (As) in food is a threat for human health, and among all cereal rice is the most important source of the metalloid through diet. The dynamic of the element and the natural ability of rice to uptake, transport, and accumulate the metalloid at grains have motivated important research to be carried out in this regard. Thus, the aim of this chapter is to draw a synthesis on the main factors which ultimately determines As content in rice, from its uptake from the soil solution to its transport, metabolism, and final accumulation in grain. The element is of natural occurrence, varying in concentration, and its chemical species are modified due to a range of factors, such as the soil redox state. Among all As species, arsenite [As (III)] is the most common in anoxic conditions, such as the ones found in flooded paddy rice fields, which form is preferred for rice root uptake. Rice is naturally efficient in As(III) uptake, compared to most crops already studied, which is mainly due to its improved ability to uptake and transport silicon, especially with the aid of aquaporins, as silicic acid and arsenite are chemically analogous. Similarly, the second most important As form, arsenate [As(V)], is mainly uptaken and transported through the phosphate way. After being uptaken, As is transported either via xylem or phloem and is loaded to the grain. Within rice tissues, the element can be metabolized, being reduced, biomethylated, complexed to other elements, or even sequestered into vacuoles. The knowledge regarding the mechanisms of As uptake, transport, and metabolization in rice allows one to draw strategies in order to mitigate the content of this element in the grains, either via management practices or also via breeding and biotechnological approaches.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Arsenic (As) is listed as a human carcinogen since 1980 by the International Agency for Research on Cancer. Its carcinogenicity is mainly related to the exposure to its inorganic forms (iAs), arsenite [As(III)] and arsenate [As(V)], which are classified as group 1 carcinogenic. The organic species (oAs) monomethylarsonic acid [MMA(V)] and dimethylarsinic acid [DMA(V)] are also potentially carcinogenic for human and are classified in group 2B (revised by Guillod-Magnin et al. 2018). Numerous studies have associated the chronic exposition to high levels of As to health adverse effects, with damages to different organs and systems. Among the diseases associated to As exposure, the most important are keratosis; skin, bladder, and lung cancers; damaged intellectual (brain) function; bronchiectasis; cardiac coronary diseases; and diabetes (revised by Lai et al. 2015).
Rice (Oryza sativa L.) presents a high ability in accumulating As, resulting in a concentration ten times higher than any other cereal crops already studied (revised by Guillod-Magnin et al. 2018). Thus, in populations where rice is the major dietary source, the exposure to As can be considered expressive, and the ingestion of this element can represent a significant health risk factor (revised by Islam et al. 2017).
In addition to health problems caused to humans, As presents a negative impact on rice growth and development. The As toxicity results in inhibition in adenosine triphosphate (ATP) synthesis and also culminates in oxidative stress, resulting in a significative reduction in grain yield. Furthermore, under As toxicity conditions, plants may present stunted growth, brown spots, and burned leaves (revised by Bakhat et al. 2017).
Arsenic concentration in soil varies due to its geological genesis and anthropogenic activities, which include metal mining and foundry, As-containing pesticide applications, wood conservants, food additives, and the use of contaminated water in irrigation. Arsenic exists in the environment in different inorganic and organic forms. In paddy flooded soils, the ones where rice is grown in many parts of the world, As(III) is the dominant species, comprising 63% of total As in soil, followed by As(V) with 36%, and finally the methylated (organic) forms DMA(V) and MMA(V) (revised by Mitra et al. 2017). The high concentration of iAs in anaerobic soils associated to the high ability of rice plants in capturing this element constitutes a current and highly relevant threat.
Thus, considering the menace of As contamination in rice grains, it becomes imperative to thoroughly summarize the information already available in the literature and draw a model to synthetize the mechanisms of uptake, transport, and metabolism in planta. In this chapter, the proteins involved in the uptake of the different As forms and their association with the uptake of other elements are presented. Chemical modifications in As molecules after being uptaken by the plant will also be described. The plant tolerance mechanisms as well as the hyperaccumulation phenomena are described in details. Furthermore, the translocation from root to shoot, mobilization via phloem, and sulfur’s influence on these processes are also addressed.
2 Arsenic Species and Factors Which Affect Its Bioavailability from Soil
The total amount of As present in a given area depends on several factors. The first is related to soil genesis, being soils formed from As-rich bedrocks naturally high in As (Punshon et al. 2017). Other factors comprise any force which increases the element in an area, including natural inputs, e.g., through volcanic emissions, and anthropogenic sources, e.g., the use of As-based pesticides and metal mining, among many others (Zhao et al. 2010). In general, the higher is the total As in soil, the higher plants uptake the element; however, when As level is too high, this correlation became not valid, as high levels of the metalloid in the soil result in the restriction of the absorption of any element by plants (reviewed by Punshon et al. 2017). However, As can be present in different species, i.e., chemical forms, which strongly affect the uptake, translocation, and accumulation of the metalloid in plants and their organs (Marin et al. 1992). The most relevant As species in the context of As rice accumulation are the inorganic and more toxic As(III) and As(V) and the organic forms DMA and MMA (reviewed by Zhao et al. 2010).
A vast range of factors, including physicochemical and biological, govern the species, mobility, and bioavailability of As in soil-water systems (Kumarathilaka et al. 2018a). Rice is grown under flooded conditions in a vast area around the world, and it has a profound impact on the As biogeochemistry and bioavailability to the crop. The anaerobic conditions affect As mobility mainly by influencing As species, soil redox potential, and pH (Yamaguchi et al. 2011). In aerobic soils, As(V) is predominant; however, in anaerobic conditions, As is reduced and As(III) becomes the prevailing species. Due to its lower sorption capacity, it is followed by its desorption from soil minerals. This gives the element more mobility; however, As(V) and organic species can still be found in anaerobic soils, but in lower proportions (Stroud et al. 2011). Bacteria which reduce As(V) and oxide As(III) are also found in the soil. Arsenate reduction by microorganisms occurs through two main ways: As(V) as a final acceptor of electrons during the anaerobic respiration and the detoxification, in which As(V) is reduced to As(III) and pumped to the external side of the microbial cell (Zhao et al. 2010).
Arsenic also interacts with several elements in the soil, more importantly with sulfur (S), iron (Fe), silicon (Si), and phosphorus (P) (Zhao et al. 2010). In the geological environment, As often occurs combined with sulfur-rich minerals (Majzlan et al. 2014), and its release from these minerals depends on natural or anthropogenic processes, such as rock weathering. Regarding Fe, anaerobic conditions provoke its dissolution, and being Fe an important site for As binding, this results in higher As mobility (Yamaguchi et al. 2011). On the other hand, Fe plaque formation in rice roots has possibly also an important role, although not completely established yet, in regulating As absorption and reduction (Seyfferth 2015). Rice genotypes which release more oxygen through their aerenchyma at roots promote Fe plaque formation at the rhizosphere, which decreases As uptake. However, the oxygen released by roots can also oxidize As(III) back to As(V), which is more absorbed by the Fe plaques, i.e., the Fe plaque could keep As in proximity to the roots (reviewed in Awasthi et al. 2017). The presence of As(III)-oxidizer bacteria, such as Acidovorax and Hydrogenophaga in the Fe plaque which are involved in the As transformation, also influences the concentration of this element in the plant tissues (Hu et al. 2015). The abundance of AsO-Bac (arsenite-oxidizing bacteria) in the root ferric plaques was negatively correlated with As concentration in roots, straw, and grain, indicating that the microorganisms from the iron plaque were actively catalyzing the As transformation and decreasing the absorption of the element by rice (Hu et al. 2015).
