Abstract
Biosorption is a physicochemical process that uses nonliving biomass to remove metal ions. The process is promising and innovative biotechnology, which has excellent economic feasibility for metal removal and/or recovery due to its efficiency, simplicity, cost-effectiveness, and abundance of biomass when compared to industrial synthetic adsorbents. Practically, most biomaterials have an affinity for metal species. By using a low-value biosorbents such as a waste stream, two sustainable goals can be achieved in a single process: reducing the waste stream and removing/recovering metals from industrial effluents. In literature, several studies have investigated the efficiency of various biosorbents to remove/recover metals from solution. This chapter critically reviews different aspects of biosorption research such as the characteristics of an ideal biosorbents, the key main parameters affecting the metal uptake performance, different metal uptake mechanisms, the process advantages and drawbacks, and potential of biosorption as a possible industrial process. It also summarizes key recent developments in these areas and the significance of biosorption in wastewater treatment processes and in the environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Metals tend to maintain indefinitely, circulate, and finally accumulate throughout the food chain, which increases the serious health hazard threat involved and eventually jeopardizes human life. In many cases, the technologically and economically important heavy metals are the same as those considered a real threat to the environment because of their accumulation in the environment caused by man’s activities (Al-Saydeh et al. 2017). High levels of contaminated heavy metal wastewater are produced by metal plating operations, mining and metallurgy industries, metal processing, battery or galvanizing and accumulator manufacturing operations, thermal power generation (especially coal-fired plants), as well as nuclear power generation (Abbas et al. 2014). The effluents from these industries and mine tailings might be the major discharge source of various combinations of toxic heavy metals. Therefore, it would be ideal to have an on-site treatment facility for the elimination of heavy metals from wastewater. It is practical from a policy standpoint that those who are responsible for metal pollution should be in charge of its remediation. Such a treatment facility that can reduce the heavy metals waste streams would be more efficient than treating large volumes of a mixture of heavy metals in a general sewage plant.
The impact of heavy metals on the environment is well known, where the big three metals, mercury, lead, and cadmium, are in the limelight (Sulaymon et al. 2010). The significant use of mercury and lead in various industries has been significantly reduced. In contrast to mercury and lead, cadmium is toxic and abundant in its distribution throughout the world. The use of cadmium in different industries has been steadily increasing in the past few years. It is widely used in many industrial processes such as metallurgical alloying, mining, smelter operations, metal plating, electroplating, paints, pigments, electronics, and nickel-cadmium batteries. The annual production of cadmium was 24,900 mt for the whole world in 2015 (Brown et al. 2017).
Recently, cadmium has attracted much attention because of its extremely serious health hazard. Its toxic effects are particularly prominent in higher trophic level animals, mainly humans. It tends to accumulate in the human body where it has a half-life of 10–30 years. The toxicity of cadmium is responsible for kidney damage, bone disease, as well as cancer. Cadmium tends to accumulate in the human body at an average of 30 mg for an American male. It is mainly deposited in the kidneys, 33%, and in the liver, 14% (Naja and Volesky 2009). Therefore, the study of cadmium uptake and recovery from metal-bearing effluent by an innovative metal uptake method is of high potential interest. Since metals are recycling in the environment, the only effective way of preventing heavy metals poisoning is metal removal and/or recovery. Conventional treatment methods are expensive and ineffective in certain cases. An alternative efficient and environment-friendly method in detoxification of waste effluent should be studied extensively due to the demand of metal species. An effective and economical process to remove/recover toxic heavy metals from industrial wastewater is in extreme demand, which can substantially decrease the overall process cost. Ideally, metal recovery should be investigated in parallel with the removal process.
The use of a biosorption method, using microorganisms or their products to remove toxic heavy metals, represents a promising technology. It can be a strong and inexpensive candidate for remediation of heavy metal-contaminated wastewater. The biosorption key process parameters should be studied extensively to improve the performance of the process.
11.2 Metal Uptake Processes
11.2.1 Conventional Metal Uptake Methods
Physicochemical processes are used for heavy metal uptake from metal-contaminated wastewater effluent. Conventional technologies, such as neutralization, precipitation, chemical oxidation or reduction, ion exchange, activated carbon adsorption, filtration, electrochemical treatment, solvent extraction, and evaporation recovery, and membrane technologies, such as electrodialysis and reverse osmosis, have been used to treat metal-bearing solutions disposed from contaminated effluent. However, these technologies have significant disadvantages including low efficiency, high energy requirements, high cost, and generation of other toxic waste products (Al-Saydeh et al. 2017; Abdi and Kazemi 2015). These treatment processes may become extremely expensive or inefficient especially in dilute metal solution, i.e., less than 100 mg/L (Abbas et al. 2014). Major disadvantages of most common processes can be summarized as follows (Gadd 2009).
-
(a)
Evaporation method can be economically feasible with a highly concentrated metal solution. Evaporation lagoons are an inexpensive and an easy way to treat wastewater effluent, where the main cost of lagoons is the capital cost for suitable land. The operational cost of this technique is high, because the process is ineffective and time consuming. Also, metals cannot be selectively separated from the effluent, where a sludge of a mixture of waste metals is obtained. Consequently, another downstream sequestering method such as ion exchange or biosorption needs to be applied to recover certain metal species.
-
(b)
Membrane technology is usually applied in desalting seawater and specific food processes. The concentration of metal ion in the feed should be considerably low for a successful membrane operation. Increasing the metal concentration may lead to not only a decrease in the separation efficiency but also to membrane scaling. The higher the metal concentration, the higher the required energy for separation, and the lower the observed efficiency. The membranes are expensive materials that have a short operation time and cannot operate with various types of chemicals and solution pHs. Also, they lose their productivity with time and are subjected to decomposition in the presence of microorganisms, compaction, and scaling. All these major cost drawbacks make the membrane technology less favored for the use in wastewater treatment.
-
(c)
Precipitation and clarification technologies require a large volume of chemicals to treat wastewater effluent, which make it an expensive technology. The metal concentration after the precipitation process is often higher than the acceptable discharging limits. Moreover, metal cyanide complexes cannot be treated by this method, thus they must be separated by other methods prior to the precipitation process. In this process, one type of wastewater is converted to another, i.e., sludge with metal hydroxides. Although the waste volume is reduced, the impurities concentration can still be unacceptably high. Other drawback of the process is the poor settling and filtration properties of the resulting sludge.
-
(d)
Activated carbon is the most widely used commercial adsorbent, since it has a good adsorptive capacity for organic compounds. However, the activation and regeneration process of the used carbon are a costly step of the carbon adsorption process. The activation process must be carried out after each regeneration process of the saturated carbon. The uptake capacity decreases after each activation/regeneration cycle.
-
(e)
Ion-exchange resins have been used extensively in wastewater treatment; however, few factors still limit their application. They are often cannot tolerate thermal and chemical changes such as the effects of oxidation, where organic materials can cause fouling of the resins. Chemical decomposition can take place when metallic species in the solution break the polymer chains or modify the functional groups. The metal uptake capacity of resins is affected by the presence of calcium or magnesium ions in the effluent stream. Moreover, ion-exchange resins are usually more expensive than biosorbent materials. It is well known that the most expensive biosorbent, e.g., biosorbent derived from freshwater alga, Chlorella vulgaris, is still less expensive than the cheapest ion-exchange resins. A quantitative comparison between metal uptake capacity by biosorbent materials and ion-exchange resins shows that biosorbents can reach similar or even higher uptake capacities than synthetic ion-exchange resins (Wang and Chen 2009).
Water quality standards in many countries clearly emphasize on controlling or reducing the level of heavy metals in discharge effluent to a certain limit. Although industrial processes have certain standard limits, drinking water quality has a much lower limit that may significantly increase the cost of treatment. The acceptable safety limits for cadmium intake have little general agreement among different health organizations. In the United States, the water standard level of cadmium in drinking and waste discharge has been set at 5 and 100 μg/L, respectively (Zhao et al. 2002; Bazrafshan et al. 2015).
11.2.2 Biological Treatment Method
The main concerns of early studies of metal uptake have been solely on nutritional and toxicological aspects accompanied by the presence of metal. Alternative metal uptake and/or recovery methods have been investigated, which are based on metal sequestering properties of natural biomaterials. The potential use of certain types of microorganism in contaminated wastewater treatment has being studied intensively (Al-Saydeh et al. 2017; Abbas et al. 2014; Abdel-Ghani and El-Chaghaby 2014; Mehta and Gaur 2005; Fiset et al. 2008; Abdi and Kazemi 2015; Ayangbenro and Babalola 2017; De Gisi et al. 2016). The use of various microorganisms or their products to remove toxic heavy metals has given promising results as an inexpensive remediation method for heavy metal-contaminated wastewater. In literature, biosorbent originating from fungal biomass or yeast has been shown to be effective in heavy metal uptake. A variety of yeast species show the ability to remove cadmium from solution. Preliminary screening of different biomass types for their affinity of cadmium removal showed few dissimilarities and an interesting potential regarding the biomass of a common yeast Saccharomyces cerevisiae.
11.2.2.1 Biosorption and Bioaccumulation
It is important to define an appropriate terminology related to the metal uptake by various types of microorganisms. The metal accumulation by active cells is usually referred to as bioaccumulation. This process depends on the metabolic activity of the cell, which in sequence can be significantly affected by the presence of metallic ions. Many studies on metal uptake by biomass have been conducted with living organisms for environmental, toxicological, and pharmaceutical purposes rather than in terms of industrial application. Bioaccumulation, which has been mainly studied by microbiologists for its toxicological importance, is less desirable for metal removal and/or recovery design process (Abbas et al. 2014). This can be due to the requirement of the cells to be active which eventually means a special culture environment.
