Abstract
Carbon monoxide (CO) is a colorless, tasteless, odorless, dangerous gas, which exists anywhere people live, and it is slightly lighter than air. The prognosis of CO poisoning varies depending on the exposure situation of CO gas, and some suffer from disorientation, urinary incontinence, energy loss, or even death after a full recovery from CO poisoning. The pathogenesis of acute CO poisoning has not been clearly identified, and the treatment protocol has also not been established.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Carbon monoxide (CO) is a colorless, tasteless, odorless, dangerous gas, which exists anywhere people live, and it is slightly lighter than air. The prognosis of CO poisoning varies depending on the exposure situation of CO gas, and some suffer from disorientation, urinary incontinence, energy loss, or even death after a full recovery from CO poisoning. The pathogenesis of acute CO poisoning has not been clearly identified, and the treatment protocol has also not been established.
CO goes through the lungs [1] to combine with hemoglobin (Hb) in a high affinity (200–250 times stronger than oxygen), [2] to shift Hb oxygen dissociation curve to left, and [3] to be coupled with the cytochrome c in mitochondria, and cause breathing difficulties of organ, which induces organ failure by low oxygen transport (hypoxia). When CO-Hb level goes up to more than 20%, it increases the impact on the brain and heart [1], to make big damage on the brain, which consumes oxygen the most, followed by cardiac muscle, and skeletal muscle [2, 3]. And, after fully recovering from the consciousness disorders once in an acute phase, psychoneurotic symptoms may be caused sometimes (delayed neuropsychological sequelae; DNS). Therefore, the treatment is important for eliminating CO from the body as soon as possible.
2 Implementation Criteria in Each Hospital and Institute
The criteria for implementation of hyperbaric oxygen therapy (HBOT) for acute CO poisoning differ according to hospitals and institutes. So far, the criteria for implementation (Table 7.1) by Colignon and Lamy (1986) [4] is the only one published as the world standards. However, HBOT has been performed for severe cases and normobaric oxygen therapy (NBOT) for the mild cases, which clearly suggests that the method of treatment varies depending on the severity of the cases. Therefore, the severity of the cases should be equal when comparing the therapeutic effects between HBOT and NBOT. Kusuba et al. [5] classified the implementing facilities into three ranks; facility where HBOT is rarely performed (A rank), the intermediate facilities (B rank), and facility where HBOT is frequently performed (C rank), respectively (Table 7.2). According to this classification, the therapeutic effect was compared considering the severity, and a new interpretation has been added to the application of HBOT and NBOT [5].
3 Pathophysiology of CO Poisoning
As oxygen delivery is controlled by inhaled oxygen concentration, Hb level, and blood flow volume, CO gas reduces the amount of Hb that can transport oxygen as anemia by combining with Hb at the high affinity (left shift of the oxygen dissociation curve). If the oxygen consumption is not remediated instantly, hypoxia continues for long term to induce tissue acidosis, and the patient lapses in delayed neuropsychological sequelae (DNS) of CO poisoning.
In CO poisoning, myelin basic protein (MBP), acetaldehyde, and malondialdehyde (MDA) are produced as antigens [6], which has been thought to cause DNS of CO poisoning as a result of immunological reactions [7]. Therefore, treatment against immune response has started to be used as the treatment of DNS of CO poisoning.
3.1 CO-NO-O2 Competitive Coupling
3.1.1 Hemoglobin (Hb)
Since carbon monoxide competitively binds to Hb in red blood cells with 200–250 times higher affinity than oxygen [8], it has been said that CO-Hb indicates the severity of CO poisoning.
However, CO-Hb level can be easily dropped down by O2 inhalation, thus oxygen inhalation is considered to be a possible effective treatment (both NBO and HBO). Carbon monoxide diffuses from the alveoli in the body; CO-Hb level can change due to various factors related with CO uptake and release impacts (Table 7.3), and a small amount of CO is directly oxidized into CO2. Due to the factors affecting the Hb level in the body, the average half-life (t1/2) of CO-Hb varies widely between 27 and 464 min (Table 7.4) [9,10,11]. NBOT is similar to HBOT in that it can reduce the level of CO-Hb, but the difference in the therapeutic effect between the two treatments is being questioned. Some think NBOT is sufficient for CO poisoning and it can improve oxygen transport capacity in the blood. If CO gas remains in peripheral tissues, however, hypoxic state continues there, which suggests that HBOT would be better indicated in severe CO poisoning.
CO is taken in from the outside of the body. Also, when heme is degraded by heme oxygenase into Fe and biliverdin, CO is produced in the body. Although CO directly up-regulates the activity of nitric oxide synthase (NOS) enzymes, NO radicals (•NO) compete with CO in the active site at the cellular level. Thus, CO inhibits NO from binding to heme protein, which increases the NO concentration both inside and outside of platelets and also in endothelial cells [12,13,14].
3.1.2 Cytochrome c oxidase (CCO)
Cytochrome c oxidase (CCO) is the final enzyme in a mitochondrial electron transport chain. CO incorporated into the tissues competitively binds with various intracellular proteins that use O2, CO, or •NO gases as ligands. CO inhibits mitochondrial respiration once it binds to CCO. In the cascade of electron transport for producing ATP (Fig. 7.1), CO inhibits CCO in the final stage (site III) and stops the cells from functioning [15, 16]. Although the binding capacity of CO to CCO is not as strong as to O2 or •NO, it has great significance when the cells go into the low oxygen status. Synthesized endogenous CO reduces cellular respiration by 30% [17]. When CO-Hb level reaches 20%, the injuries due to shock, postoperative ileus, organ transplantation, and ischemia reperfusion have been reported to be prevented [18,19,20]. However, exogenous CO combines with CCO and inhibits mitochondrial respiration [21], which might selectively induce apoptosis in brain nerve cells [22, 23]. The inflammatory and apoptotic effects of CO occur as a result of the synthesis of stress-dependent protein [24, 25]. Free radicals produced from mitochondria that are exposed to CO are key stressers to induce necrosis or apoptosis of nerve cells [26, 27]. On the other hand, stabilization and activation of hypoxia-inducible factor-lα regulates genes involved in cell proliferation, differentiation, and survival [28], and reduces the organ response to the injury, which is considered to be “protective” [24].
