Abstract
Taurine is essential for the development and function of the central nervous system, retina, and cardiovascular system. It is a naturally occurring amino acid, abundantly found in the retina. It has been shown to exhibit antioxidant, neuroprotective, and osmoregulatory functions in the retina. We used conditionally immortalized rat retinal capillary endothelial cells (TR-iBRB), in vitro, to investigate the effects of oxidative stress, high glucose (HG) and hypertonic conditions on taurine transport. TR-iBRB cells pre-treated with tumor necrosis factor alpha (TNF-α) showed a significant increase in [3H]taurine uptake rate, which, however, decreased when treated with taurine (50 mM). Addition of paeonol and propranolol to TNF-α pre-treated cells had no significant effect on [3H]taurine uptake, but the addition of 10 mM taurine caused a reduction. The uptake rate decreased under HG conditions, in contrast to that under hypertonic conditions. [3H]Taurine uptake increased with pre-incubation time. Additionally, uptake of [3H]taurine and mRNA expression of taurine transporter (TauT) decreased significantly under hypertonic and HG conditions, following pre-incubation with 10 mM taurine, 1 mM paeonol, and 0.1 mM propranolol. [3H]Taurine uptake was significantly inhibited in the presence of taurine transporters such as taurine and β-alanine. Results indicate that oxidative stress and hypertonic conditions increased taurine uptake in iBRB cell lines, whereas HG conditions reduced the uptake rate. Taurine may be useful in stabilizing the microenvironment in cells affected by oxidative stress as well as hypertonic and HG conditions. Moreover, taurine may play a key role in maintaining taurine concentrations in the taurine transporter system of retinal cells.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Taurine protects photoreceptor cells and regulates calcium transport and signal transduction, by inhibiting protein phosphorylation (Lombardini 1991). Taurine concentration in the retina is 100 times more than that in the serum, suggesting a role for taurine in the retina. Insulin resistance and failure of pancreatic β cells characterize type 2 diabetes mellitus (T2D). Taurine regulates glucose and lipid homeostasis under normal, pre-diabetic, and diabetic conditions (Borck et al. 2018). Diabetes is associated with activation of aldose reductase (AR), hyperglycemia, intracellular osmotic stress, oxidative stress, and tissue damage (Nakashima et al. 2005). In addition, taurine may be used as a therapeutic supplement to prevent type-I and II diabetes, and enhance osmoregulation in the retina. Taurine significantly decreased blood glucose levels and related mortality rates, as well as symptoms of heart failure, whereas commonly used anti-diabetic drugs did not reduce mortality rates related to diabetes (Chen et al. 2016). Similarly, taurine may play a key role in the regulation of cell volume, osmoregulation, and membrane stabilization also act as a neurotransmitter, neuromodulator and an anti-oxidant (Olson and Martinho 2006). It has been identified as a strong osmolyte with hypertonicity, and a signal inducer for the taurine transporter system in the glial cells, renal cells and epithelial cells (L’Amoreaux 2012).
The retina, which is composed of neural tissue, has a blood retinal barrier (BRB) which is essential for vision. BRB plays a key role in maintaining retinal functions and homeostatic regulation of the nutrient supply to the retina from blood (Kubo et al. 2016). Based on structural and functional properties, taurine transporter (TauT) is categorized as a member of the solute carrier (slc6a) family and functions as a Na+ and Cl− dependent neurotransmitter transporter (Chen et al. 2004). The influx and efflux of solutes such as taurine is mediated by TauT. Hypertonicity upregulates and HG down regulates TauT in several cell types such as astrocytes, hepatocytes and retinal cells (El-Sherbeny et al. 2004).
In previous study, already investigated the change of taurine transport in variable conditions (Kang et al. 2009). And in the present study investigated characteristics of the taurine transport system under hypertonic, HG and oxidative stress conditions following pre-treatment with the drugs: taurine, paeonol, and propranolol, using (TR-iBRB) cell lines, in vitro. Furthermore, mRNA expression under those conditions was also used to elucidate regulating mechanisms. Our results indicated that the taurine transporter was upregulated under hypertonic and oxidative stress conditions and down regulated under HG condition in TR-iBRB cells.