Arsenite (as AsO33−) is chemically similar to silicate (SiO44−) and As(V) (AsO43−) analogous to phosphate (PO43−) (Zhao et al. 2010). It means that these molecules compete at the same time for retention sites on soil mineral surfaces and for transporters for uptake and translocation in the rice plant (reviewed by Bakhat et al. 2017). Phosphorus is a macronutrient, essential for plant growth, playing important physiological roles (Shen et al. 2011), and Si is a beneficial element, which helps plants to fight biotic and abiotic stresses (Meharg and Meharg 2015). Furthermore, rice is a Si accumulator, being able to contain Si at about 10% of the shoot dry weight, thanks to its highly efficient Si transporters (Yamamoto et al. 2012). The fact that As(III) and SiO44−, and As(V) and PO43−, compete for binding sites in soil would mean that supplying both SiO44− and PO43− as fertilizers would help to release more As in the soil solution, which would be more available to plant acquisition. However, research has suggested that the competition for transporters in the plant is more important in the context of As uptake, transport, and accumulation; hence, the application of SiO44− and PO43−, along sulfur (S) fertilizers, is highly recommended aiming decrease As accumulation by crops (Li et al. 2009; Bakhat et al. 2017).
3 Arsenic Uptake Mechanisms
The iAs uptake from the soil by plants depends on the amount and the chemical form of the element. In aerobic soils, the prevailing form is As(V), strongly sorbed in minerals from the soil, such as Fe oxides. In anaerobic soils, As(V) is reduced to As(III), followed by the desorption from the soil minerals, due to its low sorption capacity, compared to As(V); this is why As(III) is predominant in anaerobic conditions (Suriyagoda et al. 2018). As can also occur in different organic forms in the soil, being the most common the DMA(V) and MMA(V) (revised by Lomax et al. 2012).
Even though the most common arsenic forms in the soil are As(III) and As(V), in rice grains the prevailing types are As(III) and DMA(V). It is not clear yet whether plants are able to convert iAs in oAs. What is well known is the significant role that microorganisms have in this modification and that plants grown in soils without MMA(V) and DMA(V) do not present As methylated forms in their constitution (revised by Ma et al. 2017).
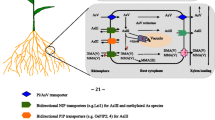
Comparing to other cereals, rice is the one which exhibits the highest capacity to uptake and accumulate As in different organs and grains. This behavior is due to the mobility of reduced As(III) present in anaerobic soils, characteristic of the irrigated (flooded) rice cultivation system in addition to the efficient As(III) capture mechanisms present in rice (Lomax et al. 2012). The uptake of the different As species by rice plants takes place through different processes, involving diverse proteins. In magnitude, rice plants uptake from soil As(III) > As(V) > DMA(V) > MMA(V). The capture of As(V) and As(III) occurs due to the similarity of these molecules with phosphate and silicate, respectively. Arsenate enters the root cells through phosphate transporter proteins (PHT) and As(III) through aquaglyceroporin transporter proteins from various classes and mainly via nodulin 26-like intrinsic protein, an aquaporin. DMA(V) and MMA(V) also use aquaporins to enter in the root cells (revised by Awasthi et al. 2017; Punshon et al. 2017; Suriyagoda et al. 2018). Arsenate is analogous to phosphate, interfering in phosphate metabolism (such as phosphorylation and ATP production). Arsenite binds to the protein’s sulfhydryl groups, affecting their structures or catalytic capacity (reviewed in Awasthi et al. 2017).
In rice, the gene family Pht1 (OsPT1-OsPT13) encodes for Pi transporters which are located on the plasma membrane (Ye et al. 2017). The As(V) uptake is performed through the transporters OsPHT1;8 (OsPT8) (Wu et al. 2011; Wang et al. 2016), OsPHT1;1 (OsPT1) (Kamiya et al. 2013) and OsPHT1;4 (OsPT4) (Cao et al. 2017; Ye et al. 2017). Rice mutants for ospt8 showed reduction of 33–57% in As(V) uptake and increased significantly the tolerance to this form of the metalloid, with a 100-fold or higher increase in root length, demonstrating that the uptake of As(V) via OsPHT8 exercises a pronounced toxic effect in root elongation (Wang et al. 2016). The ospt1 rice mutant presented a reduction in 60% in the As content in the plant (Kamiya et al. 2013). Further, ospt4 mutants accumulated 16–32% less As and still observed that the xylem of the mutants presented 20–40% less As comparing to the wild types (Cao et al. 2017).
It has been reported that PHT transporters are unidirectional, carrying out the influx of As(V) from soil to root cells (revised by Abbas et al. 2018). In addition to PHT proteins, other proteins can act on As(V) uptake, such as the transcription factor phosphate starvation response 2 (OsPHR2) and phosphate transporter traffic facilitator 1 (OsPHF1) (Wu et al. 2011). The As(V) transport from roots to shoots also occurs through different PHTs (revised by Awasthi et al. 2017).
Aquaporins are membrane channels belonging to the major intrinsic protein (MIP) family. MIPs are classified into three subgroups: aquaporins (AQPs) or specific water channels; glycerol facilitators (GlpFs) permeable to small solutes, such as glycerol; and aquaglyceroporins, a channel permeable to water, glycerol, and small solutes (revised by Thomas et al. 2002). Plant aquaporins are classified in four subfamilies according to their location and homology: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), nodulin 26-like intrinsic membrane proteins (NIPs), and small and basic intrinsic proteins (SIPs) (Ma et al. 2008).
Aquaporins present an aromatic/arginine (ar/R) region, which acts as a filter for subtract selection. According to the ar/R region, NIPs were divided into NIP I, NIP II, and NIP III. Members belonging to these three subgroups present ability to uptake As(III), however, in different quantities (Ma et al. 2008).
In rice, low silicon 1 (OsLsi1, also named OsNIP2;1) is a silicic acid NIP III aquaporin transporter, which can also inadvertently capture As(III), MMA(V), and DMA(V). In terms of magnitude, OsLsi1 captures As(III) > MMA(V) > DMA(V) (Punshon et al. 2017). Other members of the NIP family also capture As(III), however, at lower rates than OsLsi1 (Ma et al. 2008). OsLsi1 transporter is bidirectional performing in root cells the influx and efflux of As(III), i.e., As(III) can be transported depending on the difference of As concentration between roots cell and soil (revised by Abbas et al. 2018). OsLsi2 is a transporter which is not involved in the As(III) influx to the root cells, but instead in the efflux of this molecule to xylem, being directly associated with the As accumulation in rice grains (Ma et al. 2008). OsLsi1 and OsLsi2 are located on the plasma membrane of root exodermic cells, where the Casparian strips are found. However, with different polar localization, since OsLsi1 locates at the distal side of the cell and OsLsi2 at the proximal side (revised by Zhao et al. 2009; Chen et al. 2017). Rice oslsi1 mutants present a significant decrease in As(III) uptake, and oslsi2 mutants show an expressive reduction of As(III) content in xylem, aerial organs, and grains. Thus, it is verified that OsLsi2 has a more pronounced impact on the As(III) accumulation in rice grains than OsLsi1 (Ma et al. 2008).