The metal transfer from the surrounding environment into the cell may be achieved by active transport accompanied with metabolic activities. However, it is possible that a passive metal uptake by the cell can be a part of the process of metal transportation throughout the cell wall, membrane, and into the cell interior, where it becomes practically a part of the cell structure or a metabolic product (Dhankhar and Hooda 2011). Recently, attention has turned to the usage of inactive, dead microorganisms as potential industrial tools for metal uptake and/or recovery. Biosorption is a property of certain types of inactive, nonliving, microorganism to uptake heavy metal ions from contaminated solutions even at low concentrations. Biosorbent, derived from biomass, can show chemical substance properties, i.e., an ion exchange of biological origin.
The term biosorption is generally used for the metabolically independent passive metal ion uptake by dead biomass. It should be distinguished from bioaccumulation, which is mainly an active metabolically dependent process that takes place in living microorganisms. Metabolically active cells are used in bioaccumulation, which is opposite to biosorption and requires a different approach for investigation (Abdi and Kazemi 2015). Frequently, biosorption and bioaccumulation terms are used as an exchangeable term for the metal uptake process since the main mechanism is not known. The use of inactivated microorganisms is not subjected to physiological constraints. It eliminates some undesirable issues related to the maintenance of an active microbial culture such as nutrient supply, toxicity thresholds, and thermal inactivation of the microorganism and cross contamination by other microorganisms. Living cells cannot resist the toxic effect of a heavy metal effluent, which may result in cell death. Therefore, passive biosorption may be ideal for such a case. Moreover, inactive brewer’s yeast cells of S. cerevisiae were significantly more efficient than those of active cells in metal uptake capacity of copper, zinc, and nickel for 1.3, 14, and 16 times, respectively (Machado et al. 2009). Also, cadmium uptake by inactive biomass of Bacillus cereus was found to be more than those of living yeast cells (Ganguly et al. 2011).
The major challenges in the biosorption field are to select the potential types of biomass from a very large pool of available and inexpensive biomaterials and to define the predominant mechanism that controls the biosorption operation. Therefore, biosorption studies have been scattered in several different research contributions. Few extensive reviews are available in the literature dealing with large number of biomaterials that have been tested for their metal uptake capacity under various conditions.
11.2.2.2 Biosorption Advantages
The biosorption process has many advantages including low amount of biological sludge for disposal, high efficiency in the treatment of low concentration effluent, and no nutrients required as for the case of bioaccumulation. The use of dead biomass in wastewater applications shows some advantages since the biosorption application and the production of the microbial cells can be a separate process, which can be optimized to increase the metal uptake. Furthermore, the biomass is an active chemical formulation, which is the main raw source to produce efficient biosorbent material. These biosorbents can be greatly efficient, selective, and inexpensive. These properties give biosorbents the advantages over commercial ion-exchange resins and activated carbons. Several potential properties of biosorbents make metal uptake by biosorption an economical and feasible treatment process. The major advantages of this innovative treatment technology are (Abbas et al. 2014):
-
Dilute metal solution can be selectively treated.
-
Biosorbent has very low affinity for calcium and magnesium ion species and other light metals in general.
-
Effluent discharge concentrations meet the environmental limits.
-
Biosorption systems present low capital cost and very low operational costs.
-
Biosorption systems can operate over a wide range of pH values (pH 3–9).
-
Biosorption systems are effective over a temperature range of 4–90 °C.
-
The cost of toxic sludge disposal is reduced substantially by converting pollutant metals to a metal product solution.
Conversely, the main disadvantages of biosorption process can be its ineffectiveness at highly concentrated metal-bearing solutions as well as being undefined technology when compared with well-established technologies, e.g., ion exchange.
11.2.2.3 Comparison Between Biosorption and Conventional Methods
The commercial feasibility of biosorption may be improved by a combination of different approaches including the use of waste industrial biomass, regeneration of the biomass after each biosorption cycle, and recycle and sale of the eluted metal species. Biosorption is eventually suitable as a polishing process in wastewater treatment. By applying biosorption, it is possible to obtain high standard drinking water quality, e.g., initial metal concentrations of 1–100 mg/L can be reduced to final metal concentrations of <0.01–0.1 mg/L (Abbas et al. 2014). A higher uptake affinity for the metal at low residual concentration is obtained in the biosorption process. It is recommended to use another pretreatment technique such as precipitation, for concentrated metal solutions, when the metal concentration is higher than 100 mg/L. This is to avoid unreasonably fast exhaustion of the sorption capacity. However, this approach has the main drawback that is represented by generation of toxic hazard sludges from which it may not be feasible to recover the metals.
11.2.2.4 Economic Feasibility of Biosorption
The main advantages of biosorption when compared to the conventional methods are wide range of environmental operating conditions and strong economical alternative due to the inexpensive abundant raw biosorbent materials. These materials include waste products from other industries, e.g., fermentation by-product, or naturally abundant biomass such as marine algae. The cost of the biosorption process strongly depends on the cost of the biosorbent material itself that can remove metal ions. The capacity of the biosorbent to remove metals plays an important role in efficiency and the feasibility of the process. Also, selectivity of the process for a certain metal species is an important issue for selecting the most appropriate technique. The cost of raw biomass varies from no cost, i.e., just the collecting cost, to USD $16/kg (Ngo et al. 2015). Eventually, harvesting and drying practices are the major costs of marine algal biomass types. The cost is much lower than that of commercial activated carbon of USD $20/kg (Rafatullah et al. 2010).
The overall feasibility of the biosorption process is determined by several factors such as biosorbent uptake capacity, process kinetics, the effect of other metal ion presence, the performance of the biosorbent, regeneration ability, and the possibility of metal recovery. The biosorption process is an economically feasible and technically effective technology for metal uptake and recovery. The capital and operational cost of the biosorption process is very competitive with existing treatment technologies.
Recovery of metals from solution may be beneficial since the metal may be subsequently desorbed from the biomass and recovered for reuse. The biosorbent can be regenerated, incinerated, or stored in landfills. The reuse of the biosorbent can significantly increase the economic advantages of the process. The regeneration of biosorbent after each metal sequestering cycle is as important as the metal uptake capacity. Investigations on metal elution indicate that it is possible to obtain a significant amount of the sorbed metal species when it is released from the biosorbent material in a much smaller volume of eluting solution.
Some types of biosorbent can be reused efficiently for many cycles. This simply can be achieved by metal desorption by washing the biomass with an appropriate acid, base, or salt solution. The resulting highly concentrated metal solution can be recovered, recycled, and sold. The overall goal of the biosorption process is to concentrate the metal solution by a factor of 100 or more (Mishra 2014). Consequently, the process cost can be reduced significantly, which increases the economic advantage of the biosorption process.
11.2.2.5 Biosorption Contacting Systems
The lack of detailed design information for biosorption can be because the metal sequestering process by various biomaterials is still not fully understood. The biosorbent particles should be immobilized and processed prior to their use in the operation to provide good flow conditions and suitable pressure drop in column operation. These conditions must be optimized to maximize sorption performance (Michalak et al. 2013; Vijayaraghavan et al. 2005). Due to the fast kinetics of the sorption processes, mass transfer of the sorbed metal to the available active site can control the overall sorption rate.
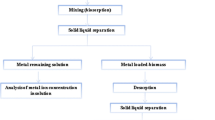
The biosorption column can be operated on two main parallel cycles consisting of loading and regeneration. The column operation can be illustrated as loading the biomass, passing the metal-containing effluent through the biosorbent where the removal of the heavy metals occurs. The biosorbent should be regenerated once it reaches the breakthrough point, i.e., when it is saturated with heavy metals. The regenerations process produces small volumes of concentrated solution of heavy metals that can be easily recovered by conventional methods. The cycle ends by backwashing of the bed with water to remove any suspended solids or trace of regeneration agents (Mishra 2014). A pair of biosorption columns as shown in Fig. 11.1 should be used in parallel to maintain a continuous biosorption process; therefore, while the biosorbent is being regenerated, the other column is undergoing the metal biosorption process. Generally, application tools for biosorption techniques are like those of ion exchange and activated carbon adsorption (Sulaymon et al. 2013a).
A schematic diagram of a biosorption process (Volesky 1990a)
The most widely used contacting configuration for sorption processes is the fixed bed reactor system and its modifications, which makes the most effective use of reactor volume. The fundamentals of deriving and evaluating the key fixed bed sorption process parameters have been studied extensively in the chemical engineering literature. The operational saturation metal uptake, the minimum theoretical bed depth, and the bed service time represent the key parameters characterizing biosorbent performance in a fixed bed column (Muhamad et al. 2010). The concentration difference, known to be the driving force in the sorption process, can be optimized. In general, the metal uptake process in a fixed bed system is substantially affected by three main regimes: the sorption equilibrium, the sorption particle mass transfer, and the flow pattern through the packed bed. The overall sorption performance of the column is determined by all these three factors, which are in turn controlled by the service time of the bed (Sulaymon et al. 2013a; Muhamad et al. 2010; Naja and Volesky 2006). At the breakthrough point, a regeneration step is required for biosorbent materials.
11.3 Selection of Biosorbent Material
Many biological materials and metabolically inactive cells, such as bacteria, fungi, and algae, have shown potential for heavy metal uptake. They can serve as the basis for producing innovative and potent biosorbent materials. Several studies have shown that different types of waste biomass have been found to be good biosorbents with a high metal uptake capacity. However, only those that have a low cost and good efficiency and selectivity for heavy metals are suitable for use in a full-scale biosorption process. An extensive search for new promising types of biomass is still proceeding in both the academic and industrial research fields.
Despite the search for the innovative technology of biosorption is mainly concerned with metal uptake capacity, the interaction between the cells and the heavy metal species, the kinetics of the metal removal process, and other essential properties of the biosorption process are still not fully understood (Abbas et al. 2014). This may serve as a guide in the search for an effective biosorbent. In the current state of the art, tedious experimental assessment of selected available types of biomass is still the main base for discovering potential biosorbents. However, it has been roughly concluded throughout the literature that certain types of biomass are more appropriate in biosorption processes than others.
