Abstract
This document provides a comprehensive overview study on the physico-chemical speciation of radioiodine observed in the atmosphere after various emissions related to nuclear activities: nuclear weapon tests, accident and incident releases, and routine discharges. The study covers different types of nuclear facilities including medical isotope production facilities (MIPFs), reprocessing plants (RPs), and nuclear power plants (NPPs). Most attention is paid to 131I which has a major human health impact in the early stages of a nuclear emergency situation with regard to inhalation. Iodine-131 combines a high yield by neutron-induced nuclear fission of 235U (2.87%) or 239Pu (3.8%), high dose coefficients, and a radioactive half-life long enough to allow for spreading at global scales and entering the food chain but sufficiently short to produce a significant dose commitment when inhaled or ingested. Reliable dose assessment requires both detailed and valid information on the physico-chemical composition of 131I present in the air. Apart from reactor explosions and fires, which produce large amounts of particles and may therefore favor the presence of iodine in particulate form at short distance, other nuclear accident scenarios will lead fairly rapidly to a dominant gaseous radioiodine proportion in the atmosphere.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Iodine
- Radioiodine
- Nuclear accident
- Thyroid
- Internal exposure
- Radiation protection
- Environmental release
- Environmental monitoring
- Routine discharges
15.1 Introduction
The goal of this chapter is to provide basic and updated knowledge about radioiodine (i.e., radioactive isotopes of iodine) speciation in the atmosphere and to provide information about the way to refine inhalation dose assessment and reduce associated uncertainties. Within this framework, different emission scenarios covering a wide range of nuclear activities and release situations are examined. Without neglecting the contribution of the 131I dose induced by ingestion, that may rapidly overwhelm that of inhalation if proper ban on sales or on foodstuff consumption are not taken rapidly after the deposition of airborne contaminant, this review focusses on the contamination of the atmosphere and the dose induced by inhalation.
Radioiodine is of major concern for public health when released in large quantities into the atmosphere, as during nuclear accidents or even significant nuclear incidents at short distances. Whether radioactive or not, iodine is necessary for the thyroid gland to synthesize hormones involved in the human body metabolism. It is considered as an essential element. Especially in areas of shortage in iodine supply in the daily diet, the human body is seeking for each iodine molecule, ion, or compound either in the breathing air or in the food. Among the various population ages, children are most sensitive to iodine deficiency and to the uptake of radioactive iodine.
The chemistry of iodine-containing species is complex, and their chemical existence once released into the atmosphere will depend on many interactions with other atmospheric compounds and will vary according to solar radiation. Iodine is a volatile element with high vapor pressures, even at relatively low temperatures. Molecular iodine (I2) sublimates quite easily even at ambient temperature and boils at 184 °C owing to this high vapor pressure. In addition, organically-bound iodine species are even more volatile, where, for example, methyl iodide (CH3I) boils at 42 °C. Particulate forms of iodine are often salt or oxide forms (e.g., CsI or IxOy such as I2O3), and iodine coexists both in these gaseous and particulate forms in the atmosphere. Even if it is expected to remain mostly in gaseous species (organic and inorganic), parts of them will condensate and nucleate to form nanoparticles, or adsorb onto atmospheric aerosols. However, the transfer kinetics related to adsorption on particles, the kinetics of the reactions with other airborne compounds and finally the kinetics of the conversion into particulate iodine by condensation and heterogeneous nucleation are not yet fully understood and are also a matter of uncertainties and biases in inhalation dose assessment.
Being a member of the halogen group, iodine is a reactive element and has a complex chemistry with other atmospheric compounds [1]: aerosols, ozone, HxOy, nitrogen oxides (NOx), volatile and semi-volatile organic compounds (VOC, SVOC), etc. In addition, these interactions are driven by light and UV radiation to form gaseous organic iodides (CH3I, CH2I2, CH3CH2CH2I, etc.) during diurnal conditions while they produce mostly inorganic gaseous species (I2, HI, IO, HOI, HIO3, IONO2, etc.) in night conditions [2, 3], in addition to aerosol-bound species (mixed iodinated aerosols, iodide salts, and iodine oxide aerosols [3] formed by reaction with ozone to give IO2, I2O3, I2O4, I2O5, etc. Gaseous iodine species such as CH3I and even more I2 are sensitive to photo-dissociation (or photolysis) under solar UV radiation. The photolysis of I2 and CH3I generate active atomic iodine (I) that will react with the previously mentioned atmospheric compounds and will be recycled through numerous intermediates in both gaseous and aerosol phases over time scales of hours to days [4]. The main final CH3I photolysis products in the air are in the form of IxOy [5]. As a consequence of photo-dissociation, the concentrations of I2 and CH3I and that of their reactions products will rapidly change between daytime and nighttime [6, 7]. According to kinetic parameters used in iodine behavior models, it has been found that I2 has a short lifetime of about 20 s before photolysis in a sunlit atmosphere [8] or that 95% of I2 released during daytime will be photo-dissociated in less than 1 min. Over a period of a few hours [9], a rapid evolution of the physico-chemical forms of iodine can be observed as a function of seasonal parameters with an almost total conversion of released I2 into INOx in winter conditions and INOx plus organic iodine in summer conditions where photolysis plays a predominant role. Based on kinetic parameters related to photo-dissociation rates, I2 is thus theoretically not expected to be found during daytime. However, iodine speciation data acquired after nuclear accidents demonstrate that gaseous inorganic iodine exists in a rather high proportion after a long range transport. Relative comparison can be made with CH3I [10] whose photo-dissociation requires between 1.1 and 4 days [6] and even 8 days (CH3I is photolyzed about 600 times more slowly than I2) [8]. Conversely, enhanced oceanic emissions of HOI (hypoiodous acid) and I2 happen during nighttime in addition to the increase of INOx as demonstrated when using kinetic parameter-based theoretical calculations [6, 7]. Filistovic and Nedveckaite [6] suggested that the atmospheric persistence of inorganic iodine species in a rather significant proportion, as observed after nuclear accidents, is less linked to I2 than to other inorganic species like HOI or IONO2 species. The global chemical complexity of iodine also encompasses iodinated reaction products which in turn will behave in a different way in the atmosphere depending on their physico-chemical features, UV radiation, and the mechanisms involved in the contamination of the environment: gas-to-particle conversion and transfer from one species to another, deposition and interception, biomass integration, re-emission of aerosol-bound iodine (liquid or solid), or gaseous re-volatilization. All the different chemical and physical forms that radioiodine (i.e., radioactive isotopes of iodine) can take in the atmosphere will influence their individual behavior, and thus their persistence in the atmosphere and the contamination of other compartments of the biosphere. The same statement stands for incident releases, and routine discharges. By extrapolation, there may ultimately be consequences for long-range deposition or transport of radioiodine with dose–response implications received in proportion to inhaled organic, inorganic, and particulate species, as well as levels of contamination of marine and continental ecosystems. Therefore, a comprehensive quantification of the exposure to radioiodine is of highest importance for radioprotection issues.
15.2 Synopsis of Radioiodine Presence in the Atmosphere
Since the beginning of the nuclear era, there has been a large variety of radioactive iodine emissions to the environment. Apart from 127I, which is the only stable isotope, there exist 42 radioactive iodine isotopes or isomers with atomic numbers between 108 and 145, and 13 of these are fission products. Thirteen iodine isotopes have a half-life longer than 1 h, and four have a half-life ranging from a few days to about 60 days (Table 15.1). Radioactive iodine isotopes are generated during the nuclear fuel life cycle (from spontaneous fission of uranium in soil to energy production and reprocessing of spent nuclear fuel) or by various techniques involved in radiopharmaceutical production like neutron bombardment. Among the different released and transported radioactive iodine isotopes in the atmosphere, most attention is paid to 131I. Iodine-131 combines a high yield by (thermal) nuclear fission of 235U (2.88%) or 239Pu (3.8%), high dose coefficients, and a radioactive half-life long enough to let it spread at worldwide scale and enter the food chain but also sufficiently short to produce a high dose commitment when inhaled or ingested. Major decay of 131I into 131Xe takes place through β and γ emissions with a short half-life of 8.02 days. The thermal fission of 235U also produces another iodine isotope of interest, namely 129I with a ratio 131I/129I = 3.59 [15] according to the most recent 2017 OECD “Joint Evaluated Fission and Fusion File” (JEFF-3.3). Only 129I has a very long half-life (T1/2 = 1.57·107 years), and is also the only radioactive iodine isotope to be naturally produced by nuclear reactions of atmospheric Xe under the impact of cosmic radiation [16], and to a lesser extent in soil by spontaneous fission of 238U, thermal neutron-induced fission of 235U (fission yield 0.9%), or by neutron activation reactions of tellurium isotopes in soil. However, its natural inventory estimated in total to ~ 230 kg (ca. 1.5 TBq) is largely overwhelmed by anthropogenic emissions from nuclear activities [2, 16].