Some studies have reported the involvement of PIP aquaporins, including OsPIP2;4, OsPIP2;6, and OsPIP2;7 in As(III) uptake and transport. Other transporters, such as OsNRAMP1 (natural resistance-associated macrophage protein 1), have also been suggested as having roles in the uptake and transport of As(III) (revised by Awasthi et al. 2017).
The uptake of methylated (organic) As forms (oAs) is slower than the uptake of the inorganic forms; however, the translocation of the methylated forms is faster than the others. Some organic As species are transported from the soil to xylem via influx Si transporters (Lsi1) and efflux Si transporters (Lsi2) (revised by Abbas et al. 2018). The mechanisms of movement in planta are discussed deeper in the Sect. 5 of this chapter.
4 Arsenic Metabolism in Plant
4.1 Arsenate Reduction
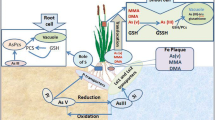
Speciation analysis of As in plant tissues demonstrates that the most prevailing form found is As(III), even in plants exposed to As(V) (Zhao et al. 2009), demonstrating a high As(V) reduction capacity. In a study with plants grown in hydroponic conditions with nutritional solution supplemented with As(III) or As(V), it was verified that As(III) remained stable; however, As(V) was rapidly reduced to As(III). Thus, As(III) represented from 92% to 99% of the total amount of As found in rice roots (Xu et al. 2007). Both roots and shoots are able to reduce As(V); however, roots are more important, considering that As(III) is the most abundant form found in xylem of many different plant species (reviewed in Zhao et al. 2010).
Rice presents two genes encoding for As(V)-reductase, viz., OsACR2.1 and OsACR2.2, which are involved in the reduction of As(V) (Duan et al. 2007). The products of these genes were purified from E. coli and demonstrate the capacity to reduce As(V) to As(III) in vitro, being OsACR2.1 the one which presented higher reductase activity comparing to OsACR2.2; however, both presented phosphatase activity. The expression of OsACR2.1 in the plant increased significatively after the As(V) exposure, while OsACR2.2 was expressed only in roots exposed to As(V) and in less quantity comparing to OsACR2.1. The genes, OsHAC1;1 and OsHAC1;2 (high arsenic content 1), were also identified as As(V)-reductases in rice (Shi et al. 2016). OsHAC1;1 and OsHAC1;2 were able to reduce As(V) to As(III) and, when expressed in E. coli deficient in As(V)-reductases, reestablished the As tolerance. When OsHAC1;1 and OsHAC1;2 were overexpressed, a significant increase in the As(III) efflux to the medium and lesser accumulation of As in rice were reported. Furthermore, a decreased amount of As was identified in the grains of the studied lines.
Glutaredoxin also plays an important role in As(V) reduction, regulating the As(III) levels in the cell; however, the complete mechanism is not well known yet (reviewed in Zhao et al. 2009). Two rice glutaredoxins, OsGrx_C7 and OsGrx_C2.1, were expressed in E. coli mutant strains, in yeast, and in A. thaliana and were important in response to As stress (Verma et al. 2016). OsGrxs expressed in E. coli promoted tolerance to As(V) and As(III) and in yeast demonstrated less As(III) intracellular accumulation and higher extracellular glutathione (GSH) accumulation. An in vitro experimentation showed that purified OsGrxs glutaredoxin presents similar activities of GSH-disulfide oxidoreductase, glutathione reductase, and As(V)-reductase.
Recently, it was reported the role of the vacuolar phosphate transporter (VPT) in As(V) tolerance in A. thaliana (Luan et al. 2018). The vpt1 mutant presented tolerance to As toxicity and lesser As accumulation, while VPT1-overexpressing lines were more sensible to the stress and presented more As accumulation, comparing to the corresponding wild type. For plants which were grown under optimal Pi conditions, the loss of function of the VPT1 increased the levels of Pi in the cytosol, which suppress the expression of the PHT1-type (phosphate transporter1), decreasing the As accumulation, which is chemically similar to Pi and enters into the cell through Pi transporters.
4.2 Arsenic Complexation and Sequestration
4.2.1 Methylation
Biomethylation of the As species has been considered a potential detoxification mechanism in microorganisms. This process results in the conversion of inorganic and toxic As to the less toxic MMA(V), DMA(V), trimethylarsenic oxide [TMAO(V)], and trimethylarsine [TMAs(III)] (Rahman and Hassler 2014). The intermediate trivalents are structurally different from the pentavalent ones, and they are more reactive and less carcinogenic (reviewed in Bastías and Beldarrain 2016). It was demonstrated that As(III) is tenfold more toxic than As(V) and that monomethylarsonite [MMA(III)] and dimethylarsonite [DMA(III)] are more toxic than As(V) and As(III) (Rahman and Hassler 2014). However, As(V) and As(III) are more toxic than MMA(V) and DMA(V). Finally, TMAO is the less toxic As form, among the most common forms.
The As methylation mechanism was described via Challenger, in studies with fungus (Zhao et al. 2010). In this system, As(III) is the initial substrate for methylation catalyzed by S-adenosylmethyltransferase (ArsM) using as methyl group donor, S-adenosyl-L-methionine. ArsMs are able to methylate As(III) sequentially in mono-, di-, and trimethyl generating as the final compost the TMA gas, which is volatilized. Enzymes involved in As methylation were detected in prokaryotic and eukaryotic cells (reviewed in Chen et al. 2017). The encoding gene for As(III) S-adenosyl methionine methyltransferase (ArsM) was identified in the bacteria Rhodopseudomonas palustris and catalyzes the formation of methylated As intermediates, viz., DMA(V) and TMAO, resulting in TMAs(III), a gas, as the final product. In the seaweed Cyanidioschyzon merolae, two ArsM enzymes were identified, and in mammals, AS3MT, homologous to the ArsM bacterial, was also identified. Transgenic rice expressing RpArsM (Rp – Rhodopseudomonas palustris) was able to produce methylated As and presented tenfold more volatile As compared to wild type (Meng et al. 2011). Arsenic accumulation in rice grains was lower in T1 generation, including As(III) and As(V) concentration.
Rice grain may contain organic and inorganic As forms. Arsenite and DMA are the more typical ones. In addition to these forms, rice can present As(V), MMA, and occasionally tetramethylarsonate. There are still reports of the presence of arsenobetaine (AsB) and arsenocholine (AsC) (reviewed in Bastías and Beldarrain 2016). However, some investigations showed that plants supplied with only iAs in hydroponic conditions presented small amounts of methylated species in their tissues and xylem, giving evidences on the As biomethylation in plants, even though at low levels (reviewed in Zhao et al. 2010). However, it has been reported that rice is not able to methylate inorganic As in vivo; thus the methylated species should have been originated from the rhizosphere, via microbial methylation (Lomax et al. 2012; Jia et al. 2013).
