Abstract
Skin is a large organ that is susceptible to damage by external forces, chronic inflammation, and autoimmune reactions. In general, tissue damage causes alterations in both the configuration and type of cells in lesional skin. This phenomenon, called tissue remodeling, is a universal biological response elicited by programmed cell death, inflammation, immune disorders, and tumorigenic, tumor proliferative, and cytoreductive activity. During this process, changes in the components that comprise the extracellular matrix are required to provide an environment that facilitates tissue remodeling. Among these extracellular matrix components, periostin (a glycoprotein secreted predominantly by dermal fibroblasts) has attracted much attention. In normal skin, periostin localizes mainly in the papillary dermis and basement membrane of the epidermis. However, it is expressed at higher levels in the dermis of lesional skin of those with atopic dermatitis, scars, systemic/limited scleroderma, melanoma, and cutaneous T cell lymphoma; expression is also increased by damage caused by allergic/autoimmune responses. Furthermore, periostin induces processes that result in development of dermal fibrosis; it also activates or protracts the immune response. The aim of this review is to summarize recent knowledge about the role of periostin in the pathogenesis of dermatoses.
The material in this chapter has been adapted from Murota et al. [33] with permission.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Skin is a large organ that covers the body surface and is exposed to the extraneous environment constantly [33]. Therefore, while forming a strong barrier that prevents invasion of foreign components, the skin also functions as a site for immune responses against various external and/or internal stimuli. The skin is divided into two compartments: the epidermis and the dermis. The dermis is roughly divided into an interstitial fibrous component and a cellular component made of the interstitial component. Mainly, the interstitial component comprises collagen fibers , although it also contains other elements such as extracellular matrix (ECM ) components, elastic fibers, and substrates. In addition to physically supporting the cellular components, the ECM provides a foothold for attachment of cellular substrates [3].
Periostin, a component of the ECM , is an N-glycoprotein that was identified initially as osteoblast specific factor-2 [44]. Subsequent studies revealed that its expression is confined to the periosteum and periodontal ligament ; therefore, it was renamed periostin [17]. From the embryonic through neonatal stages of development, periostin is expressed mainly in the dermis, the basement membrane of the epidermis, and in hair follicles; however, its expression is reduced in adults, where it is limited mainly to the basement membrane, papillary dermis, and hair follicles [47]. Periostin binds to other ECM components such as type 1 collagen, fibronectin , and tenascin-C , and is involved both in maintaining the structure of the dermis and in tissue fibrosis . Interestingly, periostin harbors the characteristics of a matricellular protein. Such matricellular proteins (MPs ) are found in the non-structural components of the ECM and are important transmitters of information between the extracellular and intercellular components. Mainly, MPs are expressed during and after birth and are required for proper growth and development. Moreover, their expression is regulated in several post-natal conditions. Some MPs are expressed primarily during development and in response to injury and wound healing [24]. For example, integrins , a type of MP, are translocated to cell surfaces where they play a role in immune cell rolling and adhesion [24, 47]. Previous reports show that periostin harbors several functional domains, including a cysteine-rich EMI domain and four tandem-fascilin-like domains. With respect to the function of MPs, the EMI domain is important for association between proteins while the tandem-fascilin-like domain is important for binding to integrins αvβ3 and αvβ5 [24]. Moreover, mRNA splicing of the C-terminal domain of MPs yield several variants [24]. The type and number of these splice variants varies in each organ; however, the characteristic variant present in skin is unclear [24]. The role of periostin as a unique MP was first analyzed in studies of cardiovascular disease [6]. Subsequently, its role in diseases such as kidney fibrosis, asthma, and skin was examined. The role of periostin in skin has been recognized throughout of skin tissue repair. Mast cells play an important role in this process; histamine secreted by mast cells induces production of periostin by fibroblasts [31, 48, 55]. Interestingly, recent reports show that periostin also plays a role in atopic dermatitis , scleroderma , and skin carcinoma.
Here, we review the function of periostin in the skin and discuss recent studies of its involvement in the pathology of skin diseases.