Respiratory chain in mitochondria [22]. The mitochondrial respiratory chain indicating sequence of electron transport, three sites of energy coupling (oxidative phosphorylation), and location of acting CO
Since CO gas binds tightly with CCO in the mitochondria, it cannot be easily released from the peripheral tissues. It disrupts the electron transport chain at the cellular level, which leads to an excessive production of reactive oxygen species with disturbance of adenosine triphosphate (ATP) synthesis. Thus, CO stops oxygen metabolism in the mitochondria [22, 29]. Treatment with HBO assists to inhibit lipid peroxidation caused by CO exposure [30] and prevents white blood cells from adhering to small blood vessels (microvasculature) [31], then helps prevent the injury of central nervous system. If the treatment is inadequate and residual CO gas continues to remain with the state of low oxygen in peripheral tissues, it results in an increased permeability of blood vessels and may cause pulmonary and cerebral edema. Long-term hypoxia may cause an impairment of consciousness and induce a vicious cycle that worsens the condition after CO exposure. CO also induces neutrophil-platelet aggregation/activation to deteriorate tissue injuries and may lead to an intermittent type CO poisoning which is characterized by the dysfunction of critical organs (Fig. 7.2) [32].
Mechanism of perivascular injury by CO [32]. nNOS neuronal nitric oxide synthase, NO nitric oxide, NO2 nitrogen dioxide, HNO2 nitrite
3.2 Priming of Blood and Perivascular Injury
After entering the blood vessels through the lungs, CO acts on red blood cells, platelets, and neutrophils. It forms nitrotyrosine around the blood vessels and promotes a chain of activation and degranulation of polymorphonuclear neutrophils (PMNs) as shown in Fig. 7.3 [33]. Accordingly as the walls of blood vessels are injured, capillary leakage may occur around the aorta, lungs, skeletal muscles, and brain [13]. The injury of blood vessel walls can cause tissue edema, circulatory disorders, and an insufficiency of coagulation system [31]. Infiltrated neutrophils promote a rapid activation of the synthesis of reactive oxygen species as well as •NO-generating substances. Oxidative stress has a critical role in neurological injuries [7, 34, 35]. On the other hand, CO has a beneficial role to healthy human bodies because of its complex functions in metabolism and inflammatory reactions. The physiological role of CO is becoming more and more clearly [22], thus how to treat CO poisoning has been reconsidered.
Heart and brain injuries caused by CO are considered as a result from the combination of hypoxic/ischemic stress, damage around the blood vessels, and excitotoxicity. By acting on the platelets, PMNs, and Hb in the blood, CO gas stimulates N-methyl-d-aspartate (NMDA) excitatory neurons in the brain and activates neural nitric oxide synthase (nNOS) to increase the concentration of brain nitrite [36,37,38]. Dysfunction of mitochondria and excessive perivascular oxidative stress caused by CO inhibit the reuptake of glutamate. Arachidonic acid release in the neurophysiological pathway is also inhibited by CO. Hypoxic/ischemic stress followed by an increase of reactive oxygen species can cause nervous activation and present its effect on neurons due to an exacerbation of excitotoxicity [39].
Activation of platelets by CO induces the aggregation of PMNs and stimulates a degranulation to release myeloperoxidase (MPO) into the blood plasma. After the degranulation, MPO deposits on the blood vessel walls and increases the amount of nitrite production, where the expression of adhesion molecules on the endothelial cells is enhanced and PMN adhesion is thus increased. Adhered PMNs activate the production of xanthine oxidase, thereby increasing the amount of oxidants to cause brain lipid peroxidation. Products of lipid peroxidation reaction and myelin basic protein together form additives, denaturing the myelin basic protein into immunological substances, and consequently lymphocytes are primed to start the immune response in the brain. As a result, survivors from CO gas intoxication suffer from learning disabilities because of the brain damage.
CO combines with much of heme proteins (hemoglobin; Hb) with 250-times higher affinity than oxygen, shifts the oxygen dissociation curve to the left, and disturbs oxygen supply to the peripheral tissues [8, 40, 41]. CO also combines with myoglobin in the muscle; however, it is unknown whether its interaction with cellular proteins does any harm in the physiological pathway [42].
3.3 Relation with Blood Flow
CO gas permeated into the tissues promotes NO production and increases micro-circulation of the blood flow by dilatation of blood vessels in the peripheral tissues. It is recognized as a partial compensation for the hypoxic stress [43]. This vasodilatation breaks the balance of blood flow distribution in the peripheral tissues, causing a steal phenomenon, which decreases blood flow in an area to induce another hypoxia. Therefore, aerobic metabolism is hindered, and metabolic acidosis occurs in this area. In each chemical reaction of the pathological cascade, injuries of nervous system or dysfunction of various organs occur as a manifestation of the cytotoxic effects of CO as shown in Fig. 7.2 [32], which is considered to cause late-onset brain injuries. When the CO-Hb level increases to about 9%, vasodilatation of retina and choroidal vessels occurs [44] along with the systemic adverse effects, such as tissue hypoxia, kidney failure, heart failure, coma, and pulmonary edema. Also, the organs which require oxygen the most, i.e., the brain and heart, fall into dysfunction first and foremost. CO injures the tissue or nerve surrounding blood vessels, which causes neurological dysfunction or loss of function in the end (Fig. 7.3).
3.4 Neurotransmitter and Excitotoxicity
CO poisoning increases the activity of NMDA neurons and nNOS. Neurologic sequelae have been confirmed by using animal models in two processes (blood vessels around MPO deposition and excitotoxicity) [45].
3.4.1 Neurotransmitter
Upon, activated heme oxygenase-2 (HO-2) increases endogenous CO synthesis from heme, and the CO thus produced plays a neurotransmitter role as mediates nerve signal transduction by activating guanylate cyclase [46]. It has not been determined, however, whether exogenous CO, one of the sources of environmental pollution, affects the neurotransmitting function in a similar manner. HO-2/CO coordinates the secretory stimulation of vasopressin in hypothalamus, which plays a role on long-term potentiation action of the upper cervical ganglia and hippocampus [47].
3.4.2 Excitotoxicity
In CO poisoning, the excitatory neurotransmitter increases in the brain, and the receptor is activated by the excitatory amino acid such as NMDA, metabotropic, d-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and kainic acid [48]. In particular, the activation of NMDA receptor causes nervous injuries due to the excitotoxicity [46]. Then, NMDA receptor antagonism reduces the degeneration of neurons due to CO in the hippocampus and the lack of memory. The activation of NMDA induces calcium influx and stimulates nNOS (type I), which means NMDA-induced excessive NO production causes neuronal damages [45, 49]. Therefore, Ca channel blocker (nimodipine) is considered to be a treatment candidate drug for preventing nerve cell death, learning disabilities, and hippocampal pathology [50].