Rice genome presents genes which encode for proteins with the UbiE/Coq5 motif, belonging to the same protein family as the bacterial genes ArsM (Norton et al. 2008). However, no As methyltransferase enzyme has been identified so far. Rice absorbs methylated As through the OsLsi1 transporter, which transports SiO44− and As(III) (Li et al. 2009).
A recent study demonstrated that the reduction and complexation at roots are important steps for As(V) and MMA(V) metabolism in rice, but not for DMA(V) (Mishra et al. 2017). As(V) and MMA(V) induced the thiols synthesis in rice, and a diversity of complexes As(V)-thiol and MMA(V)-thiol was formed in rice roots, including various homologous of GSH and phytochelatins (PC). Furthermore, the predominance of MMA(III) in roots and shoots was reported and demonstrated that during the growth period, the rice plant can accumulate high levels of MMA(III) in tillers, which can also be transported to grains.
4.2.2 Tolerance and Detoxification Mechanisms
After entry into cells, metals and metalloids form complexes, by binding to metals, which include GSH, PCs, metallothioneins, nicotianamine, organic acids, and amino acids. GSH and PCs present high affinity for binding to metals due to their thiol groups (reviewed in Zhang et al. 2018). As(III) possesses high affinity with the sulfhydryl (-SH) groups of the thiol peptides of GSH and PCs, and when complexed to them, As(III) is detoxified in the plant (Pal and Rai 2010; Batista et al. 2014). GSH is synthetized in two steps: in the first, the dipeptide γ-glutamylcysteine (γ-EC) is produced from L-glutamate and L-cysteine, in an ATP-dependent reaction, catalyzed by γ-EC synthetase (γ-ECS). GSH is synthetized in two steps: 1) the dipeptide γ-glutamylcysteine (γ-EC) is produced from L-glutamate and L-cysteine, in an ATP-dependent reaction, catalyzed by γ-EC synthetase (γ-ECS); 2) one glycine is added to the C-terminal dipeptide portion, thus producing glutathione, in an ATP-dependent reaction, catalyzed by glutathione synthetase (Hell and Bergmann 1990). PCs are synthetized through the transpeptidation of the GSH γ-glutamylcysteinyl peptides by the catalytic activity of the PC synthase (PCS) (Pal and Rai 2010). Rice genotypes exposed to stress by As(V) and As(III) presented differences in the expression of some transporters, such as GST (Glutathione S-transferase – catalyzes the conjugation of the reduced form of glutathione with toxic substrates for detoxification) and γ-ECS including the PCS which were upregulated in the genotype which accumulated more As(V) and As(III) (Rai et al. 2011).
It was demonstrated that the CRT transporter (chloroquine resistance transporter) like from rice, OsCLT1, presents an important role in the GSH homeostasis, probably mediating the exportation of γ–EC and GSH from plastids to the cytoplasm. The Osclt1 mutant exhibited lower PC contents, when treated with As, resulting in lower As accumulation in roots and high or similar accumulation in shoots (Yang et al. 2016). Overexpression of genes encoding for enzymes involved in PC synthesis increases the tolerance and accumulation of metals in plants. The overexpression of OsPCS1 under the control of the 35S promoter from CaMV (Cauliflower mosaic vírus) in rice decreased As accumulation in grains (Hayashi et al. 2017). The reduction of As accumulation in rice grains was attributed to the expression of OsPCS1 in other tissues, blocking the As translocation to grain by the action of OsABCC1, which acts sequestering As into vacuole. T-DNA knockout lines of OsPCS1 presented sensitivity to higher As(III) accumulation, highlighting even more the importance of PCs in As translocation (Uraguchi et al. 2017). Two genes encoding for PCS were identified in rice, viz., OsPCS1 and OsPCS2, being the first more sensitive to As activation than the latter (Hayashi et al. 2017).
Inside the vacuole, As is mainly sequestered when in PC-As or GSH-As complexes, in this way, the transporters responsible for sequestering these complexes present a crucial role in As detoxification (Batista et al. 2014; Zhang et al. 2018). As(III) sequestering by the vacuole is mediated by a transporter, member of the ABC transporter family (ATP binding cassette) (reviewed in Briat 2010). After being chelated by PC, As can be sequestered to vacuole, mediated by the C-type ABC transporter (ABCC). In rice, a similar ABC transporter, OsABCC1, is needed for the As(III)-PC vacuolar sequestering and As detoxification, reducing As accumulation in grains (Batista et al. 2014; Song et al. 2014). Knockout of OsABCC1 increased As sensitivity. In roots, OsABCC1 is expressed in the exodermis and pericycle. However, Osabcc1 did not present decreases in As accumulation comparing to the wild type, probably due the absence of OsABCC1, resulting in rise of As toxicity and inducing the biosynthesis of thiol compounds which bind As in the cytoplasm. On the other hand, in shoots, Osabcc1 presented decreases in As accumulation in the node I but increased the As allocation to flag leaf and grain. These findings suggest that OsABCC1 constraints As translocation to grain by sequestering the element into the vacuoles of the phloem companion cells in rice nodes.
A recent work demonstrated that sequestering As into vacuole of root and phloem cells inhibits the As translocation for the grain (Deng et al. 2018). For this purpose, a transgenic rice line expressing ScYCF1 (Saccharomyces cerevisiae yeast cadmium factor) and OsABCC1 genes, the ones involved in As sequestering into the vacuole, under the control of the RCc3 promoter (root-specific cDNA clone3) in the radicular cortex and internodal phloem cells and the bacterial gene for γ-glutamylcysteine synthetase controlled by the maize ubiquitin, was tested. These transgenic plants presented decreased As translocation from root to shoot and from internodal phloem to the grain. It was reported also a decrease in 70% in As accumulation in rice without affecting agronomic traits.
Sulfur helps in As detoxification through binding As(III) to thiol-rich peptides. This mechanism also contributes in keeping As in roots, restricting its translocation to tillers (Rai et al. 2011). The involvement of S in preventing As toxicity was also demonstrated in other study, in which S treatment (5.0 mM) in plants increased As accumulation in roots due the complexing of As with thiolic ligands as nonprotein thiols and PCs, which restricted As translocation to shoot (Dixit et al. 2015). Moreover, enzymes involved in the S assimilation pathway and downstream thiolic metabolites were upregulated with the increase of S supplementation. The decrease of the transcription of Lsi2, which product is an As(III) transporter, is probably the reason for the lower As content in the shoot.
Through As(III) complexing with thiol groups for transportation to the vacuole, a strategy is to express ABC transporters and PCS simultaneously, which can maximize As sequestering to vacuole as well as the utilization of root-specific promoters which can control the expression of genes in the development of rice genotypes presenting low As grain content (Chen et al. 2017).
4.2.3 Hyperaccumulation
The engineering for As hyperaccumulation depends basically on the development of plants which are more tolerant to As in a way to accumulate biomass and plants which have the ability to store significantly more As in the surface or any organ or tissue which do not direct As for the grain (Dhankher et al. 2002). In these plants, the formation of complexes As(III) with GSH and PC and the transportation into the cellular vacuoles of roots and shoots constitute an important mechanism to deal with As stress (Souri et al. 2017). Despite diverse plants are able to hyperaccumulate and detoxify high levels of potentially toxic elements, few species are known as As hyperaccumulators, such as Pteris vittata, P. criteca, P. longifolia, P. umbrosa, and Pityrogramma calomelanos (reviewed in Kumar et al. 2015).