2 Methods
2.1 Cell Culture
TR-iBRB cells (provided previously from Prof. T. Terasaki) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Grand Island, NY, USA); supplemented with 10% fetal bovine serum (FBS); (Invitrogen, Grand Island, NY, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin (Invitrogen, Grand Island, NY, USA). Cells were seeded on type I collagen coated culture dishes (Biocoat, Kennebunk, ME, USA) incubated at 33 °C, and cultured in a humidified atmosphere of 5% CO2 and 95% air as described previously (Kang et al. 2002).
2.2 In Vitro [3H]Taurine Uptake Study in TR-iBRB Cells
[3H]Taurine uptake at the inner blood retinal barrier cell lines was evaluated, as previously reported (Kang et al. 2009; Jung et al. 2013). In addition, iBRB cells (1 × 105 cells/well) were seeded on type I collagen coated 24 well plates (Biocoat, Kennebunk, ME, USA) and the uptake was carried out by washing with extracellular fluid (ECF) buffer (pH 7.4) at 37 °C. In addition, 200 μL ECF buffer containing [3H]taurine (5.2 nM) at 37 °C, was used both in the presence and absence of inhibitors (1 mM except β-alanine). Following the designated time period (5 min), uptake was terminated by removing the solution and washed with 1 mL ice-cold ECF buffer (Tamai et al. 1995). Next, the cells were dissolved in 1 N NaOH overnight at room temperature. The following day, measurement of radioactivity and protein assay was conducted using a liquid scintillation counter (LS6500, Beckman Instruments Inc., Fullerton, OH, USA), and DC protein assay kit (Bio-Rad, Hercules, CA, USA) with bovine serum albumin as a standard (Kang et al. 2002). To investigate the effect on [3H]taurine under normal, hypertonic, HG and oxidative stressed conditions by iBRB cells under pre-treatment conditions for 24 h and 4 h respectively. The pre-treatment was done with normal media (280 mOsm/kg) which was added to 100 mM of sucrose to produce hypertonic conditions (390 mOsm/kg) and to 25 mM of glucose to produce HG conditions, were used for pre-incubation (Kang et al. 2009). iBRB cells were pre-incubated with cold taurine (10 mM), paeonol (1 mM), and propranolol (100 μM) as necessary, to evaluate the effects of these compounds under hypertonic, HG and oxidative stressed conditions.
In addition, the cell-to-medium ratio (μl/mg protein) was calculated by using the following equation:
2.3 Real-Time Reverse Transcription Polymerase Chain Reaction
For gene silencing purposes, TR-iBRB cell lines were seeded on type-I collagen coated 6 well plates (3 × 105cells/well) and incubated for 24 h at 33 °C. Total cellular RNA was extracted by washing the cells with phosphate-buffered saline (PBS). The cells were pre-treated for 24 h under isotonic, hypertonic and HG conditions with or without inhibitors for 4 h under oxidative stress (TNF-α) conditions. Isolation of total RNA from cultured cells was carried out using the RNeasy kit (Qiagen, Velencia, CA, USA) according to manufacturer’s guidelines. Single-stranded cDNA was prepared from 1.0 μg of total RNA using a High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was performed using rat TauT or GAPDH-specific primers in the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with TaqMan® Gene Expression Master Mix (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer’s protocols. Polymerase chain reactions were investigated using a gene amplification system (MyCycler, BioRad Inc., Hercules, CA, USA). (Lee and Kang 2015).
2.4 Statistical Analysis
Unless otherwise indicated, all data are presented as mean ± SE. Statistically significant differences were calculated using the unpaired two-tailed student’s t-test. A Significance level of p < 0.05 was used.
3 Results
3.1 Inhibitory Effect of Various Compounds on [3H]Taurine Uptake by TR-iBRB Cells
To characterize the taurine transport system of iBRB cells, compounds listed in Table 1 were used to examine their inhibitory effect on [3H]taurine uptake, in vitro. Taurine transporter inhibitors such as, 1 mM taurine and 0.5 mM B-alanine strictly inhibited [3H]taurine uptake by 97% and 89% respectively. Verapamil, paeonol, clonidine, and gamma amino butyric acid (GABA) also decreased [3H]taurine uptake by 85%, 58%, 40% and 53% respectively. In contrast, other compounds such as imperatorin, propranolol, acetyl L-carnitine (ALC), citrulline, L-leucine, probenecid, para-aminohippuric acid (PAH) and tetraethyl ammonium (TEA) made no marked difference to [3H]taurine uptake (Table 1).
