Abstract
Taurine displays anti-tumor activity in some kinds of human cancers. However, the underlying mechanisms are poorly understood. Epstein-Barr virus-related nasopharyngeal carcinoma (NPC) is a distinctive type of head and neck cancer in Southeast Asia with the highest incidence in South China. We examined an apoptosis-inducing effect of taurine against NPC cells (HK1 and HK1-EBV) to clarify the mechanisms of anti-tumor effects of taurine by immunocytochemical methods. We observed that taurine induced cleavage of caspase-9/3 in a concentration-dependent manner, suggesting the involvement of mitochondrial apoptotic signals. Both PTEN and p53 activation were detected in a dose-dependent manner after taurine treatment in NPC cells. In conclusion, taurine may play an anti-tumor role by activating tumor suppressor PTEN and p53.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Taurine (2-aminoethanesulfonic acid) is a natural amino acid, expressed widely in mammalian tissues (Schaffer and Kim 2018). Several studies have demonstrated that taurine possesses anti-tumor properties through inhibiting cell proliferation and inducing apoptosis in certain cancers by upregulating pro-apoptotic and downregulating anti-apoptotic proteins (Choi et al. 2015; Ibrahim et al. 2018; Tang et al. 2015; Tu et al. 2018; Zhang et al. 2014).
Nasopharyngeal carcinoma (NPC) is a distinctive type of head and neck malignancy, with the incidence rising to 15–50 cases per 100,000 in the southern regions of China (McDermott et al. 2001) while the frequency in Caucasians in other countries is less than one case per 100,000 person-years (Yao et al. 2017). The etiology of NPC is multifactorial. Latent Epstein–Barr virus (EBV) infection, environmental carcinogens, and genetic factors play important roles in the development of NPC (Tsao et al. 2014). There is an urgent need to develop novel preventive and therapeutic strategies.
Our recent study has demonstrated that taurine can inhibit the proliferation and induce apoptosis in NPC cell lines (HK1 and HK1-EBV) in a concentration-dependent manner, as detected by MTT assay and flow cytometer (He et al. 2018). More importantly, taurine exerts a significant suppression of cell proliferation in NPC cell lines without toxic effects on normal nasopharyngeal epithelial cells under 32 mM of taurine for 48 h. Therefore, we decided upon the conditions of taurine treatment (0–32 mM for 48 h) for the current experiments.
In this study, we investigated the effects of taurine on apoptosis in NPC cells by detecting cleaved caspase 9/3 by immunocytochemistry. Tumor suppressor proteins, such as phosphatase and tensin homolog (PTEN) and p53, are important in promoting apoptosis. To clarify the anti-cancer mechanism, we examined the protein levels of PTEN and p53 in taurine-treated cells.
2 Methods
2.1 Cell Lines
Human NPC cell lines (HK1 and HK1-EBV) were a kind gift from Professor Sai-Wah Tsao (Hong Kong University) (Li et al. 2006; Lo et al. 2006; Tsang et al. 2010). Cells were maintained at 37 °C in a 5% CO2 incubator. NPC cells were cultured in RPMI1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco). Taurine (purity ≥98.5%) was purchased from Nacalai Tesque Inc. (Kyoto, Japan). All cell lines were treated with taurine at various concentrations (0–32 mM, dissolved in medium) for 48 h.
2.2 Immunocytochemistry (ICC)
Cells were fixed on slides with 4% (v/v) formaldehyde in phosphate buffered saline (PBS) for 10 min at room temperature after the treatment of taurine and washed three times with PBS. The cells were treated with 1% (v/v) Triton X-100 for 10 min, and then incubated with 5% (w/v) skim milk for 60 min at room temperature. ICC was performed by incubation with rabbit monoclonal cleaved caspase-9 (1:100, Cell Signaling Technology Inc., 20750S, Dancers, MA, USA), cleaved caspase-3 (1:100, Cell Signaling Technology Inc., 9661S), anti-PTEN (1:100, Cell Signaling Technology Inc., 9188S), or mouse monoclonal anti-p53 (1:100, Santa Cruz Biotechnology, sc-47,698, San Diego, CA, USA) overnight at room temperature. Then, they were incubated with fluorescent secondary antibodies Alexa Fluor 594–labeled goat anti-rabbit IgG or Alexa Fluor 594-labeled goat anti-mouse IgG (1:400, Thermo Fisher Scientific, Massachusetts, USA) at room temperature for 2 h. Nuclei were stained with DAPI (Southern Biotech, Birmingham, USA), and the stained cells were examined under a fluorescence microscope (BX53, Olympus, Tokyo, Japan). The numbers of positively-staining cells were analyzed using the ImageJ software (ver. 1.48).