In order to develop plants able to accumulate As in their surface, the co-expression of two bacterial genes was performed in A. thaliana, viz., arsC and γ-EC, under the control of the leaf-specific promoter of the rubisco’s small subunity (Dhankher et al. 2002). The gene arsC produced the reduction of As(V) to As(III) in leaves and conferes to the plant the potential of complexing As with the thiol groups in the surface. The product of the γ-EC gene acts in the synthesis of the γ-EC dipeptide, the first enzyme involved in the PC synthesis. The hybrid line ArsC9 + γ-EC presented a substantially lower concentration of As when comparing to the corresponding wild type.
Oxidative stress induced by the reactive oxygen species production is one of the main toxic effects of As; however, strong oxidative forces in hyperaccumulating plants can constitute an important detoxification strategy (Souri et al. 2017). Souri and co-workers (2017) describe diverse studies which show a positive correlation between an improved antioxidant capacity, especially in the cover regions of the plant, and the tolerance to As and other metals in hyperaccumulating plants.
4.2.4 Molecular Strategies for Developing Rice with Less As Grain Content
Chen and co-workers (2017) described some genes in rice that can be used for genome editing using the CRISPR/Cas9 (cluster regularly interspaced short palindromic repeats-associated nuclease Cas9) technology, aiming the development of a rice genotype with lower As accumulation. OsPht1:8, Lsi1, and Lsi2 are involved in the uptake and As transportation, and OsPT4 was also reported as a potential candidate for obtaining rice genotypes with less As content (Ye et al. 2017). OsPT4 overexpressing lines presented sensitivity to As when grown in hydroponic system supplemented with As(V). However, OsPT4 CRISPR lines presented an opposing phenotype. Regarding As accumulation, OsPT4-ox increased twofold in this element in shoots and roots, As absorption in 23–45% and 22–30% in straw and grain. In OsPT4-cr lines, As accumulation decreased 17–30% comparing to the wild type.
However, other strategies can be applied, based on the understanding of the expression profile of genes differentially expressed in rice when subjected to As stress, taking into account the differences in absorption and As metabolism in rice genotypes. In this regard, the identification of rice genotypes contrasting for As accumulation, association, and QTL mapping is also important (Tuli et al. 2010). Diverse studies have focused on the transcriptional, metabolic, and proteic alterations in plants under stress by As; the mechanisms involving the transportation, translocation, and detoxification of hyperaccumulator plants comprise an important source of information to deal with this stress (Souri et al. 2017).
5 As Translocation in planta
After being captured by rice roots, As may be translocated to many different parts of the plant, just as occur to many other elements, including essential, beneficial, and even other toxic elements. It is an important part of the “journey of this element from soil to the rice grain” (Awasthi et al. 2017). The literature on this theme is growing constantly; however, some mechanisms are still unknown, and others were only studied in arabidopsis or other species; thus for rice these aspects have to be further investigated (Kumarathilaka et al. 2018b).
It is important to mention that the uptake from soil and translocation within the plant may be performed by similar mechanisms, i.e., same transporters (Kumarathilaka et al. 2018b). However, as the uptake was discussed in the third section of this chapter, in this part, we aim to present and discuss the mobilization of this element after being captured by plants. This mobility deserves special attention, because understanding the features of the process can be a key knowledge to drive efficient management and breeding strategies (including biotechnological approaches) in the aim of mitigating the content of As in rice grains, which is, ultimately, the last objective of investigating the problem. In other words, the literature has suggested an expressive number of strategies which could be applied in order to decrease As in rice grains, being the translocation of the element already captured by plant to other tissues in detriment to the grain one of the most promising (Punshon et al. 2017).
An important motivation for this is that although rice is remarkably efficient in As uptake from soil when comparing to most other crops, as already discussed in Sect. 3 of this chapter, fortunately from the human consumption point of view, most of these As do not reach the grain, being “dispersed during the journey” from soil to grain (Lindsay and Maathuis 2017). In fact, after being captured by plant, firstly most of the As remains in the roots, and secondly it is accumulated in stems – with special importance of the upper nodes and leaves, then grain husks, and finally the grain (Zhao et al. 2009; Batista et al. 2014; Chen et al. 2015, 2017). Among the reasons for that is the As metabolism in the plant, which was discussed in Sect. 4 of this chapter, in which As is complexed to other forms, affecting its mobility, or sequestered. Part of the remaining points will be addressed here.
As earlier exposed in this chapter, As can be present in different chemical forms (presenting different levels of toxicity), which strongly determines differences during the mobilization between tissues and organs (Marin et al. 1992; Awasthi et al. 2017). The fact that As(III) is analogous to silicate and As(V) to phosphate means that for translocation in planta, As shares the pathways of the respective analogous elements, just as occur for uptake (reviewed by Suriyagoda et al. 2018).
An interesting contrast between As uptake and translocation is that the As species most efficiently captured from the soil (the inorganic forms) are not the most efficiently translocated inside the plant and especially to the grain, being in this case the organic forms, mainly DMA (reviewed by Zhao et al. 2013 and Awasthi et al. 2017). To magnify this contrast, it has been shown that regarding uptake, DMA(V) is captured from soil in a rate of one twentieth of that of As(III); however the translocation to the rice grain is around 100-fold more efficient (Abedin et al. 2002; Lomax et al. 2012).
The mobility of As species in rice plants follows the order: DMA(V), followed by MMA(V), and only then the inorganic forms (reviewed by Kumarathilaka et al. 2018b). To illustrate it, Zhao et al. (2012), by labeling As(III) with 73As radioactive tracer, showed that only 10% of the As(III) captured by roots were distributed to shoot, mainly in leaves and stems, and about 3.3% reached the grain. Literature has suggested reasons for that. One is that while As(III) is translocated to rice grain primarily via the phloem pathway, DMA is transported to grain via both the phloem and xylem pathways (Carey et al. 2010). Also, from feeded cut flag leaves, DMA and MMA were efficiently translocated to rice grain, whereas As(V) was reduced to As(III) and retained in this tissue (Carey et al. 2011a, b). Other reasons are related to As metabolism, in which As(III) is suggested to be readily complexed by thiol compounds such as PCs and sequestered in the vacuoles, resulting in decreased mobility within the plant tissue (Liu et al. 2010; Song et al. 2010), whereas for DMA(V), there is a lack of complexing (Kumarathilaka et al. 2018b) (see Sect. 4 for a more detailed discussion on As metabolism).
5.1 As Mobilization from Root to Shoot
In general, the translocation of As from root to shoot can be considered inefficient in rice, being the ratio shoot/root of As concentrations ranging from 0.11 to 0.31 (Marin et al. 1992; Zhao et al. 2009). Considering that As is also toxic to the plant (Sharma 2012), it has been suggested that keeping the metalloid away from photosynthetic tissues is possibly an important strategy that lets some plant species tolerate As (Lindsay and Maathuis 2017).
After arsenic is uptaken by roots, many transporters are involved in its translocation. The element uses both active and passive ways to move along the plant, i.e., transporters that, respectively, use and do not use energy of the metabolism; however the extent of the contribution of each of these types in moving As is a question to be still answered (Kumarathilaka et al. 2018b). All major forms of As can be translocated from roots to shoot via xylem, being As(III) the prevailing form in this sap (reviewed by Zhao et al. 2009 and Suriyagoda et al. 2018).