3.5 Cardiac Damage
The tissue with high O2 consumption has a steep oxygen gradient inside the cells, which facilitates CO reuptake into mitochondria [51]. In the most O2-sensitive tissues such as the heart, vasoconstriction is a typical disorder easily affected by an increase of CO-Hb levels. For example, coronary arterial reflux to the systemic circulation is an important factor for heart failure in addition to hypoxia [42, 52]. In the cases of severe CO poisoning, arrhythmia, cardiomyopathy, myocardial infarction, and sudden cardiac arrest may occur. Accordingly, CO poisoning could be the cause of acute cardiac death [53,54,55].
The decrease in neutrophil MPO index (MPO/cell) which is the proof of platelet–neutrophil interaction and the simultaneous increase in intravascular MPO levels is a high-risk factor for an acute coronary syndrome among the patients with moderate to severe CO poisoning [3, 56]. The patients who have suffered from an acute myocardial injury in CO poisoning carry the high risk of cardiovascular death for 10 years after exposure. To control the myocardial risks, ECG and myocardial marker in the blood plasma are useful monitors. Chest X-ray examination can be used for the evaluation of myocardial function in the case of emergency. It enables the evaluation of pulmonary injury by smoke inhalation or pulmonary congestion/alveolar infiltration due to myocardial injury [53]. Since HBOT improves the hypoxia of myocardial cells in CO poisoning, it is performed not only for the purpose of the treatment of consciousness disorder but also for the improvement of cardiac contractility.
4 Symptoms of CO Poisoning
Symptoms of CO poisoning include redness or cherry-red color of the skin, which is not specific to the disease, and some various cold-like symptoms, such as slight fever, tachycardia, tachypnea, mild headache, nausea, and coma (Table 7.5). Without knowing the fact of CO exposure, differentiating a disturbance of consciousness from other diseases such as cerebrovascular disease, diabetic coma, or drug poisoning is difficult. It may cause considerable delay in identifying the source of CO contamination or correctly diagnosing for treatment [23], which means status hearing is crucial.
Although correlation between clinical symptoms and CO-Hb levels (2–10%) is well known (Table 7.6), CO-Hb levels do not always match with the severity due to various factors including oxygen insufflations during transportation, duration of exposure, or medications which the victims take [10, 11]. Even CO exposure at 5–10% or lower CO-Hb level may cause some subtle changes in visual or auditory function, level of consciousness, occupational or learning ability [57, 58], and there may be some abnormality identified in higher brain functions by objective test such as auditory evoked potentials. These nonspecific initial symptoms appear in the course of time after the CO exposure, and as the CO-Hb level increases, it leads to respiratory insufficiency or consciousness disturbance, and finally to a circulatory collapse followed by death. Patients exposed suddenly with high concentration of CO, however, lose the consciousness immediately.
The patients with coronary artery disease may decrease exercise capacity and have early myocardial contraction frequency and symptoms of myocardial ischemia at the low CO-Hb level of 2–6% [55, 59]. In addition, exercise tolerance decreases in patients with chronic obstructive pulmonary disease [60], and complications such as angina, pulmonary edema, gastrointestinal bleeding, and acute renal failure may occur.
5 Laboratory Test of CO Poisoning
Measurement of blood CO-Hb level is a standard diagnostic test, but due to the similar optical absorption characteristics among O2-Hb, CO-Hb, and fetal hemoglobin (HbF), CO exposure cannot be identified by pulse oximetry [61, 62]. In recent years, it became possible, however, to diagnose CO poisoning before arriving the hospital using new models which can detect CO-Hb. On the other hand, metabolic acidosis and lactic acidosis, which reflects the decrease in oxygen transportation capacity, are indicators to evaluate the severity. Especially in the case of consciousness disturbance, metabolic acidosis with wide anion gap, or unexplainable lactic acidosis, it is important to suspect CO poisoning regardless of the CO-Hb levels.
In the case of acute myocardial injury showing circulatory depression due to CO exposure, both the ECG which reflects myocardial ischemia and cardiac marker levels (troponin and creatine kinase: CK-MB fraction) which reflect organ ischemia are good monitoring tools (Table 7.7). In addition, the echocardiography which is able to sensitively detect myocardial wall motions is also a useful examination tool for diagnosing myocardial injury in CO poisoning. Chest X-ray can be one of the best examinations to urgently evaluate pulmonary edema or alveolar infiltration due to smoke inhalation or myocardial injury [53].
The degree of brain damage is determined by how much a victim has been exposed to the CO gas. And consciousness disturbance or abnormal findings in CT or MRI results are considered due to the following two situations; “an initial brain injury on exposure”; and “a late-onset brain injury caused by CO residues in the tissues (delayed neuropsychological sequelae (DNS) of CO poisoning).” In brain CT (computed tomography) and MRI (magnetic resonance imaging), abnormalities of low density or high intensity are found in the wide area including the globus pallidus, putamen, thalamus, the caudate nuclei, substantia nigra, fornix, hippocampus, corpus callosum, and cortex [63,64,65]. These clinical symptoms do not always reflect abnormal findings on the images, and the nerve injuries by CO are not much completely with anatomic areas. However, since these changes are observed in the regions where oxygen required in the brain blood stream and the nerves in CO poisoning [66, 67], the neurotoxicity of CO can be assumed to coincide with the pathophysiology around the surrounding blood vessels. It is a fact that symptoms and radiographic findings disappear by the repeated HBOT. It is interesting that the prognosis is bad in a case with identified abnormality in acute CO poisoning. But, it is not clear whether HBOT is necessary until they disappear.
From research using EEG, it is said that EEG is also deeply related to the course of the CO poisoning, and EEG is a test to detect the characteristic findings of ischemic lesions in cerebral basal ganglia. As EEG test is a good prognostic indicator [3, 68], it is suggested that consciousness disturbance and the duration of CO gas exposure must be emphasized as the criteria of severity and selecting treatment methods.
6 Diagnosis of CO Poisoning
As the symptoms of CO poisoning are similar to that of a cold, CO poisoning is sometimes overlooked from unawareness of the exposure to CO gas [23]. Thus, emergency doctors should always bear the possibility of CO poisoning in their minds, and it is important to obtain the information necessary to grasp the situations such as “the family in the same house had a headache at the same time,” “the car was idling in a small garage,” or “to inhale the smoke at the fire scene” for the diagnosis. To diagnose CO poisoning, the situation of the scene will be the most important basis in addition to the CO-Hb levels and clinical signs. The classical cherry-red findings on the face and trunk are never specific, and in the case of consciousness disturbance, it is necessary to differentiate it from cerebrovascular disease, diabetic coma, or drug poisoning.