2 Role of Periostin in Wound Healing and Scar Tissue Formation
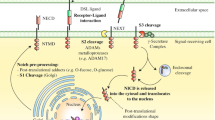
Skin damage, which is often caused by trauma, inflammation , or tumor progression, initiates a series of physiological processes, including production of humoral factors, mediators, cytokines, and growth factors. In addition, cells involved in tissue repair (e.g., mastocytes and T cells) are recruited to damaged skin; these cells then induce production of various MPs [52]. The wound healing process is divided into several phases: inflammatory, proliferative, and remodeling/regenerative [47, 52]. The inflammatory phase begins after cessation of bleeding due to formation of blood clots. During the inflammatory phase, cells necessary for tissue repair are recruited into the site of damage where they proliferate and differentiate to generate “granulation tissue”. This process is characterized by angiogenesis, tissue balls, and invasion of inflammatory cells [47, 52]. In particular, mast cells and Th2 cytokines contribute to wound healing during tissue remodeling from the inflammatory to the proliferative stages [51] (Fig. 10.1). Histamine secreted by mast cells induces production of periostin by activating extracellular signal-regulated kinase 1/2 via the H1 receptor, which is expressed by dermal fibroblasts [55]. Comprehensive DNA microarray analyses revealed that Th2 type cytokines (IL-4/IL-13) induce production of periostin by lung fibroblasts, resulting in binding of periostin to other matrix molecules such as tenascin-C , fibronectin , and collagen V [43]. Similarly, IL-4/IL-13 might contribute to production of periostin by dermal fibroblasts; however, because the expression of periostin is lower during the inflammatory phase than during other phases, it is thought that periostin is involved mainly in tissue repair from the proliferation stage onwards.
Schematic illustrating the relationships between periostin and tissue remodeling . Fibroblasts and endothelial cells secrete periostin in response to a traumatic wound. Histamines (derived mainly from degranulated mast cells), Th2 cytokines, and fibrogenic cytokines (e.g., TGFβ, CTGF, and PDGF) promote production of periostin by fibroblasts. Periostin induces differentiation of dermal fibroblasts into myofibroblasts , which then remodel the extracellular matrix . In addition, periostin, together with other matricellular proteins , activates immune cells
During the proliferative phase, an emergency matrix comprising fibrin and fibronectin forms a temporary scaffold, which is required for tissue remodeling [14]. At the end of the inflammatory phase, macrophages infiltrating the granulation tissue produce TGF-β , which promotes wound healing [4]. TGF-β activates dermal fibroblasts and induces their migration into granulation tissue where they transform into myofibroblasts ; these cells are capable of producing ECM components [15], including type I and III collagen, which are major components of the dermal ECM and contribute to maintaining skin elasticity and strength. Activated fibroblasts and myofibroblasts also produce tenascin, fibronectin, various proteoglycans, and periostin. As remarked previously, periostin binds to other ECM components and plays a role in maintaining tissue structure and in fibrosis. At the same time, it contributes to activation of dermal fibroblasts and to transformation of fibroblasts to myofibroblasts [8].
During the remodeling/regenerative phase, the surface of the granulation tissue of the skin ulcer is covered by a newly generated epidermis; this process is called re-epithelialization. In normal skin, periostin is localized in the dermis (just under the epidermis in a region termed the papillary dermis); however, after wounding it is distributed widely in all dermal layers of the scar/lesion [36]. Previously, it was estimated that periositin affects re-epithelializiation in Postn knockout mice, which exhibits delays in wound healing, and re-epithelialization in the remodeling/regenerative phase ([35, 47]). It suggests that periostin induces proliferation and differentiation of epithelial cells, resulting in appropriate re-epithelialization.
Overproduction of ECM components (e.g., type I and III collagen, periostin, and tenascin) occurs in hypertrophic scars and keloids , and the ratio of I/III collagen in these lesions is higher than that in normal scar tissue [39]. Keloid is characterized by abnormal deposition of ECM components such as versican, which is expressed at low levels in normal skin. A previous report shows extensive and intense staining of periostin in abnormal scar and keloid tissue [36]. This indicates that periostin contributes to the pathogenesis of hypertrophic scars and to keloid formation. Zhang et al. argue that expression of periostin in keloid cells is higher in hypoxic environments than under normal conditions [56]. Moreover, inhibiting periostin reduces the number and proliferative capacity of keloid cells, and production of collagen fibers, under hypoxic conditions [56]. Thus, periostin plays an important role during the proliferative and remodeling/regenerative phase of wound healing, and its aberrant expression contributes to abnormal scarring after wounding of the skin.