15.3 Physiological Aspects and Health Impact
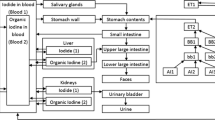
Iodinated compounds exhibit a high affinity with different organs (mainly the thyroid gland) whose metabolism is essential for the development and functioning of the body. Iodine is an essential component of the thyroid hormones thyroxine (T4) and triiodothyronine (T3), which regulate metabolic processes and are critical to growth and development. The iodine concentration in the thyroid of an adult is about 500 μg g−1, and 80–90% of iodine present in the body of an adult is contained in its thyroid. The World Health Organization (WHO) recommends a daily iodine intake of 150 μg for an adult (and 200 μg for a pregnant or breastfeeding woman). Large regional variations are observed, from less than 20 μg days−1 to more than 10,000 μg days−1. Inhalation of air contaminated with radioiodine, i.e., during the plume presence, or ingestion of contaminated foodstuff within days or even a few weeks after deposition lead to internal irradiation of the thyroid gland and increases the risk of thyroid cancer. WHO-recommended daily iodine intakes converted into gaseous-only 131I2 and CH3131I activities would be equivalent to about 0.69 and 0.35 TBq, respectively. Thus the mass of radioiodine that could be incorporated by an individual, as a consequence of a nuclear accident, would be much lower than his stable iodine intake. The thyroid will readily absorb 5–90% of the incorporated radioiodine, depending on the dietary intake of stable iodine, with highest thyroid uptake in the case of a stable iodine deficiency. Most of the remaining radioiodine intake is quickly excreted in urine. The thyroid gland is a small organ of about 15 cm3–20 cm3 only, for an adult. When inhaled or ingested, 131I will thus concentrate in this gland where a relatively high specific dose will be delivered over about one month, mainly through β− radiation of mean energy 182 keV with 100% yield and maximum emission at 606 keV (90% intensity) prone to irradiate thyroid tissues up to 2 mm range [17].
In comparison, 129I decay emits β− particles with a maximum energy of 151 keV, 100% (mean 37 keV). Because of the low levels of 129I activity produced in nuclear reactors and released in the environment in case of an accident (and its low specific activity of 6.5·106 Bq·g−1 in general), the dose induced by 129I exposure remains negligible compared to that from 131I. However, because of its very long half-life, the study of 129I is a matter of interest for 131I dose reconstruction and in the framework of radiological waste disposal. Indeed, in a long-term basis after the closure of a repository 129I as well as 36Cl, 79Se, and 99Tc are expected to be a major dose source for humans due to their high mobility in the environment and anionic nature. In the safety assessment of the spent nuclear fuel in a long-term basis, 129I and 36Cl are classified as the first (top) priority radionuclides [18].
Following the Chernobyl accident (April 1986), radio-induced hypothyroidism appeared for high dose exposures (about 10 Gy and above), while radio-induced thyroid cancer incidence increased about 3–5 years [19] after exposure among children to doses higher than 100 mGy (Fig. 15.1a) [21,22,23]. The younger the age at exposure, the higher the risk (Fig. 15.1b). After this accident, it has been found that children having stable iodine deficiency experienced at least twice as high thyroid exposure as compared with children in the case of a dietary iodine sufficiency. An increase of cancer risk with thyroid dose was also reported among “Chernobyl liquidators” exposed at adult age [24].
(a) Temporal change of the yearly thyroid cancer number in children aged <15 when exposed to the Chernobyl accident. Reprinted with permission, © IRSN. (b) Relative Risk (RR) of thyroid cancer according to the age at exposure to the Chernobyl accident, after [20]
Iodine-containing species in the atmosphere are usually categorized into gaseous inorganic species with I2 (elemental iodine, also called molecular iodine) as the representative species; gaseous organic species with CH3I (methyl iodide) as the representative, and particulate iodine (aerosol-bound). Once inhaled, these species will deposit in different proportions in the respiratory tract and integrate partly or almost totally the thyroid gland. Biokinetic effects are integrated in a single parameter named dose coefficient (dose per unit of intake, expressed in sievert per incorporated becquerel of radionuclide). Dose coefficients are published by the International Commission on Radiological Protection (ICRP) for the three representative iodine-containing species (Table 15.2). According to Morgan et al. [27], the average CH3I deposition in the respiratory tract is 72% (53–92%) of intake [27]. The deposition of inhaled I2 is even higher (>90%). For both vapors, the absorption of deposited activity to blood is complete and very rapid. About a third of the activity transferred to blood concentrates in the thyroid within 24 h. The deposition of particulate iodine depends on the size distribution of the aerosol. Gaseous organic and inorganic iodine species have higher inhalation dose coefficients than aerosol-bound because of their larger deposition in the respiratory tract: For elemental iodine vapor (I2), it is assumed that 100% are deposited in the respiratory tract [28], while regarding the aerosol-bound fraction, about half of the aerosol with an activity median aerosol diameter (AMAD) of 1 μm will deposit. Pathways of particle clearance rely on exhalation, dissolution in the lung fluids, transport in the alimentary tract, and absorption in blood. For the particulate fraction, dose coefficients vary with the dissolution rate of the deposited particulate species in the lung fluids: reference absorption types fast, moderate, or slow (F, M, S) are considered by the ICRP. Type F (rapid and complete absorption) is recommended by default for 131I when no specific information is available for members of the public [25]. The fraction of an ingested activity that is absorbed into blood is quantified according to f1 values; 1 corresponding to complete absorption is recommended for ingested iodine.
Inhalation dose coefficients for aerosols also differ according to the aerosol size due to differential deposition in the airways. For workers, an AMAD of 5 μm is commonly assumed while the default value for the public is 1 μm. The ICRP provides dose coefficients according to aerosol sizes in the range of nm to about 10 μm (Fig. 15.2). The dose coefficient variation is about a factor of 3. Assuming a case for which the 131I fraction is supported only by fine aerosols (i.e., typically <0.01 μm), as occurs when gaseous species nucleate or condensate to form nanoparticles, will result in a 2.2 times increase in the inhalation dose from aerosol-bound 131I species compared to the 1 μm aerosol reference size usually considered for members of the public.
Change in the thyroid equivalent dose coefficients for inhalation of 131I, as a function of the aerosol diameter. After: [29]
In addition, inhalation dose is age-dependent. Table 15.2 gives the dose coefficients for an adult only and Table 15.3 the change in thyroid equivalent dose coefficients with age. The radio-sensitivity of the thyroid gland is especially high for children; among them babies (age <1 year) represent the highest-risk population, while several studies, including that of the survivors of the atomic bombings, have detected no increase in relation to exposures received after the age of 20 years. More detailed information can be found in the ICRP 68 or in its updated version ICRP 137, for workers (occupational intake) and in the ICRP 71 for members of the public (ICRP 68 and ICRP 71 are based on the same bio-kinetic models).
Note that the dose coefficient for 129I is higher than for 131I whatever the species as a result of the five times higher dose coefficient per unit of uptake for 129I than for 131I. A material is assigned to Type F when the deposited materials are readily absorbed into blood from the respiratory tract. Type M is assumed for deposited particulate forms that have intermediate rates of absorption into blood from the respiratory tract, as may be the case of iodine trapped in irradiated fuel fragments. A material is assigned to Type S if deposited materials are relatively insoluble in the respiratory tract. In practice, and according to the EU directive 96-29 [31], aerosol-bound iodine isotopes are assigned to absorption type F. After a substantial release of radioiodine in the environment, the most realistic dose assessment for exposed individuals will be based on in vivo measurement of thyroid retention (Fig. 15.3), regardless of the physico-chemical form of incorporated iodine and takes into account the actual thyroidal uptake of the measured person.
Neglecting the Gaseous form of 131I in Prospective Dose Assessment Leads to an Inhalation Thyroid Dose Underestimation
Assuming that the airborne 131I activity level deduced from aerosol filter measurements corresponds to the total atmospheric iodine content would result in an underestimation of the inhalation dose by a factor of 6.4 (for an adult and aerosol type F of 1 μm). This clearly shows that the gaseous fraction of iodine, although more tedious to monitor compared with the particulate fraction, cannot be omitted and requires assumptions on the speciation or at least fractionation of iodine between gaseous and particulate species. When prospectively evaluating the potential dose to the population from exposure to a radioactive plume of a given activity level, and assuming an average 131I speciation of 30% in particulate form (AMAD 1 μm, absorption Type F), 35% for organic and 35% for inorganic 131I gaseous species, and ICRP inhalation dose coefficients for an adult will lead to about twice as much higher dose estimate than for the consideration of the particulate form only. In the absence of information regarding the gas/particle fractionation, it is therefore recommended to assume 50% particulate and 50% gas in the occupational intakes of radionuclides series published by the International Commission on Radiological Protection [28]. Regarding the gaseous speciation, when it is not possible to distinguish inorganic and organic species, the ICRP [28] recommends adoption of the deposition rate for elemental iodine. Based on half-lives and inhalation dose coefficients associated to the various iodine isotopes, those to which attention should be paid first during a nuclear reactor accident are 131I, 132I, and 133I, as well as the precursor of 132I, i.e., 132Te. Indeed, more accurate estimates of radioiodine health impact should also consider decay of tellurium isotopes, especially for inhalation dose assessment in the first few days after a nuclear accident release. Tellurium isotopes present in the releases or in the environment will give the following radioiodine: 129I by filiation of 129mTe (T1/2 = 33.6 days) and 129Te (T1/2 = 69.6 min), 131I by filiation of 131mTe (T1/2 = 30.0 h) and 131Te (T1/2 = 25.0 min), 132I by filiation of 132Te (T1/2 = 3.2 days; γmax = 228.2 keV with 88.2% abundance), 133I by filiation of 133mTe (T1/2 = 55.4 min) and 133Te (T1/2 = 12.5 min), and 134I by filiation of 134Te (T1/2 = 41.8 min). As for radioiodine with a short half-life of tens of minutes, other tellurium-induced radioiodine isotopes have a too short half-life and can therefore be neglected, since it takes usually several hours after the start of the accident when releases start. Exposures to gas or vapor forms of tellurium are relatively unusual compared with exposures to particulate forms in the environment. From aircraft samplings performed at short distance over the block IV of the Chernobyl NPP, Borisov et al. cited in [32] reported that the gaseous 132Te component varied only 1–8%. It is therefore recommended to consider a particulate form of M-type for tellurium in the absence of specific information [33]. Once inhaled, a part of 132Te (T1/2 = 3.2 days) will deposit in the respiratory tract where it will decay to give 132I. The contribution to the thyroid dose from the inhalation of 132Te is substantially higher (by a factor >10) than that from the intake of the same activity of 132I, which is in radioactive equilibrium with 132Te in air [34]. In practice, it means that the inhalation dose contribution from direct intake of 132I can be neglected compared with 132I induced by the intake of 132Te and despite its screening in the respiratory tract. Regarding the Chernobyl accident, Balonov et al. [35] estimated the contribution of 132I to the thyroid doses as being about 30% for person who did not use stable iodine prophylaxis and about 50% for persons who took KI pills on 26–27 April. Regarding the Fukushima accident, Shinkarev et al. [34] demonstrated that on March 12, 2011, the contribution of short-lived radioiodine (133I and 132I induced by the intake and radioactive decay of 132Te) to the thyroid dose from inhalation of the plume might be as great as 30–40% of the dose from 131I, while it could have reached about 10% on March 15, i.e., the day of the main releases. However, the dose from 132I was not considered in the thyroid dose estimation from radioiodine due to the lack of information [36]. In addition to 131I, 132Te, and 132I, other short-lived iodine isotopes (123I, 130I, 135I) can be considered to refine inhalation dose assessment, but this requires prompt measurements. Globally, the contribution of the inhalation exposure pathway to the thyroid dose may be dominant during the first few weeks after the beginning of the releases and as far as the application of dietary consumption restrictions, especially for fresh milk and vegetables, is ensured. No significant health effect is expected for 129I, on account of much lower levels of activity, long radioactive half-life, and rapid biological half-life.
