Abstract
Poly (vinyl alcohol) (PVA) hydrogels are widely used in biomimetic cartilage materials for its good biocompatibility and super shock absorbing properties. However, the small pore size, in general, a few micrometers, of pure PVA hydrogels prepared through freezing-thawing method can not provide the suitable microenvironment for the proliferation of chondrocytes, restricting the application of hydrogels in artificial cartilage. In order to solve this barrier, here, agarose is introduced as porogen to prepare the macroporous PVA hydrogels through freezing-thawing method. The obtained PVA hydrogel have the pore size of 20–200 μm, and macropores have good connectivity. The mechanical properties of the macroporous hydrogels are tested using uniaxial compression and tension experiments and the results show that the mechanical properties of macroporous PVA hydrogels are dependent on the preparation parameters, e.g. the duration of freezing, number of freezing-thawing cycles and the temperature of thawing. After optimization, the mechanical properties of the macroporous PVA hydrogels are closer to those of natural articular cartilage and the obtained hydrogels may be used as the artificial replacement materials.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Articular cartilage is a dense connective tissue covering the articular surface and the main physiological functions of articular cartilage include facilitating the even distribution of loads, enlarging the load bearing surface of the joint, reducing contact stress and cushioning vibration. Articular cartilage can not self-heal when the defect covers a large area as it is an avascular and aneural tissue in a harsh biomechanical environment. At present, bone marrow stimulation [1] and tissue transplantation (including periosteal transplantation [2], perichondral transplantation [3], osteochondral transplantation [4], and chondrocyte transplantation [5]) are the main methods for the treatment of cartilage injury. However, these methods have such disadvantages as expensive cost, inflammation or lacking of donors [6].
Replacement of the damaged articular cartilage with suitable artificial materials provides another option. Poly (vinyl alcohol) (PVA) hydrogel has been considered as a very interesting and promising material for articular cartilage replacement [7]. But the neat PVA hydrogels can not provide the sufficient bioactivity as the pores of PVA hydrogels are only a few micrometers [8]. To generate large pores in PVA hydrogels, the porogen such as polyethylene glycol [9], dichloromethane [10] and gelatin sponges [11] and the composites porogen consisting of poly (lactic-co-glycolic acid) and sodium chloride micro-particle [12] have been attempted.
In this communication, agarose (AG), a polysaccharide obtained from agar and used for a variety of life science applications, was used as a novel porogen to fabricate macroporous PVA hydrogel (mPVA). We also investigated the effects of preparation parameters (the duration of freezing, number of freezing-thawing cycles, the temperature of thawing) on the mechanical properties of mPVA.
2 Experiments
2.1 Materials
The PVA with a degree of polymerization of 1750 ± 50 was purchased from Sinopharm Chemical Reagent Co., Ltd., China. The agarose (G-10) was provided by Gene Company Ltd., Spain. The water used in experiments is deionized water.
2.2 Preparation of Macroporous PVA Hydrogels
PVA solution (15 wt.%) was prepared by dissolving the PVA in the deionized water and heated at 95 °C for 2 h under stirring. PVA/AG solutions were prepared by adding 4 wt.% AG powder (based on water) into the PVA solution (15 wt.%) with continuous stirring and the stirring was maintained 2 h at 95 °C until the mixtures became transparent. The mPVA were prepared by freezing-thawing method with different duration of freezing, number of freezing-thawing cycles and the temperature of thawing. Finally, AG used as porogen was removed by washing with deionized water.
2.3 Mechanical Test
Compression and tension experiments of hydrogel were carried out on 2 kN Sans Universal Testing Machine (Shenzhen SANS Testing Machine Co., Ltd., CMT-4204, China) [13,14,15]. The compression experiments were performed on the 4 mm × 4 mm × 4 mm hydrogels with a strain rate of 30%/min at room temperature. The tension experiments were performed on the 50 mm × 4 mm × 2 mm section of barbell shaped hydrogel samples with strain rate of 600%/min. Each experiment repeats at least three times.
2.4 Scanning Electron Microscopy (SEM)
The hydrogel slices were freeze-dried on a freeze drier (BK-FD10S, BIOBASE) for 16 h. Then the obtained hydrogel samples were coated with gold on a magnetron ion sputter metal coating device (Vacuum Device MSP-1s, Japan), and the surface morphology was examined on a scanning electron microscopy (SEM, FEI Quanta 200, FEI, USA).
3 Result and Discussion
3.1 Effect of Preparation Parameters on Mechanical Properties
The formation of macropores in the hydrogel will inevitably affect its mechanical properties. And it is necessary to maintain certain mechanical properties for artificial articular cartilage. The mechanical properties of mPVA can be modulated by adjusting the duration of freezing, number of freezing-thawing cycles and the temperature of thawing. The mechanical experimental results of hydrogels prepared with different parameters are presented in Figs. 1, 2 and 3. The stress values of tensile tests are presented at strains of 0.5, 1.0 and 1.5 and the stress values of compression tests are presented at strains of 0.2, 0.4, 0.6 and 0.8.
