Abstract
Methane (CH4), an important greenhouse gas (GHG), contributes ~33.0% to the total global GHGs emissions and accounts for 15–20% to the global warming. As the second most important human-generated GHG after CO2, CH4 is strongly linked with various climate phenomena. Most of the wetlands from tropics to temperate have been reported to have significantly enhanced emissions of CH4 during recent years. In wetland, microbial communities are a major determining factor in controlling the carbon cycle. The terrestrial wetlands are also among the key CH4 emitters and play a major role to climate change. The role of wetland expansion in CH4 emissions and its consequences on climate change and global warming might be a major concern for the future world. The methanogens and methanotrophs, two physiologically different microbial communities, seem to be crucial for future research investigations while comparing the CH4 production and consumption in wetland ecosystems. Anthropogenic disturbances related to wetlands are likely to influence the altering of microbial community composition of methanogens and methanotrophs and consequently net CH4 flux. The terrestrial wetlands have been reported to act as a source and sink for atmospheric CH4. Therefore, recent concerns about CH4 emission from terrestrial wetlands could be addressed properly because it is one of the major causes in contributing the status of CH4 in the environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Methane (CH4), a potent GHG, contributes about one third to the worldwide greenhouse gas emissions (Singh and Gupta 2016). It has 25 times more warming potential than CO2 over a 100-year time scale (Bridgham et al. 2013; Fazli et al. 2013; Forster et al. 2007), and little changes in its concentration could have a large consequences in the environment, climate and human being. Bridgham et al. (2013) reported that human alone contributes ~18% of total CH4 which makes it second most important greenhouse gas after CO2 (Singh and Strong 2016). The global warming contribution of CH4 is 15–20% (Tiwari et al. 2015). CH4 molecules that absorb the infrared radiation emitted from the earth become energized and start to emit heat in all directions (Fazli et al. 2013; Nema et al. 2012). The present concentration of CH4 is 2.5 times higher than observed in ice cores dated to the period of AD 1000–1750 (Amstel 2012). Agriculture and fossil fuel together account for 230 Tg CH4/year and are dominant natural source of methane emission, i.e. wetland is 174 (~100–231) Tg/year. Wetland emissions thus react to global warming and wetting. The anthropogenic CH4 is produced by different sources and includes energy production, landfills, waste, cattle and milk production, agriculture and biomass burning, etc. (Amstel 2012; Bridgham et al. 2013; Denman et al. 2007; Wang et al. 2004).
The CH4 emissions from the wetlands are the largest biogenic source of CH4 budget, contributing to one third of total growing atmospheric emissions from various sources (Bhullar et al. 2014; Bridgham et al. 2013). CH4, being the second most anthropogenic GHG after CO2, is strongly associated with climate feedbacks. The degree to which wetlands expansion and CH4 emissions will evolve and consequently driven climate feedbacks is thus a question of major concern. Besides, potential feedbacks between global change perturbations and CH4 emissions from wetlands, climate change, CO2 level and deposition of sulphate and nitrogen are also the major apprehensions of methane emission (Bridgham et al. 2006; Zhuang et al. 2006). In an estimation, the developing nations currently contribute approx. three-quarters of direct GHG emissions and seems to represent the fast-growing GHG emission sources in the coming decades (Boateng et al. 2017).
1.1 Wetlands and Methane Emissions
Wetlands occupy 3.8% of the Earth’s land surface, amounting to 20–40% of global CH4 emissions (Aselmann and Crutzen 1989; Ciais et al. 2013; Solomon et al. 2007). Despite of being a major source, wetlands are among the most prominent sources of unexplained spatial and temporal variability in global methane emission estimates (Bousquet et al. 2006). The main CH4 emitting sites in wetlands are the littoral zones where helophytes form a channel for methane production via sediment–root–stem–atmosphere continuum (Bergstrom et al. 2007). Bergstrom et al. (2007) reported that the dense vegetation of emergent macrophytes in natural wetlands may account 90% of the methane emission. However, it was supposed that anthropogenic sources are to be the only driver responsible for the increasing atmospheric CH4 burden from the late seventeenth century (Taylor et al. 2011). Paddy fields are one of the important sources of CH4 (Fazli et al. 2013; Tyagi et al. 2010) and responsible for 15–20% of total anthropogenic CH4 emission (Li et al. 2011; Xu et al. 2007) with an estimated 25–100 Tg CH4/year (Xu et al. 2007).
A very significant variation in CH4 emission across different types of wetland could be due to the variations in time, space and the factors operating within the wetland ecosystem (Kirschke et al. 2009; Melton et al. 2013). The main processes controlling the seasonal and inter-annual variations in wetland CH4 emission includes carbon availability, rate of decomposition, wetland inundation and temperature (Yvon-Durocher et al. 2014). Other controls are the presence of macrophytes (Laanbroek 2010), organic C decomposition rates (Miyajima et al. 1997), and pH (Singh et al. 2000), etc. Methane emitted from natural wetlands is a significant component of atmospheric methane budget. Biogeochemistry and atmospheric inversion models estimate the total wetland emissions to be 100–230 Tg CH4 /year, under the present climate condition (Denman et al. 2007; Tang et al. 2010). Although wetlands occupy only 2–6% of Earth’s land surface (Whiting and Chanton 2001), they significantly contribute a larger proportion of the total carbon stored in terrestrial reservoir (Schlesinger 1991). Zhang et al. (2017) reported that the climate change-induced enhancement in boreal wetland and tropical CH4 emissions would be the dominate anthropogenic CH4 emissions source by 38–56% at the end of the twenty-first century. The various reports suggested that climate mitigation policies must be in legislation to balance the wetland CH4 feedbacks to maintain average global warming below 2 °C (Zhang et al. 2017). The wetland may play a crucial role in atmospheric methane concentration in coming decades because of the huge stocks of organic carbon and mineral stored under anaerobic conditions in both boreal and tropical regions. In an estimate, carbon storage in histosols (wetland soil type composed of mainly organic materials) ranges from 3% to 68% of the total soil organic carbon reservoir (Post et al. 1982). The combination of elevated water tables, high productivity and lower decomposition rate has led to significant carbon storage in histosols (Gorham 1991) and contributes global methane balance.