Ideally, either an abundant natural material or a waste biomass is the source of raw biosorbent material where potential biosorbent can be renewable and economically feasible. Natural marine algae present enormous amounts of inexpensive biomass capable of being the raw material for providing biosorbents. Another continuous source of biosorbent is specifically cultured biomass produced by simple fermentation. Although the cost of this type of biomass may be high compared to the natural one, the cost is still considered to be competitive with those of conventional technologies such as ion-exchange resins. Alternatively, waste biomass that is generated in large quantities as waste from large-scale industrial fermentation processes, e.g., pharmaceutical, biochemical, and enzyme production, can be used as biosorbent. This biomass can create a possible disposal problem. For example, large quantities of microbial biomass have been periodically disposed into the oceans after being a by-product from enzyme production in Japan. The use of waste biomass as a source of biosorbent material can strongly increase the economic competitiveness of the biosorption technology since the biomass is inexpensive, recoverable at the end of fermentation, and produced in large amounts.
An ideal biosorbent material, which is used in the removal and/or recovery of metals from contaminated solution, can be characterized as follows (Gadd 2009):
-
1.
Cost-effective, inexpensive waste or abundant raw biomass material, serving as the source for a new category of biosorbent.
-
2.
High metal uptake and rapid kinetics of the biosorption process.
-
3.
Ability to be regenerated: usually it is achieved in a two-step process consisting of a metal elution from the biosorbent stage, which gives a high concentrated metal solution, and a simple washing stage to eliminate any trace of eluting reagent and complete the regeneration stage of biosorbent to be used in another cycle with minimal loss of the biosorbent.
-
4.
Metal selectivity: if desirable, a wide range of heavy metals can be removed without interference from common light metals. Some types of biosorbents can remove most of heavy metals without specific priority, while others can be specific for certain types of metals only.
-
5.
High adaptability, operated under a wide range of pH values, temperatures, and other process conditions and arrangements.
-
6.
Concentration independence, good heavy metal uptakes for low concentrations, less than 10 mg/L, as well as for high concentrations, higher than 100 mg/L.
-
7.
Suitable mechanical properties, particle size, hardness, density, and shape that can be used in different continuous flow configurations.
-
8.
High resistance for organics: low levels (less than 5000 mg/L) do not affect metal uptake.
11.4 Biomass Cell Wall
The cell wall is the external cellular skeleton of the microorganism cell, which is in a direct contact with the external environment especially with soluble liquid medium materials. Because of the negatively charged cell walls, this interaction is very important in the case of metallic species. The cell wall surface consists of a mixture of monovalent and divalent groups that can exchange a cation by another, e.g., H+, which in turn depends on the strength of the individual ligand complex.
11.4.1 Cell Wall Composition
Little progress has been done in identifying the exact role of several cell wall components in metal binding. Understanding of the chemistry of metal-ligand interactions could clarify the preferential impact of various ligands on metal species. Park et al. (2010) related the nature of binding and strength of interaction of individual metal ion species to the different types of functional groups located at the cell wall surface. The behavior of metal biosorption by different types of microorganisms depends on the chemical composition of the cell. The cell walls can be considered as a complex ion-exchange material like a commercial resin. However, the ion binding capacity totally depends on the presence of active functional groups and the structure of the cell wall. The number and type of binding sites depends on the chemical composition of the cell wall membrane. Different microorganisms present great varieties of cell wall types that may have different metal uptake affinities (Sulaymon et al. 2013b). Eventually, most of the sequestered metals are found on the cell walls.
The structure of algal cell wall is complex since more than ten layers can be found in some algal cells. Most of the algal cells are covered by mucilaginous layer that has a significant metal sorption capacity because it contains uronic acid. Alginate is a common term for a family of linear polysaccharides consisting of 1,4-linked β-d-mannuronic and α-glucuronic acid component randomly configured in a block-wise way along the chain (Wang et al. 2014; Davis et al. 2003). Using formic acid, ethanol, and triethylphosphite to modify amino, carboxyl, phosphate groups, Velkova et al. (2015) studied the binding of zinc by S. cerevisiae brewer’s yeast cells. They found the biosorption process depends on the presence of carboxyl, phosphate, amino, sulfhydryl, and hydroxyl groups, where carboxylic groups are the most important followed by amino and phosphate groups. The green freshwater alga Chlorella was found to contain uronic acids that may be the reason behind its chelating ability of polysaccharides. In addition, the brown alga Sargassum fluitans contains carboxyl groups of alginates and the sulfate groups of fucoidan. It plays a critical role in the complexation of heavy metals (Mehta and Gaur 2005; Davis et al. 2003).
The structure of the cell wall in most fungal cells has two main phases: (a) an outer layer containing glucans, mannans, or galactans and (b) an inner microfibrillar layer that shows crystalline characteristics, which are presented by the parallel configuration of chitin chains, cellulose chains, or in some yeast of non-cellulosic glucan. A continuous transition between both layers exists (Gow et al. 2017). The microfibrils of phycomycetes consist of cellulose, which also contains uronic acids, while in the higher fungi, they are made up of chitin. Chitin consists of a polymer of N-acetyl-d-glucosamine, which has lost a minority of the acetyl groups. An extensive review of chitin chemistry can be found in the literature (Zargar et al. 2015). These chains are connected by proteins, lipids, and polysaccharides that can act as active sites in binding metal ions (Vianna et al. 2000; Pagnanelli et al. 2000). In addition, the fungal cell walls contain pigments, polyphosphates, and inorganic ions. The formation of a complex between the metal species and the chitin has been proposed.
Chitin and chitosan have been suggested as the main components in the sequestering of metals since they are found in different quantities in the fungal cell wall. Generally, they are natural polysaccharides that are widely distributed in fungal biomass (Gow et al. 2017). The active part of the cell wall in binding metal species consists of polysaccharide. Therefore, the capability of the biomass in sequestering metal ion species depends on the polysaccharide content in the biomass (Davis et al. 2003). Even in the absence of biomass, polysaccharides have been shown to have the ability of sequestering metals (Gupta and Diwan 2017). Stereochemical variation in the polysaccharide structures can make significant difference in the affinity for the metallic species. Consequently, it substantially affects the biosorption performance by biosorbent materials. The chemical composition of three different common fungal cells is shown in Table 11.1.
The polyphosphate and carboxyl groups on the cell wall surface of S. cerevisiae are active in the metal sequestering. This view explains the improved metal uptake by microorganisms grown in synthetic medium since they have higher phosphate content in the cell wall. The phosphoryl groups form stable complexes, while carboxyl groups are involved in metal binding only after phosphoryl groups are saturated. A strong relationship was found between the decrease in metal uptake by fungal and algal biomass and the degree of blocking of carboxyl groups by esterification in the pretreated biomass (Velkova et al. 2015). Similarly, the heavy metal uptake capacities by seaweed algae were found to be proportional to the carboxyl group’s content and related to the electronegativity of studied metal species (Sulaymon et al. 2013b; Davis et al. 2003). In addition, the selectivity of ion-exchange resins such can be due to the presence of carboxyl groups in their synthetic matrix as it has been attributed by their manufacturer.
Several active negatively charged chemical groups can attract the metal species and sequester them such as acetamido groups of chitins; structural polysaccharides of fungi; amino and phosphate groups in nucleic acids; amino, amido, sulfhydryl, and carboxyl groups in proteins; hydroxyls in polysaccharides; and carboxyls and sulfates in the polysaccharides of marine algae. Nevertheless, research concerning using yeast as biosorbent of heavy metals indicated the importance of cell wall protein in binding cobalt, copper, and cadmium. Therefore, the more protein and/or sulfhydryl group content in the yeast, the more metal binding capacity may be obtained in the biosorption process (Michalak et al. 2013; Davis et al. 2003).
11.4.2 Location of Metal Bound
The place where metal uptake is occurring is not completely defined. The metal sequestering may take place on sites on the cell lipopolysaccharides or by certain chelates, siderophores, in the cell walls. Another proposal is that the uptake process strongly depends on the protein existence in the cell wall. Other researchers refer it to the presence of polysaccharides. Knowledge of metal uptake location in the cell can provide efficient evident direction about the significance of binding groups if it is related to data about which biomolecules build up the different parts of the cell. Electro-micrographs of bacterial, fungal, and algal cells in the literature indicate that the cell wall plays a critical role in metal ion (Dhankhar and Hooda 2011). Biomass residue resulting from the cell breaking may still be of interest in the biosorption process since the active binding sites are presented at cell walls (Volesky 1990a).
Furthermore, metal species are found to deposit all around the budding cells and inside the cell itself. The slow penetration by small ionic species throughout very porous membrane into the cell interior has been observed (Davis et al. 2000). This indicates that some intracellular components participate in the biosorption process. Biochemical or other physicochemical mechanisms may be involved in metal binding on or in the cell walls.
11.5 Metal Uptake Mechanism
Despite the extensive literature available on metal-biomass interaction, few studies have been conducted on examination of well-defined metal sequestering by a specific mechanism as opposed to the overall uptake where several types of removal mechanisms may occur simultaneously. For a better understanding of the dominant mechanisms in metal sequestering by biomass, more detailed and accurate knowledge about biomolecule structure and binding sites represented by different types of cell is needed. By revealing the different mechanisms behind biosorption phenomena by various biosorbent materials, the effect of different influencing parameters can be better estimated. Furthermore, determining the controlling mechanisms in metal biosorption allows one to scale-up the process that can be used in application fields (Abbas et al. 2014).
11.5.1 Classification of Binding Mechanisms
Biosorption may involve many different physicochemical mechanisms that are affected by the surrounding environmental conditions, the type of a metal, its ionic form, and the active sites responsible for metal binding. Several possible physicochemical mechanisms including adsorption, complexation, coordination, chelation, microprecipitation, ion exchange, and ion entrapment, because of the concentration gradient and diffusion through cell walls and membranes, may take place as shown in Fig. 11.2. A single combination of different mechanisms can contribute to the overall metal uptake process by biosorption.