Transporters are required, though, to load the element inside xylem. As(V) is presumably loaded through Pi transporters and As(III) by aquaporins (Verbruggen et al. 2009). Wu et al. (2011) performed an extensive study investigating the contribution of the Pi transport pathway in As mobilization in rice, and they found that the overexpression of the phosphate transporter OsPht1;8 increased the xylem loading of As(V). Other regulators of phosphate transport, viz., OsPHF1 (phosphate transporter traffic facilitator 1) and PHR2 (phosphate starvation response 2), also have an effect on As(V) transport (Wu et al. 2011). On the other hand, for As(III) the OsLsi2 (OsNIP2;1) transporter was found as involved in xylem loading (Ma et al. 2008; Fleck et al. 2013) as well as OsNRAMP1 – natural resistance-associated macrophage protein transporter (Tiwari et al. 2014). For organic As, much less is known about the translocation within the plant, comparing to iAs (Zhao et al. 2013). A recent study has shown that OsPTR7 (OsNPF8.1), a putative peptide transporter in rice, is involved in DMA(V) long-distance transport and thus accumulation in rice grain (Tang et al. 2017) also suggested for MMA(V) (Kumarathilaka et al. 2018b). An important point to highlight is that although As(V) and As(III) share, respectively, the phosphate and silicate ways, both As forms are less mobile than phosphate and silicate.
5.2 Phloem Transport
The last step regarding the accumulation of As in rice is the translocation to the grain. Arsenic, as well as most of other elements, including nutrients, is transported to rice grains mainly via the phloem (Carey et al. 2010, 2011a, b), by transporters situated in upper steam nodes (Song et al. 2014; Chen et al. 2015). Xylem plays a minor role in delivering nutrients, which includes other elements such as As, to developing seeds, possibly due to limited transpiration rate of these tissues (Bauer and Hell 2006).
For phloem loading, OsPHT1;8 and OsPHT1;1 have been suggested for As(V) and OsPTR7 for DMA(V) and MMA(V); however for As(III), transporters have still to be investigated, being suggested that inositol transporters (INT), already found in arabidopsis (Duan et al. 2016), could play similar role in rice (reviewed by Kumarathilaka et al. 2018b). Finally, for grain loading, As(V) is transported through OsPHT1;8 and DMA(V), and MMA(V), through OsPTR7. Once more, for As(III) inositol transporters (INT) and OsNIP2;2 can only be suggested as transporters, as further studies are required in rice to better elucidate this mechanism (reviewed by Kumarathilaka et al. 2018b).
Stem nodes have been proved to be of high importance for As storage and transport in rice. They have been suggested to act as a filter restricting As(III) distribution to grains being Lsi2 (OsNIP2;1) the transporter which plays a decisive role in this process (Chen et al. 2015). Also, OsABCC1, located at the tonoplast in the phloem cells from the node, was shown as responsible for the sequestration of As(III)–PC (As complexed) in the vacuoles (Song et al. 2014).
It is well known that there is a symplastic discontinuity between the maternal (plant) and the filial (seed) tissues, which could affect the As deposition in grains. DMA(V) has been proved to be several times more mobile to grain than any inorganic As form (Carey et al. 2010). Furthermore, DMA(V) not only reaches the grain more expressively but also permeates readily in the endosperm, whereas As(III) shows a strong tendency to accumulate in the ovular vascular traces, located on the surface of the grain (reviewed by Zhao et al. 2013).
In summary, rice grains, especially polished rice, tend to present a higher content of DMA(V) in comparison with As(III), As(V), and MMA(V). The positive aspect of this information is that DMA(V) is expressively less toxic for human consumption than the inorganic forms, and in addition to the small amount of total As which usually reaches the rice grain, it is suggested that DMA(V) in rice would not be a very high cause of concern regarding health risk (Zhao et al. 2013).
5.3 Sulfur’s Influence on As Accumulation in Rice
As earlier discussed, As and S present a strong relationship in soil (Majzlan et al. 2014). In the same way, in the journey from soil uptake to the final deposit, S has been found to affect As acquisition, translocation, and accumulation in rice (Zhang et al. 2011; Dixit et al. 2015; Hashimoto and Kanke 2018). The element plays a crucial role in As regulation through complexation of As by S-containing ligands GSH and PCs (Mishra et al. 2013). These As-thiol complexes are subsequently transported to vacuoles as an As detoxification process (Song et al. 2010).
Considering the importance of S in As regulation and taking also into account that it is an essential nutrient for plant growth, S mineral fertilization has been broadly suggested as an important mitigation alternative for reducing As in rice grain (Bakhat et al. 2017).
6 Final Considerations and Future Perspectives
Arsenic in rice has been considered a menace for consumers worldwide, deserving especial attention regarding the development of different strategies in the aim of mitigate this situation. Numerous strategies have been suggested with this aim, such as related to water and soil management, e.g., supplying silicate and phosphate fertilizers and the cultivation under rainfed conditions, and postharvest management, e.g., polishing the grain and even cooking it with an extra volume of water. On the other hand, another group of strategies comprise the development of improved rice cultivars, able to deal with the As in the soil, either by minimizing the absorption from soil or by the reduction of the translocation to the grain, through sequestering the element into other tissues.
Understanding the mechanisms involved in the uptake and transport of As in rice, as presented and discussed in the present chapter, is of fundamental importance for rice breeders to define specific targets in breeding programs. These genotypes can be obtained through biotechnology techniques, such as plant transformation and gene editing. Mutation breeding is also a powerful tool. In addition, it could be achieved through conventional approaches, via hybridizations, generation advancing, and selection of superior lines, requiring, though, genetic variability in rice germplasm. It has to be pointed out that more than probably, integrating different breeding approaches, in addition to improvements in management, will lead to advances in mitigating the problem of the presence of As in rice, one of the most important staple food for humankind.