15.4 Management of Nuclear Emergency Situations with Regard to Radioiodine
An effective decision-making process is required in the emergency phase of nuclear events. When a release of radioactive material occurs or is about to happen, prompt protective actions can avoid or mitigate the potential consequences. These actions can be decided and set up preventively in the perspective of a possible future release, or in response to an ongoing release and combine sheltering, administration of stable iodine tablets, evacuation if necessary, and later possible restrictions on foodstuff consumption. In any case, a specific evaluation would be necessary in order to propose protective actions to set up correspondingly to the situation, to the public authorities. For radioactive iodine impact due to the passage of the plume, one has to evaluate the corresponding projected thyroid dose. This protection strategy is driven by comparing those projected doses to the corresponding action guide level (50 mSv as for instance in France, Germany, and many other countries). Transboundary harmonization on reference levels is not yet fully operational. Nevertheless, in Europe, the Herca-Wenra approach proposes that in the very early stages of the crisis, if the analysis of the situation is shared, the bordering countries align themselves with the decisions taken by the country where the accident occurs [37, 38].
From a general point of view, there are several factors that influence the assessment of the radioiodine-induced inhalation dose during an atmospheric release:
-
Magnitude of the release, physico-chemical forms of iodine (gaseous inorganic, gaseous organic, aerosol), granulometry of aerosol-bound iodine species;
-
Dispersion conditions such as height of the release, meteorological conditions (wind speed, direction, atmospheric stability, boundary layer thickness, presence of rain, fog, etc.), local topography (rural, urban, etc.) and depletion processes (dry deposition, wet scavenging etc.);
-
Exposure scenario of individuals such as time of exposure, breathing rate, possible indoor protection factor;
-
Biokinetics of the incorporated materials, which depend on factors related to the age and gender (see previous section).
The remaining parameters and the modelings have to be chosen by experts performing dose assessments. Due to major unknowns in number of the parameters and modeling simplifications, especially in the acute phase of the emergency, the projected dose assessment is subject to a significant uncertainty level. In general, a balance is made between model complexity and timely response. Emergency response employs substantial simplifying assumptions that make the evaluation of the consequences become tractable and available rapidly:
-
Generally, a Gaussian dispersion modeling is used, with constant and uniform deposition velocities, sometimes with uniform wind speed and direction; gaseous and particulate iodines are generally associated with different dry or wet deposition velocities;
-
Representative persons are considered: it consist of a virtual individual, with constant breathing rate, with no benefit of any protection factor during the whole time period of exposure. This fictitious individual is sometimes supposed to remain located at different distances downwind on the plume centerline, where the air-activity concentrations are the highest. For reactor accidents, a 1–2 years old child is the ICRP age category for which the calculated dose is the highest.
Emergency planning ensures the availability of potassium stable iodine (KI) tablets by a pre-distribution performed in the near to medium fields around a nuclear power plant. In France, this distance is set to 20 km. Their administration must be organized as soon as possible in the case of a nuclear accident involving significant radioiodine releases to the atmosphere, to saturate the thyroid and protect it from exposure to radioiodine [39]. Administration is recommended when the thyroid equivalent dose is expected to be higher than 50 mSv. This procedure is complementary to others protective actions such as population sheltering or evacuation. The efficiency of the protection is at its highest when KI tablets are administrated at the time of radioiodine intake and therefore distributed at the start of the accident releases or even better by anticipation; ideally 2 h before inhalation of contaminated air (Table 15.4). ICRP recommends a biological half-life of iodine of 80 days, however a more recent empirical study suggested a biological half-life as short as 66 ± 6 days [41]. From Eq. 15.1, an effective half-life of iodine in the human body (for adults with intact thyroid function) of about 7 days can be deduced (Teff being the effective half-life, Tbio the biological half-life, and Tphys the physical half-life).
However, the efficiency of the KI tablets is about 1 day after ingestion (Fig. 15.4). The following populations are targeted for priority KI tablet administration since they are at highest risk for negative health effects to the thyroid from radioiodine: newborns (< 1 month), infants (1 month–3 years), children (3–12 years), adolescents (12–18 years), pregnant and breastfeeding women [40]. Considering KI tablets of 65 mg (equivalent to 50 mg of I), the dosages are as follows: newborns: ¼ of a KI tablet, dissolved in a liquid; infants: ½ of a KI tablet dissolved in a liquid; children: one tablet; and from 12 years including pregnant women up to 40 years: two KI tablets. In the event of prolonged or repeated exposure by inhalation, public health authorities may advise taking KI tablets more than once. Under such circumstances, neonates (<1 month) and pregnant or breastfeeding women should not be given repeated doses of KI and other protective actions should be considered such as early evacuation, for these particular groups [42]. The administration of iodine would concern in all cases only the emergency and short-term phases. Indeed, if the iodine levels are still too high beyond a few weeks, it also means that the area is very contaminated and that for reasons of external dose, evacuation is advised.
Efficacy of KI as a function of the period between administration and 131I intake. After: [40]
Beside sanitary preoccupations, the management of nuclear emergency situations integrates the communication to mass media. The omission of the gaseous component could be publicly perceived as a deliberate intention to minimize the situation as far as the gaseous predominance will be reminded, and even if levels of activities are of no concern for public health. A mistrust feeling may spread leading to undermine confidence in governmental and expert institutions [43].
15.5 Anthropogenic Releases and Discharges
Anthropogenic releases and routine discharges of radioiodine in the environment can be categorized in five main sources, namely (1) nuclear weapon tests, (2) nuclear reactor accidents, (3) routine discharges from radiopharmaceutical production1 and use in nuclear medicine, (4) routine discharges1 from the nuclear fuel reprocessing industry, and (5) routine dischargesFootnote 1 from the nuclear power production industry.
15.5.1 Nuclear Weapon Tests (NWT)
First large-scale radioiodine emissions occurred during the nuclear weapons testing era (1945–1980). In total, it is estimated that 0.28–0.98·1012 Bq (or TBq) of 129I and about 6.7–7.8 and even 12.2·105 PBq [44] (1 PBq = 1015 Bq) of 131I were released by ca. 530–543 NWT (depending on accounting methods) performed in the atmosphere [45, 46] and at ground-level [47, 48]. An approximate rate of 0.17 and 0.28 g of 129I per kiloton TNT equivalent is produced by a nuclear detonation [2], from fission of 235U and 239Pu, respectively. The total yield of atmospheric nuclear weapons tested is about 440 megatons (Mt) among which ca. 182 Mt. came from fission [48]. Globally, the fraction of radionuclides injected by atmospheric detonations into the troposphere quotes about 5% only, the rest into the stratosphere where the residence time of aerosol-bound radionuclides has been estimated 1–2 years. This duration is long enough to allow for a complete decay of 131I before reaching lower tropospheric layers. However, the remaining 5% in the troposphere is of significance for the deposition of short-lived radionuclides such as 131I. This percentage is also closely linked to the bomb yield and latitude because the tropopause height varies with latitude. In the case of a 1 Mt detonation in equatorial region, the percentage of radionuclides released in the troposphere will rise to 65%. Iodine-131 was the most important isotope in fallout with regard to health impact, as demonstrated for instance in the surroundings of the Nevada Test Site (NTS) with an excess risk in thyroid cancers in the US [49]. However, on a global scale, the contribution of 131I to the total effective dose equivalent commitment to the world’s population from atmospheric nuclear testing has been estimated in the range 1.4–3.8%, far below the contribution of 14C (70%) [47, 50]. Among radioiodine, only 129I from this period is still present in the environment.