Metal biosorption mechanisms: bold line, mechanism probably significant in biosorption by Sargassum biomass; dashed line, secondary importance binding relation (Volesky and Schiewer 1999)
An understanding of the metal-ligands interaction mechanism may substantially clarify the current expansive definition of biosorption mechanism in the literature. The proposal of ion exchange as the main mechanism in the metal uptake for a certain process may not eventually contradict the claim that chelation is the relevant mechanism. In addition, the overall mechanism of ion exchange can be related to the sub-mechanisms of complexation or adsorption and vice versa (Limousin et al. 2007).
Complexation plays an important role in metal-ligand and sorbate-sorbent interactions. A complex is a polyatomic molecule that consists of one or more central atoms surrounded by ligands, which are attached to it. Complexes can be neutral, positively charged, or negatively charged. Microprecipitation is the deposition of electrically neutral material at the surface of the biomass. It does not essentially involve a type of bond between the biomass surface and the deposited material. However, it may be assisted by the binding of metal species to different active binding sites that may play a role as nucleation sites for further precipitation. Microprecipitation depends on interactions between the solute and the solvent and occurs when the local solubility is exceeded. In general, metal ions precipitate as salts in the form of phosphates, sulfides, carbonates, oxides, etc. This can play a vital role in the metal uptake process that should be given a special concern (Davis et al. 2003).
11.5.2 Ion Exchange
Although different uptake mechanisms can be involved in the biosorption process, substantial evidence suggests ion exchange is the main mechanism of metal biosorption. Recent studies revealed that uptakes of divalent metals were accompanied by the release of ionic species from the biosorbents (Michalak et al. 2013).
Freshwater alga biomass pretreated with a solution of Ca2+ and Na+ has been found to release cations of these light metals into the solution along with the ability to remove the heavy metal cations. The relative quantities of heavy metals adsorbed by the biomass are approximately equal to the amount of light metals released into the solution (Sulaymon et al. 2013b). Similar results are obtained with fungal biomass (Dhankhar and Hooda 2011). Many studies clearly show that the ion-exchange mechanism is strongly involved in the binding of metal species on the surface of cell walls that contains alginate. Chemical analysis such as electron microscopy, infrared spectroscopy, X-ray dispersion, and diffraction analysis can be used to show that ion exchange is the main mechanism in the biosorption phenomenon. The IR spectra of original and used biomass samples indicate no shifts in the major bands. However, the change in intensities may be explained as changes in concentrations rather than structural changes. These results can be another evidence of the ion-exchange phenomenon in the biosorption process (Michalak et al. 2013).
Sulaymon et al. (2013b) found that the amount of released light ions (K, Na, Ca, Mg) from the native alga was higher in cadmium and copper metal solutions than in lead and arsenic metal solutions. Insignificant change in potassium release was found in the four investigated metals. It was concluded that ion exchange is responsible for the sorption process. In addition, Singleton and Simmons (1996) found that the binding of Ag by S. cerevisiae causes ions to be released into the solution with a stoichiometry of 1.5:1 silver adsorbed to ion released. This stoichiometry does not change strongly with biomass concentration. The main ions released are hydrogen followed by magnesium and calcium.
Ion exchange, rather than sorption to free sites, is the main relevant predominant mechanism in biosorption (Davis et al. 2003). Since the overall charge of the biomass material must be neutral, any binding of one cation must be accompanied by either a stoichiometric release of other cations or by the binding of anions. Therefore, it is unlikely that free anions will be bound to the biomass since the biomass itself is a negatively charged surface. The natural selectivity of biosorbents, which has been observed but never clarified, can now be better understood. The well-established and developed principle of ion exchange can be applied now in biosorption. This can be used as a good tool by researchers in this field.
11.5.3 Binding Forces
The forces between atoms or molecules can be categorized into two main classifications: chemical and physical. Chemical forces occur over short distances (0.1–0.2 nm). Chemical bonds are strong with energy values reported in literature ranges between 40 and 400 kJ/mol. Covalent bonds are formed by the sharing of electron clouds between nonionic molecules. Physical forces can be classified into two main types: electrostatic and London-van der Waals forces. The energy of physical sorption is reported as 2–40 kJ/mol in the literature. In such a bond, the electrons remain in their original situations. Electrostatic forces extend over a long range and are the strongest among various physical bonds. The force magnitude is proportional to each ion charge and inversely proportional to the square of the distance between the ions. The mechanisms and forces that are probably the most important in the case of metal binding are already illustrated in Fig. 11.2. The driving force of this bonding is the affinity of the sorbate species to the sorbent, supported by physical electrostatic attraction between metal ions and biomass ligands (Volesky and Schiewer 1999).
The amount of released protons increased with an increase in the binding strength of the metal ion species. However, proton release did not indicate covalent binding since part of the protons may have also been bound by electrostatic attraction forces and thus may be easily replaceable by electrostatically bound ions (Abbas et al. 2014). It can be concluded that in cases where no protons were involved, e.g., most alkaline and alkaline earth metals, electrostatic attraction was the dominant binding mechanism, while covalent binding took place accompanied by proton release (Naja and Volesky 2011). They showed that the ionic and covalent metal binding can be characterized by cation displacement experiments. In addition, covalent bonding was observed at low concentrations, whereas electrostatic interactions become significantly important at higher concentrations. The researchers concluded that the ability of the cell to form covalent bonds was probably the main limiting factor of metal uptake. Despite electrostatic interaction of metal species and wall ligands of S. cerevisiae, these bonds show an increased covalent character.
The electrostatic force binding alone is weaker than the binding force involving covalent bonds, where it occurs between positively charged ions and negatively charged surface sites. Sulfate groups can assist in forming covalent bonds between adjacent chains of fucoidans and other sulfated polysaccharides. Copper was adsorbed not only by ion exchange but also by additional covalent bonding with the carboxyl groups of Vaucheria pectins (Naja and Volesky 2011).
Furthermore, the metal sequestering mechanism by algae varies with different metals from relatively weak electrostatic bonding observed in alkali and alkaline earth (Ca, Na) to stronger bonding for certain metals with oxygen, nitrogen, and sulfur containing ligands forming a covalent bond (Cu). A complex redox reaction may take place with some noble metals (Davis et al. 2003; Naja and Volesky 2011). To better understand the reasons why some ions bind stronger than others, it is essential to consider the properties of different metals. Such knowledge can be used as the basis to predict how strongly an ion may bind to a different biomass. Also, it can be used to have a better understanding of the metal sequestering mechanism.
11.6 Pretreatment, Recovery, and Regeneration
The affinity of different sorbate species for the sorbent is a very critical principle in the biosorption process. For the case of a sorbate “A” being sorbed by a “B”-saturated biosorbent, the theory of ion exchange indicates that there are two possibilities depending on the respective affinities of A and B for the biosorbent. These two possibilities can reflect the performance of the biosorbent. In favorable operation, when species A is more strongly bound to the surface than B, i.e., affinity of A > affinity of B, then the required contact period is small. However, if the affinity of B is greater than that of A, this period can be extended further, and it is obviously an unfavorable operation. Biosorption can be defined in the light of this effect as a process where toxic heavy metals may be exchanged for nontoxic species. The positions of protons, heavy metals, and light metals in the affinity series are the main guide to determine whether the ionic forms and regenerates are suitable or not.
The biomass can be regenerated and reused in several sorption-desorption cycles in the biosorption process. The number of these cycles strongly related to the physical properties of the biosorbent, where biomass immobilization may improve its properties such as particle size, particle strength, and chemicals resistance. Well-prepared biosorbents are like ion-exchange resins in many features, such as their convenient application in metal removal/recovery processes (Park et al. 2010). Thus, using the raw biomass as biosorbent may not be practical in biosorption processes since it may not have the required characteristics for the process.
A good eluting agent must be effective, inexpensive, non-damaging to the biosorbent, and environmentally friendly. For a biosorbent, the smallest possible elutant (liquid) volume is desired. The elution efficiency depends on the binding strength of the desorbed ion to the biosorbent surface and is determined by the ratio of the metal desorbed into the solution after the elution process to the metal initially bound to the biomass. The elution efficiency decreases with an increase in pH. Not all sites created by desorbing protons are effective for metal uptake. This can be because the binding of metal ions can significantly reduce the surface charge density, hence reducing the effectiveness of the residual sites.
The biosorbent can be prepared in different ionic forms such as protonated (H-form) or saturated with Ca2+, Mg2+, Na+, etc. by washing it with mineral acids, salts, and/or bases. The capacity of the biosorbent was found to increase more than three times by converting the biomass Rhizopus arrhizus from H- to Na- or Ca/Mg forms by washing it with a solution of NaHCO3 and hard water, respectively (Michalak et al. 2013). Different mineral acids and few salt solutions, e.g., CaCl2, showed a metal elution, or desorption, efficiency close to 100%. An insignificant difference between the elution efficiency of CaCl2 and that of Ca(NO3)2 was found. The cost of CaCl2 is almost half of Ca(NO3)2. In addition, the nitrate residuals in the environment are considered a potential risk, while this is not the case for chlorides since a higher level of chlorides can be tolerated (Davis et al. 2000).
Desorption can be also achieved by acid wash, e.g., HCl or H2SO4. The use of acid-washed biosorbent may have a negative effect on metals with low affinities for the carboxyl groups, which cannot replace the strongly bound proton from the H-saturated biosorbent. Neutralization of these groups may be required before the loading stage to obtain an effective sorption of heavy metals (Park et al. 2010). Concentrated acids may have a pronounced negative effect on the structure of alginate chains and the hydrogen bonding capacity of the biomass by destroying them, as well as by simultaneous hydrolysis of polysaccharides. This may explain the lowered metal uptake capacity in certain processes (Horsfall et al. 2006). However, relatively diluted acid did not cause any significant damage to the cross-linked biomass in two operating cycles. The HCl, 0.1 and 1.0 N, wash of the biomass can release the entire bound metal (Park et al. 2010). Hydrochloric acid, 0.1 N HCl, can effectively remove cadmium, copper, and zinc from the biomass. This desorption process was completed without damaging the structure of the biomass. Various studies show that acid wash pretreatment improves metal uptake capacity of various biomasses. Protonation process ensures homogeneity of biomass surface, which leads to uniform surface behavior. Protonation increases the metal uptake of copper and cadmium using B. lentus (about 30 mg/g), while it has insignificant effect on S. cerevisiae (<5 mg/g). This can be due to the higher lipid content in B. lentus, which may be involved in metal binding (Vianna et al. 2000). In addition, the acid pretreatment can lead to a release of large amounts of intracellular components, which can bind metal species due to the presence of different functional groups and may reduce the amount of metal bound by the biomass. Non-protonated biomass of B. lentus and A. oryzae shows a higher metal uptake capacity than those of treated biomass at a pH value of 4.5 (Vianna et al. 2000). This can be related to the differences among the original cell wall polymers in various microorganisms. Therefore, the dilute acid, HCl, has been established as an efficient elutant.