References
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Amjad M, Hussain M, Natasha (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15:59
Abedin MJ, Feldmann J, Meharg AA (2002) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128:1120–1128
Awasthi S, Chauhan R, Srivastava S, Tripathi RD (2017) The journey of arsenic from soil to grain in rice. Front Plant Sci 8:1007
Bakhat HF, Zia Z, Fahad S, Abbas S, Hammad HM, Shahzad AN, Abbas F, Alharby H, Shahid M (2017) Arsenic uptake, accumulation and toxicity in rice plants: possible remedies for its detoxification: a review. Environ Sci Pollut Res Int 24:9142–9158
Bastías JM, Beldarrain T (2016) Arsenic translocation in rice cultivation and its implication for human health. Chil J Agric Res 76:114–122
Batista BL, Nigar M, Mestrot A, Rocha BA, Barbosa Júnior F, Price AH, Raab A, Feldmann J (2014) Identification and quantification of phytochelatins in roots of rice to long-term exposure: evidence of individual role on arsenic accumulation and translocation. J Exp Bot 65:1467–1479
Bauer P, Hell R (2006) Translocation of Iron in plant tissues. In: Barton LL, Abadia J (eds) Iron nutrition in plants and Rhizospheric microorganisms, 1st edn. Springer, Switzerland, pp 279–288
Briat J (2010) Arsenic tolerance in plants: “Pas de deux” between phytochelatin synthesis and ABCC vacuolar transporters. Proc Natl Acad Sci U S A 107:20853–20854
Cao Y, Sun D, Ai H, Mei H, Liu X, Sun S, Xu G, Liu Y, Chen Y, Ma LQ (2017) Knocking out OsPht1;4 gene decreases arsenic uptake by rice plants and inorganic arsenic accumulation in rice grains. Environ Sci Technol 51:12131–12138
Carey AM, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Charnock JM, Feldmann J, Price AH, Meharg AA (2010) Grain unloading of arsenic species in rice. Plant Physiol 152:309–319
Carey AM, Norton GJ, Deacon C, Scheckel KG, Lombi E, Punshon T, Guerinot ML, Lanzirotti A, Newville M, Choi Y, Price AH, Meharg AA (2011a) Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol 192:87–98
Carey AM, Lombi E, Donner E, de Jonge MD, Punshon T, Jackson BP, Guerinot ML, Price AH, Meharg AA (2011b) A review of recent developments in the speciation and location of arsenic and selenium in rice grain. Anal Bioanal Chem 402:3275–3286
Chen Y, Moore KL, Miller AJ, McGrath SP, Ma JF, Zhao FJ (2015) The role of nodes in arsenic storage and distribution in rice. J Exp Bot 66:3717–3724
Chen Y, Han Y-H, Cao Y, Zhu Y-G, Rathinasabapathi B, Ma LQ (2017) Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front Plant Sci 8:268
Deng F, Yamaji N, Ma JF, Lee S, Jeon J, Martinoia E, Lee Y, Song W (2018) Engineering rice with lower grain arsenic. Plant Biotechnol J 16:1691–1699
Dhankher OP, Li Y, Rosen BP, Shi J, Salt D, Senecoff JF, Sashti NA, Meagher RB (2002) Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and γ-glutamylcysteine synthetase expression. Nat Biotechnol 20:1140–1145
Dixit G, Singh AP, Kumar A, Singh PK, Kumar S, Dwivedi S, Trivedi PK, Pandely V, Norton GJ, Dhankher OP, Tripathi RD (2015) Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J Hazard Mater 298:241–251
Duan G, Zhou Y, Tong Y, Mukhopadhyay R, Rosen BP, Zhu Y (2007) A CDC25 homologue from rice functions as an arsenate reductase. New Phytol 174:311–321
Duan G-L, Hu Y, Schneider S, McDermott J, Chen J, Sauer N, Rosen BP, Daus B, Liu Z, Zhu YG (2016) Inositol transporters AtINT2 and AtINT4 regulate arsenic accumulation in Arabidopsis seeds. Nat Plants 2:15202
Fleck AT, Mattusch J, Schenk MK (2013) Silicon decreases the arsenic level in rice grain by limiting arsenite transport. J Plant Nutr Soil Sci 176:785–794
Guillod-Magnin R, Brüschweiler BJ, Aubert R, Haldimann M (2018) Arsenic species in rice and rice-based products consumed by toddlers in Switzerland. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 35:1164–1178
Hashimoto Y, Kanke Y (2018) Redox changes in speciation and solubility of arsenic in paddy soils as affected by sulfur concentrations. Environ Pollut 238:617–623
Hayashi S, Kuramata M, Abe T, Takagi H, Ozawa K, Ishikawa S (2017) Phytochelatin synthase OsPCS1 plays a crucial role in reducing arsenic levels in rice grains. Plant J 91:840–848
Hell R, Bergmann L (1990) γ-Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta 180:603–612
Hu M, Li F, Liu C, Wu W (2015) The diversity and abundance of As(III) oxidizers on root iron plaque is critical for arsenic bioavailability to rice. Sci Rep 5:13611
Islam S, Rahman MM, Rahman MA, Naidu R (2017) Inorganic arsenic in rice and rice-based diets: health risk assessment. Food Control 82:196e202
Jia Y, Huang H, Zhong M, Wang F, Zhang L, Zhu Y (2013) Microbial arsenic methylation in soil and rice rhizosphere. Environ Sci Technol 47:3141–3148
Kamiya T, Islam MR, Duan G, Uraguchi S, Fujiwara T (2013) Phosphate deficiency signaling pathway is a target of arsenate and phosphate transporter OsPT1 is involved in As accumulation in shoots of rice. Soil Sci Plant Nutr 59:580–590
Kumar S, Dubey RS, Tripathi RD, Chakrabarty D, Trivedi PK (2015) Omics and biotechnology of arsenic stress and detoxification in plants: current updates and prospective. Environ Int 74:221–230
Kumarathilaka P, Seneweera S, Meharg A, Bundschuh J (2018a) Arsenic speciation dynamics in paddy rice soil-water environment: sources, physico-chemical, and biological factors-a review. Water Res 140:403–414
Kumarathilaka P, Seneweera S, Meharg A, Bundschuh J (2018b) Arsenic accumulation in rice (Oryza sativa L.) is influenced by environment and genetic factors. Sci Total Environ 642:485–496
Lai PY, Cottingham KL, Steinmaus C, Karagas MR, Miller MD (2015) Arsenic and rice: translating research to address health care providers’ needs. J Pediatr 167:797–803
Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150:2071–2080
Lindsay ER, Maathuis FJ (2017) New molecular mechanisms to reduce arsenic in crops. Trends Plant Sci 22:1016–1026
Liu WJ, Wood BA, Raab A, McGrath SP, Zhao FJ, Feldmann J (2010) Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol 152:2211–2221
Lomax C, Liu WJ, Wu L, Xue K, Xiong J, Zhou J, McGrath SP, Meharg AA, Miller AJ, Zhao FJ (2012) Methylated arsenic species in plants originate from soil microorganisms. New Phytol 193(3):665–672
Luan M, Liu J, Liu Y, Han X, Sun G, Lan W, Luan S (2018) Vacuolar phosphate transporter 1 (VPT1) affects arsenate tolerance by regulating phosphate homeostasis in Arabidopsis. Plant Cell Physiol 59:1345–1352
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A 105:9931–9935
Ma L, Wang L, Jia Y, Yang Z (2017) Accumulation, translocation and conversion of six arsenic species in rice plants grown near a mine impacted city. Chemosphere 183:44e52
Majzlan J, Drahota P, Filippi M (2014) Parageneses and crystal chemistry of arsenic minerals. Rev Mineral Geochem 79:17–184
Marin AR, Masscheleyn PH, Patrick WH (1992) The influence of chemical form and concentration of arsenic on rice growth and tissue arsenic concentration. Plant Soil 139:175–183