3.2 Time Course Effect of Taurine and TNF-α on [3H]Taurine Uptake at the TR-iBRB Cells
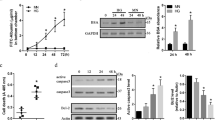
Taurine transport activity in TR-iBRB cells was examined in vitro. Unlabeled taurine at a concentration of 50 mM and 20 ng/mL TNF-α were used for pre-treatment for 24 h. [3H]Uptake rate decreased significantly with increasing pre-treatment time in the presence of taurine. However, although the uptake rate increased until and up to 4 h after pre-treatment with TNF-α, there was no increase in the uptake rate after 4 h (Fig. 1).
Time-course effect of TNF-α or unlabeled taurine pre-treatment on [3H]taurine uptake by TR-iBRB cells at 37 °C for 5 min. Cells were pre-treated with 20 ng/mL TNF- α (open circles), or 50 mM unlabeled taurine (closed circles) for the time periods indicated in the figure. Each time point represents the mean ± SE (n = 3–4). ∗p < 0.05, ∗∗p < 0.001 significantly different from time = 0
3.3 Time Course Effect of Hypertonicity and HG on [3H]Taurine Uptake in TR-iBRB Cells
To investigate the effect of hypertonic (390 mOsm/kg) and HG (25 mM) conditions, taurine uptake in iBRB cells was examined in vitro. [3H]Taurine uptake in TR-iBRB cells was significantly increased and decreased during 24 h of incubation with hypertonic media and HG media respectively (Fig. 2).
[3H]Taurine uptake by TR-iBRB2 cells. Uptake was performed for 5 min at 37 °C after pre-incubation in hypertonic culture medium (open circles) or high glucose culture medium (closed circles). Each point represents the mean ± SE (n = 4). ∗∗∗p < 0.001 and ∗p < 0.05, significantly different from control
3.4 Cold Inhibition Effect of Taurine on the Hypertonicity, High Glucose and TNF-α Conditions
[3H]Taurine uptake in iBRB cells was examined under various conditions after pre-treatment. Cells were pre-incubated under hypertonic, high glucose and oxidative stress conditions for 24 h and 4 h respectively. After pre-incubation, [3H]taurine was evaluated following pre-treatment with unlabeled 10 mM taurine as a cold inhibitor. Cold inhibition by 10 mM taurine caused [3H]taurine uptake to significantly drop by more than 95% in 5 min under all conditions (Fig. 3).
TR-iBRB cells were pre-incubated with hypertonic media, high glucose media, or TNF- α containing media for 24 h and 4 h respectively. After a designated pre-incubation time, the uptake of [3H]taurine was performed in the presence of unlabeled taurine (10 mM) for 5 min at 37 °C. Each column represents the mean ± SE (n = 3–4). ∗p < 0.05 and ∗∗∗p < 0.001, significantly different from the control; ###p < 0.01, significantly different from the hypertonic media; +++p < 0.001, significantly different from the high glucose media; γγγp < 0.001, significantly different from the TNF- α media
3.5 The Pre-treatment Effect of Taurine, Paeonol and Propranolol of Taurine Transport Function at Normal Condition
[3H]Taurine uptake in TR-iBRB cells was monitored to investigate the effect of unlabeled taurine (10 mM), paeonol (1 mM) and propranolol (100 μM) under normal conditions. Similarly, to clarify the transport system of taurine under normal conditions, mRNA expression level was evaluated during 24 h after pre-treatment. The uptake of [3H]taurine was reduced by 67%, 30% and 20% following pre-treatment with unlabeled taurine, paeonol and propranolol for 24 h under normal conditions (Fig. 4a). The expression of TauT mRNA was reduced by 43%, 59% and 19% under normal conditions for 24 h following pre-treatment with taurine, paeonol and propranolol respectively (Fig. 4b).