Although clinical symptoms of headache and dizziness have a constant relationship with CO-Hb levels (2–10%), it is important to keep in mind that its relationship is different in reality. Because the blood CO-Hb level declines in the course of time or due to oxygen insufflations during transportation, the case of CO-Hb levels less than 10% on arrival could be a severe CO poisoning case [69].
Since a CO-Hb level in the arterial blood is almost same as that of the venous blood, the CO-Hb level should not necessarily tested with the arterial blood. When an increase in oxygen partial pressure of venous blood is confirmed, it is expected that respiratory enzyme is inhibited due to CO poisoning. It is important to make note in the outbreak situations and the clinical symptoms. In the case of fire, it may be complicated with the symptoms of hydrogen cyanide [22], and there are more cases of mixed gas poisoning accompanying chemical choking by other gas such as hydrogen cyanide, carbon dioxide, or hydrogen sulfide. Therefore, the blood measurement of CO-Hb is important in the diagnosis.
Brain CT (computed tomography)/MRI (magnetic resonance imaging)/SPECT (the single photon emission computed tomography) have been reported in wide areas of lesions [63, 65], which is evidence in those tests (Table 7.8). CT can’t capture the changes of cerebral blood flow, but it is to detect abnormalities like edema of the basal ganglia and white matter. While MRI can detect those abnormalities in more sensitive than CT, SPECT is more sensitive to changes in white/gray matter and detection of delayed change, and better in cerebral blood flow than CT or MRI. The effect of HBOT can be assessed, with increasing sensitivity, by using CT, MRI, SPECT, respectively [22]. However, these CO-associated findings do not reflect nerve injuries and anatomical area [64]. These findings are suggested to match with pathophysiology of the blood vessels surrounding.
7 Treatment for CO Poisoning
Treatment for CO poisoning is based on general management of poisoning and is special to CO poisoning.
7.1 General Management of CO Poisoning
7.1.1 Primary Treatment for CO Poisoning
As to direct treatment for CO poisoning, pure oxygen insufflations, artificial respiration, and HBOT are used to: [1] prevent its invasion; (2) eliminate from the body; and (3) antagonize the toxins absorbed (Table 7.9). The oxygen insufflations are basic treatment, and the methods are separated into atmospheric pressure and high pressure. CO poisoning is one of the most common indications for HBOT, HBOT quickly increases the oxygenation in tissues and at the same time rapidly isolates CO from the hemoglobin, which is the most theoretical treatment.
7.1.2 Intensive Care for Life Support
As the CO poisoning complicate various diseases, in severe CO poisoning cases, the treatment includes improving or preventing cerebral edema and activating cerebral metabolism. In addition, the multimodal treatment is required to address DIC or circulation failure, and so on. HBOT improves cardiovascular diseases, reduces the mortality rate, and prevents autoimmune neurological sequela [70]. It is said to reduce the mortality rate further when using hypothermia therapy together with HBO after CO exposure, however, it is unlikely that HBOT helps to improve the prognosis when the brain has been injured by hypoxia [27].
7.2 Treatment by HBO
Since HBOT has the two effective points: (1) high oxygen partial pressure promoting the exclusion of CO from the body, and (2) high dissolved oxygen increasing oxygen supply. HBOT is used for recovery from CO poisoning and prevent intermittent-CO poisoning. HBOT or NBOT is selected according to the triage criteria by Colignon and Lamy (1986) [4], and for patients presenting with any symptoms, HBOT is performed instantly. Even if the consciousness is recovered or the electroencephalogram (EEG) are normalized, it is recommended to continue every day for 1–2 weeks to prevent late-onset of brain damage [22, 71] (Table 7.1). The therapeutic effects of HBOT have been discussed for many years; the combined treatment of HBO with thyrotropin-releasing hormone (TRH) or Ca channel blocker (Nimodipine) inhibits neuronal degeneration, learning disabilities, and hippocampal neuronal cell death caused by CO, and is meaningful as a method for treatment of acute CO poisoning [51, 72, 73]. However, there are reports that both confirm and deny the HBOT from the standpoint of evidence-based medicine.
7.2.1 Effects of HBOT
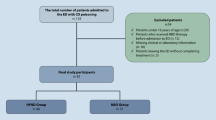
In a retrospective multicenter comparison study of HBOT and NBOT, it is difficult to unify (1) background of the patient, (2) evaluation of patient, and (3) management of HBOT (pressure, duration, and times). Effectiveness of HBOT is not determined in acute CO poisoning because evaluation criteria for therapeutic effect is unequal between each center (Fig. 7.4) [74,75,76]. As evaluation of the treatment effects differ according to the HBOT practices and implementation criteria, comparative research is never an ideal method. The prognosis and treatment of acute CO poisoning cases in Japan was surveyed by Kusuba et al., and the institutions were divided into three groups: FULL RECOVERY (where symptoms or abnormal findings disappeared completely); IMPROVED (where symptoms or abnormal findings were improved; and UNCHANGED (where any changes in symptoms or abnormal findings were confirmed), by using their own HBOT implementation criteria for acute CO poisoning. Consequently, it is concluded that HBOT is more effective than NBOT [5] (Fig. 7.5). Interestingly, it is reported that there was no difference in ratio of effective to ineffective between each rank (group) in HBOT cases, however in NBOT cases, the ratio of ineffective cases was higher than that of HBOT cases [5] (Fig. 7.6). This report minimized the difference between the comparison groups by simplifying the evaluation criteria and comparing within the same institutions, which makes it the large retrospective comparison test (RCT) with the highest reliability in the world. Hyperbaric oxygen administration has been proven to relieve the symptoms of poisoning in animal experiments [77], and it was reported that oxygen administration, brain anti-edema therapy, and brain activation therapy for recovery of consciousness were the therapeutic effects of HBOT [22, 73, 78, 79], whose active implementation was discussed. From these things, it needs to be promoted that HBOT should be prioritized over NBOT in treating severe acute CO poisoning.
Comparison of prognosis between types of treatment administered among institutions with and without an HBOT chamber [5]. (Pearson’s Chi-Square Test, n = 1667)
Prognosis of cases included in the study [5]. (Pearson’s Chi-Square Test, n = 1667)
Comparison of prognosis between groups A, B, and C among institutions equipped with HBOT chambers [5]. (Cochram-Armitage test, n = 967)
HBOT reduces the acute mortality and decreases the occurrence of neurological sequelae and the neurological injuries due to immunity; however, it does not reduce the injuries caused by hypoxia [27, 70]. Therefore, there is another idea that HBOT is effective in the patients with CO poisoning who fall under one of the following categories: with consciousness disturbance; with the CO-Hb level of more than 25%; or more than 36 years old. Considering the mechanism of hypoxia in tissue, just because the CO-Hb level decreases does not mean that it has been cured. Since a small amount of residue of the CO gas in the tissue can cause a various injuries, HBOT is considered to be a reasonable choice for severe CO poisoning.