2 Overview of the Methane Emissions and Methane-Producing Bacteria
The bacterial clusters involved in the emission and reduction are crucial in the methane flux of soil. The study explores that solutions are required to be developed to decrease the emission rate or encourage consumption of CH4 by methanotrophic bacteria to minimize its concentration from flooded soils, particularly to the rice fields.
The methanogens and methanotrophs are actively involved in the biogeochemical cycling of CH4 in soil (Fazli et al. 2013). The methanogenic bacteria are accountable for releasing CH4. They are obligate anaerobes and active in flooded, swampy areas (Pazinato et al. 2010). However, the methanotrophs are aerobic microorganisms, ubiquitous in nature and mostly active in oxic soil. Methanogens and methanotrophs have been reported from several environmental conditions likely sludge digesters (Hwang et al. 2008), lakes (Antony et al. 2012), peatland (Godin et al. 2012), freshwater and marine sediments (Newby et al. 2004) and rice soil (Fazli et al. 2013; Wang et al. 2010).
2.1 Methanogens
The methanogens are obligate anaerobes (Garcia 1990) that belong to kingdom Euryarchaeota of Archaea domain (Ferry 2010). Borrel et al. (2011) reported that methanogenic group consists 31 genera under the phylum Euryarchaeota based on 16S rRNA sequence analysis (Rosenzweig and Ragsdale 2011). Methanogens produce CH4 through diverse metabolic pathways termed as methanogenesis (Singh 2009). The methanogenesis includes acetoclastic methanogenesis and hydrogenotrophic methanogenesis pathway to release CH4, i.e. the conversion of acetate to CH4 and CO2 and H2 and CO2 to CH4, respectively (Conrad et al. 2006; Dubey 2005). In fact, methanogens are engaged in the biodegradation of organic compounds anaerobically in wetlands and rice fields (Rosenzweig and Ragsdale 2011). The 16S rRNA analyses showed that methanogenic archaea can be classified under three important groups, i.e. group I contains of Methanobacterium and Methanobrevibacter, group II comprises Methanococcus and group III includes Methanospirillum and Methanosarcina. They multiply in anaerobic environments, for example, swampy areas, sediments, flooded water, the digestive tract, etc. (Dubey 2005). Most of the methanogens thrive in mesophilic conditions and actively function from 20 to 400 °C temperature range (Dubey 2005). The methanogens have also been reported from extreme environmental conditions such as deep hydrothermal vents sustaining at temperatures >100 °C. Methanogenic Archaea generally takes acetate (contributing up to 80% of total CH4 production) as carbon source. In addition, H2/CO2 and formats also contribute 10–30% in CH4 release (Dubey 2005).
2.1.1 Methanogens in Paddy Soil
The paddy rhizosphere is a vital habitat for methanogens (Ma and Lu 2011) due to the decay of paddy roots and the liberation of H2 and CO2, which provides nutritional support to microbes (Watanabe et al. 2010). Das et al. (2011) and Datta et al. (2013) reported that higher populations of acetoclastic methanogens are found in Indian rice soil than hydrogenotrophic methanogens. The pathway of methanogenesis in rice fields has been investigated globally. But the detailed information about methanogenic population in paddy soil is limited. First of all, Rajagopal et al. (1988) isolated and characterized the methanogenic Archaea from Louisiana paddy soils and elucidated about the presence of strains similar to Methanobacterium and Methanosarcina. Joulian et al. (1997) showed the existence of methanogenic bacterial population in the paddy soils of the Philippines, France and the United States. In addition, Reichardt et al. (1997) reported that the root extracts of adult paddy plants were rich in methanogenic bacteria. Four genera Methanobacterium, Methanosarcina, Methanobrevibacter and Methanoculleus were isolated from Italian paddy fields (Fetzer et al. 1993). Asakawa et al. (1995) reported that only couple of strains (Methanobrevibacter arboriphilus and Methanosarcina mazei) have been identified in rice fields. Similarly, Adachi (1999) reported Methanobrevibacter and Methanobacterium spp. from Japanese paddy soil.
2.1.2 Methanogenesis
The CH4 is released in the anoxic layers of rice soil by methanogenic breakdown of organic substances (Dubey 2005). The anoxic conversion of organic matter takes mainly four steps: (1) action of hydrolytic organisms on polymers, (2) action of fermentative bacteria on organic compound for acid formation, (3) action of syntrophic bacteria or homoacetogenic on fermentations metabolites for acetate formation and (4) liberation of CH4 from H2/CO2, acetate, etc. Emancipation of CH4 from the organic matter also involves various important coenzymes, some of which are solely found in methanogenic archaea. At least nine methanogen-specific enzymes are used in the mechanism of CH4 removal from H2 and CO2 (Dubey 2005).
2.1.3 Factors Affecting Methane Production
Methanogens are influenced by variety of natural as well as anthropogenic factors. It has been reported that acetoclastic methanogenesis is accountable above two third of the CH4 liberation and remaining portion of CH4 is emitted by hydrogenotrophic methanogens (Das and Adhya 2012). Moreover, at elevated temperatures (40–50 °C), the phenomenon methanogenesis is shown by hydrogenotrophic methanogenic archaea. In addition, the expanding CO2 level favours hydrogenotrophic methanogenesis in the environment (Das and Adhya 2012). For instance, Wang et al. (2010) reported the following methanogenic archaea in a Chinese rice field: Methanomicrobiales, Methanosaetaceae, Zoige cluster I (ZC-I), Methanosarcinaceae and Methanocellales.