(a) Regulation of taurine transport and (b) TAUT mRNA expression in TR-iBRB cells. Cells were cultured with a pre-treatment of unlabeled 10 mM taurine, 1 mM paeonol, and 0.1 mM of propranolol for 24 h. [3H]Taurine uptake was performed for 5 min at 37 °C. Each column represents the mean ± SE (n = 3–4). ∗∗p < 0.01 and ∗∗∗p < 0.001, significantly different from the control
3.6 Effect of Taurine Uptake and mRNA Expression Level at TNF-α Condition in TR-iBRB Cells
The pre-treatment effect of TNF-α in the presence of 10 mM taurine, 1 mM paeonol, and 0.1 mM propranolol on the rate of [3H]taurine uptake and mRNA expression in TR-iBRB cells was investigated. Both mRNA expression and [3H]taurine uptake showed a significant increase of more than 120% in the TNF-α pre-treated cells 4 h after treatment. Similarly, following the addition of taurine, paeonol and propranolol to the TNF-α containing media, a significant decrease was noticed only in taurine in the TNF-α containing media. Propranolol and paeonol did not show any significant effect. However, mRNA expression and [3H]taurine uptake value was markedly reduced in the pre-treated cells for 4 h in the presence of TNF-α together with taurine, paeonol and propranolol (Fig. 5).
(a) The rate of [3H]taurine uptake and (b) mRNA expression level in TR-iBRB cells. Cell were pre-treated with 20 ng/ml TNF-α, 10 mM of taurine, 1 mM of paeonol, and 0.1 mM propranolol for 4 h. [3H]taurine uptake was performed for 5 min at 37 °C. Each column represents the mean ± SE (n = 4–5). ∗∗p < 0.01, significantly different from the control; #p < 0.05 and ###p < 0.001 significantly different from TNF-α
3.7 The Pre-treatment Effect of Taurine, Paeonol and Propranolol of Taurine Transport Function at Hypertonic Condition
To clarify the transport system of taurine under hypertonic conditions, the [3H]taurine uptake and mRNA expression were monitored for 24 h following pre-treatment. [3H]taurine uptake and mRNA expression under hypertonic condition (390 mOsm/kg), showed a significant increase compared to that under isotonic conditions (280 mOsm/kg). Exposure of TR-iBRB cells to the hypertonic condition, for 24 h, following treatment with 10 mM taurine, 1 mM paeonol, 0.1 mM propranolol, caused taurine uptake and mRNA expression level to decrease significantly, compared to the hypertonic conditions (Fig. 6).
(a) Regulation of taurine transport and (b) TAUT mRNA expression in TR-iBRB cells. Cells were cultured under hypertonic conditions and pre-treated with 10 mM taurine, 1 mM paeonol, and 0.1 mM of propranolol for 24 h. [3H]Taurine uptake was performed for 5 min at 37 °C. Each column represents the mean ± SE (n = 3–4). ∗∗∗p < 0.001, significantly different from the control; ###p < 0.001 significantly different from hypertonic condition
3.8 The Pre-treatment Effect of Taurine, Paeonol and Propranolol of Taurine Transport Function at High Glucose Condition
[3H]Taurine uptake and mRNA expression under HG conditions was investigated. The cells pre-treated with HG conditions were treated with taurine, paeonol, and propranolol and evaluated for 24 h for both mRNA and [3H]taurine uptake. Studies demonstrated that the taurine uptake rate was decreased by 87% and mRNA expression level reduced by 84% under the high glucose condition. HG condition combined with taurine, paeonol and propranolol pre-treated cells showed a significant reduction in the [3H]taurine uptake rate by 71%, 33%, and 21% and, in mRNA expression level by 43%, 34% and 35% respectively, compared to the HG condition alone (Fig. 7).
(a) Regulation of taurine transport and (b) TAUT mRNA expression in TR-iBRB cells. Cells, cultured under high glucose conditions, were pre-treated with unlabeled 10 mM taurine, 1 mM paeonol, and 0.1 mM of propranolol for 24 h. [3H]Taurine uptake was performed for 5 min at 37 °C. Each column represents the mean ± SE (n = 3–4). ∗∗∗p < 0.001, significantly different from the control; #p < 0.05 and ##p < 0.01, significantly different from high glucose conditions
4 Discussion
The present in vitro study demonstrated that taurine transport in the inner blood-retinal barrier (iBRB), under oxidative stress, hypertonic and HG conditions, is regulated by the taurine transporter (TauT) system. TR-iBRB cells play a crucial role as a tool for screening the transport of compounds to the retina. TauT is described as a Na+ and Cl− dependent transporter. [3H]Taurine uptake was time and concentration dependent with a Km value of 22.2 μM, and is regulated by a low affinity (22.2 μM) transporter in iBRB cells (Kang et al. 2009; Tomi et al. 2006).