Effect of individual freezing duration and number of freezing-thawing cycles on tensile strength of mPVA. The individual freezing durations are 8 h, 12 h and 16 h in (a), (b) and (c), respectively. N: freezing-thawing cycle time. The freezing temperature is −20 °C. The thawing temperature is 18 °C and the individual thawing time is 8 h.
Effect of individual freezing duration and number of freezing-thawing cycles on compression strength of mPVA. The individual freezing durations are 8 h, 12 h and 16 h in (a), (b) and (c), respectively. N: freezing-thawing cycle time. The freezing temperature is −20 °C. The thawing temperature is 18 °C and the individual thawing time is 8 h.
Effect of thawing temperature on tension (a, b) and compression (c, d) strength of mPVA. The thawing temperature is 18 °C in (a), (b) and −3 °C in (c) and (d), respectively. N: freezing-thawing cycle time. The individual freezing duration is 16 h and the freezing temperature is −20 °C. The thawing time is 8 h.
As shown in Fig. 1, the tensile strength of mPVA prepared with 8 h and 12 h individual freezing duration firstly increases and then decreases with the number of freezing-thawing cycles, the strength reaches the strongest when the numbers of freezing-thawing cycles are 4 (Fig. 1a, b). The tensile strength of mPVA prepared with 16 h freezing time increases with the increase of number of freezing-thawing cycles (Fig. 1c). Figure 2 shows the relationship between the compressive strength of mPVA and the number of freezing-thawing cycles when the individual freezing durations are 8 h, 12 h and 16 h, respectively. The compressive strength of mPVA increases firstly and then decreases with the increase of number of freezing-thawing cycles. The compressive strength of mPVA with 8 h and 12 h individual freezing duration reaches the strongest when the numbers of freezing-thawing cycles are 4 (Fig. 2a, b), and that of mPVA with 16 h individual freezing duration reaches the strongest when the numbers of freezing-thawing cycles are 3 (Fig. 2c).
Figure 3 shows the effect of thawing temperature on the mechanical properties of mPVA. The thawing temperatures are designated as 18 °C and −3 °C. Other parameters, including freezing temperature (−20 °C), individual freezing duration (16 h), and individual thawing duration (8 h), are the same. Tensile strength increases with the increase of number of freezing-thawing cycles. This is true no matter the thawing temperature is 18 °C or −3 °C. But when the number of freezing-thawing cycles is fixed, the tensile strength of hydrogels at thawing temperature 18 °C is higher than that at thawing temperature −3 °C (Fig. 3a, b). From Fig. 3c, d, it can be found that the compressive strength of the hydrogel prepared with 18 °C thawing temperature is 5122 kPa (strain = 0.8, N = 3), and the compressive strength is only 2706 kPa (strain = 0.8, N = 3) when the thawing temperature is −3 °C.
In brief, the hydrogel with the best mechanical properties is obtained when the number of freezing-thawing cycles is 4 (for tension) or 3 (for compression), the individual freezing duration is 16 h and the thawing temperature is 18 °C.
3.2 Morphology Characterization
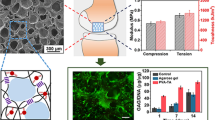
The micromorphology change of PVA hydrogels before and after the addition of AG is shown in Fig. 4. The honeycomb-like structure can be observed for the neat PVA hydrogel and the diameters of pores range from 2 to 3 μm (Fig. 4a). In contrast, macropores range in 20–200 μm are generated in PVA hydrogels when 4 wt.% AG is introduced (Fig. 4b), which means the macroporous PVA hydrogels are prepared successfully using AG as porogen. The AG particles exist in the pores, indicating that the introduced AG acts as the poregen. The AG particles in the pores need to be removed to leave space for the cell growth and proliferation. Fortunately, The AG particles can be removed simply by washing. The macroporous PVA hydrogel after the removal of AG is shown in Fig. 4c. Small pores (as indicated by the blue arrows) can also be observed on the walls of the original pores, as Fig. 4d shows, which means the macropores have good connectivity, which may facilitate the migration of cells, the diffusion of nutrients and the removal of waste products.
4 Conclusion
Macroporous PVA hydrogel is successfully prepared by using AG as porogen through freezing-thawing method. The pore size increases from less than 3 μm to 20–200 μm when AG is introduced, which meets the requirements of chondrocyte implantation. And the macropores have good connectivity, which may facilitate the migration of cells, the diffusion of nutrients and the removal of waste products. The mechanical properties of macroporous PVA hydrogels can be modified by adjusting the preparation parameters, and the proposed freezing-thawing parameters are 16 h individual freezing duration, 3 cycles of freezing-thawing and 18 °C thawing temperature.