The treatment of biomass with alkaline acetone shows an increase in the overall negative charge of the cell walls. This can be due to the dissociation of ionic groups thus increasing the total number of active sites suitable for binding of metal species. Pretreated biosorbent by NaOH or other alkaline solution shows an increase in the metals uptake capacity by different biomass (Vianna et al. 2000). Similar observations were confirmed by Velkova et al. (2015), where NaOH-treated S. cerevisiae yeast cell gave the highest zinc uptake when compared with other methods that have an order of untreated biomass CaCl2, H3PO4, and H2O2, which gave the lowest metal uptake.
Therefore, not all chemical pretreatment methods improve the metal uptake capacity. Few chemical modifications are known to remove proteins, which contains several components that are capable of binding metals such as amino, thiol, and phosphate groups. Removal of proteins may decrease the ability to bind metals. Chemical modifications are likely to change the composition of biomass by introducing different new chemical groups to the biomass. The degree of chemical modification for a given biomass cannot be predicted. Therefore, the best modification configuration and experimental conditions must be evaluated for each type of biomass due to the difference in the chemical composition.
11.7 Parameters Affecting the Biosorption Process
The amount of metal bound on the biosorbent depends on many environmental factors and especially the selected biosorbent. The physiological state of the organism, its nutrients status, growing medium conditions, the type of chemical modification, and the specific surface properties of the microorganism, e.g., particle size and the type of active binding site responsible for the metal uptake, are all essential parameters influencing the performance of a biosorbent. Environmental conditions during the biosorption process such as solution pH, temperature, biosorbent concentration, the metal of interest, its ionic form, its initial concentration, the presence of other cations and/or anions, and concentration of other impurities are the main environmental factors that play an important role in the biosorption process (Abbas et al. 2014; Pagnanelli et al. 2000). Practically, solution pH and the presence of other cations have a significant effect on the efficiency of biosorption.
11.7.1 Solution pH
Many studies have shown the important role of the solution pH in the biosorption process. Changes in the initial pH values have a great effect on the metal uptake that may indicate different possible binding mechanisms. The pH value of the metal-bearing solution can affect the biosorption process in three different ways. Firstly, the binding active sites may be altered, where the availability of free binding sites depends on the pH. At a low pH, all the active sites are protonated, and complete desorption of the bound metal ion species can be obtained. Therefore, acid treatment is a method for regeneration of the sorbent material or recovery of the bound metal species. A two-unit decrease in the pH value can in some cases result in an approximately 90% reduction of metal binding. Secondly, extremely low pH values, which are used in desorption processes, i.e., regeneration, may have a negative effect by damaging the structure of the biosorbent material. Thirdly, the dissociation of the metal ion in the aqueous solution is pH dependent. The presence of metals in aqueous solutions is typically in the form of hydrate cations in solvation shells at low pH, while hydroxides may form at a higher pH. The formation of metal oxide and hydroxide complexes and/or precipitates is frequently referred to as hydrolysis, i.e., decomposition by water (Farhan and Khadom 2015; Schiewer and Patil 2008).
Few biosorption studies thought that at higher pH values, a combined sorption-microprecipitation mechanism might be responsible for the accumulation of heavy metals inside the cells and/or on the cell walls; however most heavy metals start to precipitate at pH >5.5 (Schiewer and Patil 2008). The study of the biosorption phenomena becomes more complicated when metals tend to form insoluble microprecipitates, because the predominant mechanism will not be defined accurately. However, from the process application viewpoint, it may desirably increase the metal uptake, thus increasing the apparent overall uptake capacity of biosorbent materials. The pH range of metal precipitation varies with respect to the metal and its salts. Typically, investigation of the pH effects over a value range of more than 7.0 is impossible because metal precipitation takes place and interferes with the process (Hadi et al. 2003). For example, addition of sodium hydroxide (NaOH), which may be used to adjust pH, to a lead nitrate solution can result in the formation of insoluble complexes of Pb(NO3)2Pb(OH)2 and Pb(NO3)25Pb(OH)2. The precipitation of these salts starts at pH values above 5.0 and 6.7 for lead and nickel, respectively (Davis et al. 2000). However, this effect was neglected in many studies. Increasing the pH to more than 9.0, like in research work described by Zouboulis et al. (1999), Al-Homaidan et al. (2014), and Fadel et al. (2017), can precipitate the metal where the biosorption is not the technology responsible for metal uptake. Therefore, the pH dependence of biosorption represents a limitation of the biosorption process for the treatment of wastewaters since the solution pH should be kept below the value of precipitation.
The uptake capacity by different types of biomass increases significantly with pH increases from 2 to 6 and levels off at a pH greater than 6.0 (Sulaiman 2015). In acidic solutions with a pH of 2.0, protonation of the fungal cell wall takes place. Increasing the pH, the negative charge density may increase on the cell wall surface because of the deprotonation of the metal sequestering sites and hence increase biosorption (Say et al. 2001). The low pH value of 3.5 was unfavorable, whereas the maximum uptake capacity decreases significantly when compared to that at pH 6.0 (Farhan and Khadom 2015). In addition, Vianna et al. (2000) found that metal sequestering was low for B. lentus, A. oryzae, and S. cerevisiae under acidic conditions at pH values of 2.5 and 3.5. The metal uptake was much higher at a pH value of 4.5 than those at pH of 2.5 and 3.5. The metal uptake substantially depends on pH, and the predominant uptake mechanism most likely involves electrostatic attraction to negatively charged binding sites on the cell walls. Figure 11.3a, b shows the effect of solution pH on the metal uptake.
Metal uptake depends on the attraction of the sorbate species to the solid surface and its lyophobic behavior, which indicates that sorption increases with a decrease in solubility. Since the solubility of many metal complexes in aqueous solution decreases with an increase of pH, this may give another possible explanation to the increasing of metal sorption as pH values increase. Moreover, hydrolyzed ion species have less hydration, i.e., less energy is required for the removal of the hydrated species upon binding (Davis et al. 2000). A further increase of pH leads to a decrease of the metal complexes solubility to a level of precipitation.
The theory of acid-base equilibria can be applied to biosorption, which states that in the pH range of 2.5–5.0, the binding of heavy metals is determined mainly by the state of dissociation of the weakly acidic groups. The most significant functional group at pH 4.5 may be the phosphate, carboxyl, and sulfate and amino groups, at the fungal cell wall surface (Say et al. 2001). Low pH values indicate high H+ ion concentrations, which may have a competition effect between the protons and the metal ions. At high pH values, a lowered competition by protons for the same active binding sites takes place, since most of the biomass cell will be in the unprotonated state. The rate of proton uptake was found to depend on solution pH, where faster proton uptake rates were observed in more acidic solutions (Sulaymon et al. 2013b). A rapid drop in pH may occur during the first hour in non-adjusted pH metal-bearing solutions (Hadi et al. 2003). This can be because small quantities of polysaccharides may leach into the solution from the biomass. Thus, it can interrupt the kinetics of metal uptake by interfering with the solution pH (Davis et al. 2000).
Consequently, the pH must be controlled during the whole period of contact until the sorption equilibrium is obtained. The pH can be adjusted with acid and/or base to keep its value close to the optimum pH during the first few hours of the sorption process. Although pH control or buffering of the solution is essential in biosorption studies, it may strongly affect the metal uptake process. The additive for pH control should be selected carefully to avoid any interference with the biosorption process such as reduction of the metal uptake capacity (Sulaiman 2015).
The optimum pH for metal uptake varies with different biomass types and metals of interest. For example, Hadi et al. (2003) found that the optimum pH for the removal of cadmium by S. cerevisiae and K. fragilis is 5.0, which was close to the cadmium precipitation limit, while this value was found within a range of 5.0–6.0 for lead, zinc, chromium, cobalt, cadmium, and copper using the same yeast cell S. cerevisiae cells (Sulaiman 2015). Similarly, the optimum pH was found between 5.0 and 6.0 for copper metal uptake using red algae (Palmaria palmata) and beer draff (Li et al. 2011). The optimum solution pH was found to be 6.0 to achieve maximum metal uptake of different ion species by fungal biomass (Say et al. 2001). The optimal pH values were found within a range of 4 and 5 for uptake of lead, copper, cadmium, and arsenic using algae (Sulaymon et al. 2013b). Finally, a pH value of 4.5 was found to be the optimum in another study (Davis et al. 2000). These results indicate a competition between heavy metal ions and protons for the available cell wall binding sites. At pH values higher than 5.0, the cadmium hydroxides may start to precipitate.
11.7.2 Solution Chemistry
The solution chemistry of metals plays a great role in the biosorption investigation since it is related to their hydration and hydrolysis reaction. The large charge-to-size ratio of cations results in an increase in hydration energy if no reaction beyond the coordination of water molecules to the cation occurs:
In aqueous systems, metal or metalloid oxides are generally covered with surface hydroxyl groups, so that hydroxylated oxide particles may be considered as polymeric oxo acids or oxo bases. The charge of the metal or metalloid hydrated oxides depends on the solution pH with proton transfers at the amphoteric surface. Therefore, the surface chemistry of metal oxides and hydroxides should be taken into consideration (Abbas et al. 2014; Sulaiman 2015).