15.5.2 Nuclear Reactor Accidents
In the case of a nuclear reactor accident, radioactive iodine isotopes account for the second largest fraction of emissions, second only to xenon isotopes. In addition, radioiodines belong to the most hazardous class of radionuclides that are released, and are responsible for 80–90% of the dose received in the first hours after the start of the accident, primarily due to inhalation of 131I contaminated air (i.e., during the initial emergency situation, and before taking into consideration ingestion of contaminated food). While the dose contribution through contaminated food was significant after Chernobyl, it has been suggested that inhalation was more significant than ingestion in the Fukushima case [51]. Radioiodine and radiotellurium isotopes (see previous section) to be considered in such scenarios have half-lives typically ranging between hours and about 10 days (see Table 15.1). For a loss-of-coolant accident (LOCA), the radioiodine released, as ranked by decreasing order, are: 131I, 133I, 135I, 132I, and to a lesser extent 134I, 125I, 129I, and 130I. The speciation of the iodine species present in the containment (before release in the environment) may differ depending on the accident conditions. Gaseous iodine can be released and aerosols iodine can be also produced by reaction between gaseous iodine and other species such as fission products (Cs, Cd, Ag etc.) or ozone or other air radiolysis products [52]. Various techniques (filtration, adsorption) are deployed including wet purification step to retain the different iodine-containing species, but gaseous CH3I, unlike I2, is not significantly absorbed by a liquid phase and requires dedicated filtration steps.
At Windscale Works, Sellafield (U.K.), the graphite-moderated reactor (pile 1) using a gas coolant was operating for the production of plutonium and other materials for the UK weapon program, when it experienced a graphite fire in 1957. The fire caused the release of 0.74–1.8 PBq 131I in the environment (Table 15.5). The damaged (1979) unit 2 of the Three Mile Island (TMI, USA) NPP was a pressurized water reactor (PWR). Despite melting of the fuel, the reactor vessel maintained its integrity, and the damaged fuel was retained inside [69] thus limiting the release into the environment. The unit 4 at the Chernobyl NPP was a graphite-moderated power reactor with pressure channels (RBMK) and the Fukushima Daiichi reactors (units 1, 2, 3, and 4) were BWRs. The Chernobyl and Fukushima accidents were associated with significant core meltdowns, steam or hydrogen explosions, and partial or total loss of the confinement. They were responsible for the highest amounts of radioactive iodine released to the environment (Table 15.5). Estimates of 129I releases during Windscale and TMI accidents are difficult since the 129I generated signal was blurred or encompassed in the global fallout signal [70]. In the case of the Windscale accident, the available activity of 129I was only about 1.5·10−8 times that of 131I. Health consequences were undoubtedly established for the young population exposed to 131I after the Chernobyl accident. The main radionuclides investigated in Europe were 99Mo, 103Ru, 106Ru, 110mAg, 125Sb, 129mTe, 132Te, 131I, 134Cs, 137Cs, 140Ba, 141Ce, and 144Ce. Other radionuclides (60Co, 63Ni, 90Sr, 95Nb, 95Zr, 154,155Eu, 238,239,240,241Pu, 241Am, and 242,243,244Cm) were also observed in Poland for instance (see Chaps. 1 and 2).
The major released radionuclides from the Fukushima accident were 132Te (T1/2 = 3.2 days), 131Te (T1/2 = 24.8 min) and their respective progeny 132I and 131I, as well as 134Cs (T1/2 = 2.06 years) and 137Cs (T1/2 = 30 years). In its source term assessment, the French Institute for Radiological Protection and Nuclear Safety (IRSN) retains the following decreasing importance of released radioiodine (in Bq): 131I, 132I, 133I, 135I, 130I, 134I, 132mI, 129I, and 128I, for a total amount of 182 PBq. After the Fukushima accident, a cocktail of radionuclides was detectable in Japan among them 129mTe, 132Te, 129I, 131I, 132I, 133I, 134Cs, 136Cs, 137Cs (see [71] for a more complete list). Cesium isotopes (134 and 137), particulate and gaseous 131I were detectable worldwide. At a limited number of European locations equipped with high-volume aerosol samplers and low-level gamma-ray spectrometry, it was also possible to find in addition 132Te and 132I [72], 129Te, 129mTe, and ultra-traces of 136Cs [73] and 140La [74,75,76].
The immediate aftermath of the Fukushima accident has not yet been fully clarified because no systematic data on exposures of the general public to short-lived radionuclides, especially 131I, were available soon after the releases [36]. Indeed, no fission products could be monitored during 12 days because of blackout of power supply; only dose rates were measured [77]. The lack of information on ambient 131I concentrations in the early stage of an accident can later be partly filled, step by step, but requires strong assumptions on the relationship between 131I and other radionuclides having longer half-lives and on their deposition conditions, once deposited on soil or integrated into the biomass. Among them, 129I is the most interesting since, as an iodine isotope, it is assumed to behave similarly [78] to 131I and its long half-life makes it possible to investigate retrospective evaluation of 131I deposition density and 131I external exposure, long after the accident [79, 80] and even if the uncertainty in the 129I/131I ratio may reach 20% [81] to 30% [82]. Nevertheless, it has to be considered that the 129I background may not be equal to zero when subtracting the pre-accident concentrations, because of 129I from global fallout and 129I fallout from a reprocessing plant (RP), at local or regional scales. The inverse reconstruction of the 131I airborne activities and inhalation dose to the thyroid from 129I deposition may thus be highly uncertain because of additional assumptions on deposition mechanisms. Finally, taking advantage of the affinity of the thyroid gland with iodine, the internal dose from radioiodine, whether inhaled or ingested, is generally evaluated subsequently by in vivo counting methods [83] (thyroid monitoring [84] or whole-body counting) but the drawback is that no distinction between intake from inhalation or from ingestion can be done without additional hypothesis. The knowledge of the iodine concentrations in the air if not in real-time at least within a short delay after sampling is therefore a strong tool for emergency situation management.
15.5.3 Routine Discharges
15.5.3.1 Radiopharmaceutical Production
Medical isotope production facilities (MIPFs) have the highest 131I release authorizations (up to 780 and even 1600 GBq years−1 for the two most important ones located in Europe). The most widely produced radioiodine is 125I for radio-immuno-analysis, therapy and diagnostic; and 131I for radiotherapy and diagnosis applications. Medical isotopes facilities also produce 133I in large quantities, 123I and 124I for medical imaging and diagnosis. Up-to-date and detailed information on iodine isotopes for medical purpose can be found in [17]. To cope with an increasing demand of radiopharmaceuticals, the production has raised and is expected to grow [85, 86]. Consequently and despite various abatement techniques [85], the number of 131I detections in the atmosphere close to production units is also increasing [87]. The January–February 2017 European-scale 131I detection event ensued from a combination of routine releases from the main European MIPFs and poor atmospheric dispersion conditions [88].
15.5.3.2 Nuclear Medicine Hospitals
Iodine-131 is a frequently used radionuclide in nuclear medicine with therapeutic (rather than diagnostic) applications. The activity of 131I involved in diagnosis ranges 0.19–3.7 MBq, while it can reach 1.8–9.2 GBq per treatment for a thyroid cancer. A standard amount of 123I administered for diagnosis is 110–220 MBq and will result in an effective dose of 0.5–2.2 mSv, which is close to the average annual natural exposure [17]. One day after the administration of a 131I capsule (NaI) for therapeutic purpose, a patient still exhales 131I, mainly (94% to almost 100%) in organically bound form [89, 90]. Depending on the diagnostic or therapeutic purpose of the treatment, a patient will stay 1–3 days at the hospital before leaving. The first case corresponds to an ambulatory stay for which about 70% of the administrated 131I activity will be excreted in the municipal sewerage system once the patient returns home and taking into account the radioactive decay. In the second case (therapeutic), the percentage of iodine entering the sewerage system is usually insignificant if the nuclear-medicine hospital is (as it is usually the case) equipped with septic tanks in which urines from lavatories are distinctly collected and stored for 131I decay up to 3 months before discharge to municipal sewerage. Regarding excretion, more than 90% of iodine loss from the body is due to renal clearance of iodide [28]. However, part of urines may be directly released in the hospital showers and thus may escape without significant decay before leaving the hospital. Excretion through feces is about 10–12% that of urinary excretion, but feces are usually drained out directly without storage and significant decay as for urines because of the nosocomial hazard that has to be avoided within the hospital premises [88]. Depending on the treatment, almost all the administrated iodine will finally be evacuated from the body within at least a week with an effective half-life of a dozen hours for a carcinoma thyroid, to more than 100 h for patients suffering from hyperthyroidism [91, 92].