3 Periostin and Scleroderma
Scleroderma is an autoimmune connective tissue disease in which various organs, including the skin, become fibrotic and sclerotic [34]. Sclerosis usually begins in the deep dermis and is defined as accumulation of ECM components; this accumulation arises from a vicious cycle involving excessive synthesis and attenuated degradation of ECM components. This cycle exacerbates disease progression [34]. Deposition of ECM components such as collagen, hyaluronic acid, glycosaminoglycan, and fibronectin , destroys the original structure of the skin and impairs proper function [46]. In addition, activation of myofibroblasts and resistance of fibroblasts to apoptosis are observed. Although in scleroderma, the myofibroblast precursors in lesional skin have not been determined, interstitial fibroblasts (as well as cells such as pericytes, endothelial cells, and bone marrow -derived fibroblast-progenitor cells) can differentiate into myofibroblasts [9]. Adipocytic progenitor cells have also been identified as a source of myofibroblasts [27].
Previous reports reveal that several mediators contribute to activation of fibroblasts in skin lesions. Among these, TGF-β plays a central role in sclerosis [25]. In general, the harmful impacts of over-exposure to TGF-β are suppressed by the negative feedback function provided by the orphan nuclear receptor, NR4A1 [37]. However, in scleroderma , continuous activation of TGF-β is mediated by abnormalities in its transcriptional and post-transcriptional regulation, or reduced feedback via NR4A1. Dysfunction of the feedback loop may result in high susceptibility to fibrosis [25, 37]. Moreover, connective tissue growth factor (CTGF, also known as CCN2, a member of the CCN matrix protein family), which is produced by endothelial cells stimulated with TGFβ, endothelin-1, and angiotensin II, also promotes the skin fibrosis process in co-operation with TGF-β [1, 42, 49]. Platelet-derived growth factor (PDGF), a potent mitogen for mesenchymal cells, also plays a role in scleroderma-related fibrosis [19]. PDGF is produced by endothelial cells, platelets, macrophages, and fibroblasts. Its receptors are expressed at high levels in the skin and lungs of patients with scleroderma .
The role of periostin in the pathology of scleroderma was confirmed in animal models. In mouse models, scleroderma-like skin sclerosis is induced following subcutaneous administration of bleomycin for several weeks. Surprisingly, Postn KO mice do not develop scleroderma-like skin sclerosis and show no increase of type 1 collagen expression despite increased expressions of TGFβ and CTGF (similar to those seen in the wild type) [54]. In other words, periostin is necessary for induction of TGF-β- and CTGF-mediated type 1 collagen expression during pathogenesis of scleroderma . Furthermore, TGF-β-mediated induction of myofibroblasts does not occur in Postn KO mice [54]. Periostin acts on αv-integrin expressed by fibroblasts and induces expression of type 1 collagen via the PI3K/Akt signaling pathway [54]. This suggests that periostin creates a suitable environment for fibrosis. By contrast, a recent in vitro study investigating the effects of crenolanib (an inhibitor of PDGF receptor signaling) on the fibrotic activity of TGF-β-stimulated cultured dermal fibroblasts derived from scleroderma patients found that crenolanib attenuated expression of CTGF and periostin [26]. These results indicate that periostin orchestrates the direction of the fibrotic response mediated by TGF-β , CTGF, and PDGF.
Periostin is localized throughout the dermis of the scleroderma lesion; indeed, immunostaining for periostin in scleroderma lesions is stronger than that observed in keloid and hypertrophic scars [54]. Similarly, periostin is present throughout the dermis in individuals with morphea, which is characterized by localized skin sclerosis [22]. Interestingly, another report indicates the utility of serum periostin levels as a biomarker for scleroderma disease severity [53]. Thus, periostin is involved in both the pathogenesis and pathology of scleroderma and is a possible molecular target for scleroderma treatment.