2.3 Statistic Analysis
Values were presented as means ± SD. Differences between groups were analyzed using one-way ANOVA followed by Tukey’s post hoc analysis using GraphPad Prism5 software (GraphPad Software, San Diego, CA, USA). A p-value <0.05 was considered to be statistically significant.
3 Results
3.1 Taurine Increases Cleaved Caspase-9/3 in HK1 and HK1-EBV Cells
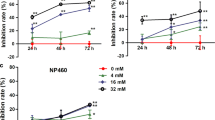
To analyze taurine-induced apoptosis, caspase activation was assessed by ICC. Treatment of the cells with taurine showed a significant increase in the levels of cleaved caspase-9 (Fig. 1a–d) and caspase-3 (Fig. 1e–h), which suggested that taurine may enhance the apoptosis-inducing effect through the mitochondrial pathway.
Taurine yields cleaved caspase-9/3 in HK1 and HK1-EBV cells. Cells were treated with taurine for 48 h and photographed to observe cleaved caspase-9 (a, b) and cleaved caspase-3 (e, f). Data represent quantitative image analysis for cleaved caspase-9 (c, d), and caspase-3 (g, h). Intensity of staining and total number of DAPI-stained cell nuclei was analyzed by ImageJ software. ∗∗p < 0.01 compared to taurine 0 mM group
3.2 Taurine Activates PTEN in HK1 and HK1-EBV Cells
To identify whether PTEN is involved in the anti-tumor effect on NPC, the levels of PTEN were assessed in HK1 and HK1-EBV cells incubated with different concentrations of taurine for 48 h by ICC (Fig. 2). Taurine dose-dependently increased the levels of PTEN compared with the control group. These results suggest that taurine may show an anti-tumor ability on NPC cells through PTEN activation.
Taurine activates PTEN in HK1 and HK1-EBV cells. Cells were treated with taurine for 48 h and (a, b) photographed to observe PTEN (red), DAPI (blue) staining using a microscope. (c, d) Data represents quantitative image analysis for taurine-induced PTEN activation. Intensity of staining and total number of DAPI-stained cell nuclei was analyzed by Image J software. ∗p < 0.05, ∗∗p < 0.01 compared to taurine 0 mM group
3.3 Taurine Activates p53 in HK1 and HK1-EBV Cells
Studies have shown that p53 causes apoptosis in response to DNA damage, which can be induced by anti-cancer drugs or radiation. p53 plays a critical role in activating apoptosis via direct activation of transcription of proapoptotic genes, such as Bcl-xL (Dudgeon et al. 2006). We found that p53 was upregulated with increasing concentrations of taurine (Fig. 3).
Taurine activates p53 in HK1 and HK1-EBV cells. Cells were treated with taurine for 48 h (a, b) and photographed to observe p53 (red), DAPI (blue) staining using a microscope. (c, d) Data represent quantitative image analysis for taurine-induced p53 activation. Intensity of staining and total number of DAPI-stained cell nuclei was analyzed by Image J software. ∗∗p < 0.01 compared to taurine 0 mM group
4 Discussion
Apoptosis is an important homeostatic mechanism balancing cell division and cell death, which is a highly attractive and widely studied area to search for more effective agents for treatment of human cancers (Cheng et al. 2005). A wide variety of in vivo and in vitro studies published in recent years suggested that many chemotherapeutic agents could induce apoptosis in different cancer cells (Lian et al. 2018). Apoptosis is mainly divided into two pathways, one is the death receptor-dependent (external) pathway and the other is the mitochondrial-dependent (inner) pathway (Fulda and Debatin 2006). Additionally, a group of cell death enzymes, cysteine aspartases (caspase), which play an important role in apoptosis, has been intensively studied. Caspase-3 is involved in the terminal phase of apoptosis (Cryns and Yuan 1998), and caspase-9 is a key player of apoptosis in the mitochondrial pathway (Li et al. 2017). Our results demonstrated that taurine increased the levels of cleaved caspase-9 and cleaved caspase-3 in a dose-dependent manner (Fig. 1), suggesting that the taurine can induce apoptosis through a mitochondrial pathway. This result is consistent with the results in colon cancer cell lines (Zhang et al. 2014).