Meharg C, Meharg AA (2015) Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ Exp Bot 120:8–17
Meng XY, Qin J, Wang LH, Duan GL, Sun GX, Wu HL, Chu CC, Ling HQ, Rosen BP, Zhu YG (2011) Arsenic biotransformation and volatilization in transgenic rice. New Phytol 191:49–56
Mishra S, Wellenreuther G, Mattusch J, Stärk HJ, Küpper H (2013) Speciation and distribution of arsenic in the non-hyperaccumulator macrophyte Ceratophyllum demersum. Plant Physiol 163:1396–1408
Mishra S, Mattush J, Wennrich R (2017) Accumulation and transformation of inorganic and organic arsenic in rice and role of thiol-complexation to restrict their translocation to shoot. Sci Rep 7:40522
Mitra A, Chatterjee S, Moogouei R, Gupta DK (2017) Arsenic accumulation in rice and probable mitigation approaches: a review. Agronomy 7:67
Norton GJ, Lou-Hing DE, Meharg AA, Price AH (2008) Rice-arsenate interactions in hydroponics: whole genome transcriptional analysis. J Exp Bot 59:2267–2276
Pal R, Rai JPN (2010) Phytochelatins: peptides involved in heavy metal detoxification. Appl Biochem Biotechnol 160:945–963
Punshon T, Jackson BP, Meharg AA, Warczack T, Scheckel K, Guerinot ML (2017) Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Sci Total Environ 581–582:209–220
Rahman MA, Hassler C (2014) Is arsenic biotransformation a detoxification mechanism for microorganisms? Aquat Toxicol 146:212–219
Rai A, Tripathi P, Dwivedi S, Dubey S, Shri M, Kumar S, Tripathi PK, Dave R, Kumar A, Singh R, Adhikari B, Bag M, Tripathi RD, Trivedi PK, Chakrabarty D, Tuli R (2011) Arsenic tolerances in rice (Oryza sativa) have a predominant role in transcriptional regulation of a set of genes including sulphur assimilation pathway and antioxidant system. Chemosphere 82:986–995
Seyfferth AL (2015) Abiotic effects of dissolved oxyanions on iron plaque quantity and mineral composition in a simulated rhizosphere. Plant Soil 397:43–61
Sharma I (2012) Arsenic induced oxidative stress in plants. Biologia 67:447–453
Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156:997–1005
Shi S, Wang T, Chen Z, Tang Z, Wu Z, Salt DE, Chao D, Zhao F (2016) OsHAC1;1 and OsHAC1;2 function as arsenate reductases and regulate arsenic accumulation. Plant Physiol 172:1708–1719
Song WY, Park J, Mendoza-Cozatl DG, Suter-Grotemeyer M, Shim D, Hortensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, Schroeder JI, Lee Y, Martinoia E (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci U S A 107:21187–21192
Song WY, Yamaki T, Yamaji N, Ko D, Jung KH, Fujii-Kashino M, An G, Martinoia E, Lee Y, Ma JF (2014) A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci U S A 111:15699–15704
Souri Z, Karimi N, Sandalio LM (2017) Arsenic Hyperaccumulation strategies: An overview. Front Cell Dev Biol 5:67
Stroud JL, Khan MA, Norton GJ, Islam MR, Dasgupta T, Zhu YG, Price AH, Meharg AA, McGrat SP, Zhao FJ (2011) Assessing the labile arsenic pool in contaminated paddy soils by isotopic dilution techniques and simple extractions. Environ Sci Technol 45:4262–4269
Suriyagoda LDB, Dittert K, Lambers H (2018) Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric Ecosyst Environ 253:23–37
Tang Z, Chen Y, Chen F, Ji Y, Zhao FJ (2017) OsPTR7 (OsNPF8. 1), a putative peptide transporter in rice, is involved in dimethylarsenate accumulation in rice grain. Plant Cell Physiol 58:904–913
Thomas D, Bron P, Ranchy G, Duchesne L, Cavalier A, Rolland J-P, Raguénès-Nicol C, Hubert J-F, Haase W (2002) Aquaglyceroporins, one channel for two molecules. Biochim Biophys Acta 1555:181–186
Tiwari M, Sharma D, Dwivedi S, Singh M, Tripathi RD, Trivedi PK (2014) Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ 37:140–152
Tuli R, Chakrabarty D, Trivedi PK, Tripathi RD (2010) Recent advances in arsenic accumulation and metabolism in rice. Mol Breed 26:307–323
Uraguchi S, Tanaka N, Hofmann C, Abiko K, Ohkama-Ohtsu N, Weber M, Kamiya T, Sone Y, Nakamura R, Takanezawa Y, Kiyono M, Fujiwara T, Clemens S (2017) Phytochelatin synthase has contrasting effects on cadmium and arsenic accumulation in rice grains. Plant Cell Physiol 58:1730–1742
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:364–372
Verma PK, Verma S, Meher AK, Pande V, Mallick S, Bansiwal AK, Tripathi RD, Dhankher OP, Chakrabarty D (2016) Overexpression of rice glutaredoxins (OsGrxs) significantly reduces arsenite accumulation by maintaining glutathione pool and modulating aquaporins in yeast. Plant Physiol Biochem 106:208–217
Wang P, Zhang W, Mao C, Xu G, Zhao F-J (2016) The role of OsPT8 in arsenate uptake and varietal difference in arsenate tolerance in rice. J Exp Bot 67:6051–6059
Wu Z, Ren H, McGrath SP, Wu P, Zhao F-J (2011) Investigating the contribution of the phosphate transport pathway to arsenic accumulation in Rice. Plant Physiol 157:498–508
Xu XY, McGrath SP, Zhao FJ (2007) Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol 176:590–599
Yamaguchi N, Nakamura T, Dong D, Takahashi Y, Amachi S, Makino T (2011) Arsenic release from flooded paddy soils is influenced by speciation, Eh, pH, and iron dissolution. Chemosphere 83:925–932
Yamamoto T, Nakamura A, Iwai H, Ishii T, Ma JF (2012) Effect of silicon deficiency on secondary cell wall synthesis in rice leaf. J Plant Res 125:771–779
Yang J, Gao MX, Hu H, Ding XM, Lin HW, Wang L, Xu JM, Mao CZ, Zhao FJ, Wu ZC (2016) OsCLT1, a CRT-like transporter 1, is required for glutathione homeostasis and arsenic tolerance in rice. New Phytol 211:658–670
Ye Y, Li P, Xu T, Zeng L, Cheng D, Yang M, Luo J, Lian X (2017) OsPT4 contributes to arsenate uptake and transport in rice. Front Plant Sci 8:2197
Zhang J, Zhao QZ, Duan GL, Huang YC (2011) Influence of sulphur on arsenic accumulation and metabolism in rice seedlings. Environ Exp Bot 72:34–40
Zhang J, Martinoia E, Lee Y (2018) Vacuolar transporters for cadmium and arsenic in plants and their applications in phytoremediation and crop development. Plant Cell Physiol 59:1317–1325
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Zhao F-J, Stroud JL, Khan MA, McGrath SP (2012) Arsenic translocation in rice investigated using radioactive 73As tracer. Plant Soil 350:413–420
Zhao F-J, Zhu YG, Meharg AA (2013) Methylated arsenic species in rice: geographical variation, origin, and uptake mechanisms. Environ Sci Technol 47:3957–3966
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Costa de Oliveira, A., Batista, B.L., Pegoraro, C., Venske, E., Viana, V.E. (2020). Mechanisms of Arsenic Uptake, Transport, and in planta Metabolism in Rice. In: Srivastava, S. (eds) Arsenic in Drinking Water and Food. Springer, Singapore. https://doi.org/10.1007/978-981-13-8587-2_14
Download citation
DOI: https://doi.org/10.1007/978-981-13-8587-2_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8586-5
Online ISBN: 978-981-13-8587-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)