Uptake of [3H]taurine was inhibited by more than 90% in the presence of typical taurine substrates, taurine, and β-alanine. These observations suggest that taurine uptake by retinal endothelial cells may occur via a carrier mediated process (Tamai et al. 1995). Similarly, in the presence of verapamil, a novel organic cationic substrate/calcium channel blocker, [3H]taurine uptake was reduced by more than 85%. Taurine transport was affected by extracellular Ca2+ and Ca2+ calcium channel blocker. Paeonol and clonidine, which are novel organic cationic substrates, also reduced the rate of [3H]taurine uptake in iBRB cells. Furthermore, GABA is a substrate of TauT, and GABA 1 mM inhibited [3H]taurine uptake by 53%. (Tomi et al. 2008). However, novel organic cationic substrates such as imperatorin, propranolol, acetyl L-carnitine (ALC) and other compounds probenecid, L-leucine and citrulline had no effect on [3H]taurine uptake by TR-iBRB cells (Table 1).
[3H]Taurine uptake was evaluated for 24 h, using cells pre-treated with unlabeled taurine 50 mM and TNF-α 20 ng/mL. The uptake rate was markedly reduced in the taurine 50 mM pre-treated cells but in the TNF-α 20 ng/mL pre-treated cells the uptake rate remained unchanged after 4 h of incubation (Fig. 1). TNF-α is a pro-inflammatory cytokine induced by cell damage. Therefore, results indicate that oxidative stress inducing agents such as TNF-α may increase taurine uptake. However, 4 h after pre-treatment with cold taurine 50 mM, taurine uptake rate was markedly suppressed by 39% in the iBRB cell line. This may be due to an increase in the concentration of taurine in the retina being suppressed by [3H]taurine transport activity (Lee and Kang 2004; Kang et al. 2002). Time course effect of hypertonic and HG conditions on [3H]taurine uptake by TR-iBRB cells resulted in an increase and a decrease in the taurine uptake rate, respectively, with pre-treatment time. Taurine acts as an antioxidant and osmolyte, and the taurine transport system may likely be involved in osmoregulation. Therefore, the induction effect may follow osmolality, where, under hypertonic stress conditions, cells initially shrink and the volume subsequently recovers during uptake (Fig. 2) (Yahara et al. 2010; Lee and Kang 2013). TR-iBRB cells were pre-incubated with hypertonic, HG and TNF-α containing media for 24 h and 4 h, after which pre-incubation 10 mM taurine was used for the uptake study. The results indicated a marked inhibition of more than 95% in taurine uptake, because the presence of taurine suppressed the uptake rate (Fig. 3). The TNF-α signal activates NF-KB transcriptional activity by nuclear translocation and therefore the TNF-α-NF-KB pathways may be involved in TauT induction. However, taurine uptake level was increased by other factors such as TNF-α and hypertonicity. High concentration of taurine suppressed the taurine uptake rate in iBRB cells (Kang 2006).
Taurine is physiologically important as an antioxidant as well as in osmoregulation and stress responses. In isotonic media, unlabeled 10 mM taurine, 1 mM paeonol, and 0.1 mM propranolol were used for pre-treatment for 24 h after which [3H]taurine uptake and TauT mRNA expression were observed. In the presence of these compounds, both uptake and mRNA expression level decreased significantly (Fig. 4a, b). The transport system of taurine is subject to adaptive regulation, and was down regulated during the pre-incubation time in the culture system, as reported in a previous report on human placental choriocarcinoma cells (JAR) (Jayanthi et al. 1995). In addition, this transport system is dependent on incubation time and concentration of taurine in TR-iBRB cells (Satsu et al. 1997). Based on the same mechanism, compounds such as paeonol and propranolol (substrates of the organic cationic transporter system) may also downregulate the taurine transport system in iBRB cells. The mRNA expression and taurine uptake rate was increased in the cells pre-treated with TNF-α for 4 h. However, in the presence of 10 mM taurine, 1 mM paeonol, and 0.1 mM propranolol with TNF-α pre-treatment for 4 h, there was a significant decrease in the uptake of [3H]taurine and mRNA expression (Fig. 5a, b). The expression level of taurine transporter (TauT) in the rat retina was downregulated with pre-treatment of organic cationic drugs such as paeonol and propranolol. The uptake of these drugs through the iBRB follows the cationic transporter system but the taurine follows the TauT. So, these drugs can affect the uptake of taurine and expression level of TauT in the iBRB. In addition, the level of taurine in the cell depends on the TauT and the taurine synthesizing enzyme CSAD/CAD (Wu 1982).