Wang et al. (2010) also stated that the types of methanogenic structure found in rice field are different due to soil type, sampling location, moisture content and temperature (Das and Adhya 2012). Sugano et al. (2005) demonstrated that before the mid-season drainage, the methanogenic communities included rice cluster I, Methanomicrobiales and Methanosarcinales, but after this period, the Methanomicrobiales were perceived. Methanomicrobiales and rice cluster I are the archaea accountable for breakdown of paddy straw under flooded environment. The water management can also influence the methanogens community composition by changing the moisture content of soil; subsequently it is an important aspect for CH4 emissions (Yao et al. 2006; Zhao et al. 2011). The alternate wetting and drying of the soil could modify the population, community structure and transcriptional functions of methanogens (Watanabe et al. 2010). Since, methanogens are more active under flooding environments as compared to dry soil (Watanabe et al. 2009). Thus, draining the soil reduces CH4 production from rice field (Khosa et al. 2011; Zhang et al. 2011). In addition, drainage might also augment the nitrous oxide (N2O) liberation (Johnson-Beebout et al. 2009; Zhao et al. 2011) due to denitrification of nitrate in anoxic and flooded situation (Fangueiro et al. 2010; Malla et al. 2005). Therefore, the issue needs more specific research to reduce the production of CH4 along with of N2O release. Ghosh et al. (2003) suggested that the use of nitrification inhibitors likely dicyandiamide might have a reducing impact on CH4 and N2O emission. Malla et al. (2005) also reported that dicyandiamide plays a significant role as a sink for CH4. Similarly, Smith et al. (1997) showed that addition of dicyandiamide after urea application could decrease N2O production up to 82%. The polymer-coated fertilizers are also potent to reduce N2O release (Akiyama et al. 2010). It has been showed that at low C:N ratio in soil improves N2O emission. As a result, C:N balance could shrink the emission, though the threshold ratio needs to be explored. The addition of fertilizers can modify the methanogens found in soil. The N fertilizer stimulates the denitrifying bacteria, which are more competent than methanogenic archaea for growth nutrients. Consequently, N fertilizers suppress CH4 production, for example, (NH4)2SO4 reduces CH4 emission than urea application (Ghosh et al. 2003).
Elevation in GHGs, especially CO2, is a serious concern. The increased concentration of CO2 in atmosphere can simultaneously decrease the methanogenic activity, reducing the CH4 oxidation in paddy fields (Das and Adhya 2012). To overcome the situation, water management could be a suppressing tool for CH4 production (Epule et al. 2011; Tyagi et al. 2010; Zhao et al. 2011). Temperature of the soil also plays an important role in CH4 production (Khalil et al. 1998; Yang and Chang 1998). Yang and Chang (1998) reported the enhanced emission of CH4 emission at temperature 4 to −37 °C. Nozhevnikova et al. (2007) also reported CH4 formation at temperature 15–20 °C in anaerobic soil.
2.2 Methanotrophs
Methanotrophs include aerobic and anaerobic CH4-oxidizing important bacterial groups. The methanotrophs have been categorized into couple of groups: type I (Gammaproteobacteria which takes CH4 adapting the RuMP pathway) and type II (Alphaproteobacteria which oxidize CH4 via the serine pathway) (Rosenzweig and Ragsdale 2011). However, Hanson and Hanson (1996) added ‘type X’ group of methanotrophic cluster, likely Methylococcus and Methylocaldum (Bowman 2006). Moreover, the type X can be considered as a subdivision of type I. Irrespective of few resemblances, the type X (having low levels of enzymes of the serine pathway) showed differences with other members of type I methanotrophs. But, information regarding the group is still lacking (Semrau et al. 2010). Methanotrophs oxidize the CH4 produced by methanogens in soil and the rhizospheric region of plants (e.g. rice) (Bodelier et al. 2005; Conrad et al. 2006) and use CH4 as sole carbon and energy source. Moreover, the CH4 consumers have a major role in regulation of CH4 production from submerged soils, such as rice fields and natural wetlands (Hoffmann et al. 2002).
2.2.1 Methanotrophy in Paddy Soil
Type I and II of methanotrophs are natural inhabitants of paddy fields and thrive in different niches based on oxygen and CH4 availability (Mayumi et al. 2010). Type I CH4 oxidizers grow in environments with high oxygen and low CH4 intensity as compared to type II methanotrophs which sustain well in poorer oxic soils (Mayumi et al. 2010). In flooded condition, the interchange of oxygen from outer environment to the root might develop an oxygen-rich environment in the root and rhizosphere which support the high growth and activity of methanotroph type II than type I (Wu et al. 2009). In the case of drained soil, the type I methanotrophs are prevalent in place of type II (Mayumi et al. 2010). Additionally, a positive correlation has been shown between methanotrophs and the age of paddy plants due to elevation in plant biomass, decrease in soil moisture content and NH4+-N concentration in tropical rice fields (Yue et al. 2007).