11.7.2.1 Cations
Most industrial effluents are likely to contain several different toxic heavy metals. In this case, biosorption involves a competitive ion exchange where several toxic heavy metals compete for a limited number of active binding sites available on the biosorbent. However, many biosorption studies are carried out only on single metal solution. Therefore, the results obtained from these studies may not be valid when applied to industrial effluent treatment. Ion species competition on available different binding site has been shown to affect biosorption by several different biomass types and metals (Sulaymon et al. 2013b; Sulaiman 2015).
The chemical form of metal ions, e.g., cationic or hydrolyzed neutral/anionic species, plays an important role in the biosorption process. The effect of other metallic ions (or even anionic co-ions) on the performance of single metal biosorption has been investigated. It gave different results from the original (single metal) sorption when a known concentration of a mixture was introduced. The metal uptake of a single metal decreases with the presence of other competing ion species. However, the overall metal uptake of different ion species has been reported to increase slightly (Say et al. 2001). The amount of reduction depends on the binding strength of various ions to the biomass. The inhibition of heavy metals binding to biomass by light or alkali metals, e.g., K and Na, is insignificant in comparison with that by heavy metals, e.g., Zn, Cu, Fe, and U. It can be generally concluded that the light metals such as alkaline and alkaline earth metals have less binding strength than heavy metal ions or radioactive elements. Therefore, light metals may be less likely to interfere with the biosorption of heavy metals (Sulaymon et al. 2013b).
The metal competition effect on biosorption is as important as the metal concentration of the metal of interest in the solution. For example, cadmium is found to have a significant effect on decreasing the biosorption of silver when compared to the presence of sodium (Volesky and Schiewer 1999). In fungal biomass of R. arrhizus, the inhibition effect of the presence of uranium is much higher on the binding of cadmium, zinc, and silver than that of these elements on the binding of uranium. In addition, zinc shows a weak binding among the heavy metals; hence it is more significantly affected by other ions. Conversely, in few cases, the presence of metals in the solution may have an influence on the biomass structural composition. For instance, copper was responsible for protein removal from S. cerevisiae, which is related to the metal concentration in the solution. The released protein may bind to the copper and prevent the biosorption process (Singliton and Simmons 1996).
11.7.2.2 Anions
An increase of anion concentration has inhibitory effects on metal uptake by S. cerevisiae, probably by forming less cationic, neutral, or anionic metal complexes. At higher anion concentrations, there is no evidence of metal uptake (Fadel et al. 2017). Hypothetically, the presence of anionic ligands can lead to one of the following:
-
1.
Formation of complexes that have higher affinity for the sorbent than the free metals, i.e., improved sorption process.
-
2.
Formation of complexes that have lower affinity for the sorbent than the free metals, i.e., reduced sorption process.
-
3.
Interaction of these anions with the biomass wall surface, which may change the texture of the active sites. This can either improve or reduce the sorption process.
The third type of interaction is less likely to occur especially with biosorbent materials that have negatively charged carboxyl and sulfate groups as the probable binding sites on their cell walls surface. This is because these functional groups are not expected to interact with anions such as sulfate, nitrate, etc. (Michalak et al. 2013).
The presence of other ligands may have a negative effect on metal binding, except OH. This indicates that the biosorbent has less affinity for the metal-ligand complexes than for the free metal ion. The effect of ligands in solution can be described as competitive with the biomass for sequestering the metal ion species. This influence may be insignificant in few cases unless these anions form strong complexes with the metal ion, e.g., EDTA, which may cause an inhibition of binding. In addition, the influence of the ligand presence is of secondary importance since considerable concentrations of strong complex are not expected in typical metal-containing industrial effluents (Michalak et al. 2013).
11.7.3 Temperature
The effect of temperature on the biosorption process is relatively small, where the temperature is critical in the energy-dependent mechanism such as metal bioaccumulation. However, energy-independent mechanisms are less influenced by solution temperature changes, since the biosorption process is a physicochemical interaction (Bazrafshan et al. 2015). In addition, its influence in the lower temperature range is slightly more prominent. Biosorption processes are typically exothermic, where the equilibrium constant decreases with a temperature increase. For most metals, the heat of reaction is found to be constant, regardless of the degree of site occupation. However, for copper the heat of reaction decreases with an increase of the site occupation degree. This may indicate that there are either different binding sites involved in the process or different types of copper complexes with biomass may be formed. The heat of reaction was in a range of 7 and 11 kJ/mol for heavy metals, while it was about 2.5 and 6 kJ/mol for light metals (Sag and Kutsal 2000).
The binding of cobalt by the brown alga Ascophyllum nodosum increases from 50% to 70% with increasing temperature within a range of 4–23 °C. The increase becomes insignificant until the temperature reaches 40 °C, whereas the temperature of 60 °C can change the structure of the sorbent and consequently reduce its uptake capacity. A 20% increase was obtained when the temperature increased from 4 to 55 °C for Spirulina algae (Volesky and Schiewer 1999). The metal uptake of lead and cadmium by the brown seaweed Cystoseira baccata in significantly changed with increasing temperature in the range of 15–55 °C (Lodeiro et al. 2006). The initial biosorption rates of Z. ramigera for Fe and Pb were found to increase with temperature in a range of 35–45 °C. Moreover, the Fe and Cr uptake by R. arrhizus was greater at higher temperatures. However, the uptake rate constants of lead by Z. ramigera and Nickel by R. arrhizus were insignificantly affected by temperature (Sag and Kutsal 2000). Using S. cerevisiae, Ting and Sun (2000) found that copper uptake was temperature independent between 10 and 50 °C. Generally, to avoid decomposition of the biosorbent materials, biosorption application processes must take place at a narrow range of temperatures, i.e., 5–50 °C. Warm water might extract polysaccharides and other cell components that have low molecular weight. It may denature some polypeptides, which may provide some amino acid metal coordination sites for metal species.
11.7.4 Initial Metal Concentration
At sufficiently high metal concentration, more active sites can be occupied when compared to low metal concentration (Mukhopadhyay et al. 2007). However, the driving force alone is not enough to replace all protons from all sites. Proton removal by a basic medium provides anion functional group sites that represent an attractive force for metal binding.
Metal uptake per unit mass weight increases with an increase in metal concentration, even though some saturation situations of few hard ions were observed at higher concentrations (Hadi et al. 2003). The initial biosorption rate of Pb, Fe, Cr, and Ni by Z. ramigera increased with increasing initial metal ion concentration, Ci, up to 300 mg/L. The same trend was observed with R. arrhizus up to 200 mg/L (Sag and Kutsal 2000). In addition, the metal uptake capacity by P. chrysosporium increased with an increase in initial metal concentration until it reached the saturation level, which was around 300 mg/L (Say et al. 2001).
11.7.5 Biosorbent Concentration
Increasing the biomass concentrations increases the biosorbed metal, because of an increase in the surface area and subsequently the available binding sites. However, the metal uptake per unit biosorbent weight decreases with increasing biosorbent concentration, which was observed by several studies (Farhan and Khadom 2015; Hadi et al. 2003; Malkoc and Nuhoglu 2005; Mukhopadhyay et al. 2007). At high biosorbent concentration, the interference between the binding sites may increase, and the driving force of metal concentration may become insufficient to completely cover the available binding sites, which result in low metal uptake per unit mass. Different hypotheses have been proposed to explain this effect such as reduced effective mixing at high biomass densities, interference between various binding sites, limited availability of metal species in the solution, and electrostatic interactions (Ting and Sun 2000).
These results show that the hypothesis of electrostatic interaction between cells as a significant factor in the biomass dependence is invalid. Therefore, it is useless to increase biomass by more than 10 g/L to purify a 100 mg/L contaminated solution. A reduction in the biomass concentration increases the metal to biomass ratio at a given metal concentration. Therefore, metal uptake capacity increases per gram of biosorbent, if it is not saturated. The same maximum uptake can be achieved with a low biomass concentration when it is saturated and with an unsaturated biomass concentration.
11.7.6 Particle Size
Ideally, decreasing the particle size increases the metal uptake due to an increase of the surface area. However, this has a negative impact on the biosorption process due the difficulty of separation and possible clogging of the process. The size distribution of biosorbent particles had insignificant effect on the cadmium uptake (Davis et al. 2000). This agrees with the hypothesis that the metal uptake does not occur exclusively on the biosorbent particle surface. Apparently, the cationic metals diffuse through the particle, and the metal uptake rate can be dominated by interparticle metal ion mass transfer.
Conversely, Leusch et al. (1995) found that the particle size and chemical modification affected the biosorption performance. They were found to have a significant but unpredictable influence on the process. The metal uptake was higher with larger particles (0.84–1.00 mm) than that of smaller particles (0.105–0.295 mm) of Sargassum fluitans and Ascophyllum nodosum. They concluded that the larger the particles, the higher metal uptake obtained. This observation was more obvious at low concentrations than at high concentrations where the differences between large and small particles were 93% and 48% for low and high concentrations, respectively. In addition, the bigger the particle, the better the equilibrium metal sorption.
11.7.7 Morphological Conditions and Age of the Cells
The morphological differences within the same type of cells can influence the sorption process. The sorption efficiency and the capacity of a biomass depend not only on the biomass species itself but also on the growth conditions, physiological state, age of the microorganism, and the carbon source that is used in the cultivation process. Different preparations of the yeast biomass have significant effect on the uptake of uranyl ion. In addition, there is strong evidence that different media and growth conditions produce different microbial culture growth characteristics and hence metal uptake capacities (Broach 2012).