4 Periostin and Atopic Dermatitis
Atopic dermatitis presents with clinical manifestations that are characteristic of age of onset and disease duration. The childhood atopic dermatitis in the typical clinical picture appears mainly at eczematous lesions of the ear, lower neck, elbow, and knee. Eczema is accompanied by strong itching, and scratching of which will lead to exacerbation of eczema. If disease duration lasts into adolescence, eczematous lesions and pruritus develop into chronic dermatitis, resulting in lichenification of the skin [21, 31], which is a major skin manifestation of chronic atopic dermatitis [31]. Chronic inflammation and addictive scratching result in acanthosis of the epidermis, prolongation of dermal papilla, proliferation of fibroblasts, and an increase in the number of thickened collagen fibers. Chronic intractable lesions are formed, which are characterized by basement membrane thickening and increased production of ECM components. This skin remodeling contributes to homing of inflammatory cells in the skin, leading to prolongation of chronic inflammation by inhibiting drug delivery to skin lesions. Tissue remodeling is not limited to atopic dermatitis; it is also seen during allergic inflammation of other organs (e.g., in asthma and allergic rhinitis). When managing the chronic inflammatory disease it is necessary to pay attention to both remodeling and symptomatic treatment. Mast cells play an important role in tissue remodeling, and their specific actions have been identified [29].
High numbers of mast cells are present in atopic dermatitis lesions [32]. Mast cells undergo degranulation upon antigen challenge, after infection followed by stimulation by scratching of the skin. As a result, various inflammatory mediators are released into the tissues, and then act on constituent skin cells and increase proliferation [30]. Among these mediators, histamine acts on the cells expressing histamine receptors; such cells include epidermal cells, fibroblasts, vascular endothelial cells, antigen-presenting cells (e.g., Langerhans cells, dendritic cells, and macrophages) and neurons. Histamine causes an itching sensation, recruits inflammatory cells, and triggers vasodilation and leakage of plasma into the tissues [30].
Histamine also activates the innate immune system and tissue remodeling by activating H1 receptors [29]. Engagement of these receptors on skin fibroblasts, vascular endothelial cells, Langerhans cells, and eosinophils results in production of inflammatory mediators and causes inflammatory cells to adhere to vascular endothelial cells; histamine also alters the composition of the ECM . This alteration of the ECM drives the pathogenesis of chronic inflammatory diseases by promoting infiltration by leukocytes [29, 30]. Histamine induces collagen synthesis by fibroblasts [32]; therefore, hardening of the skin is frequently observed in lichenoid lesions [21]. Indeed, in vitro studies show that histamine causes an increase in type 1 collagen synthesis by fibroblasts 48 h after stimulation, a response that might involve one or more second messengers [31].
Previous reports that used genome-wide association studies and quantitative mRNA expression analysis to explore genes related to atopic disease reveal that several MPs , including periostin, are expressed strongly at lesion sites [16, 50]. Both wild-type and Postn knockout (KO) mice develop atopic dermatitis-like skin inflammation after topical application of mite extract. However, unlike wild-type mice, Postn KO mice show a relatively milder skin phenotype in terms of acanthosis and infiltration by inflammatory cells [28].
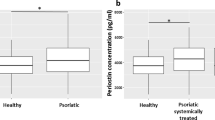
Shiraishi et al. report that periostin plays a role in chronicity of allergic skin inflammation by inducing Th2 chemokines, such as TSLP, production by fibroblasts and keratinocytes [38]. Indeed, we confirmed that large amounts of periostin are deposited in lesions in patients with atopic dermatitis [55] (Fig. 10.2). It is thought that periostin plays a role in tissue remodeling as well as in the chronic pathology of atopic dermatitis and lichenification (Fig. 10.3).