7.2.2 Prognosis in the NBOT Group
As described in the implementation criteria for HBOT by Colingnon and Lamy [79], HBOT group is more severe than NBOT group. It is suggested that the patients with mild acute CO poisoning tend to be transported to the facilities without a HBOT instrument, and the patients with any severity, severe to mild, to the facilities with a HBOT system. Therefore, the installation of HBOT equipment makes difference in the patients’ severity. Also, according to the Cochrane review, the implementation of HBO to an acute CO poisoning only shorten the half-life of CO and is not recommended as a routine treatment [80]. Even the clinical policy of American College of Emergency Physicians, HBOT has no high-quality evidence but is just one of the choices [81]. HBOT was believed to have the same therapeutic effect as NBOT [9,10,11]; however, Kusuba et al. [5] observed the difference in the percentage of non- effectiveness by NBOT among the facilities (Fig. 7.6). To be assessed, on the other hand, no differences in both therapeutic effects of HBOT and NBOT group cannot negate the effect of HBOT therapy, which can show the prognosis of severe and mild acute CO poisoning patients could be the same. Consequently, it means the marked therapeutic effects of HBOT.
7.3 Historical Transition of HBOT
7.3.1 Efficacy of HBOT
Many clinical experiences were reported about effectiveness of HBO for CO poisoning [82,83,84]. In 1895, Ha1dane showed preventive effect of HBO for CO poisoning in animal experiments [32]. Since Smith reported the effective results of HBO in 1962 [85], HBOT became to be used for acute CO poisoning. Although HBO was recognized then as only and absolute treatment by this poisoning case, tissue damage due to CO poisoning had not fully elucidated yet, and HBOT was not generalized as an effective treatment. The HBOT triage criteria (Table 7.1) for CO poisoning was first published by Colignon and Lamy (1986) [4], which has generalized HBOT as a treatment for CO poisoning. In 1995, Ducasse et al. [74] and Thom et al. [86] reported in their two RCTs that HBO was more effective treatment than NBO in mild cases. Although one RCT was reported in 1999 [72] which denied the effect of HBO for acute CO poisoning, it had the problems that there was no consideration for the involvement of alcohol and medication and that the duration of oxygen administration was short as 60 min, even at 2.8 ATA [32]. In 2002, Weaver et al. [73] reported that HBO suppressed DNS of CO poisoning and improved its neurological prognosis better than NBOT, and reported in the RCT that HBOT has positive treatment effects for higher order functions.
After these reports, there were a report recommending HBOT [87], and other studies were conducted to compare the neuropsychological functions between the HBO groups (which received HBO treatment three times within 24 h from the discovery) and the NBO groups (which received NBO two times for 125 min as control). Compared to the NBO, it was found that HBO inhibited the intermittent-CO poisoning and improved the neurological prognosis, which has changed the understanding of the treatment in Japan, and the idea has become popular that HBOT is an effective treatment for an acute CO poisoning. This RCT was internationally valued as the most designed clinical trial in acute CO poisoning, and Sheridan et al. [88] stated that HBOT could be a prospective treatment for severe CO poisoning. However, at this time, it suggests that it is important to let experienced specialists make the judgment of effect and to accumulate the results of clinical treatment to determine the effectiveness of HBOT. The high CO-Hb level and the sustained cerebral ischemia in the early stage of acute CO poisoning may induce a delayed neuronal apoptosis, and thus it might be possible to determine the early transition to an intermittent-CO poisoning.
7.3.2 Unnecessary-Theory of HBOT
The randomized comparative test (RCT) in 1989 by Raphae1 et al. [89] was the very first relevant trial report. However, in the patients without consciousness disturbance there was no difference found between the NBO group (with 6-h pure oxygen inhalation) and the HBO group (1-h treatment under 2 ATA) in the outcome of the neurological function one month after treatment. And, in the patients with consciousness disturbance, there was no difference shown in its therapeutic effect between the patients who received the HBO treatment once and twice. In addition, Scheinkestel et al. [90] reported that about half of the CO poisoning patients who had been treated with HBOT received an additional treatment, and they considered that HBOT was not necessary but NBOT was sufficient. According to these argument which designated HBOT unnecessary, it was considered that “there is no evidence that HBO is more effective than NBO groups (22) in CO poisoning,” and also in Japan, the theory designating HBOT unnecessary became the majority. However, in the discussion about HBOT, it was shown that treatment procedures of each individual were so different that the randomized controlled trial was difficult. As HBOT was performed in only once or twice, which means the severity of patients was mild in this RCT, it seemed to be difficult to estimate the effect of HBOT [89]. According to the reports, those articles which question the evaluation of the effect of HBOT could not be the conclusion [79, 91, 92].
7.4 HBOT Procedure
Weaver et al. (2002) [73] reported that three times of HBOT within 24 h (3 ATA for 60 min at the first time, and 2 ATA for 100 min at second and third times) could suppress the incidence of neuropsychological (cognitive) failure, and had recommended the implementation of HBOT. However, it is still unknown which procedure of HBOT is the most effective for acute CO poisoning, which is one of the causes making it questionable for the effectiveness of HBOT. As a result by Weaver et al. [73], the Pan-European Committee in 2004 had put out the treatment guideline for CO poisoning. If a patient has a history of neurological symptoms or impaired consciousness, HBO (at 2.5 ATA for 90 min) treatment should be carried out for 1 to 3 times as soon as possible. If a patient is a pregnant woman or a child, also HBO is recommended even in a mild case, otherwise NBO for more than 12 h is recommended. In addition, the treatment pressure of 2.5–3.0 ATA is recommended also in the United States. In Japan, HBO for 60 min at 2 ATA is a standard HBOT procedure, which was introduced by Raphael et al. as mentioned earlier (1989) [89]. In contrast, Weaver et al. [73] insisted that it is important to perform long-running HBO with high pressure multiple times within 24 h in the early phase. This points the characteristics of their treatment procedure.