15.5.3.3 Sewage, Waste Water Treatment Plants, and Sludge Incinerators
The patient-to-sewage pathway either from hospitals or from home may thus represents a widespread source of 131I to the environment at many locations. Finally, taking account of the activity of urines that escape to the decay tank and that of feces excretion, an estimate of about 20% can be retained for the activity that will be released in the municipal sewer system for patients treated for therapeutic purposes. In addition, excreta may be exempt from regulations that address disposal of radioactivity into municipal sewerage. Wastewater treatment plants (WWTP) settled in watershed area equipped with nuclear-medicine hospitals will thus receive a part of 131I excreted by patients through hospital effluent or from domestic effluents, once returned at home. Iodine-131 entering the WWTP will be covalently bound to organic matter, a reaction that is essentially irreversible [93]. According to Kitto et al. [94], water treatment process and chemical forms (e.g., organic or inorganic) of 131I in the waste influence the ability of the isotope to concentrate in the sludge. The authors confirm that most 131I emission to the atmosphere from a WWTP came originally from excreta entering the plant, then concentrated in dried sludge and finally burned in the WWTP incinerator. According to Hormann and Fischer [95], 20% at most of the 131I inflow activity is retained in the sludge. However, the real amount of remaining 131I greatly depends on the internal structure of the WWTP and time spent by the sludge in the circulation processes prior to their incineration or further treatments, which make it possible for radioactive decay [96]. Other syntheses have reported a 131I retention in sludge ranging 2% to about 20% [97, 98]. Incineration of dried sewage sludge inside WWTP is increasingly applied and encouraged to reduce their transportation cost outside from the plant, to increase the yield of sludge driers or for heating of the premises. Despite the physical decrease of 131I during its residence time inside the plant plus filtration and scrubbing systems to clean the fumes before discharge, WWTP incinerators are prospective 131I re-emission source to the atmosphere. Typical atmospheric 131I emission is about 1% of the amount entering the plant as wastewater [99] and typical airborne 131I activity of several mBq m−3 at 300 m from a WWTP incinerator were observed [100]. Typical daily emissions of gaseous 131I have been estimated between 15 and 60 kBq [94] from such facilities but are of no concern for public health. They are worth mentioning however, since they can explain the detection of 131I in the atmosphere close to medical facilities/hospitals or WWTPs.
15.5.3.4 Reprocessing Plants (RPs)
Nuclear fission of 235U and 239Pu produces 129I and 131I among other radionuclides. At the end of a 4-year activity, the core of a PWR 1300 MW reactor contains about 400 PBq of 131I and 75 GBq of 129I. According to the time spent between de-fueling and reprocessing (about 8–10 years), all 131I has totally disappeared by radioactive decay, whereas 129I is almost entirely present during reprocessing operation. This iodine isotope remains stored in the fuel pellets until the dissolution and solvent extraction steps involved in the reprocessing of spent nuclear fuel which leads to the release of significant amounts of 129I. Despite trapping and filtration of the fumes, a small proportion (ca. 1%) of 129I is emitted to the atmosphere. Main RPs currently in operation are located in La Hague (France) since 1966, in Mayak (Russian Federation) since 1948, and in Rokkasho (Japan) [101] since 2000. Other RPs have ceased operation, such as in the USA: West Valley since 1980, Hanford (1944–1988), Savannah River (1954–1989); in Europe: Sellafield (UK, 1951–ended November 2018 for the Thermal Oxide Reprocessing Plant—THORP), Marcoule (France, 1959–1997), Karlsruhe (Germany, 1970–1990); Tokai-mura (Japan, 1977–2006); Seversk and Zheleznogorsk (Russian Federation, 1956 and 1964–1995). Other plants are likely to be still in operation at the Mayak industrial complex, and in India (Trombay, Tarapur, and Kalpakkam). Detailed estimated amounts of 129I released to the atmosphere are summarized in [102] and in [103]. Due to its long half-life, 129I accumulates in the environment including atmosphere, hydrosphere, and biosphere and will continue to accumulate. The estimate of the naturally produced 129I amount is ca. 250 kg, while the input from anthropogenic activities can be evaluated to more than 6500 kg [2], among which 99% originates from reprocessing activities [104]. It has been assumed by [102] that the average atmospheric 129I releases from the Savannah River facilities was at 58% in a gaseous form. By 2012, the reprocessing plant in La Hague had discharged 4677 kg 129I into the English Channel and 77 kg (~2%) of 129I into the atmosphere, in addition to the 1634 kg 129I into the Irish Sea and 197 kg (~12%) into the atmosphere from the Sellafield RP. Yearly 129I atmospheric release authorizations for La Hague and Sellafield RPs are 18 GBq years−1 and 70 GBq years−1, respectively. Actual releases represent about 30–34% for la Hague [105] and 14% for Sellafield, of those authorizations. In addition, the 131I release of Sellafield was about 1.2% of the yearly authorization (37 GBq years−1) [106]. Despite of a higher amount of 129I released by the Fukushima accident (129I/131I ratio isotopic ratio = 31.6 ± 8.9, as of March 15, 2011) [82], it was impossible to discern the Fukushima-derived 129I, while 131I was easily detectable in Europe after the arrival of the contaminated air masses [107]. This was due to the 129I background originating from European RP discharges [108, 109]. Today most 129I, if not directly deposited on or discharged to the ocean, has migrated in the marine environment. At a more or less local scale, 129I can also be found in the terrestrial environment close to reprocessing facilities or waste disposal repositories. Based on stable iodine distribution in soils, it is apparent that soil-iodine enrichment is limited to about 100–120 km from coastal areas. It can be hypothesized that the same impact can be found for 129I [110].
15.5.3.5 Nuclear Power Plants (NPPs)
In comparison with fuel reprocessing, the nuclear energy industry has much limited authorizations and much lower actual radioiodine discharges to the atmosphere since most iodine remains basically trapped in the fuel rods. Despite the various techniques used to purify the fumes, residual radioiodine activities are released to the atmosphere (about 15 iodine isotopes but mainly 131I and 133I). However, no significant increase in the 129I by AMS measurement technique and no detection of 131I activity in the surroundings of NPPs have been reported [111] to our knowledge, in routine operations. Nedveckaite et al. [112] investigated the reason for thyroid disorders around the Ignalina nuclear power plant and concluded that thyroid disorders ensued from a stable iodine supply deficiency. Taking the case of the French NPPs (pressurized water reactor technology) as an example, the yearly authorized release for radioiodine is 0.4 GBq years−1 for each reactor. The average actual discharge for each reactor is estimated to be ca. 0.016 GBq years−1. This estimate is based on accountancy rules established in France according to which the detection limits are considered as being reached even when the measurement result remains below. The actual discharges are likely to be smaller. Additionally, it is supposed that most 131I (CH3I) discharges occur during gaseous trap efficiency tests.
The contribution of routine emissions to the atmosphere from these different industries or activities (MIPF, NPP, sewage sludge incinerators, etc.) has recently been investigated on the occasion of the early 2017 European-scale detection event of airborne 131I [88]. The expected contributions of various typical routine releases to the ambient atmospheric concentration during this detection event were ranked as follows: MIPF > sewage sludge incinerators > NPP > spontaneous fission of uranium in soil.
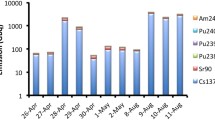
Between routine discharges and accident releases, there have been several 131I release incidents within or close to authorization limits, in the recent years from nuclear reactors or MIPFs. Borisov et al. [113] reported the use of combined filter and charcoal packs for the complex analysis of air nearby the St Petersburg NPP (probably in March 1992) with 60–70% of total 131I in gaseous form associated with contamination level of 104 Bq m−3 [113]. On August 22, 2008, at the Institut des RadioEléments (IRE) in Fleurus (Belgium), 47 GBq of elemental iodine were released at once [114], which corresponded to the yearly authorization limit. This incident was rated 3 on the INES scale (International Nuclear and radiological Event Scale), but no airborne 131I measurement has been reported. In the time interval between September 8 and November 16, 2011, 342 GBq of 131I, compared to the 1600 GBq yearly authorized, were released from an activation facility at the Institute of Isotopes Ltd., Hungary [115]. This incident was rated 1 on the INES. It led to detections in the range of a few to several tens of μBq m−3 in Europe, and even in animal thyroids [84]. Other 131I detections in the μBq m−3 to tens μBq m−3 range occur from time to time as, for example, in April 2010 in Sweden and Germany; in March 2011 (the week prior to the F1NPP accident) in several countries in Europe; in September to October 2011 as previously indicated; in February 2012 in several European countries; in December 2013 in Scandinavia; in March and May 2015 in Sweden, Finland, Norway, Poland, France, Russian Federation; in January–February 2017 and in January–February 2018, in Europe. Figure 15.5 shows the contribution of various 131I source emissions. Iodine-131 induced by the spontaneous fission of uranium has been added for comparison. Its contribution to ambient airborne activity is much lower than any other anthropogenic emissions [88] and is out of reach of conventional HPGe gamma spectrometry detectors. At distance from the various emission points, only large-scale releases (on the order of magnitude of several tens GBq) can be detectable by low-level gamma spectrometry combined with high-volume aerosol sampling. However, close to routine discharge points (MIPFs, nuclear-medicine hospitals, sewage incinerators), 131I may be detected occasionally.
Source apportionment of particulate 131I in the air over Europe in January/February 2017 based on source term estimates. Taken from [88]. Reprinted with permission from the American Chemical Society, © 2018
15.6 Conclusion
The presence of radioiodine in the environment is of major concern for radioprotection issues for workers and members of the public. Regardless of the iodine isotope (129I, 131I, 132I); of the situation (routine release, incident, or accident); and the nuclear activity (radiopharmaceutical production and use, nuclear energy production, nuclear reprocessing), an overall dominant iodine gaseous fraction has to be considered in the global atmosphere at distance from the emission source. The dominancy of the radioiodine gaseous fraction is about 4–5 times higher compared to the aerosol-bound fraction. This pattern applies for Three Mile Island, Chernobyl, and Fukushima Daiichi NPP accidents at more or less long distances from the release points, i.e., where the concentrations of the different radioactive iodine-containing species have significantly decreased because of (1) plume dispersion, (2) deposition mechanisms (dry + wet) involved along the route of the contaminated air masses and according to different deposition velocities linked to the different iodine-containing species, (3) photochemical reaction of gaseous iodine with ozone and NOx leading to oxide particles [8, 116], and (4) the sorption kinetics of gaseous iodine onto ambient aerosols, to a lesser extent.