Younger S. cerevisiae and K. fragilis yeast cells (24 h) were found to bind cadmium slightly more than the older one (72 h). Thus, younger yeast cells were more effective in sequestering cadmium ions (Hadi et al. 2003). This can be due to the decrease of protein in the cell wall with age and increase in carbohydrate content with respect to the same period. In addition, the structure of alginates differs between young and old tissues as well as between different parts of the same plant. Also, the sulfate groups and uronic acids content in algal biomass differ with respect to season and geographical area even within the same species (Wang et al. 2014). Table 11.2 shows the chemical structure for young and old cells for the whole cells and isolated cell walls of the S. cerevisiae yeast. Protein represents higher concentration in young cells and the whole cells than that of old cells and isolated cell walls. This may indicate that protein is involved in the metal uptake by S. cerevisiae.
11.8 Kinetics and Modeling of Biosorption
11.8.1 Kinetics of Biosorption
The kinetics of the biosorption process is very important to identify the required contact time between the biosorbent materials and the metal-bearing solution to achieve a desirable metal uptake. The faster the metal uptake by the biosorbent, the more desirable the biosorption since shorter contact time is needed. This means that smaller equipment is required which directly reduces not only the capital cost but also the operating cost. Both equilibrium and kinetics studies of the process are important for evaluation of biosorption performance and the process design. Furthermore, the desorption process, which can determine the ability of biosorbents to be reused, is another important feature.
Biosorption involves essentially very rapid sorption reaction mechanisms. Typically, the biosorption of heavy metals has two main phases: an initial fast (<4 s) metal uptake related to surface binding on the cell walls, followed by a much slower uptake. This may be an indication that a different secondary metal binding mechanism occurs in the second slow phase, which represents the diffusion of ions into the cell structures (Park et al. 2010).
In addition, in many biosorption systems, there are many indications that most of the metal biosorption occurs in a matter of 5–15 min after solid-liquid contact (Abbas et al. 2014). The metal uptake kinetics is usually very fast, in the order of minutes or even of seconds. The copper uptake by Aspergillus niger occurred very fast with 70% of the final uptake taking place within 30 min. Additional metal uptake takes place slowly over several hours. Also, the maximum cadmium uptake by S. cerevisiae and K. fragilis yeast cells was achieved within 5 min. Insignificant levels of metal uptake were observed after this period, i.e., less than 10% (Hadi et al. 2003). Consequently, the rapid kinetics enhances the use of biosorbents in continuous mode contactors.
11.8.2 Modeling of Biosorption
Models are essential tools for scale-up from laboratory scale to an application process scale. Accurate models can assist in interpreting experimental data and predicting the change in the system under different conditions to assess the efficiency and feasibility of a biosorption process. It is a relatively simple task to obtain a single equilibrium sorption isotherm in a laboratory. A small amount of the required sorbent is supplied to a solution of the sorbate. The amount of metal, M, bound per mass of biosorbent is called metal uptake, q. The uptake depends not only on the sorbent material, X, but also on the metal concentration, C f, of all sorbates in addition to other parameters such as solution pH. The sorption isotherm is a plot of the metal uptake, q, and the residual equilibrium concentration of remaining sorbate in the solution, C f. Increasing the metal concentration in the solution, the binding increases from zero until it reaches its maximum as shown in Fig. 11.3b. The environmental conditions in the sorption system should be controlled especially the pH. The time required to attain the sorption equilibrium can be determined by a preliminary sorption kinetics test. Ideally, the sorption equilibrium is established once the sorbate concentration becomes unchanged in the solution. In a few cases, the equilibrium is established after 6 h of metal-biomass contact (Say et al. 2001). It is favorable that a sorbent material possesses a high metal uptake capacity and a high affinity for the metal ion species, which is indicated by a steep slope of the isotherm curve at low residual metal concentrations. Although many experimental studies have been published on metal biosorption, a much greater effort should be made to develop models that can predict the metal uptake phenomenon (Davis et al. 2003). Conventional models cannot effectively describe the metal uptake. More studies are required to develop and modify the existing mathematical models.
In the literature, the most common sorption isotherm models used are a simple Langmuir’s model or Freundlich’s model, where the metal uptake is obtained as a function of the final equilibrium metal concentration without indicating the pH of the solution or the presence of other ions. A recent extensive review of most used isotherm models can be found in Abbas et al. (2014), Park et al. (2010), and Limousin et al. (2007).
11.8.3 Limitations of Conventional Models
Conventional models are widely applied due to their simplicity and sufficiency of describing experimental data at various operating conditions. However, they are unable to predict the effect of critical parameters such as pH, ionic strength, metal competition, and more complex uptake behavior (Abbas et al. 2014). They do not take into consideration the electrostatic interaction since they assume that the cell wall consists of a homogenous network of functional groups (Limousin et al. 2007). Do not have a meaningful physical interpretation of the biosorption process. Not only the results cannot be extrapolated, but also no conclusions can be drawn for systems operating under various conditions. These simple conventional models do not consider the effects of any external environmental factors. In addition, an irregular pattern may be represented by the biosorption isotherms because of the complex structure of the biosorbent, its multiple heterogeneous active sites, and the complex solution chemistry of some metallic species. Different researchers of the biosorption phenomenon rarely have considered this issue. They regularly force smooth isotherm curves through scattered experimental points and/or use the earlier simplistic sorption models to fit those experimental data points.
Despite several indications of ion exchange as the main mechanism of biosorption by nonliving biomass, most studies of biosorption continue to analyze experimental data obtained by means of models originally developed for sorption on granular activated carbon. The models for activated carbon cannot identify if biosorbents may operate on different ionic cycles, which are determined by the types of acids and/or bases used for pretreating the biomass in the regeneration step. Therefore, the elementary difference between ion exchange and sorption on granulated activated carbon limits the use of these models in biosorption.
Despite the critical role of solution pH in sorption processes, it is usually neglected in most mathematical descriptions of the process. The complex structure of biosorbent materials, which may consist of multiple active binding sites, makes the modeling of the pH effect in the biosorption process difficult (Abbas et al. 2014). Consequently, isotherms have been studied separately for each pH value. Such a step is essential since both Langmuir and Freundlich isotherm models do not consider the effect of pH. Several attempts have been made in literature to lump the effects of several parameters including the pH into one empirical parameter.
11.8.4 Ion-Exchange Isotherm
Ion-exchange isotherm model is difficult to apply due to the complex structure of the biomass when compared to a synthetic ion-exchange resin. Volesky and Schiewer (1999) proposed a model based on the ion exchange of two sites for metal uptake by Sargassum fluitans, which consists of two main active groups mainly carboxyl and sulfate. It can predict the equilibrium binding of cadmium, copper, and zinc by seaweed biomass as a function of pH and the final metal concentration. The proton concentration is integrated into the model independently as a variable so that the prediction of metal uptake by biosorption at different solution pH values becomes an easy calculation. However, this model neglects the effect of metal ion hydrolysis in aqueous solution on biosorption performance. Yang and Volesky (1999) proposed a model that considers ion exchange between protons in the same biomass and hydrolyzed uranium ion as a simple competition for available binding sites where no reverse reactions take place. It assumed that due to the uranium hydrolysis in aqueous solution, the total uranium ion concentration is not necessarily the same as the free uranium ion concentration. The model can predict the uranium and proton binding based on the pH value and total uranium concentration. Sulaymon et al. (2013a) applied a general ion-exchange equilibrium model to describe lead, copper, cadmium, and arsenic uptake by algae.
The Langmuir equation and the ion exchange constant for the uptake of a metal ion, M, replacing a proton, H, on an active site, B, can be explained as follows:
Therefore,
The main difference between the two methodologies is that the Langmuir isotherm assumes that all sites are initially unoccupied and neglects any possibility of a reverse reaction of the displaced ion, i.e., proton. The ion-exchange approach assumes that all active binding sites are initially occupied and the number of free sites remains constant. The difference between the two approaches was especially obvious at low metal concentrations, since the effect of the reverse reaction involving the displaced ions is pronounced at these concentrations (Limousin et al. 2007). Figure 11.4 represents the most generalized description of the ion-exchange sorption equilibrium isotherm for binary systems.
Typical ion-exchange equilibrium isotherm (Leusch et al. 1995)
Although the ion-exchange model is probably closer to reality than that of the Langmuir model, it is not very satisfactory. Even though constant many free sites may be reasonable as an assumption for a constant pH system, it may be invalid for system with varying pH. The modeling of commercial ion-exchange resin can be simply achieved, since these materials contain only one active group. However, biosorbents are complex materials that contain several heterogeneous active groups. In addition, since the metal uptake increases with an increase in pH, modeling the competitive binding of metals and protons by using only a metal-proton-ion-exchange constant is not appropriate (Abbas et al. 2014).
11.9 Conclusions
Heavy metals are environmentally toxic but can be precious materials at the same time in many cases. Conventional removal/recovery methods are usually expensive and ineffective at low metal concentrations. Biosorption has evolved as a cost-effective and environment-friendly polishing process, which has been studied extensively by different research areas. A wide range of publications is available in the literature, which has investigated the various factors affecting the biosorption technology, including operational and environmental parameters, where solution pH was found to play the most critical role. Most studies were focused on the kinetics and equilibrium of biosorption, the uptake capacity of different sorbate using various biosorbents, recovery of biosorbed metals, and modeling of the essential parameters. Batch biosorption process has been studied extensively with few continuous biosorption attempts and biosorption reactor. Despite many experimental studies investigated the biosorption process, several technical issues need to be addressed to commercialize the technology, which is required to meet the industrial demands. Detailed economic analyses are required for large-scale industrial wastewater, taking into consideration the recovery, separation, and purification issues. Simplified mathematical models to describe real-life cases of multi-metal systems are required, where different biosorption mechanisms should be tested to reveal the most dominant one(s). Finally, efficiency and selectivity of biomaterials should be the main criteria to examine the new biosorbents. Ideal biosorbents should be abundantly available and/or waste material to decrease the overall cost of the process, which is the key element that kept the biosorption process an attractive option for the last decade.