HBOT procedures for acute CO poisoning vary in Japan [55, 57,58,59], and even the combination of the therapeutic pressure and duration of HBO is not standardized internationally. In the first 24 h on the first day of arrival to hospital, HBOT is performed 1–3 times at 2.5–3.0 ATA for 60 min, and for the following days it is continuously performed at 2.0 ATA for 60 min for 7–14 times referring to clinical symptoms (Fig. 7.7). As the prognosis becomes worse, the frequency of HBOT increases. HBOT had no significant effect even when the frequency increased over 14 times. In contrast, as the frequency of HBOT of cases with complete recovery (full recovery) is as low as seven times, the rationale for HBOT frequency for acute CO poisoning is 7–14 times (Table 7.10). To address the sequelae from CO poisoning after the acute phase, patients have been followed up including high-order neurofunctional evaluation by the multidisciplinary team formed with the department of Neurosurgery, Neurology, and Rehabilitation.
7.5 Investigating Issues in the Treatment
HBO treatment method has some investigating issues. As HBOT is often performed for 7–14 times in Japan, there is expectation that the ongoing HBO might suppress the occurrence of DNS of CO poisoning. In the treatment of acute CO poisoning, HBOT has still many issues right now. It is worthy of attention as to whether HBOT can prevent DNS of CO poisoning, what can be the procedure criteria, based on what criteria the treatment can be terminated, whether the half-life of CO-Hb level or improvement of the results of CT/MRI can be the criteria, or whether NBO is inadequate as a treatment. Also, it is still unknown whether neurological sequelae can occur even if a patient is without consciousness disturbance in acute poisoning, even if the patient receives a rapid and appropriate emergency treatment [73, 86], or whether the pathological apoptosis similar to a late-onset neurological necrosis can occur by an transient cerebral ischemia [78]. As the expectations of HBOT involve the evaluation of the acute poisoning, clinical findings to guess the prognosis by many clinicians have been known empirically (Table 7.11). In the accumulation of evidence, the issues would be solved scientifically in the near future.
References
Prockop LD, Chichkova RI. Carbon monoxide intoxication: an updated review. J Neurol Sci. 2007;262:122–30.
Chung HT, Choi BM, Kwon YG, Kim YM. Interactive relations between nitric oxide (NO) and carbon monoxide (CO): heme oxygenase-1/CO pathway is a key modulator in NO-mediated antiapoptosis and anti-inflammation. Methods Enzymol. 2008;441:329–38.
Henry CR, Satran D, Lindgren B, Adkinson C, Nicholson CI, Henry TD. Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA. 2006;295:398–402.
Colignon M, Lamy M. Carbon monoxide poisoning and hyperbaric oxygen therapy. In: Schmutz J, editor. Proceedings of the 1st Swiss symposium on hyperbaric medicine. Basel: Foundation for Hyperbaric Medicine; 1986. p. 51–68.
Kusuba Y, Taki K, Ohta A. Questionnaire results of hyperbaric oxygen therapy for acute carbon monoxide poisoning in Japan. Undersea Hyperb Med. 2012;39:639–45.
Thiele GM, Tuma DJ, Willis MS, Miller JA, McDonald TL, Sorrell MF, et al. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol Clin Exp Res. 1998;22:1731–9.
Thom SR, Bhopale VM, Fisher D, Zhang J, Gimotty P. Delayed neuropathology after carbon monoxide poisoning is immune-mediated. Proc Natl Acad Sci U S A. 2004;101:13660–5.
Douglas CG, Haldane JS, Haldane JB. The laws of combination of haemoglobin with carbon monoxide and oxygen. J Physiol. 1912;44:275–304.
Weaver LK, Howe S, Hopkins R, Chan KJ. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;117:801–8.
Burney RE, Wu SC, Nemiroff MJ. Mass carbon monoxide poisoning: clinical effects and results of treatment in 184 victims. Ann Emerg Med. 1982;11:394–9.
Myers RAM, Jones DW, Britten JS. Carbon monoxide half-life study. In: Kindwall EP, editor. Proceedings of the Eighth International Congress on Hyperbaric Medicine. Flagstaff, AZ: Best Publishing; 1987. p. 263–6.
Thom SR, Xu YA, Ischiropoulos H. Vascular endothelial cells generate peroxynitrite in response to carbon monoxide exposure. Chem Res Toxicol. 1997;10:1023–31.
Thom SR, Fisher D, Xu YA, Garner S, Ischiropoulos H. Role of nitric oxide-derived oxidants in vascular injury from carbon monoxide in the rat. Am J Phys. 1999;276:H984–92.
Thom SR, Fisher D, Xu YA, Notarfrancesco K, Ischiropoulos H. Adaptive responses and apoptosis in endothelial cells exposed to carbon monoxide. Proc Natl Acad Sci U S A. 2000;97:1305–10.
Palacios-Callender M, Quintero M, Hollis VS, Springett RJ, Moncada S. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc Natl Acad Sci U S A. 2004;101:7630–5.
Xu W, Liu L, Charles IG, Moncada S. Nitric oxide induces coupling of mitochondrial signalling with the endoplasmic reticulum stress response. Nat Cell Biol. 2004;6:1129–34.
D’Amico G, Lam F, Hagen T, Moncada S. Inhibition of cellular respiration by endogenously produced carbon monoxide. J Cell Sci. 2006;119:2291–8.
Mazzola S, Forni M, Albertini M, Bacci ML, Zannoni A, Gentilini F, et al. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J. 2005;19:2045–7.
Zuckerbraun BS, Otterbein LE, Boyle P, Jaffe R, Upperman J, Zamora R, et al. Carbon monoxide protects against the development of experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2005;289:G607–13.
Emerling BM, Platanias LC, Black E, Nebreda AR, Davis RJ, Chandel NS. Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling. Mol Cell Biol. 2005;25:4853–62.
Chance B, Erecinska M, Wagner M. Mitochondrial responses to carbon monoxide toxicity. Ann N Y Acad Sci. 1970;174:193–204.
Jain KK. Carbon monoxide and other tissue poisons. Seattle: Hogrefe & Huber; 1999.
Barret L, Danel V, Faure J. Carbon monoxide poisoning, a diagnosis frequently overlooked. J Toxicol Clin Toxicol. 1985;23:309–13.
Dolinay T, Szilasi M, Liu M, Choi AM. Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med. 2004;170:613–20.
Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–8.
Piantadosi CA, Zhang J, Levin ED, Folz RJ, Schmechel DE. Apoptosis and delayed neuronal damage after carbon monoxide poisoning in the rat. Exp Neurol. 1997;147:103–14.
Gilmer B, Kilkenny J, Tomaszewski C, Watts JA. Hyperbaric oxygen does not prevent neurologic sequelae after carbon monoxide poisoning. Acad Emerg Med. 2002;9:1–8.
Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A. 2007;104:5109–14.
Brown SD, Piantadosi CA. Recovery of energy metabolism in rat brain after carbon monoxide hypoxia. J Clin Invest. 1992;89:666–72.
Thom SR. Antagonism of carbon monoxide-mediated brain lipid peroxidation by hyperbaric oxygen. Toxicol Appl Pharmacol. 1990;105:340–4.
Thom SR. Functional inhibition of leukocyte B2 integrins by hyperbaric oxygen in carbon monoxide-mediated brain injury in rats. Toxicol Appl Pharmacol. 1993;123:248–56.
Thom SR. Carbon monoxide pathophysiology and treatment. In: Neuman TS, Thom SR, editors. Physiology and medicine of hyperbaric oxygen therapy. Philadelphia: Sunders Elsevier; 2008. p. 321–48.
Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–70.
Hirayama A, Noronha-Dutra AA, Gordge MP, Neild GH, Hothersall JS. S-nitrosothiols are stored by platelets and released during platelet-neutrophil interactions. Nitric Oxide. 1999;3:95–104.
Thom SR, Bhopale VM, Han ST, Clark JM, Hardy KR. Intravascular neutrophil activation due to carbon monoxide poisoning. Am J Respir Crit Care Med. 2006;174:1239–48.
Thom SR, Fisher D, Manevich Y. Roles for platelet-activating factor and ∗NO-derived oxidants causing neutrophil adherence after CO poisoning. Am J Physiol Heart Circ Physiol. 2001;281:H923–30.
Zhao S, Zhang Y, Gu Y, Lewis DF, Wang Y. Heme oxygenase-1 mediates up-regulation of adhesion molecule expression induced by peroxynitrite in endothelial cells. J Soc Gynecol Investig. 2004;11:465–71.
Sohn HY, Krotz F, Zahler S, Gloe T, Keller M, Theisen K, et al. Crucial role of local peroxynitrite formation in neutrophil-induced endothelial cell activation. Cardiovasc Res. 2003;57:804–15.
Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14:2924–32.
Gibson QH, Olson JS, McKinnie RE, Rohlfs RJ. A kinetic description of ligand binding to sperm whale myoglobin. J Biol Chem. 1986;261:10228–39.
Haldane J. The Action of Carbonic Oxide on Man. J Physiol. 1895;18:430–62.
Favory R, Lancel S, Tissier S, Mathieu D, Decoster B, Neviere R. Myocardial dysfunction and potential cardiac hypoxia in rats induced by carbon monoxide inhalation. Am J Respir Crit Care Med. 2006;174:320–5.
Meilin S, Rogatsky GG, Thom SR, Zarchin N, Guggenheimer-Furman E, Mayevsky A. Effects of carbon monoxide on the brain may be mediated by nitric oxide. J Appl Physiol (1985). 1996;81:1078–83.
Resch H, Zawinka C, Weigert G, Schmetterer L, Garhofer G. Inhaled carbon monoxide increases retinal and choroidal blood flow in healthy humans. Invest Ophthalmol Vis Sci. 2005;46:4275–80.
Thom SR, Fisher D, Zhang J, Bhopale VM, Cameron B, Buerk DG. Neuronal nitric oxide synthase and N-methyl-D-aspartate neurons in experimental carbon monoxide poisoning. Toxicol Appl Pharmacol. 2004;194:280–95.
Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor--still lethal after eight years. Trends Neurosci. 1995;18:57–8.
Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–50.
Hiramatsu M, Yokoyama S, Nabeshima T, Kameyama T. Changes in concentrations of dopamine, serotonin, and their metabolites induced by carbon monoxide (CO) in the rat striatum as determined by in vivo microdialysis. Pharmacol Biochem Behav. 1994;48:9–15.
Rodriguez-Alvarez J, Lafon-Cazal M, Blanco I, Bockaert J. Different routes of Ca2+ influx in NMDA-mediated generation of nitric oxide and arachidonic acid. Eur J Neurosci. 1997;9:867–70.
Yang JQ, Zhou QX. Protective effect of nimodipine against cerebral injury induced by subacute carbon monoxide intoxication in mice. Acta Pharmacol Sin. 2001;22:423–7.
Jones DP, Kennedy FG. Intracellular oxygen supply during hypoxia. Am J Phys. 1982;243:C247–53.
Winston JM, Roberts RJ. Influence of carbon monoxide, hypoxic hypoxia or potassium cyanide pretreatment on acute carbon monoxide and hypoxic hypoxia lethality. J Pharmacol Exp Ther. 1975;193:713–9.
Johnson CD. Carbon monoxide toxicity with neurological and cardiac complications. Bol Asoc Med P R. 2005;97:315–22.
Hubalewska A, Pach D, Pach J, Sowa-Staszczak A, Winnik L, Huszno B. Clinical status of carbon-monoxide-poisoned patients and the results of rest 99mTc-MIBI and 99mTc-Amiscan heart scintigraphy performed in the acute phase of intoxication and stress-rest 99mTc-MIBI scintigraphy six month later. Przegl Lek. 2004;61:213–6.
Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, et al. Short-term effects of carbon monoxide exposure on the exercise performance of subjects with coronary artery disease. N Engl J Med. 1989;321:1426–32.
Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, et al. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol. 1998;31:352–8.
Luria SM, McKay CL. Effects of low levels of carbon monoxide on visions of smokers and nonsmokers. Arch Environ Health. 1979;34:38–44.
Hudnell HK, Benignus VA. Carbon monoxide exposure and human visual detection thresholds. Neurotoxicol Teratol. 1989;11:363–71.
Sheps DS, Herbst MC, Hinderliter AL, Adams KF, Ekelund LG, O'Neil JJ, et al. Production of arrhythmias by elevated carboxyhemoglobin in patients with coronary artery disease. Ann Intern Med. 1990;113:343–51.
Kurt TL, Mogielnicki RP, Chandler JE. Association of the frequency of acute cardiorespiratory complaints with ambient levels of carbon monoxide. Chest. 1978;74:10–4.
Hampson NB. Pulse oximetry in severe carbon monoxide poisoning. Chest. 1998;114:1036–41.
Perrone J, Hoffman RS. Falsely elevated carboxyhemoglobin levels secondary to fetal hemoglobin. Acad Emerg Med. 1996;3:287–9.
Gale SD, Hopkins RO, Weaver LK, Bigler ED, Booth EJ, Blatter DD. MRI, quantitative MRI, SPECT, and neuropsychological findings following carbon monoxide poisoning. Brain Inj. 1999;13:229–43.