Exemptions to this scheme (i.e., a dominant radioiodine particulate fraction) may temporarily occur when a high amount of aerosol is produced as during explosions and fires affecting a nuclear reactor or its outer superstructures. At short distances, the release features and/or accident scenario therefore control the distribution of radioiodine, and it is difficult to predict from where and when it will return in favor of the gaseous fraction. This is all more important than at such distances the airborne radioiodine activity levels are likely to be a matter of health issues, and the resulting inhalation dose assessment will be sensitive to the detailed speciation of iodine between gaseous inorganic and organic species, plus aerosol-bound species. During explosions and fires, which is typically what happened during the Chernobyl and Fukushima accident, the amount of particles is likely to enhance the proportion of aerosol-bound iodine. This is also typically what was observed during atmospheric nuclear weapon detonations. In the Fukushima-Daiichi NPP case, explosion phases alternated with venting, scrubbing, and leaking phases, which exhibited a higher gaseous proportion. Methyl iodide (CH3I) and elemental iodine (I2) have higher dose coefficients than aerosol-bound species since they settle at 70% and 100%, respectively. At a more detailed stage, is thus of primary importance to know or assess the gaseous chemical speciation (organic and inorganic species) to derive a realistic dose assessment in the early stage of an accident. Indeed, if the gaseous fraction remains unmonitored or ignored, the inhalation dose can be underestimated by a factor of about 6. Recent accident or incident situations involving 131I releases show the need to shift to a higher sampling frequency for most accurate source term estimates and in the case of an undeclared or a priori unknown release, to locate the source location with less uncertainty.
Among the gaseous species, organic gaseous iodine (CH3I as a representative) is the dominant 131I species in the atmosphere after an accident situation while it is expected to remain lower than elemental iodine (I2) during a normal operation. Differences in I2 and HOI contributions were noticed depending on the type of accident (TMI and Chernobyl). In addition to the contribution of iodine-containing species to the inhalation dose assessment, it is important to consider the contribution of 132I induced by decay of 132Te which could lead to a 30% underestimation in the inhalation thyroid dose. Apart from the three main iodine chemical species for which inhalation dose coefficients have been established, little is known about the 131I in the form of hypoiodous acid (HOI) in the atmosphere. Moreover, no inhalation dose coefficient exists for this species. There are some indications of a significant contribution (up to 40%) of HO131I to the total 131I content in the atmosphere when dealing with routine discharges and up to 10% during accident releases. Due to the high photo-dissociation rate of I2, it has even been proposed to replace I2 by HOI and IONO2 species with regard to inorganic species to be considered [6]. More investigations on routine releases are welcome. By default, the following distribution can be adopted in the absence of detailed information: 25% for particulate iodine species, 25% for gaseous inorganic species, and 50% for gaseous organic species.
Other topics of interest are directly or indirectly linked to the increasing use of radiopharmaceuticals for the purpose of both diagnostics and treatments of malignant tumors, and the increasing number of patients. The routine radiopharmaceutical production exhibits by far the highest routine releases, and some recent incidents in Europe have highlighted this source. At a lesser extent, atmospheric discharges from waste water treatment plants equipped with incinerators and located on watersheds with nuclear-medicine hospitals can be considered as possible source of 131I emission to the atmosphere, despite cleaning techniques applied to the fumes. Preliminary investigations have already been carried out in Germany [95], Poland, and in the USA [94] but remain limited in number. However, their impact in terms of dose to the residents living nearby is also worth to be assessed.
Despite the Chernobyl and Fukushima accidents, there is still a lack of information and sampling capabilities dedicated to the knowledge of iodine in the atmosphere and especially regarding its gaseous component.
Notes
- 1.
Incident releases can be integrated in these categories.
References
Saiz-Lopez A, Fernandez RP, Ordóñez C, Kinnison DE, Martín JCG, Lamarque JF, et al. Iodine chemistry in the troposphere and its effect on ozone. Atmos Chem Phys. 2014;14:13119–43.
Hou X, Hansen V, Aldahan A, Possnert G, Lind OC, Lujaniene G. A review on speciation of iodine-129 in the environmental and biological samples. Anal Chim Acta. 2009;632:181–96.
Trincal J. Modélisation du comportement de l’iode dans l’atmosphère. PhD thesis, Université de Lille, France. 2015.
Landis JD, Hamm NT, Renshaw CE, Dade WB, Magilligan FJ, Gartner JD. Surficial redistribution of fallout 131iodine in a small temperate catchment. Proc Natl Acad Sci U S A. 2012;109:4064–9.
Kulyukhin SA, Kulemin VV, Rumer IA, Konovalova NA. Gas-phase UV photolysis of CH3 131I. Radiochemistry. 2005;47:296–300.
Filistovic V, Nedveckaite T. Photochemical aspects of the behavior of the atmospheric radioiodine after the Chernobyl accident. J Radioanal Nucl Chem. 1999;242:75–80.
Saiz-Lopez A, Plane JMC, Cuevas CA, Mahajan AS, Lamarque JF, Kinnison DE. Nighttime atmospheric chemistry of iodine. Atmos Chem Phys. 2016;16:15593–604.
Jenkin ME, Cox RA, Candeland DE. Photochemical aspects of tropospheric iodine behaviour. J Atmos Chem. 1985;2:359–75.
Trincal J, Cantrel L, Cousin F, Fevre-Nollet V, Lebegue P. Impact of atmospheric species reactivity on radioactive gaseous iodine transport in severe accident conditions. WIT Trans Ecol Environ. 2015;198:77–86.
Chameides WC, Davis DD. Iodine: its possible role in tropospheric chemistry. J Geophys Res Atmos. 1980;85:7383–98.
Brookhaven National Laboratory. Interactive chart of nuclides, NuDat 2.7. 2019. https://www.nndc.bnl.gov/nudat2/. Accessed Jan 2019.
Chu SYF, Ekström LP, Firestone RB. Table of radioactive isotopes. 2019. http://nucleardata.nuclear.lu.se/toi/nucSearch.asp. Accessed Jan 2019.
ICRP 38. Radionuclides transformations, energy and intensity of emissions. ICRP Publication 38; 1983.
Laraweb. Library for gamma and alpha emissions. 2019. http://www.nucleide.org/Laraweb/index.php. Accessed Jan 2019.
OECD. JEFF-3.3. 2017. http://www.oecd-nea.org/dbdata/jeff/jeff33/index.html. Accessed Jan 2019.
Fan Y, Hou X, Zhou W. Progress on 129I analysis and its application in environmental and geological researches. Desalination. 2013;321:32–46.
Shirakami Y. Radioactive Iodine. In: Kaiho T, editor. Iodine chemistry and applications. Hoboken: Wiley; 2015. p. 605–23.
Haapanen A. Results of monitoring at Olkiluoto in 2008 – environment: Posiva Working Report 2009-45. 2009.
UNSCEAR. Sources and effects of ionising radiation (Report to the General Assembly). New York: United Nations; 2000.
Veiga LHS, Holmberg E, Anderson H, Pottern L, Sadetzki S, Adams MJ, et al. Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiat Res. 2016;185:473–84.
Ivanov V, Kashcheev V, Chekin S, Maksioutov M, Tumanov K, Menyajlo A, et al. Results of the thyroid cancer epidemiological survey in Russia following the Chernobyl accident. In: Thyroid cancer and nuclear accidents: long-term aftereffects of Chernobyl and Fukushima. Amsterdam: Academic Press; 2017. p. 88–95.
Ivanov VK, Kashcheev VV, Chekin SY, Maksioutov MA, Tumanov KA, Vlasov OK, et al. Radiation-epidemiological studies of thyroid cancer incidence in Russia after the Chernobyl accident (estimation of radiation risks, 1991-2008 follow-up period). Radiat Prot Dosim. 2012;151:489–99.
Tronko M, Brenner AV, Bogdanova T, Shpak V, Oliynyk V, Cahoon EK, et al. Thyroid neoplasia risk is increased nearly 30 years after the Chernobyl accident. Int J Cancer. 2017;141:1585–8.
Kesminiene A, Evrard AS, Ivanov VK, Malakhova IV, Kurtinaitise J, Stengrevics A, et al. Risk of thyroid cancer among Chernobyl liquidators. Radiat Res. 2012;178:425–36.
ICRP 71. Age-dependent doses to members of the public from intake of radionuclides. Part 4. Inhalation dose coefficients. ICRP Publication 71; 1995.
ICRP 68. Dose coefficients for intakes of radionuclides by workers. ICRP Publication 68; 1994.
Morgan A, Morgan DJ, Evans JC, Lister BAJ. Studies on the retention and metabolism of inhaled methyl iodide-II: metabolism of methyl iodide. Health Phys. 1967;13:1067–74.
ICRP 137. Occupational intakes of radionuclides. ICRP Publication 137; 2017.
ICRP 66. Human respiratory tract model for radiological protection. ICRP Publication 66; 1994.