2.2.2 Factors Affecting Methanotrophs Activity
Methanotrophic activity is affected by various factors such as type of plants species, variety of the plants, pattern of crop rotation and other environmental constrains (Min et al. 2002; Xuan et al. 2011). The specific cultivar of rice has influenced the CH4 consuming activity and methanotrophs level in paddy roots and rhizosphere as reported by Win et al. (2011). However, another study reported that paddies have no significant impact on methanotrophs population (Wu et al. 2009). The community composition of soil methanotrophs can be affected by type and crop rotation pattern including Verrucomicrobia (Xuan et al. 2011) which might be due to the production of different root exudates affecting the soil microbial community (Doornbos et al. 2012). Wu et al. (2009) reported that type I methanotrophs are sensitive to environmental factors. However, type II methanotrophs showed more stability (Vishwakarma and Dubey 2010). The pH of the medium significantly alters the community of methanotrophs and CH4 production in soil. The optimum condition of CH4 oxidation may be between pH level 6 and 8 in paddy soil (Min et al. 2002), which ultimately assists in the alleviation of methane. Paddy soil having pH <6 needs to be adjusted for better crop productivity. Results suggested that addition of crop residues, lime, pyrite and other organic amendments may improve the population of methanotrophs in rice fields and crop productivity (Li et al. 2011; Singh et al. 2010). Amendment of N fertilizer (urea) may inhibit the methanotroph population; however, the addition of N and K together (e.g. potassium chloride) or the combination of N, P, K and crop residues stimulates the growth of methanotroph abundance (Zheng et al. 2008).
3 Mechanistic Pathways of Methane Emission
For a better understanding of the processes which involved in the process of CH4 emission from paddies, a brief introduction of plant and soil chemistry is essential. Carbon is the basic prerequisite for methanogenic growth generated from three basic sources: the death of crop root tissue, decay of both fresh organic matter and humus and carbohydrate exudates (Wassmann et al. 2000). The methanogens can produce CH4 either from the H2 or CO2 (Wassmann et al. 2000) as follows:
Or
Summary line
Schütz et al. (1989) explained CH4 emission from paddies via three pathways including diffusion (<1%), ebullition (10%) and plant-mediated transport (90%) from rice plant itself. The rice plants have an efficient gas exchange system between the anaerobic soil and the troposphere which can change the exchange pathway according to soil condition and CH4 concentration (Holzapfel-Pschorn et al. 1986; Wassmann et al. 2000). In rice growing in the temperate region, the main route of CH4 (>90%) emission is plant transport (Dubey 2005), while in the tropics, CH4 evolution takes place by the process of ebullition (transportation of gas in the form of bubbles) particularly in the early months of the season and high organic input (Dubey 2005). The process of ebullition of CH4 flux is also commonly observed in natural wetlands (Dubey 2005) and found to be significant in the case of high fertilization (Sass et al. 2000). Dubey (2005) also reported that in the case of unvegetated plant and plant with undeveloped aerenchyma, ebullition plays a key role in CH4 emission (Dubey 2005). However, CH4 emission restricted to the surface layer and the rate of emission is regulated by the concentration of CH4, porosity of the soil, temperature of the soil and plant aerenchyma (Li 2000). Methane diffusion through the soil is a very slow process as the rate of diffusion of CH4 is extremely low in liquid phase (~104 times slower than diffusion through the gas phase) and thus hardly contributes to the total CH4 flux (Aulakh et al. 2000). The CH4 diffusion phenomenon across the flooded soil and overlying water of the paddy field to the atmosphere is a function of wind speed, surface water concentration of CH4 and CH4 supply to the surface water (Dubey 2005).
4 Adaptive Measures Controlling CH4 Emission
From the centuries, European wetlands have been continuously drained for agricultural and other industrial needs. In estimation, more than 50% of all the peatlands in Europe were lost due to anthropogenic interference (Nivet and Frazier 2004; Jerman et al. 2009). However, with the increasing importance of the wetland functions, utilization and approaches towards wetland conservation have now been changed from Europe to all over the world. The major restoration strategies along these include cessation of agricultural practices, protection, conservation and re-establishment of wetland and its hydrology (Rosenthal 2003). The malpractices of wetland exploitation in agriculture in Europe have reversed to land subsidence and sequestered atmospheric CO2 as peat accretes (He et al. 2015).
Wetlands are the biggest non-anthropogenic resource of atmospheric CH4 and key global carbon reservoir. Therefore, characterizing the belowground wetland microbial communities which participate in carbon dynamics might be a broad area of research to understand the microbial importance and their responses to changing land and climate. Wetlands cover 5–8% of the total land area of the Earth (Jerman et al. 2009) and support various ecosystem services, viz. wildlife habitat, flood control, water purification, etc. Wetland, as a major terrestrial carbon reservoir, covers 20–30% of the global soil carbon pool (Jerman et al. 2009) and plays an important role in global carbon cycling. However, wetlands are continuously shrinking due to agricultural, urbanization, population growth and industrial insurgency (Jerman et al. 2009), releasing stored carbon into the atmosphere and enhancing global climate change. In addition to reversing land subsidence, the high primary production and low rate of decomposition in restored wetlands may result in a net atmospheric CO2 sequestration, allowing them to act as ‘carbon farms’.
Climate and land use changes directly affect ecosystem processes by influencing the plant community composition (Sutton-Grier and Megonigal 2011), nutrient availability, organic carbon concentration and nutrient cycling in wetlands (Mitsch et al. 2013; Petruzzella et al. 2013; Singh et al. 2018). In addition, transport of oxygen in the root tissue may alter the accessibility of oxygen in the sediment, resulting into methanogenesis suppression or CH4 oxidation (Sutton- Grier and Megonigal 2011).
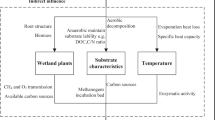
Recent concern of global warming has developed interest in the role of terrestrial ecosystems in minimizing CH4 levels (Chan and Parkin 2000). Terrestrial systems function as net sources or sinks for atmospheric CH4. Methane flux measured at the soil/atmosphere interface is the result of CH4 oxidation and methanogenesis (Knowles 1993). A negative CH4 flux (consumption of CH4 by soil) occurs when the magnitude of the CH4 uptake is larger than the process of methanogenesis and generally found in arable land, when conditions are predominately aerobic (Hansen et al. 1993). A positive CH4 flux indicates net CH4 production and occurs when the magnitude of the methanogenic process is larger than CH4 uptake and predominates in anaerobic condition such as paddies and wetlands (flooded or water saturated) (Lauren and Duxbury 1993). The process of CH4 flux is supported by soil, wetland systems and mixture of anaerobic and aerobic sites. The natural sources of CH4 include wetlands, oceans, hydrates, geological sources, termites, animals, wildfires, etc. (Fig. 5.1).