PTEN, identified in 1997 in chromosome 10q23, is a well-known tumor suppressor gene. PTEN is inactivated in patients with advanced cancers, and has gradually become a hot issue in cancer research in the past decades (Lee et al. 2018; Que et al. 2018). The main function of PTEN is negative regulation of PI3K/Akt/mTOR pathway, which plays an important role in the regulation of many important signaling pathways, including cell proliferation and metastasis (Chen et al. 2016; Jiang and Liu 2009). We evaluated PTEN expression after taurine treatment and found that PTEN was upregulated in a dose-dependent manner (Fig. 2). PTEN suppresses Akt activation, which inhibits pro-apoptotic molecules. Increases in PTEN leads to apoptosis. Taurine may induce apoptosis via the PTEN/Akt pathway.
p53 also known as TP53, is one of the most widely studied tumor suppressor genes and plays an important role in cell cycle regulation (Poon 2014). Inactivation or a mutation in p53 can damage its DNA-binding properties and transcription factor function, thereby driving tumorigenesis (Wang et al. 2017). Inhibition of the p53 pathway can accelerate cancer progression and cause resistance to chemotherapy and radiotherapy (Malkin et al. 1992; Wan et al. 2018). p53 generates multiple pro-apoptotic signals in its network, via regulation of target genes, such as PTEN (Freeman and Espinosa 2013). It is suggested that the accumulation of PTEN in the nucleus leads to p53 stabilization and p53-mediated apoptosis of liver cancer cells (Zhang et al. 2016). In this study, we demonstrated that taurine promoted an upregulation of both PTEN and p53 in a concentration-dependent manner (Figs. 2 and 3, respectively). Taurine may have an impact on the p53 network of pro-apoptotic signaling, including the PTEN/Akt pathway.
The results of the present study have provided novel evidence demonstrating that taurine can upregulate the expressions of PTEN and p53. Although further study is needed to clarify the activation pathway of PTEN and p53 by taurine, this study indicates that taurine may exert an anti-tumor effect by upregulating the expression of PTEN and p53 in NPC.
5 Conclusion
In summary, our current work demonstrates that taurine can induce apoptosis in nasopharyngeal carcinoma cells via a mitochondrial pathway through activation of PTEN and p53. These results may provide a new therapeutic strategy for patients with nasopharyngeal carcinoma.
References
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J, Wen X (2016) The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front Biosci (Landmark Ed) 21:1084–1091
Cheng YL, Lee SC, Lin SZ, Chang WL, Chen YL, Tsai NM, Liu YC, Tzao C, Yu DS, Harn HJ (2005) Anti-proliferative activity of Bupleurum scrozonerifolium in A549 human lung cancer cells in vitro and in vivo. Cancer Lett 222:183–193
Choi EJ, Tang Y, Lee CB, Cheong SH, Park PJ, Moon SH, Kim EK (2015) Investigation of antioxidant and anticancer potential of taurine by means of multiple chemical and biological assays. Adv Exp Med Biol 803:179–189
Cryns V, Yuan J (1998) Proteases to die for. Genes Dev 12:1551–1570
Dudgeon C, Kek C, Demidov ON, Saito S, Fernandes K, Diot A, Bourdon JC, Lane DP, Appella E, Fornace AJ Jr, Bulavin DV (2006) Tumor susceptibility and apoptosis defect in a mouse strain expressing a human p53 transgene. Cancer Res 66:2928–2936
Freeman JA, Espinosa JM (2013) The impact of post-transcriptional regulation in the p53 network. Brief Funct Genomics 12:46–57
Fulda S, Debatin KM (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25:4798–4811
He F, Ma N, Midorikawa K, Hiraku Y, Oikawa S, Zhang Z, Huang G, Takeuchi K, Murata M (2018) Taurine exhibits an apoptosis-inducing effect on human nasopharyngeal carcinoma cells through PTEN/Akt pathways in vitro. Amino Acids 50(12):1749–1758
Ibrahim HM, Abdel Ghaffar FR, El-Elaimy IA, Gouida MS, Abd El Latif HM (2018) Antitumor and immune-modulatory efficacy of dual-treatment based on levamisole and/or taurine in Ehrlich ascites carcinoma-bearing mice. Biomed Pharmacother 106:43–49
Jiang BH, Liu LZ (2009) PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res 102:19–65