ICRP 72. Age-dependent doses to the members of the public from intake of radionuclides - part 5 compilation of ingestion and inhalation coefficients. ICRP Publication 72; 1996.
EUR-lex. Council Directive 96/29/Euratom of 13 May 1996 laying down basic safety standards for the protection of the health of workers and the general public against the dangers arising from ionizing radiation. 1996. https://eur-lex.europa.eu/eli/dir/1996/29/oj. Accessed Jan 2019.
Ogorodnikov BI, Budyka AK, Pazukhin ÉM, Krasnov VA. Aerosol emissions from the destroyed power-generating unit of the Chernobyl nuclear power plant in 1986 and 2003-2005. Atomic Energy. 2006;100:264–70.
ICRP. ANNEX B: deposition, characterisation, and sampling of radioactive aerosols. Ann ICRP. 2002;32:139–306.
Shinkarev SM, Kotenko KV, Granovskaya EO, Yatsenko VN, Imanaka T, Hoshi M. Estimation of the contribution of short-lived radioiodines to the thyroid dose for the public in case of inhalation intake following the Fukushima accident. Radiat Prot Dosim. 2015;164:51–6.
Balonov M, Kaidanovsky G, Zvonova I, Kovtun A, Bouville A, Luckyanov N, et al. Contributions of short-lived radioiodines to thyroid doses received by evacuees from the Chernobyl area estimated using early in vivo activity measurements. Radiat Prot Dosim. 2003;105:593–9.
Tokonami S, Hosoda M. Thyroid equivalent doses for evacuees and radiological impact from the Fukushima nuclear accident. Radiat Meas. 2018;119:74–9.
European Commission. Radiation Protection No. 165; Medical effectiveness of iodine prophylaxis in a nuclear reactor emergency situation and overview of European practices. 2010. https://ec.europa.eu/energy/sites/ener/files/documents/165.pdf.
HERCA. New European approach for cross-border emergency preparedness. 2014. http://www.herca.org/herca_news.asp?newsID=41. Accessed Jan 2019.
Reiners C, Schneider R. Potassium iodide (KI) to block the thyroid from exposure to I-131: current questions and answers to be discussed. Radiat Environ Biophys. 2013;52:189–93.
Ministry of Health and Long-Term Care (Canada). Potassium Iodide (KI) Guidelines. 2014. http://www.health.gov.on.ca/en/pro/programs/emb/rhrp/docs/ki_guidelines.pdf. Accessed Jan 2019.
Kramer GH, Hauck BM, Chamberlain MJ. Biological half-life of iodine in adults with intact thyroid function and in athyreotic persons. Radiat Prot Dosim. 2002;102:129–35.
WHO. Use of potassium iodide for thyroid protection during nuclear or radiological emergencies. Geneva: WHO; 2011. https://www.who.int/ionizing_radiation/pub_meet/tech_briefings/potassium_iodide/en/.
Petrova K, Jankovec M, Fojtíkova I, Hůlka J. New challenges in crisis communication − the results of sociological survey in the Czech Republic. In: Proceedings of the RICOMET 2017 conference: social and ethical aspects of decision-making in radiological risk situations. IAEA: Vienna, Austria. 27−29th June 2017.
Izrael YA. Radioactivity in the environment, vol. 3. New York: Elsevier; 2002.
Hu Q, Moran JE. Iodine. In: Atwood DA, editor. Radionuclides in the environment. Chichester: Wiley; 2010. p. 165–78.
NCRP. 129I: evaluation releases from nuclear power generation. Report NCRP no. 75. Bethesda: National Council on Radiation Protection and Measurements; 1983.
Prăvălie R. Nuclear weapons tests and environmental consequences: a global perspective. Ambio. 2014;43:729–44.
UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation. Reports to the General Assembly of the United Nations. New York: United Nations; 1982.
Gilbert ES, Huang L, Bouville A, Berg CD, Ron E. Thyroid cancer rates and 131I doses from Nevada atmospheric nuclear bomb tests: an update. Radiat Res. 2010;173:659–64.
UNSCEAR. Report to the general assembly (Annex B—Exposures from man-made sources of radiation). 1993. http://www.unscear.org/docs/reports/1993/1993c_pages%2091-120.pdf. Accessed Jan 2019.
Steinhauser G, Chávez-Ortega M, Vahlbruch J-W. Japanese food data challenge the claimed link between Fukushima’s releases and recently observed thyroid cancer increase in Japan. Sci Rep. 2017;7:10722.
Colombani J, Gregoire AC, Morin S. Main findings of the IRSN experimental programs performed on iodine chemistry in severe accident conditions. In: Proceedings of the International OECD/NEA-NUGENIA/SARNET Workshop April 1, 2015. Marseille (France). 2015.
Nikipelov BV, Romanov GN, Buldakov LA, Babaev NS, Kholina YB, Mikerin EI. Accident in the southern Urals on 29 September 1957. International Atomic Energy Agency Report INFCIRC-368, Vienna; 1989.
Wakeford R. A double diamond anniversary - Kyshtym and Windscale: the nuclear accidents of 1957. J Radiol Prot. 2017;37:E7–E13.
Garland JA, Wakeford R. Atmospheric emissions from the Windscale accident of October 1957. Atmos Environ. 2007;41:3904–20.
McNally RJQ, Wakeford R, James PW, Basta NO, Alston RD, Pearce MS, et al. A geographical study of thyroid cancer incidence in north-west England following the Windscale nuclear reactor fire of 1957. J Radiol Prot. 2016;36:934–52.
Levin RJ, De Simone NF, Slotkin JF, Henson BL. Incidence of thyroid cancer surrounding three mile island nuclear facility: the 30-year follow-up. Laryngoscope. 2013;123:2064–71.
Rogovin M, Frampton GT, Cornell EK, DeYoung RC, Budnitz R, Norry P. Three Mile Island: a report to the commission and the public. NUREG/CR 1250 volume 1. Washington: US NRC; 1980. 184 pp
UNSCEAR. Exposures and effects of the Chernobyl accident (Annex J). New York: United Nations; 2000.
IAEA. The Fukushima Daiichi accident. Technical volume 1/5. Description and context of the accident. Vienna: IAEA STI/PUB/1710. 2015.
IRSN. Information report, 22 March 2011. 2011. www.irsn.fr/EN/news/Documents/IRSN_fukushima-radioactivityreleased-assessment-EN.pdf. Accessed Jan 2019.
Mathieu A, Korsakissok I, Quélo D, Groëll J, Tombette M, Didier D, et al. Atmospheric dispersion and deposition of radionuclides from the Fukushima Daiichi nuclear power plant accident. Elements. 2012;8:195–200.
MEXT. Radioactive substance emission data, press release, October 20, 2011. 2011. www.meti.go.jp/press/2011/10/20111020001/20111020001.pdf. (in Japanese). Accessed Jan 2019.
NISA. Evaluation of the status of reactor cores in units 1–3 of the Fukushima Daiichi nuclear power plant. 2011. http://www.meti.go.jp/press/2011/06/20110606008/20110606008-2.pdf. (in Japanese). Accessed Jan 2019.
Povinec PP, Gera M, Holý K, Hirose K, Lujaniené G, Nakano M, et al. Dispersion of Fukushima radionuclides in the global atmosphere and the ocean. Appl Radiat Isot. 2013;81:383–92.
Saunier O, Mathieu A, Didier D, Tombette M, Quélo D, Winiarek V, et al. An inverse modeling method to assess the source term of the Fukushima nuclear power plant accident using gamma dose rate observations. Atmos Chem Phys. 2013;13:11403–21.
Steinhauser G, Brandl A, Johnson TE. Comparison of the Chernobyl and Fukushima nuclear accidents: a review of the environmental impacts. Sci Total Environ. 2014;470–471:800–17.
UNSCEAR. Sources, effects and risks of ionizing radiation. UNSCEAR 2013 Report, Volume I, Scientific Annex A. Levels and Effects of Radiation Exposure due to the Nuclear Accident after the 2011 Great East-Japan Earthquake and Tsunami New York. 2014.
Yamada M. A brief review of environmental impacts and health effects from the accidents at the three Mile Island, Chernobyl and Fukushima Daiichi nuclear power plants. Radiat. Emerg. Med. 2012;1:33–9.
Gallagher D, McGee EJ, Mitchell PI, Alfimov V, Aldahan A, Possnert G. Retrospective search for evidence of the 1957 Windscale fire in NE Ireland using 129I and other long-lived nuclides. Environ Sci Technol. 2005;39:2927–35.
Steinhauser G. Fukushima’s forgotten radionuclides: a review of the understudied radioactive emissions. Environ Sci Technol. 2014;48:4649–63.
Lozano RL, Hernandez-Ceballos MA, Adame JA, Casas-Ruiz M, Sorribas M, San Miguel EG, et al. Radioactive impact of Fukushima accident on the Iberian Peninsula: evolution and plume previous pathway. Environ Int. 2011;37:1259–64.
Knetsch GJ. Environmental radioactivity in the Netherlands. Results in 2011. RIVM report 610891004/2013. 2013.
De Vismes OA, Gurriaran R, Cagnat X, Masson O. Fission product activity ratios measured at trace level over France during the Fukushima accident. J Environ Radioact. 2013;125:6–16.
Gudelis A, Druteikiene R, Lujaniene G, Maceika E, Plukis A, Remeikis V. Radionuclides in the ground-level atmosphere in Vilnius, Lithuania, in March 2011, detected by gamma-ray spectrometry. J Environ Radioact. 2012;109:13–8.