5 Conclusions and Future Prospects
This manuscript emphasizes the aspects of methanogenesis and CH4 oxidation in different wetlands and the environment. The CH4 has been recognized as one of the most important GHG in the atmosphere. Because of the strict anaerobic environment for CH4 generation, natural wetlands are considered as the main sources of biogenic CH4. Off all the wetland, tropical wetlands are the largest natural contributor of global CH4 budget. Continuous increase in atmospheric CH4 and other GHG level are predicted to raise global temperature with several implications. The assessment of climatic changes by CH4 and other GHG can be assessed only by measuring the quantity of the production, oxidation and emission of CH4 from all the natural and anthropogenic sources and characterizing their responses on the plants and animals. The available database on CH4 flux to the atmosphere is insufficient in relation to the large variety of climatological and edaphological factors that would allow to extrapolate data at a global scale and to design more precise models on the impact of the global climatic change leading to a better forecast of future state of affairs. The increasing demands of rice due to population load could lead to further expansion of the areas used for rice cultivation and, therefore, would add to higher CH4 level. As a result, rice cultivation would put a massive load on future global warming. Therefore, the research should not be focused only on rice cultivation but also in the development of technologies for better analysis of CH4 production and its oxidation. Besides, it is imperative to develop possible mitigation approaches to diminish and/or suppress emissions of this hydrocarbon in a sustainable manner.
References
Adachi K (1999) Isolation of hydrogenotrophic methanogenic archaea from a subtropical paddy field. FEMS Microbiol Ecol 30:77–85
Akiyama H, Yan X, Yag K (2010) Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta-analysis. Glob Chang Biol 16:1837–1846
Antony CP, Murrell JC, Shouche YS (2012) Molecular diversity of methanogens and identification of Methanolobus sp. as active methylotrophic archaea in Lonar Crater Lake sediments. FEMS Microbiol Ecol 81:43–51
Asakawa S, Agakawa-Matsushita M, Morii H, Yago Y, Hayano K (1995) Characterization of Methanosarcina mazei TMA isolated from a paddy field soil. Curr Microbiol 31:34–38
Aselmann I, Crutzen PJ (1989) Global distribution of natural fresh-water wetlands and rice paddies, their net primary productivity, seasonality and possible methane emissions. J Atmos Chem 8:307–358
Aulakh MS, Bodenbender J, Wassmann R, Reenberg H (2000) Methane transport capacity of rice plants. II. Variations among different rice cultivars and relationship with morphological characteristics. Nutr Cycl Agroecosyst 58:367–375
Bergstrom I, Makela S, Kankaala P, Kortelainen P (2007) Methane efflux from littoral vegetation stands of southern boreal lakes: an upscaled regional estimate. Atmos Environ 41:339–351
Bhullar GS, Edwards PJ, Venterink HO (2014) Influence of different plant species on methane emissions from soil in a restored Swiss wetland. PLoS One 9(2):e89588
Boateng KK, Obeng GY, Mensah E (2017) Rice cultivation and greenhouse gas emissions: a review and conceptual framework with reference to Ghana. Agriculture 7:1–14
Bodelier PLE, Meima-Franke M, Zwart G, Laanbroek HJ (2005) New DGGE strategies for the analyses of methanotrophic microbial communities using different combinations of existing 16S rRNA-based primers. FEMS Microbiol Ecol 52:163–174
Borrel G, Jézéquel D, Biderre-Petit C, Morel-Desrosiers N, Morel JP, Peyret P, Fonty G, Lehours AC (2011) Production and consumption of methane in freshwater lake ecosystems. Res Microbiol 162:832–847
Bousquet P et al (2006) Contribution of anthropogenic and natural sources to atmospheric methane variability. Nature 443:439–443
Bowman J (2006) The methanotrophs – the families methylococcaceae and methylocystaceae. Prokaryotes 5:266–289
Bridgham SD, Megonigal JP, Keller JK, Bliss NB, Trettin C (2006) The carbon balance of North American wetlands. Wetlands 26:889–916
Bridgham SD, Cadillo-Quiroz H, Keller JK, Zhuang Q (2013) Methane emissions from wetlands: biogeochemical, microbial, and modelling perspectives from local to global scales. Glob Chang Biol 19:1325–1346
Chan ASK, Parkin TB (2000) Evaluation of potential inhibitors of methanogenesis and methane oxidation in a landfill cover soil. Soil Biol Biochem 32:1581–1590
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V et al (2013) Carbon and other biogeochemical cycles. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, p 1535
Conrad R, Erkel C, Liesack W (2006) Rice Cluster I methanogens, an important group of Archaea producing greenhouse gas in soil. Curr Opin Biotechnol 17:262–267
Das S, Adhya TK (2012) Dynamics of methanogenesis and methanotrophy in tropical paddy soils as influenced by elevated CO2 and temperature interaction. Soil Biol Biochem 47:36–45
Das S, Ghosh A, Adhya TK (2011) Nitrous oxide and methane emission from a flooded rice field as influenced by separate and combined application of herbicides bensulfuron methyl and pretilachlor. Chemosphere 84:54–62