References
Caplan, A.I.: Mesenchymal stem cells. J. Orthop. Res. 9(5), 641–650 (1992)
Ito, Y., Fitzsimmons, J.S., Sanyal, A., Mello, M.A., Mukherjee, N., O’Driscoll, S.W.: Localization of chondrocyte precursors in periosteum. Osteoarthr. & Cartil. 9, 215–223 (2001)
Bouwmeester, P.S.J.M., Kuijer, R., Homminga, G.N., Bulstra, S.K., Geesink, R.G.T.: A retrospective analysis of two independent prospective cartilage repair studies: autogenous perichondrial grafting versus subchondral drilling 10 years post-surgery. J. Orthop. Res. 20(2), 267–273 (2002)
Hangody, L., Karpati, Z.: A new surgical treatment of localized cartilaginous defects of the knee. J. Orthop. Trauma 37, 237–243 (1994)
Brittberg, M., Lindahl, A., Nilsson, A., Ohlsson, C., Isaksson, O., Peterson, L.: Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 331(14), 889–895 (1994)
Zeng, L., Yao, Y., Wang, D., Chen, X.: Effect of microcavitary alginate hydrogel with different pore sizes on chondrocyte culture for cartilage tissue engineering. Mater. Sci. Eng., C 34(1), 168–175 (2014)
Ma, R., Xiong, D., Miao, F., Zhang, J., Peng, Y.: Novel PVP/PVA hydrogels for articular cartilage replacement. Mater. Sci. Eng., C 29(6), 1979–1983 (2009)
Gutiérrez, M.C., García-Carvajal, Z.Y., Jobbágy, M., Rubio, F., Yuste, L., Rojo, F., Ferrer, M.L.: Poly (vinyl alcohol) scaffolds with tailored morphologies for drug delivery and controlled release. Adv. Func. Mater. 17(17), 3505–3513 (2007)
Miao, T., Miller, E.J., Mckenzie, C., Oldinski, R.A.: Physically crosslinked polyvinyl alcohol and gelatin interpenetrating polymer network theta-gels for cartilage regeneration. J. Mater. Chem. B 3(48), 9242–9249 (2015)
Scholten, P.M., Ng, K.W., Joh, K., Serino, L.P., Warren, R.F., Torzilli, P.A., Maher, S.A.: A semi-degradable composite scaffold for articular cartilage defects. J. Biomed. Mater. Res., Part A 97A(1), 8–15 (2015)
Ng, K.W., Torzilli, P.A., Warren, R.F., Maher, S.A.: Characterization of a macroporous polyvinyl alcohol scaffold for the repair of focal articular cartilage defects. J. Tissue Eng. Regen. Med. 8(2), 164–168 (2014)
Cao, Y., Xiong, D., Wang, K., Niu, Y.: Semi-degradable porous poly (vinyl alcohol) hydrogel scaffold for cartilage repair: evaluation of the initial and cell-cultured tribological properties. J. Mech. Behav. Biomed. Mater. 68, 163–172 (2017)
Zhang, W., Liu, L.F., Xiong, Y.J., Liu, Y.F., Yu, S.B., Wu, C.W.: Effect of in vitro storage duration on measured mechanical properties of brain tissue. Sci. Rep. 8, 1247 (2018)
Zhang, W., Zhang, R.R., Feng, L.L., Li, Y., Wu, F., Wu, C.W.: Mechanical response of brain stem in compression and the differential scanning calorimetry and FTIR analyses. ASME J. Appl. Mech. 83(9), 091005 (2016)
Zhang, W., Zhang, R.R., Wu, F., Feng, L.L., Yu, S.B., Wu, C.W.: Differences in the viscoelastic features of white and grey matter in tension. J. Biomech. 49(16), 3990–3995 (2016)
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (11772086, 51775541, 11572080, 51811530309), the Natural Science Foundation of Liaoning Province (201800935), and the Fundamental Research Funds for the Central Universities in China (DUT18ZD302).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Wang, S., Li, H., Qi, Z., Yin, M., Wu, C., Zhang, W. (2020). Effects of Freezing-Thawing Cycles on Mechanical Strength of Poly (Vinyl Alcohol) Hydrogels. In: Wahab, M. (eds) Proceedings of the 13th International Conference on Damage Assessment of Structures. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-13-8331-1_58
Download citation
DOI: https://doi.org/10.1007/978-981-13-8331-1_58
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8330-4
Online ISBN: 978-981-13-8331-1
eBook Packages: EngineeringEngineering (R0)