Lujanienė G, Byčenkienė S, Povinec PP, Gera M. Radionuclides from the Fukushima accident in the air over Lithuania: measurement and modelling approaches. J Environ Radioact. 2012;114:71–80.
Doizi D, Reymond la Ruinaz S, Haykal I, Manceron L, Perrin A, Boudon V, et al. Analytical measurements of fission products during a severe nuclear accident. EPJ Web Conf. 2018;170:08005.
Xu S, Zhang L, Freeman SPHT, Hou X, Shibata Y, Sanderson D, et al. Speciation of radiocesium and radioiodine in aerosols from Tsukuba after the Fukushima nuclear accident. Environ Sci Technol. 2015;49:1017–24.
Muramatsu Y, Matsuzaki H, Toyama C, Ohno T. Analysis of 129I in the soils of Fukushima prefecture: preliminary reconstruction of 131I deposition related to the accident at Fukushima Daiichi nuclear power plant (FDNPP). J Environ Radioact. 2015;139:344–50.
Pietrzak-Flis Z, Krajewski P, Radwan I, Muramatsu Y. Retrospective evaluation of 131I deposition density and thyroid dose in Poland after Chernobyl accident. Health Phys. 2003;84:698–708.
Yang G, Tazoe H, Yamada M. Can 129I track 135Cs, 236U, 239Pu, and 240Pu apart from 131I in soil samples from Fukushima prefecture, Japan? Sci Rep. 2017;7:15369.
Miyake Y, Matsuzaki H, Fujiwara T, Saito T, Yamagata T, Honda M, et al. Isotopic ratio of radioactive iodine (129I/131I) released from Fukushima Daiichi NPP accident. Geochem J. 2012;46:327–33.
Kim E, Kurihara O, Tani K, Ohmachi Y, Fukutsu K, Sakai K, et al. Intake ratio of 131I to 137CS derived from thyroid and whole-body doses to Fukushima residents. Radiat Prot Dosim. 2015;168:408–18.
Steinhauser G, Merz S, Kübber-Heiss A, Katzlberger C. Using animal thyroids as ultra-sensitive biomonitors for environmental radioiodine. Environ Sci Technol. 2012;46:12890–4.
Doll CG, Sorensen CM, Bowyer TW, Friese JI, Hayes JC, Hoffmann E, et al. Abatement of xenon and iodine emissions from medical isotope production facilities. J Environ Radioact. 2014;130:33–43.
Karpov Institute of Physical Chemistry. Activity report. 2017. http://www.rosatom.ru/upload/iblock/334/334f8a20cd4f2b02cb25e58b48190bd8.pdf. (in Russian). Accessed Jan 2019.
Ageevа NV, Kim VM, Vasilieva KI, Katkova MN, Volokitin AA, Polyanskaya ON. Long-term monitoring airborne I-131 in the surface layer in Obninsk city. Radiation & Risk. 2015;24:96–107.
Masson O, Steinhauser G, Wershofen H, Mietelski JW, Fischer HW, Pourcelot L, et al. Potential source apportionment and meteorological conditions involved in airborne 131I detections in January/February 2017 in Europe. Environ Sci Technol. 2018;52:8488–500.
Schomäcker K, Sudbrock F, Fischer T, Dietlein M, Kobe C, Gaidouk M, et al. Exhalation of 131I after radioiodine therapy: measurements in exhaled air. Eur J Nucl Med Mol Imaging. 2011;38:2165–72.
Sudbrock F, Fischer T, Zimmermanns B, Drzezga A, Schomäcker K. Exhalation of 131I after radioiodine therapy: Dosimetric considerations based on measurements in exhaled air. J Environ Radioact. 2017;166:162–5.
Ravichandran R, Binukumar JP, Al Saadi A. Estimation of effective half life of clearance of radioactive iodine (131I) in patients treated for hyperthyroidism and carcinoma thyroid. Indian Journal of Nuclear Medicine. 2010;25:49–52.
Willegaignon J, Malvestiti LF, Guimarães MI, Sapienza MT, Endo IS, Neto GC, et al. 131I effective half-life (Teff) for patients with thyroid cancer. Health Phys. 2006;91:119–22.
Yeager CM, Amachi S, Grandbois R, Kaplan DI, Xu C, Schwehr KA, et al. Microbial transformation of iodine: from radioisotopes to iodine deficiency. Adv Appl Microbiol. 2017;101:83–136.
Kitto ME, Fielman EM, Fielman SE, Gillen EA. Airborne 131I at a background monitoring site. J Environ Radioact. 2005;83:129–36.
Hormann V, Fischer HW. The physicochemical distribution of 131I in a municipal wastewater treatment plant. Internal report, University of Bremen; 2017. pp. 1–17.
Hormann V, Fischer HW. A simple compartment model for the dynamical behavior of medically derived 131I in a municipal wastewater treatment plant. Environ Sci Technol. 2018;52:9235–42.
Fenner FD, Martin JE. Behavior of Na131I and Meta (131I) Iodobenzylguanidine (MIBG) in municipal sewerage. Health Phys. 1997;73:333–9.
Titley JG, Carey A, Crockett GM, Ham GJ, Harvey MP, Mobbs SF, et al. Investigation of the sources and fate of radioactive discharges to public sewers, environment agency R&D technical report P288. Bristol: Environment Agency; 2000.
Masson O, Baeza A, Bieringer J, Brudecki K, Bucci S, Cappai M, et al. Tracking of airborne radionuclides from the damaged Fukushima Dai-ichi nuclear reactors by European networks. Environ Sci Technol. 2011;45:7670–7.
Kitto ME, Fielman EM, Hartt GM, Gillen EA, Semkow TM, Parekh PP, et al. Long-term monitoring of radioactivity in surface air and deposition in New York state. Health Phys. 2006;90:31–7.
Japan Nuclear Fuel Limited. Reprocessing. 2019. https://www.jnfl.co.jp/en/business/reprocessing/. Accessed Jan 2019.
Reithmeier H, Lazarev V, Rühm W, Nolte E. Anthropogenic 129I in the atmosphere: overview over major sources, transport processes and deposition pattern. Sci Total Environ. 2010;408:5052–64.
Eslinger PW, Napier BA, Anspaugh LR. Representative doses to members of the public from atmospheric releases of 131I at the Mayak production association facilities from 1948 through 1972. J Environ Radioact. 2014;135:44–53.
Xing S, Hou X, Aldahan A, Possnert G, Shi K, Yi P, et al. Iodine-129 in snow and seawater in the Antarctic: level and source. Environ Sci Technol. 2015;49:6691–700.
Orano. Rapport d’information sur la sûreté nucléaire et la radioprotection du site AREVA la Hague (in French). 2014.
Sellafield Ltd. (2015) Monitoring our Environment. Discharges and environmental monitoring. Annual report.
Zhang L, Hou X, Xu S. Speciation of 127I and 129I in atmospheric aerosols at Risø, Denmark: insight into sources of iodine isotopes and their species transformations. Atmos Chem Phys. 2016;16:1971–85.
Daraoui A, Riebe B, Walther C, Wershofen H, Schlosser C, Vockenhuber C, et al. Concentrations of iodine isotopes (129I and 127I) and their isotopic ratios in aerosol samples from northern Germany. J Environ Radioact. 2016;154:101–8.
Xu S, Freeman SPHT, Hou X, Watanabe A, Yamaguchi K, Zhang L. Iodine isotopes in precipitation: temporal responses to 129I emissions from the Fukushima nuclear accident. Environ Sci Technol. 2013;47:10851–9.
Fuge R. Iodine deficiency: an ancient problem in a modern world. Ambio. 2007;36:70–2.
Hou XL, Dahlgaard H, Nielsen SP, Kucera J. Level and origin of Iodine-129 in the Baltic Sea. J Environ Radioact. 2002;61:331–43.
Nedveckaite T, Motiejunas S, Kucinskas V, Mazeika J, Filistovic V, Jusciene D, et al. Environmental releases of radioactivity and the incidence of thyroid disease at the Ignalina nuclear power plant. Health Phys. 2000;79:666–74.
Borisov NB, Budyka AK, Verbov VV, Ogorodnikov BI. Monitoring of radioiodine in air. J Aerosol Sci. 1994;25:271–2.
Vandecasteele CM, Sonck M, Degueldre D. Rejet accidentel d’iode-131 par l’IRE sur le site de Fleurus: retour d’expérience de l’autorité de sûreté belge. Radioprotection. 2011;46:159–73. (in French)
European Commission. Technical Report HU-12/01. 2012. https://ec.europa.eu/energy/sites/ener/files/documents/tech_report_hungary_2012_en.pdf. Accessed Jan 2012.
Garland JA. The adsorption of iodine by atmospheric particles. Journal of Nuclear Energy. 1967;21:687–700.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Masson, O. et al. (2019). Radioiodine Releases in Nuclear Emergency Scenarios. In: Steinhauser, G., Koizumi, A., Shozugawa, K. (eds) Nuclear Emergencies. Current Topics in Environmental Health and Preventive Medicine. Springer, Singapore. https://doi.org/10.1007/978-981-13-8327-4_15
Download citation
DOI: https://doi.org/10.1007/978-981-13-8327-4_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8326-7
Online ISBN: 978-981-13-8327-4
eBook Packages: MedicineMedicine (R0)