Datta A, Santrac SC, Adhya TK (2013) Effect of inorganic fertilizers (N, P, K) on methane emission from tropical rice field of India. Atmos Environ 66:123–130
Denman KL, Brasseur G, Chidthaisong A et al (2007) Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 541–584
Doornbos RF, Cornelis van Loon L, Bakker PAHM (2012) Impact of root exudates and plant defense signalling on bacterial communities in the rhizosphere: a review. Agron Sustain Dev 32:227–243
Dubey SK (2005) Microbial ecology of methane emission in rice agroecosystem: a review. Appl Ecol Environ Res 3(2):1–27
Epule ET, Peng C, Mafany NM (2011) Methane emissions from paddy rice fields: strategies towards achieving a win-win sustainability scenario between rice production and methane emission reduction. J Sustain Dev 4(6):188–196
Fangueiro D, Chadwick D, Dixon L, Grilo J, Walter N, Bol R (2010) Short term N2O, CH4 and CO2 production from soil sampled at different depths and amended with a fine sized slurry fraction. Chemosphere 81:100–108
Fazli P, Man HC, Shah UKM, Idris A (2013) Characteristics of methanogens and methanotrophs in rice fields: a review. Asia Pac J Mol Biol Biotechnol 21(1):3–17
Ferry JG (2010) The chemical biology of methanogenesis. Review article. Plane Space Sci 581:775–1783
Fetzer S, Bak F, Conrad R (1993) Sensitivity of methanogenic bacteria from paddy soils to oxygen and desiccation. FEMS Microbiol Ecol 12:107–115
Forster P, Ramaswamy P, Artaxo P et al (2007) Changes in atmospheric constituents and in radiative forcing. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 129–234
Garcia IL (1990) Taxonomy and ecology of methanogens. FEMS Microbiol Rev 87:297–308
Ghosh S, Majumdar D, Jain MC (2003) Methane and nitrous oxide emissions from an irrigated rice of North India. Chemosphere 51:181–195
Godin A, McLaughlin JW, Webster K, Packalen M, Basiliko N (2012) Methane and methanogen community dynamics across a boreal peatland nutrient gradient. Soil Biol Biochem 48:96–105
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182–195
Hansen S, Maehlum JE, Bakken LK (1993) N2O and CH4 fluxes in soil influenced by fertilization and tractor traffic. Soil Biol Biochem 25:621–630
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 62:439–471
He S, Malfatti SA, McFarland JW, Anderson FE, Pati A, Huntemann M, Tremblay J, Glavina del Rio T, Waldrop MP, Windham-Myers L, Tringe SG (2015) Patterns in wetland microbial community composition and functional gene repertoire associated with methane emissions. mBio 6(3):e00066–e00015
Hoffmann T, Horz HP, Kemnitz D, Conrad R (2002) Diversity of the particulate methane monooxygenase gene in methanotrophic samples from different rice field soils in China and the Philippines. Syst Appl Microbiol 25:267–274
Holzapfel-Pschorn A, Conrad R, Seiler W (1986) Effects of vegetation on the emission of methane from submerged paddy soil. Plant Soil 92:223–233
Hwang K, Shin SG, Kim J, Hwang S (2008) Methanogenic profiles by denaturing gradient gel electrophoresis using order-specific primers in anaerobic sludge digestion. Appl Microbiol Biotechnol 80(2):269–276
Jerman V, Metje M, MandicMulec I, Frenzel P (2009) Wetland restoration and methanogenesis: the activity of microbial populations and competition for substrates at different temperatures. Biogeosciences 6:1127–1138
Johnson-Beebout SE, Angeles OR, Alberto MCR, Buresh RJ (2009) Simultaneous minimization of nitrous oxide and methane emission from rice paddy soils is improbable due to redox potential changes with depth in a greenhouse experiment without plants. Geoderma 149:45–53
Joulian C, Escoffier S, Lemer J, Neue HU, Roger PA (1997) Population and potential activities of methanogens and methanotrophs in rice fields: relation with soil properties. Eur J Soil Biol 33:105–166
Khalil MAK, Rasmussen RA, Shearer MJ, Dalluge RW, Ren L, Duan CL (1998) Factors affecting methane emissions from rice fields. J Geophys Res Atmos 103(D19):25219–25231
Khosa MK, Sidhu BS, Benbi DK (2011) Methane emission from rice fields in relation to management of irrigation water. J Environ Biol 32:169–172
Kirschke S et al (2009) Methane and nitrous oxide fluxes from a farmed Swedish Histosol. Eur J Soil Sci 60:321–331
Knowles R (1993) Methane: process of production and consumption. In: Peterson GA, Baenzinger PS, Luxmoore RJ (eds) Agricultural ecosystem effects on trace gases and global climate change. ASA, Madison, pp 145–178
Laanbroek HJ (2010) Methane emission from natural wetlands: interplay between emergent macrophytes and soil microbial processes. A mini-review. Ann Bot 105:141–153
Lauren JC, Duxbury JM (1993) Methane emissions from flooded rice amended with a green manure. In: Peterson GA, Baenzinger PS, Luxmoore RJ (eds) Agricultural ecosystem effects on trace gases and global climate change. ASA, Madison, pp 183–192
Li CS (2000) Modelling trace gas emission from agricultural ecosystem. Nutr Cycl Agroecosyst 58:259–267
Li D, Liu M, Cheng Y, Wang D, Qin J, Jiao J, Li H, Hua F (2011) Methane emissions from double-rice cropping system under conventional and no tillage in southeast China. Soil Tillage Res 113:77–81
Ma K, Lu Y (2011) Regulation of microbial methane production and oxidation by intermittent drainage in rice field soil. FEMS Microbiol Ecol 75:446–456