Localization of periostin in lesional skin in atopic dermatitis . A skin specimens from a normal control (non-atopic dermatitis) (upper left), from atopic dermatitis non-lesional skin (upper right), and an atopic dermatitis acute lesion (lower left) 72 h after a scratch test with Derf1 (lower right). Periostin was stained with alkaline phosphatase (red). Magnification, ×100. (This figure was reproduced from Murota et al. (with permission from Elsevier Publishing Group))
Schematic illustrating the relationship between periostin and atopic dermatitis . In allergic dermatoses, chronic inflammation triggers tissue remodeling and continuous activation of dermal fibroblasts or keratinocytes. Periostin activates keratinocytes, which then secrete chemokines such as TSLP; these cytokines induce continuous activation of immune cells
As described above, we confirmed that histamine derived from mast cells induces periostin production by fibroblasts via the H1 receptor, and that expression of type 1 collagen occurs via an autocrine pathway [55]. These new findings about the relationship between mast cells and tissue remodeling have increased our understanding of the mechanism underlying lichenification. Thus, therapeutic strategies that target mast cells could regulate tissue remodeling, which might in turn inhibit excessive expression of periostin .
5 Periostin and Melanoma
Melanoma is widely recognized as a life-threatening malignant tumor of the skin because it metastases easily to internal organs. Once melanoma gains metastatic potential and spreads to other organs, patient prognosis is poor. Thus, it is imperative that we understand how melanoma acquires metastatic and invasive characteristics. Recently, proteome and genome initiatives have increased our knowledge about several gene products such as BRAF, FAK, and ERK1, which are deregulated in invasive and metastatic melanomas [13].
In addition, the relationship between tumor cells and the surrounding ECM (called the tumoral stroma) plays an important role in invasion of proximal tissue by tumor cells and their subsequent metastasis to other organs. With respect to melanoma, analyzing the cytoskeletal structure and relationships between melanoma cells and the surrounding ECM is helpful [13]. Naka and colleagues explored factors related to development of melanoma using a quantitative proteomic analysis method called “isobaric tags for relative and absolute quantitation (iTRAQ)” [23]. They found that periostin was expressed at high levels by invasive melanomas. A relationship between melanoma and periostin was also reported by Tilman and colleagues, who found increased transcription of Postn in some melanoma cell lines [45]. Naka and colleagues observed an increased expression of periostin when melanoma cells were co-cultured with normal human dermal fibroblasts [23]. In addition, periostin derived from fibroblasts promotes proliferation of melanoma cells by increasing expression of integrins [23]. This tumor growth-promoting effect of periostin was confirmed in in vivo experiments; Postn/Rag2 double knockout mice inoculated with melanoma developed significantly smaller tumor masses than in Rag2 knockout mice [23]. Fukuda and colleagues reported that periostin acts as a chemotactic factor during metastasis of melanoma cells [11]. Their findings confirmed that periostin secreted by wounded skin promotes migration of melanoma cells into the lesion [11]. However, periostin did not affect proliferation of melanoma cells [11]. Although the impact of periostin on proliferation of melanoma cells remains unclear, these results indicate that an increase in periostin expression contributes to formation of a pro-tumor microenvironment and promotes progression of melanoma to a more serious stage. Periostin expressed in the melanoma microenvironment is thought to be derived from fibroblasts stimulated with CTGF produced by melanoma cells [18]. In summary, periostin derived from the tumor stroma appears to play a role in invasion of melanoma cells by creating a tumor microenvironment , which in turn contributes to proliferation and metastasis of melanoma cells.
The next task is to understand how MPs , including periostin, promote invasion by melanoma cells. A study by Spatz et al. reported a new animal model that closely reproduces the conditions that support melanoma invasion in vivo [40]. Such animal models will enable us to better understand how MPs involved in melanoma progression affect the motility of melanoma cells, and their interaction with the ECM , stromal cells, and blood vessels.
6 Periostin and Other Dermatoses
Several studies suggest a possible association between periostin and pathogenesis of certain dermatoses. For example, because periostin is a downstream signaling molecule for Th2 cytokines (e.g., Il-4 and Il-13 ), Bae et al. measured serum periostin levels in subjects with chronic spontaneous urticaria [5]. The results showed, somewhat surprisingly, that serum periostin levels were significantly lower in cases of severe chronic spontaneous urticaria with high levels of serum IL-13 [5]. Likewise, although the role played by periostin in the pathogenesis of dermatoses remains obscure, abnormal immunohistochemical staining for periostin has been reported in cases of pemphigus vulgaris, bullous pemphigoid, mycosis fungoides , and lichen sclerosus et atrophicus [10, 12, 20].