References
Abbas SH, Ismail IB, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: a review. J Chem Sci Technol 3(4):74–102
Abdel-Ghani NT, El-Chaghaby GA (2014) Biosorption for metal ions removal from aqueous solutions: a review of recent studies. Int J Latest Res Sci Technol 3(1):24–42
Abdi O, Kazemi M (2015) A review study of biosorption of heavy metals and comparison between different biosorbents. J Mater Environ Sci 6(5):1386–1399
Al-Homaidan AA, Al-Houri HJ, Al-Hazzani AA, Elgaaly G, Moubayed NMS (2014) Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass. Arab J Chem 7(1):57–62
Al-Saydeh SA, El-Naas MH, Zaidi SJ (2017) Copper removal from industrial wastewater: a comprehensive review. J Ind Eng Chem 56:35–44
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14(1):94
Bazrafshan E, Mohammadi L, Ansari-Moghaddam A, Mahvi AH (2015) Heavy metals removal from aqueous environments by electrocoagulation process–a systematic review. J Environ Health Sci Eng 13(1):74
Broach JR (2012) Nutritional control of growth and development in yeast. Genetics 192(1):73–105
Brown TJ, Idoine NE, Raycraft ER, Shaw RA, Deady EA, Hobbs SF, Bide T (2017) World mineral production. Br Geol Surv
Davis TA, Volesky B, Vieira R (2000) Sargassum seaweed as biosorbent for heavy metals. Water Res 34(17):4270–4278
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37(18):4311–4330
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40
Dhankhar R, Hooda A (2011) Fungal biosorption–an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ Technol 32(5):467–491
Fadel M, Hassanein NM, Elshafei MM, Mostafa AH, Ahmed MA, Khater HM (2017) Biosorption of manganese from groundwater by biomass of Saccharomyces cerevisiae. HBRC J 13(1):106–113
Farhan SN, Khadom AA (2015) Biosorption of heavy metals from aqueous solutions by Saccharomyces cerevisiae. Int J Ind Chem 6(2):119–130
Fiset JF, Blais JF, Riveros PA (2008) Review on the removal of metal ions from effluents using seaweeds, alginate derivatives and other sorbents. Rev Sci de l’eau/J Water Sci 21(3):283–308
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84(1):13–28
Ganguly A, Guha AK, Ray L (2011) Adsorption behaviour of cadmium by Bacillus cereus M116: some physical and biochemical studies. Chem Speciat Bioavailab 23(3):175–182
Gow NAR, Latge JP, Munro CA (2017) The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr
Gupta P, Diwan B (2017) Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep 13:58–71
Hadi B, Margaritis A, Berruti F, Bergougnou F (2003) Kinetics and equilibrium of cadmium biosorption by yeast cells S. cerevisiae and K. fragilis. Int J Chem React Eng 1(1):826–829
Horsfall M Jr, Ogban FE, Akporhonor EE (2006) Recovery of lead and cadmium ions from metal-loaded biomass of wild cocoyam (Caladium bicolor) using acidic, basic and neutral eluent solutions. Electron J Biotechnol 9(2):0–0
Kratochvil D, Volesky B (1998) Advances in the biosorption of heavy metals. Trends Biotechnol 16(7):291–300
Leusch A, Holan ZR, Volesky B (1995) Biosorption of heavy metals (Cd, Cu, Ni, Pb, Zn) by chemically-reinforced biomass of marine algae. J Chem Technol Biotechnol 62(3):279–288
Li Y, Helmreich B, Horn H (2011) Biosorption of Cu (II) ions from aqueous solution by red alga (Palmaria Palmata) and beer draff. Mater Sci Appl 2(02):70
Limousin G, Gaudet JP, Charlet L, Szenknect S, Barthes V, Krimissa M (2007) Sorption isotherms: a review on physical bases, modeling and measurement. Appl Geochem 22(2):249–275
Lodeiro P, Barriada JL, Herrero R, Sastre De Vicente ME (2006) The marine macroalga Cystoseira baccata as biosorbent for cadmium (II) and lead (II) removal: kinetic and equilibrium studies. Environ Pollut 142(2):264–273
Machado MD, Janssens S, Soares HMVM, Soares EV (2009) Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae: advantages of using dead biomass. J Appl Microbiol 106(6):1792–1804
Malkoc E, Nuhoglu Y (2005) Investigations of nickel (II) removal from aqueous solutions using tea factory waste. J Hazard Mater 127(1):120–128
Mehta S, Gaur J (2005) Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotechnol 25(3):113–152
Michalak I, Chojnacka K, Witek-Krowiak A (2013) State of the art for the biosorption process—a review. Appl Biochem Biotechnol 170(6):1389–1416
Mishra SP (2014) Adsorption–desorption of heavy metal ions. Curr Sci 107(4):601–612
Muhamad H, Doan H, Lohi A (2010) Batch and continuous fixed-bed column biosorption of Cd 2+ and Cu 2+. Chem Eng J 158(3):369–377
Mukhopadhyay M, Noronha SB, Suraishkumar GK (2007) Kinetic modeling for the biosorption of copper by pretreated Aspergillus niger biomass. Bioresour Technol 98(9):1781–1787
Naja G, Volesky B (2006) Behavior of the mass transfer zone in a biosorption column. Environ Sci Technol 40(12):3996–4003
Naja GM, Volesky B (2009) Toxicity and sources of Pb, Cd, Hg, Cr, As, and radionuclides in the environment. Heavy Met Environ 8:16–18
Naja G, Volesky B (2011) The mechanism of metal cation and anion biosorption. In: Microbial biosorption of metals. Springer, pp 19–58
Ngo HH, Guo W, Zhang J, Liang S, Ton-That C, Zhang X (2015) Typical low cost biosorbents for adsorptive removal of specific organic pollutants from water. Bioresour Technol 182:353–363
Pagnanelli F, Papini MP, Toro L, Trifoni M, Veglio F (2000) Biosorption of metal ions on Arthrobacter sp.: biomass characterization and biosorption modeling. Environ Sci Technol 34(13):2773–2778
Park D, Yun YS, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15(1):86–102
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177(1):70–80
Sağ Y, Kutsal T (2000) Determination of the biosorption activation energies of heavy metal ions on Zoogloea ramigera and Rhizopus arrhizus. Process Biochem 35(8):801–807
Say R, Denizli A, Arıca MY (2001) Biosorption of cadmium (II), lead (II) and copper (II) with the filamentous fungus Phanerochaete chrysosporium. Bioresour Technol 76(1):67–70
Schiewer S, Patil SB (2008) Modeling the effect of pH on biosorption of heavy metals by citrus peels. J Hazard Mater 157(1):8–17
Singleton I, and Simmons P (1996) Factors affecting silver biosorption by an industrial strain of Saccharomyces cerevisiae. J Chem Technol Biotechnol 65(1):21–28
Sulaiman MS (2015) Factors affecting biosorption of cu (ii) ions from industrial wastewater. Appl Res J 1:311–315
Sulaymon AH, Shahlaa EE, Sama MA, Al-Musawi TJ (2010) Removal of lead, cadmium, and mercury ions using biosorption. Desalin Water Treat 24(1–3):344–352
Sulaymon AH, Mohammed AA, Al-Musawi TJ (2013a) Column biosorption of lead, cadmium, copper, and arsenic ions onto algae. J Bioprocess Biotechnol 3(128):2
Sulaymon AH, Mohammed AA, Al-Musawi TJ (2013b) Competitive biosorption of lead, cadmium, copper, and arsenic ions using algae. Environ Sci Pollut Res 20(5):3011–3023
Ting YP, Sun G (2000) Use of polyvinyl alcohol as a cell immobilization matrix for copper biosorption by yeast cells. J Chem Technol Biotechnol 75(7):541–546
Velkova Z, Kirova G, Kafadarova V, Stoytcheva M, Gochev V (2015) Biosorption of Zn (II) Ions by Pretreated Waste Brewery Biomass. Int J Ind Chem 6:119–130
Vianna LNL, Andrade MC, Nicoli JR (2000) Screening of waste biomass from Saccharomyces cerevisiae, Aspergillus oryzae and Bacillus lentus fermentations for removal of Cu, Zn and Cd by biosorption. World J Microbiol Biotechnol 16(5):437–440
Vijayaraghavan K, Jegan J, Palanivelu K, Velan M (2005) Biosorption of copper, cobalt and nickel by marine green alga Ulva reticulata in a packed column. Chemosphere 60(3):419–426
Volesky B (1990a) Removal and recovery of heavy metals by biosorption. In: Volesky B (ed) Biosorption of heavy metals. CRC Press, Boca Raton, pp 7–43
Volesky B (1990b) Biosorption by fungal biomass. In: Volesky B (ed) Biosorption of heavy metals. CRC Press, Boca Raton, pp 139–171
Volesky B (2003) Sorption and biosorption. BV Sorbex, Montreal
Volesky B, Schiewer S (1999) Metals biosorption. In: Flickinger M, Drew S (eds) Encyclopedia of bioprocess technology: fermentation, biocatalysis, and bioseparation. Wiley, New York, pp 433–4531999
Wang L (2014) Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar Drugs 12(9):4984–5020
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27(2):195–226
Yang J, Volesky B (1999) Modeling uranium-proton ion exchange in biosorption. Environ Sci Technol 33(22):4079–4085
Zargar V, Asghari M, Dashti A (2015) A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev 2(3):204–226
Zhao X, Höll WH, Yun G (2002) Elimination of cadmium trace contaminations from drinking water. Water Res 36(4):851–858
Zouboulis AI, Rousou EG, Matis KA, Hancock IC (1999) Removal of toxic metals from aqueous mixtures. Part 1: biosorption. J Chem Technol Biotechnol 74(5):429–436
Acknowledgments
The authors would like to thank Zineb Bouabidi for her help with formatting the chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Hadi, B., El-Naas, M.H. (2019). Biosorption of Heavy Metals: Potential and Applications of Yeast Cells for Cadmium Removal. In: Bharagava, R. (eds) Environmental Contaminants: Ecological Implications and Management . Microorganisms for Sustainability, vol 14. Springer, Singapore. https://doi.org/10.1007/978-981-13-7904-8_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-7904-8_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7903-1
Online ISBN: 978-981-13-7904-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)