Malla G, Bhatia A, Pathak H, Prasad S, Jain N, Singh J (2005) Mitigating nitrous oxide and methane emissions from soil in rice–wheat system of the Indo-Gangetic plain with nitrification and urease inhibitors. Chemosphere 58:141–147
Mayumi D, Yoshimoto T, Uchiyama H, Nomura N, Nakajima-Kambe T (2010) Seasonal change in methanotrophic diversity and populations in a rice field soil assessed by DNA-stable isotope probing and quantitative Real-Time PCR. Microbes Environ 25(3):156–163
Melton JR et al (2013) Present state of global wetland extent and wetland methane modelling: conclusions from a model inter- comparison project (WETCHIMP). Biogeosciences 10:753–788
Min H, Chen ZY, Wu WX, Chen MC (2002) Microbial aerobic oxidation of methane in paddy soil. Nutr Cycl Agroecosyst 64(1–2):79–85
Mitsch W, Bernal B, Nahlik A, Mander Ü, Zhang L, Anderson C, Jørgensen S, Brix H (2013) Wetlands, carbon, and climate change. Landsc Ecol 28:583–597
Miyajima T, Wada E, Hanba YT, Vijarnsorn P (1997) Anaerobic mineralization of indigenous organic matters and methanogenesis in tropical wetland soils. Geochim Cosmochim Acta 61:3739–3751
Nema P, Nema S, Roy P (2012) An overview of global climate changing in current scenario and mitigation action. Renew Sustain Energy Rev 16:2329–2336
Newby DT, Reed DW, Petzke LM, Igoe AL, Delwiche ME, Roberto FF, McKinley JP, Whiticar MJ, Colwell FS (2004) Diversity of methanotroph communities in a basalt aquifer. FEMS Microbiol Ecol 48:333–344
Nivet C, Frazier S (2004) A review of European wetland inventory information. Report prepared in the framework of “A Pilot Study towards a Pan-European Wetland Inventory”, a cooperative project between Wetlands International and the Dutch Institute for Inland Water Management and Waste Water Treatment (RIZA). pp 1–262
Nozhevnikova AN, Nekrasova V, Ammann A, Zehnder AJB, Wehrli B, Holliger C (2007) Influence of temperature and high acetate concentrations on methanogenesis in lake sediment slurries. FEMS Microbiol Ecol 62:336–344
Pazinato JM, Paulo EN, Mendes LW, Vazoller RF, Tsai SM (2010) Molecular characterization of the archaeal community in an Amazonian wetland soil and culture-dependent isolation of methanogenic archaea. Diversity 2:1026–1047
Petruzzella A, Marinho CC, Sanches LF, Minello M, Esteves FDA (2013) Magnitude and variability of methane production and concentration in tropical coastal lagoons sediments. Acta Limnol Brasil 25:341–351
Post WM, Emanuel WR, Zinke PJ, Stangen- Berger AG (1982) Soil carbon pools and world life zones. Nature 298:156–159
Rajgopal BS, Belay N, Daniel L (1988) Isolation and characterization of methanogenic bacteria from rice paddies. FEMS Microbiol Ecol 53:153–158
Reichardt W, Mascarina G, Padre B, Doll J (1997) Microbial communities of continuously cropped, irrigated rice fields. Appl Environ Microbiol 63:233–238
Rosenthal G (2003) Selecting target species to evaluate the success of wet grassland restoration. Agric Ecosyst Environ 98:227–246
Rosenzweig A, Ragsdale SW (2011) Methanogenesis. In: Methods in methane metabolism, part A: methanogenesis. Academic Press Science, p 424
Sass RL, Fischer FM Jr, Huang Y (2000) A process-based model for methane emission from irrigated rice fields: experimental basis and assumption. Nutr Cycl Agroecosyst 58:249–258
Schlesinger WH (1991) Biogeochemistry, an analysis of global change. Academic, New York, p 443
Schütz H, Holzapfel-Pschorn A, Conrad R et al (1989) A 3-year continuous record on the influence of daytime, season, and fertilizer treatment on methane emission rates from an Italian rice paddy. J Geophys Res Atmos 94(D13):16405–16416
Semrau JD, DiSpirito AA, Yoon S (2010) Methanotrophs and copper. FEMS Microbiol Rev 34:496–531
Singh SN (2009) Environmental science and engineering, climate change and crops, p 384
Singh JS, Gupta VK (2016) Degraded land restoration in reinstating CH4 sink. Front Microbiol 7:923
Singh JS, Strong PJ (2016) Biologically derived fertilizer: a multifaceted bio-tool in methane mitigation. Ecotoxicol Environ Saf 124:267–276
Singh S, Kulshreshtha K, Agnihotri S (2000) Seasonal dynamics of methane emission from wetlands. Chemosphere 2:39–46
Singh JS, Pandey VC, Singh DP, Singh RP (2010) Influence of pyrite and farmyard manure on population dynamics of soil methanotroph and rice yield in saline rain-fed paddy field. Agric Ecosyst Environ 139:74–79
Singh R, Upadhyay AK, Chandra P, Singh DP (2018) Sodium chloride incites reactive oxygen species in green algae Chlorococcum humicola and Chlorella vulgaris: implication on lipid synthesis, mineral nutrients and antioxidant system. Bioresour Technol 270:489–497
Smith KA, McTaggart IP, Tsuruta H (1997) Emissions of N2O and NO associated with nitrogen fertilization in intensive agriculture, and the potential for mitigation. Soil Biol Biochem 48:96–105
Solomon S, Qin D, Manning M, Averyt K, Marquis M (2007) Climate change 2007-the physical science basis: working group I contribution to the fourth assessment report of the IPCC (4). Cambridge University Press, Cambridge
Sugano A, Tsuchimoto H, Cho TC, Kimura M, Asakawa S (2005) Succession of methanogenic archaea in rice straw incorporated into a Japanese rice field: estimation by PCR-DGGE and sequence analyses. Archaea 1:391–397
Sutton-Grier AE, Megonigal JP (2011) Plant species traits regulate methane production in freshwater wetland soils. Soil Biol Biochem 43:413–420