When hypertonic media was pre-incubated for 24 h with taurine, paeonol and propranolol, [3H]taurine uptake and TauT mRNA expression level decreased significantly in iBRB cells. However, presence of only hypertonic media induced both taurine uptake and mRNA level in TR-iBRB cells (Fig. 6a, b). Previous reports also indicate that the activity of taurine transport was upregulated by hypertonic conditions and that such upregulation was associated with increased gene expression of TauT (Satsu et al. 1999). In regard to diabetic conditions, elevated levels of glucose may evidently disturb the cellular osmoregulation mechanism. Disturbance in osmoregulation may lead to cellular dysfunction. Our previous results also reveal that [3H]taurine uptake may decrease in under 24 h in cells pre-incubated with HG media (Lee and Kang 2013; Lee and Kang 2015). Presumably, under HG conditions, pre-treatment with taurine, paeonol and propranolol, suppressed the [3H]taurine uptake rate and mRNA expression of TauT in TR-iBRB cells (Fig. 7a, b). Therefore, we contend that taurine may be useful in preventing retinal diseases such as diabetic retinopathy. An imbalance in cell volume may cause retinal diseases such as diabetic retinopathy, neurodegeneration, ischemia and macular edema, but taurine may be able to regulate cell volume in the retina (Tomi et al. 2007). Taurine is highly concentrated in the retina where it may act as an organic osmolyte. Our study demonstrates the [3H]taurine uptake as well as TauT mRNA expression may decrease during pre-incubation with various compounds. Therefore, these results indicate that taurine may play an important role in maintaining a healthy retina.
5 Conclusion
Our study demonstrates that oxidative stress and hypertonic conditions may increase taurine uptake in iBRB cell lines, whereas HG conditions may decrease such uptake. Pre-treatment with paeonol and propranolol under hypertonic and HG conditions, decreased taurine transport function, whereas these compounds had no effect on taurine transport function under the TNF-α condition. Pre-treatment effect of taurine was shown in carrier mediated transport function under all conditions. Taurine transport system in the presence of organic cationic compounds is regulated under various conditions by TauT, and may play a key role in maintaining taurine concentration in retinal cells.
Abbreviations
- iBRB:
-
Inner blood retinal barrier
- RT-PCR:
-
Real time polymerase chain reaction
- HG:
-
High glucose
- Taut:
-
Taurine transporter
- TNF-α:
-
tumor necrosis factor alpha
References
Borck PC, Vettorazzi JF, Branco RCS, Batista TM, Santos-Silva JC, Nakanishi VY, Boschero AC, Ribeiro RA, Carneiro EM (2018) Taurine supplementation induces long-term beneficial effects on glucose homeostasis in ob/ob mice. Amino Acids 50:765–774
Chen NH, Reith ME, Quick MW (2004) Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch 447:519–531
Chen W, Guo J, Xhang Y, Xhang J (2016) The beneficial effects of taurine in preventing metabolic syndrome. Food Funct 7:1849–1563
El-Sherbeny A, Naggar H, Miyauchi S, Ola MS, Maddox DM, Martin PM, Ganapathy V, Smith SB (2004) Osmoregulation of taurine transporter function and expression in retinal pigment epithelial, ganglion, and Muller cells. Invest Opthalmol Vis Sci 45:694–701
Jayanthi LD, Ramamoorthy S, Mahesh VB, Leibach FH, Ganapathy V (1995) Substrate-specific regulation of the taurine transporter in human placental choriocarcinoma cells (JAR). Biochim Biophys Acta 1235:351–360
Jung MK, Kim KY, Lee NY, Kang YS, Hwang YJ, Kim Y, Sung JJ, McKee A, Kowall N, Lee J, Ryu H (2013) Expression of taurine transporter (TauT) is modulated by heat shock factor 1(HSF1) in motor neurons of ALS. Mol Neurobiol 47:699–710