Lee YR, Chen M, Pandolfi PP (2018) The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. https://doi.org/10.1038/s41580-018-0024-z
Li HM, Man C, Jin Y, Deng W, Yip YL, Feng HC, Cheung YC, Lo KW, Meltzer PS, Wu ZG, Kwong YL, Yuen AP, Tsao SW (2006) Molecular and cytogenetic changes involved in the immortalization of nasopharyngeal epithelial cells by telomerase. Int J Cancer 119:1567–1576
Li P, Zhou L, Zhao T, Liu X, Zhang P, Liu Y, Zheng X, Li Q (2017) Caspase-9: structure, mechanisms and clinical application. Oncotarget 8:23996–24008
Lian W, Lian H, Li Q, An H, Liu S (2018) The venom of spider Haplopelma hainanum suppresses proliferation and induces apoptosis in hepatic cancer cells by caspase activation in vitro. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2018.06.022
Lo AK, Lo KW, Tsao SW, Wong HL, Hui JW, To KF, Hayward DS, Chui YL, Lau YL, Takada K, Huang DP (2006) Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia 8:173–180
Malkin D, Jolly KW, Barbier N, Look AT, Friend SH, Gebhardt MC, Andersen TI, Borresen AL, Li FP, Garber J et al (1992) Germline mutations of the p53 tumor-suppressor gene in children and young adults with second malignant neoplasms. N Engl J Med 326:1309–1315
McDermott AL, Dutt SN, Watkinson JC (2001) The aetiology of nasopharyngeal carcinoma. Clin Otolaryngol Allied Sci 26:82–92
Poon RY (2014) DNA damage checkpoints in nasopharyngeal carcinoma. Oral Oncol 50:339–344
Que WC, Qiu HQ, Cheng Y, Liu MB, Wu CY (2018) PTEN in kidney cancer: a review and meta-analysis. Clin Chim Acta 480:92–98
Schaffer S, Kim HW (2018) Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther (Seoul) 26:225–241
Tang Y, Choi EJ, Cheong SH, Hwang YJ, Arokiyaraj S, Park PJ, Moon SH, Kim EK (2015) Effect of taurine on prostate-specific antigen level and migration in human prostate cancer cells. Adv Exp Med Biol 803:203–214
Tsang CM, Zhang G, Seto E, Takada K, Deng W, Yip YL, Man C, Hau PM, Chen H, Cao Y, Lo KW, Middeldorp JM, Cheung AL, Tsao SW (2010) Epstein-Barr virus infection in immortalized nasopharyngeal epithelial cells: regulation of infection and phenotypic characterization. Int J Cancer 127:1570–1583
Tsao SW, Yip YL, Tsang CM, Pang PS, Lau VM, Zhang G, Lo KW (2014) Etiological factors of nasopharyngeal carcinoma. Oral Oncol 50:330–338
Tu S, Zhang XL, Wan HF, Xia YQ, Liu ZQ, Yang XH, Wan FS (2018) Effect of taurine on cell proliferation and apoptosis human lung cancer A549 cells. Oncol Lett 15:5473–5480
Wan J, Zhang J, Zhang J (2018) Expression of p53 and its mechanism in prostate cancer. Oncol Lett 16:378–382
Wang Z, Liao K, Zuo W, Liu X, Qiu Z, Gong Z, Liu C, Zeng Q, Qian Y, Jiang L, Bu Y, Hong S, Hu G (2017) Depletion of NFBD1/MDC1 induces apoptosis in nasopharyngeal carcinoma cells through the p53-ROS-mitochondrial pathway. Oncol Res 25:123–136
Yao JJ, Zhou GQ, Wang YQ, Wang SY, Zhang WJ, Jin YN, Zhang F, Li L, Liu LZ, Cheng ZB, Ma J, Qi ZY, Sun Y (2017) Prognostic values of the integrated model incorporating the volume of metastatic regional cervical lymph node and pretreatment serum Epstein-Barr virus DNA copy number in predicting distant metastasis in patients with N1 nasopharyngeal carcinoma. Chin J Cancer 36:98
Zhang X, Tu S, Wang Y, Xu B, Wan F (2014) Mechanism of taurine-induced apoptosis in human colon cancer cells. Acta Biochim Biophys Sin Shanghai 46:261–272
Zhang C, Jia X, Bao J, Chen S, Wang K, Zhang Y, Li P, Wan JB, Su H, Wang Y, Mei Z, He C (2016) Polyphyllin VII induces apoptosis in HepG2 cells through ROS-mediated mitochondrial dysfunction and MAPK pathways. BMC Complement Altern Med 16:58
Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Numbers JP16H05255 (MM).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
He, F. et al. (2019). Anti-Cancer Mechanisms of Taurine in Human Nasopharyngeal Carcinoma Cells. In: Hu, J., Piao, F., Schaffer, S., El Idrissi, A., Wu, JY. (eds) Taurine 11. Advances in Experimental Medicine and Biology, vol 1155. Springer, Singapore. https://doi.org/10.1007/978-981-13-8023-5_49
Download citation
DOI: https://doi.org/10.1007/978-981-13-8023-5_49
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8022-8
Online ISBN: 978-981-13-8023-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)