Mycosis fungoides (MF) comprises the majority of cutaneous lymphoma cases and accounts for up to 40% of all cutaneous lymphoma cases [41]. Usually, patients with MF exhibit a chronic clinical course and suffer persistent symptoms. Most patients with MF remain at the early patch stage; however, some are at risk of gradual progression from the patch stage to the plaque and/or tumor stage [2]. Histopathological findings suggest that the intensity of periostin-positive staining is more prominent at the early stage of disease. Recent reports identify an apparent increase in the number of tissue-infiltrating M2 macrophages in lesional skin. Monocyte-derived macrophages stimulated with periostin show phenotypic characteristics typical of tumor-associated macrophages at the early stage of MF, and express high levels of CXCL5 and CXCL10 [12]. As these chemokines affect formation of cutaneous T cell lymphoma, increased expression of periostin in the tumor microenvironment contributes to pathogenesis of MF by increasing the numbers of tissue-infiltrating macrophages.
Skin aging due to sun damage is regarded as a type of dermatoses. As mentioned above, expression of periostin is low in the papillary dermis. As skin aging continues, expression of periostin falls, resulting in less collagen production and loss of elasticity in aged skin [7].
7 Conclusion
Studies of the role of periostin in the pathogenesis of skin diseases provide important information about its actions on the ECM in the lesional microenvironment. Most interestingly, periostin affects not only tissue remodeling (as a component of the ECM), but also induces tissue inflammation during pathogenesis of several dermatoses. However, research into the role of periostin is still young. New findings in the near future will contribute to our understanding of its role in skin disease and clinical dermatoses.
Funding Source
None
Conflict of Interest
The authors declare no conflicts of interest.
References
Abraham D (2008) Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc? Rheumatology (Oxford) 47(Suppl 5):v8–v9
Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, Robson A, Calonje E, Stefanato CM, Wain EM, Wilkins B, Fields PA, Dean A, Webb K, Scarisbrick J, Morris S, Whittaker SJ (2010) Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol 28:4730–4739
Ando T, Xiao W, Gao P, Namiranian S, Matsumoto K, Tomimori Y, Hong H, Yamashita H, Kimura M, Kashiwakura J, Hata TR, Izuhara K, Gurish MF, Roers A, Rafaels NM, Barnes KC, Jamora C, Kawakami Y, Kawakami T (2014) Critical role for mast cell Stat5 activity in skin inflammation. Cell Rep 6:366–376
Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, Ross R, Sporn MB (1987) Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A 84:6020–6024
Bae Y, Izuhara K, Ohta S, Ono J, Hong GU, Ro JY, Park GH, Choi JH (2016) Periostin and Interleukin-13 are independently related to chronic spontaneous Urticaria. Allergy Asthma Immunol Res 8:457–460
Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, Arron JR, Holweg CT, Kudo A (2014) The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci 71:1279–1288
Egbert M, Ruetze M, Sattler M, Wenck H, Gallinat S, Lucius R, Weise JM (2014) The matricellular protein periostin contributes to proper collagen function and is downregulated during skin aging. J Dermatol Sci 73:40–48
Elliott CG, Forbes TL, Leask A, Hamilton DW (2015) Inflammatory microenvironment and tumor necrosis factor alpha as modulators of periostin and CCN2 expression in human non-healing skin wounds and dermal fibroblasts. Matrix Biol 43:71–84
Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ (2015) Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat Rev Nephrol 11:233–244
Fujimura T, Kakizaki A, Furudate S, Aiba S (2017) A possible interaction between periostin and CD163(+) skin-resident macrophages in pemphigus vulgaris and bullous pemphigoid. Exp Dermatol 26:1193–1198
Fukuda K, Sugihara E, Ohta S, Izuhara K, Funakoshi T, Amagai M, Saya H (2015) Periostin is a key niche component for wound metastasis of melanoma. PLoS One 10:e0129704