Kang YS (2006) The effect of oxidative stress on the transport of taurine in an in vitro model of the blood-brain barrier. Adv Exp Med Biol 583:291–298
Kang YS, Ohtsuki S, Takanaga H, Tomi M, Hosoya K, Terasaki T (2002) Regulation of taurine transport at the blood-brain barrier by tumor necrosis factor-alpha, taurine and hypertonicity. J Neurochem 83:1188–1195
Kang YS, Lee NY, Chung YY (2009) The change of taurine transport in variable stress states through the inner blood-retinal barrier using in vitro model. Biomol Ther 17:175–180
Kubo Y, Akanuma S, Hosoya K (2016) Impact of SLC6A transporters in physiological Taurine transport at the blood–retinal barrier and in the liver. Biol Pharm Bull 39:1903–1911
L’Amoreaux W (2012) The roles of taurine in the retina. Transworld Res Netw 37/661:215–254
Lee NY, Kang YS (2004) The brain-to-blood efflux transport of taurine and changes the blood-brain barrier transport system by tumor necrosis factor-alpha. Brain Res 1023:141–147
Lee NY, Kang YS (2013) The effects of bisphosphonates on taurine transport in retinal capillary endothelial cells under high glucose conditions. Adv Exp Med Biol 776:59–66
Lee NY, Kang YS (2015) The changes by hypoxia inducible factor-1alpha (HIF-1α) on taurine uptake in brain capillary endothelial cells at high glucose conditions. Adv Exp Med Biol 803:501–551
Lombardini JB (1991) Taurine: retinal function. Brain Res Rev 16:151–169
Nakashima E, Pop-Busi R, Towns R, Thomas TP, Hosaka Y, Nakamura J, Greene DA, Killen PD, Schroeder J, Larkin DD, Ho YL, Stevens MJ (2005) Regulation of the human taurine transporter by oxidative stress in retinal pigmental epithelial cells stavly transformed to overexpress aldose reductase. Antioxid Redox Signal 7:1530–1542
Olson JE, Martinho E (2006) Regulation of taurinet transport in rat hippocampal neurons by hypoosmotic swelling. J Neurochem 96:1375–1389
Satsu H, Watanabe H, Arai S, Shimizu M (1997) Characterization and regulation of taurine transport in Caco-2, human intestinal cells. J Biochem 121:1082–1087
Satsu H, Miyamoto Y, Shimizu M (1999) Hypertonicity stimulates taurine uptake and transporter gene expression in Caco-2 cells. Biochemica et Biophysica 1419:89–96
Tamai I, Senmaru M, Terasayaki T, Tsuji A (1995) Na+- and Cl− -dependent transport of taurine at the blood-brain barrier. Biochem Pharmacol 11:1783–1793
Tomi M, Terayama T, Isobe T, Egami F, Morito A, Kurachi M, Ohtsuki S, Kang YS, Terasaki T, Hosoya KI (2006) Function and regulation of taurine transport at the inner blood-retinal barrier. Microvasc Res 73:100–106
Tomi M, Tajima A, Tachikawa M, Hosoya KI (2008) Function of taurine transporter Slca6/TauT as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochemica et Biophysica Acta 2008:2138–2148
Wu JY (1982) Purification and characterization of cysteic/cysteine sulfinic acids decarboxylase and L-glutamate decarboxylase in bovine brain. Proc Natl Acad Sci U S A 79:4270–4274
Yahara T, Tachikawa M, Akanuma SI, Hosoya KI (2010) Hypertonicity enhances GABA uptake by cultured rat retinal capillary endothelial cells. Drug Metab Pharmacokinet 25:611–615
Acknowledgements
Our study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2011-0030074).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Gyawali, A., Kang, YS. (2019). The Effect of Drug Pre-treatment on Taurine Transport at the Inner Blood-Retinal Barrier Under Variable Conditions. In: Hu, J., Piao, F., Schaffer, S., El Idrissi, A., Wu, JY. (eds) Taurine 11. Advances in Experimental Medicine and Biology, vol 1155. Springer, Singapore. https://doi.org/10.1007/978-981-13-8023-5_80
Download citation
DOI: https://doi.org/10.1007/978-981-13-8023-5_80
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8022-8
Online ISBN: 978-981-13-8023-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)