Parkinson RB, Hopkins RO, Cleavinger HB, Weaver LK, Victoroff J, Foley JF, et al. White matter hyperintensities and neuropsychological outcome following carbon monoxide poisoning. Neurology. 2002;58:1525–32.
Porter SS, Hopkins RO, Weaver LK, Bigler ED, Blatter DD. Corpus callosum atrophy and neuropsychological outcome following carbon monoxide poisoning. Arch Clin Neuropsychol. 2002;17:195–204.
Shimosegawa E, Hatazawa J, Nagata K, Okudera T, Inugami A, Ogawa T, et al. Cerebral blood flow and glucose metabolism measurements in a patient surviving one year after carbon monoxide intoxication. J Nucl Med. 1992;33:1696–8.
Silverman CS, Brenner J, Murtagh FR. Hemorrhagic necrosis and vascular injury in carbon monoxide poisoning: MR demonstration. AJNR Am J Neuroradiol. 1993;14:168–70.
Murata M, Suzuki M, Hasegawa Y, Nohara S, Kurachi M. Improvement of occipital alpha activity by repetitive hyperbaric oxygen therapy in patients with carbon monoxide poisoning: a possible indicator for treatment efficacy. J Neurol Sci. 2005;235:69–74.
Rosenthal LD. Carbon monoxide poisoning. Immediate diagnosis and treatment are crucial to avoid complications. Am J Nurs. 2006;106:40–6.. quiz 46-47
Thom SR, Bhopale VM, Fisher D. Hyperbaric oxygen reduces delayed immune-mediated neuropathology in experimental carbon monoxide toxicity. Toxicol Appl Pharmacol. 2006;213:152–9.
Brvar M, Mozina H, Osredkar J, Mozina M, Noc M, Brucan A, et al. S100B protein in carbon monoxide poisoning: a pilot study. Resuscitation. 2004;61:357–60.
Scheinkestel CD, Bailey M, Myles PS, Jones K, Cooper DJ, Millar IL, et al. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: a randomised controlled clinical trial. Med J Aust. 1999;170:203–10.
Weaver LK, Hopkins RO, Chan KJ, Churchill S, Elliott CG, Clemmer TP, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1057–67.
Ducasse JL, Celsis P, Marc-Vergnes JP. Non-comatose patients with acute carbon monoxide poisoning: hyperbaric or normobaric oxygenation? Undersea Hyperb Med. 1995;22:9–15.
Scheinkestel CD, Bailey M, Myles PS, Jones K, Cooper DJ, Millar IL, et al. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: a randomized controlled clinical trial. Undersea Hyperb Med. 2000;27:163–4.
Weaver LK, Valentine KJ, Hopkins RO. Carbon monoxide poisoning: risk factors for cognitive sequelae and the role of hyperbaric oxygen. Am J Respir Crit Care Med. 2007;176:491–7.
Buckley NA, Juurlink DN, Isbister G, Bennett MH, Lavonas EJ. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev. 2011; CD002041.
Heckerling PS, Leikin JB, Terzian CG, Maturen A. Occult carbon monoxide poisoning in patients with neurologic illness. J Toxicol Clin Toxicol. 1990;28:29–44.
Hawkins M, Harrison J, Charters P. Severe carbon monoxide poisoning: outcome after hyperbaric oxygen therapy. Br J Anaesth. 2000;84:584–6.
Norman JN, MacIntyre J, Shearer JR, Smith G. Use of a one-man, mobile pressure chamber in the treatment of carbon monoxide poisoning. Br Med J. 1970;2:333–4.
Wolf, S. J., Lavonas, E. J., Sloan, E. P., Jagoda, A. S., and American College of Emergency, P. Clinical policy: Critical issues in the management of adult patients presenting to the emergency department with acute carbon monoxide poisoning. Ann Emerg Med. 2008;51:138–52.
Coric V, Oren DA, Wolkenberg FA, Kravitz RE. Carbon monoxide poisoning and treatment with hyperbaric oxygen in the subacute phase. J Neurol Neurosurg Psychiatry. 1998;65:245–7.
Turner M, Esaw M, Clark RJ. Carbon monoxide poisoning treated with hyperbaric oxygen: metabolic acidosis as a predictor of treatment requirements. J Accid Emerg Med. 1999;16:96–8.
Michiue T, Ishikawa T, Quan L, Li DR, Komatsu A, Zhao D, et al. Immunohistochemical distribution of single-stranded DNA in the brain in medico-legal autopsy cases of carbon monoxide intoxication. Chudoku Kenkyu. 2008;21:63–8.
Smith G. The treatment of carbon monoxide poisoning with oxygen at two atmospheres absolute. Ann Occup Hyg. 1962;5:259–63.
Thom SR, Taber RL, Mendiguren II, Clark JM, Hardy KR, Fisher AB. Delayed neuropsychologic sequelae after carbon monoxide poisoning: prevention by treatment with hyperbaric oxygen. Ann Emerg Med. 1995;25:474–80.
Turner M, Hamilton-Farrell MR, Clark RJ. Carbon monoxide poisoning: an update. J Accid Emerg Med. 1999;16:92–6.
Sheridan RL, Shank ES. Hyperbaric oxygen treatment: a brief overview of a controversial topic. J Trauma. 1999;47:426–35.
Raphael JC, Elkharrat D, Jars-Guincestre MC, Chastang C, Chasles V, Vercken JB, et al. Trial of normobaric and hyperbaric oxygen for acute carbon monoxide intoxication. Lancet. 1989;2:414–9.
Scheinkestel CD, Jones K, Myles PS, Cooper DJ, Millar IL, Tuxen DV. Where to now with carbon monoxide poisoning? Emerg Med Australas. 2004;16:151–4.
Thomas R. Carbon monoxide poisoning and hyperbaric oxygen. J Accid Emerg Med. 1999;16:461–2.
Weaver LK. Hyperbaric oxygen in carbon monoxide poisoning. BMJ. 1999;319:1083–4.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Taki, K., Goda, S. (2020). Evaluation of Hyperbaric Oxygen Therapy as a First-Line Treatment for Carbon Monoxide Poisoning. In: Shinomiya, N., Asai, Y. (eds) Hyperbaric Oxygenation Therapy. Springer, Singapore. https://doi.org/10.1007/978-981-13-7836-2_7
Download citation
DOI: https://doi.org/10.1007/978-981-13-7836-2_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7835-5
Online ISBN: 978-981-13-7836-2
eBook Packages: MedicineMedicine (R0)