Furudate S, Fujimura T, Kakizaki A, Kambayashi Y, Asano M, Watabe A, Aiba S (2016) The possible interaction between periostin expressed by cancer stroma and tumor-associated macrophages in developing mycosis fungoides. Exp Dermatol 25:107–112
Gaggioli C, Sahai E (2007) Melanoma invasion – current knowledge and future directions. Pigment Cell Res 20:161–172
Greiling D, Clark RA (1997) Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci 110(Pt 7):861–870
Hinz B, Gabbiani G (2003) Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol 14:538–546
Hoffjan S, Epplen JT (2005) The genetics of atopic dermatitis: recent findings and future options. J Mol Med (Berl) 83:682–692
Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A (1999) Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14:1239–1249
Hutchenreuther J, Vincent KM, Carter DE, Postovit LM, Leask A (2015) CCN2 expression by tumor Stroma is required for melanoma metastasis. J Invest Dermatol 135:2805–2813
Iwayama T, Olson LE (2013) Involvement of PDGF in fibrosis and scleroderma: recent insights from animal models and potential therapeutic opportunities. Curr Rheumatol Rep 15:304
Kakizaki A, Fujimura T, Furudate S, Kambayashi Y, Aiba S (2015) Immunohistochemical similarities between lichen Sclerosus et Atrophicus and Morphea: a case study. Case Rep Dermatol 7:39–45
Katayama I, Yokozeki H, Nishioka K (1992) Mast-cell-derived mediators induce epidermal cell proliferation: clue for lichenified skin lesion formation in atopic dermatitis. Int Arch Allergy Immunol 98:410–414
Kim MW, Park JT, Kim JH, Koh SJ, Yoon HS, Cho S, Park HS (2017) Periostin in mature stage localized scleroderma. Ann Dermatol 29:268–275
Kotobuki Y, Yang L, Serada S, Tanemura A, Yang F, Nomura S, Kudo A, Izuhara K, Murota H, Fujimoto M, Katayama I, Naka T (2014) Periostin accelerates human malignant melanoma progression by modifying the melanoma microenvironment. Pigment Cell Melanoma Res 27:630–639
Kudo A (2011) Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci 68:3201–3207
Lafyatis R (2014) Transforming growth factor beta – at the Centre of systemic sclerosis. Nat Rev Rheumatol 10:706–719
Makino K, Makino T, Stawski L, Mantero JC, Lafyatis R, Simms R, Trojanowska M (2017) Blockade of PDGF receptors by crenolanib has therapeutic effect in patient fibroblasts and in preclinical models of systemic sclerosis. J Invest Dermatol 137:1671–1681
Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, Scherer PE, Tourtellotte WG, Varga J (2015) Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol 67:1062–1073
Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, Takeuchi S, Koike K, Ono J, Noshiro H, Furue M, Conway SJ, Narisawa Y, Izuhara K (2012) Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest 122:2590–2600
Murota H, Katayama I (2009) Emedastine difumarate: a review of its potential ameliorating effect for tissue remodeling in allergic diseases. Expert Opin Pharmacother 10:1859–1867
Murota H, Katayama I (2011) Assessment of antihistamines in the treatment of skin allergies. Curr Opin Allergy Clin Immunol 11:428–437
Murota H, Katayama I (2017) Exacerbating factors of itch in atopic dermatitis. Allergol Int 66:8–13
Murota H, Bae S, Hamasaki Y, Maruyama R, Katayama I (2008) Emedastine difumarate inhibits histamine-induced collagen synthesis in dermal fibroblasts. J Investig Allergol Clin Immunol 18:245–252
Murota H, Lingli Y, Katayama I (2017) Periostin in the pathogenesis of skin diseases. Cell Mol Life Sci 74:4321–4328
Nishioka K, Katayama I, Kondo H, Shinkai H, Ueki H, Tamaki K, Takehara K, Tajima S, Maeda M, Hayashi S, Kodama H, Miyachi Y, Mizutani H, Fujisaku A, Sasaki T, Shimizu M, Kaburagi J (1996) Epidemiological analysis of prognosis of 496 Japanese patients with progressive systemic sclerosis (SSc). Scleroderma Research Committee Japan. J Dermatol 23:677–682
Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, Fukayama M, Kudo A (2011) Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One 6(4):e18410
Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, Yang L, Fujimoto M, Arima K, Suzuki S, Murota H, Toda S, Kudo A, Conway SJ, Narisawa Y, Katayama I, Izuhara K, Naka T (2012) Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol 21:331–336
Palumbo-Zerr K, Zerr P, Distler A, Fliehr J, Mancuso R, Huang J, Mielenz D, Tomcik M, Furnrohr BG, Scholtysek C, Dees C, Beyer C, Kronke G, Metzger D, Distler O, Schett G, Distler JH (2015) Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med 21:150–158
Shiraishi H, Masuoka M, Ohta S, Suzuki S, Arima K, Taniguchi K, Aoki S, Toda S, Yoshimoto T, Inagaki N, Conway SJ, Narisawa Y, Izuhara K (2012) Periostin contributes to the pathogenesis of atopic dermatitis by inducing TSLP production from keratinocytes. Allergol Int 61:563–572
Sidgwick GP, Bayat A (2012) Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol 26:141–152
Spatz A, Batist G, Eggermont AM (2010) The biology behind prognostic factors of cutaneous melanoma. Curr Opin Oncol 22:163–168
Stadler R, Stranzenbach R (2018) Molecular pathogenesis of cutaneous lymphomas. Exp Dermatol 27:1078–1083
Stawski L, Han R, Bujor AM, Trojanowska M (2012) Angiotensin II induces skin fibrosis: a novel mouse model of dermal fibrosis. Arthritis Res Ther 14:R194
Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, Mckenzie AN, Nagai H, Hotokebuchi T, Izuhara K (2006) Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 118:98–104
Takeshita S, Kikuno R, Tezuka K, Amann E (1993) Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J 294(Pt 1):271–278
Tilman G, Mattiussi M, Brasseur F, Van Baren N, Decottignies A (2007) Human periostin gene expression in normal tissues, tumors and melanoma: evidences for periostin production by both stromal and melanoma cells. Mol Cancer 6:80
Varga J, Rudnicka L, Uitto J (1994) Connective tissue alterations in systemic sclerosis. Clin Dermatol 12:387–396
Walker JT, Mcleod K, Kim S, Conway SJ, Hamilton DW (2016) Periostin as a multifunctional modulator of the wound healing response. Cell Tissue Res 365:453–465
Weller K, Foitzik K, Paus R, Syska W, Maurer M (2006) Mast cells are required for normal healing of skin wounds in mice. FASEB J 20:2366–2368
Weng CM, Yu CC, Kuo ML, Chen BC, Lin CH (2014) Endothelin-1 induces connective tissue growth factor expression in human lung fibroblasts by ETAR-dependent JNK/AP-1 pathway. Biochem Pharmacol 88:402–411
Wood SH, Ke X, Nuttall T, Mcewan N, Ollier WE, Carter SD (2009) Genome-wide association analysis of canine atopic dermatitis and identification of disease related SNPs. Immunogenetics 61:765–772
Wynn TA (2004) Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 4:583–594
Yamaguchi Y, Yoshikawa K (2001) Cutaneous wound healing: an update. J Dermatol 28:521–534
Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z, Aihara M, Takahashi K (2013) Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br J Dermatol 168:717–725
Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, Matsui S, Kudo A, Naka T, Murota H, Katayama I (2012) Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS One 7:e41994
Yang L, Murota H, Serada S, Fujimoto M, Kudo A, Naka T, Katayama I (2014) Histamine contributes to tissue remodeling via periostin expression. J Invest Dermatol 134:2105–2113
Zhang Z, Nie F, Kang C, Chen B, Qin Z, Ma J, Ma Y, Zhao X (2014) Increased periostin expression affects the proliferation, collagen synthesis, migration and invasion of keloid fibroblasts under hypoxic conditions. Int J Mol Med 34:253–261
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kuwatsuka, Y., Murota, H. (2019). Involvement of Periostin in Skin Function and the Pathogenesis of Skin Diseases. In: Kudo, A. (eds) Periostin. Advances in Experimental Medicine and Biology, vol 1132. Springer, Singapore. https://doi.org/10.1007/978-981-13-6657-4_10
Download citation
DOI: https://doi.org/10.1007/978-981-13-6657-4_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6656-7
Online ISBN: 978-981-13-6657-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)