Tang J, Zhuang Q, Shannon RD, White JR (2010) Quantifying wetland methane emissions with process-based models of different complexities. Biogeosciences 7:3817–3837
Taylor KE, Stouffer RJ, Meehl GA (2011) An overview of CMIP5 and the experiment design. Bull Am Meteorol Soc 93:485–498
Tiwari S, Singh JS, Singh DP (2015) Methanotrophs and CH4 sink: effect of human activity and ecological perturbations. Clim Chang Environ Sustain 3(1):35–50
Tyagi L, Kumari B, Singh SN (2010) Water management-a tool for methane mitigation from irrigated paddy fields. Sci Total Environ 408:1085–1090
Van Amstel A (2012) Methane. A review. J Integr Environ Sci 9(sup1):5–30
Vishwakarma P, Dubey SK (2010) Diversity of methanotrophs in urea-fertilized tropical rice agroecosystem. Indian J Microbiol 50:205–211
Wang JS, Logan JA, McElroy MB, Duncan BN, Megretskaia IA, Yantosca RM (2004) A 3-D model analysis of the slow down and inter annual variability in the methane growth rate from 1988 to 1997. Glob Biogeochem Cycles 18:GB3011
Wang G, Watanabe T, Jin J, Liu X, Kimura M, Asakawa S (2010) Methanogenic archaeal communities in paddy field soils in north-east China as evaluated by PCR-DGGE, sequencing and real-time PCR analyses. Soil Sci Plant Nutr 56:831–838
Wassmann R, Lantin SR, Neue H-U (eds) (2000) Methane emissions from major rice ecosystems in Asia. Kluwer Academic Publishers, Dordrecht, p 394
Watanabe T, Kimura M, Asakawa S (2009) Distinct members of a stable methanogenic archaeal community transcribe mcrA genes under flooded and drained conditions in Japanese paddy field soil. Soil Biol Biochem 41:276–285
Watanabe T, Hosen Y, Agbisit R, Llorca L, Fujita D, Asakawa S, Kimura M (2010) Changes in community structure and transcriptional activity of methanogenic archaea in a paddy field soil brought about by a water-saving practice – estimation by PCR-DGGE and qPCR of 16S rDNA and 16S rRNA. 19th World Congress of Soil Science, Soil solutions for a changing world 1–6 August 2010, Brisbane, Australia. Published on DVD
Whiting GJ, Chanton JP (2001) Greenhouse carbon balance of wetlands: methane emission versus carbon sequestration. Tellus 53B:521–528
Win KT, Nonaka R, Win AT, Sasada Y, Toyota K, Motobayashi T, Hosomi M (2011) Comparison of methanotrophic bacteria, methane oxidation activity, and methane emission in rice fields fertilized with anaerobically digested slurry between fodder rice and a normal rice variety. Paddy Water Environ. https://doi.org/10.1007/s10333-011-0279-x
Wu L, Ma K, Li Q, Ke X, Lu Y (2009) Composition of archaeal community in a paddy field as affected by rice cultivar and N fertilizer. Microb Ecol 58:819–826
Xu S, Jaffé PR, Mauzerall DL (2007) A process-based model for methane emission from flooded rice paddy systems. Ecol Model 205:475–491
Xuan DT, Guong VT, Rosling A, Alström S, Chai B, Högberg N (2011) Different crop rotation systems as drivers of change in soil bacterial community structure and yield of rice, Oryza sativa. Biol Fertil Soils. https://doi.org/10.1007/s00374-011-0618-5
Yang SS, Chang HL (1998) Effect of environmental conditions on methane production and emission from paddy soil. Agric Ecosyst Environ 69:69–80
Yao H, Wen Z, Xunhua Z, Shenghui H, Yongqiang Y (2006) Estimates of methane emissions from Chinese rice paddies by linking a model to GIS database. Acta Ecol Sin 26(4):980–988
Yue J, Shi Y, Zheng X, Huang G, Zhu J (2007) The influence of free-air CO2 enrichment on microorganisms of a paddy soil in the rice-growing season. Appl Soil Ecol 35:154–162
Yvon-Durocher G, Allen AP, Bastviken D, Conrad R, Gu-dasz C, St-Pierre A, Thanh-Duc N, del Giorgio PA (2014) Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 507:488–491
Zhang JS, Zhang FP, Yang JH, Wang JP, Cai ML, Li CF, Cao CG (2011) Emissions of N2O and NH3, and nitrogen leaching from direct seeded rice under different tillage practices in central China. Agric Ecosyst Environ 140:164–173
Zhang Z, Zimmermann NE, Stenke A, Li X, Hodson EL, Zhu G, Huang C, Poulter B (2017) Emerging role of wetland methane emissions in driving 21st century climate change. PNAS 114(36):9647–9652
Zhao X, Jia H, Cao J (2011) Study on mitigation strategies of methane emission from rice paddies in the implementation of ecological agriculture. Energy Procedia 5:2474–2480
Zheng Y, Zhang LM, Zheng YM, Di H, He JZ (2008) Abundance and community composition of methanotrophs in a Chinese paddy soil under long-term fertilization practices. J Soils Sediments 8:406–414
Zhuang Q, Melillo JM, Sarofim MC et al (2006) CO2 and CH4 exchanges between land ecosystems and the atmosphere in northern high latitudes over the 21st century. Geophys Res Lett 33:L17403
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tiwari, S., Singh, C., Singh, J.S. (2020). Wetlands: A Major Natural Source Responsible for Methane Emission. In: Upadhyay, A., Singh, R., Singh, D. (eds) Restoration of Wetland Ecosystem: A Trajectory Towards a Sustainable Environment. Springer, Singapore. https://doi.org/10.1007/978-981-13-7665-8_5
Download citation
DOI: https://doi.org/10.1007/978-981-13-7665-8_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7664-1
Online ISBN: 978-981-13-7665-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)