Abstract
Brassinosteroids (BRs), a class of steroidal hormones, play diverse roles in plant growth, development, signaling and defense against various biotic and abiotic stresses. It is broad spectrum key regulator in plants that participates in various molecular processes. Exogenous application of BRs vanish various constrains in the path of agricultural development. The present book chapter highlights the interaction and crosstalk of brassinosteroids with other phytohormones such as auxins, gibberellins, jasmonic acid, abscisic acid, salicylic acid, polyamines, ethylene and strigolactones in regulation of various physiological and developmental processes in plants. Various pathways reveal the versatile role of brassinosteroids in various hormonal interactions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Brassinosteroids (BRs) are endogenous steroidal phytohormones that have polyoxygenated structure and are found to regulate various physiological and metabolic processes at very low concentrations (Youn et al. 2018). It modulates various growth and development related processes such as microspore and seed germination, embryogenesis, regulation of cell division and differentiation, development and growth of thecae and pollen tubes, initiates flowering, regulate leaf senescence, vascular-differentiation, reproduction, root development, photomorphogenesis, and also respond to various biotic and abiotic stresses (Sreeramulu et al. 2013; Ahammed et al. 2014; Saini et al. 2015; Sharma et al. 2015, Li et al. 2016, 2017; Ahmad et al. 2018). In addition, BRs acts as important factors in stress modulation and defense in plants (Youn et al. 2018). Mutants deficient or plants insensitive to BRs exhibit a range of growth defects, including dwarf phenotypes (Vukašinović and Russinova 2018), photomorphogenesis in the dark, altered stomatal development and reduced male fertility (Ye et al. 2011; Kim et al. 2012). Because of their immense role for plant development and possible use as a tool for crop yield enhancement, BRs have attracted the attention of researchers in the past two decades. As a result, the BR signaling cascade is conceivably one of the preeminent characterized signaling pathways in plants (Youn et al. 2018). Endogenous regulation of BR is critical for various fundamental functions in plants. Furthermore, BRs act as a master regulator in plant disease resistance and defensive responses to pathogen attack. BRs also enhance tolerance to abiotic stress, including high temperature stress in a range of crop species (Ahammed et al. 2014). BRs maintain the polarization of cell membrane, proton pumping to apoplast and into a vacuole by stimulation of transmembrane ATPases, as well as increasing the efficiency of photosynthesis by increasing the level of CO2 assimilation through Rubisco activity. Previous studies reveal that stress ameliorative effects of BR are attributed to BR-induced enhancement in secondary metabolism in plants (Ahammed et al. 2013; Çoban and GöktürkBaydar 2016; Li et al. 2016). BRs concentration is found to be higher in pollen grains and immature seeds, whereas low concentration is observed in mature organs. BR mutant plants show various types of deformities, visualised as plant height reduction, dwarfism, dark green leaves, male sterility, delayed flowering, and senescence (Youn et al. 2018). They also stimulate the expression of alfa- and beta-tubulin genes and affect reorientation of cortical microtubules, which influence arrangement of cellulose microfibrils. Leaf senescence proved to be stimulated by this group of hormones as well. Application of low concentration of BRs promotes rooting whereas at higher concentrations, root inhibition was observed. Moreover, BRs regulate the processes of photo- and skotomorphogenesis (etiolation) and are known to have a positive impact on reproductive development and regulation of flowering time. Many reports have shown their significant role in both stress-protection and stress-amelioration (Bari and Jones 2009; Bajguz 2010). Physiological functions of plants and their responses to biotic and abiotic stresses are also elucidated regarding the dramatic recent progress in understanding the BRs-other phytohormones crosstalk.
2 Brassinosteroids-Auxins
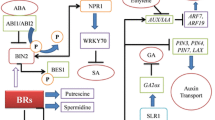
Innumerable phases of plant growth and development are regulated by BR-Auxin crosstalk (Hao et al. 2013; Saini et al. 2013; Chaiwanon and Wang 2015). Although this interaction was known for years, but the genetic and physiological evidences for exact mechanism underlying have been discovered only recently (Li et al. 2018a). These recent investigations have shown that an intact auxin signaling pathway aided by key signaling components such as BZR1 (BRASSINAZOLE-RESISTANT 1), IAAs (INDOLE-3-ACETIC ACID) and ARFs (AUXIN-RESPONSE FACTORS) is necessary for BR responses (Li et al. 2018a, b). In Arabidopsis, Oh et al. (2014) reported that BZR1 binds directly to the promoters of IAA19 and ARF7 thereby repressing the expression of IAA19 while that of ARF7 was induced (Fig. 16.1). In another investigation, microarray analysis by Youn et al. (2016) showed that in order to modify certain plant growth and developmental events, BR regulate a number of downstream target genes by using IAA19 and ARF7. Additionally, in controlling hypocotyl cell elongation, BZR1 and ARF7 besides having a usual protein-DNA interaction, these two showed a physical protein-protein interaction and co-regulates PHYB-4 ACTIVATION-TAGGED SUPPRESSOR 1 (BAS1) transcription. Moreover, BZR1 interacts directly with ARF6 (another auxin response factor). This interaction induces their mutual activity and regulates a large number of common target genes. Thus, to coordinate plant growth and development, BR and auxin pathways are integrated via its signaling components BZR1 and ARFs via multiple modes. In earlier investigations also, cooperation between BIN2 (BR INSENSITIVE 2) and ARF2 was established which showed link between BR and auxin for plant development and improvement (Vert et al. 2008). Additionally, BRX (BREVIS RADIX) which is necessary for rate limiting BR biosynthesis, positively controls the CPD (CONSTITUTIVE PHOTOMORPHOGENESIS AND DWARFISM) and DWARF4 genes (Tanaka et al. 2005). Usually, BR represses BRX expression but external application of BR can recover brx mutant defects. Reversibly, auxins strongly enhance BRX gene expression but diminishes in brx mutants. This signifies the link between BR biosynthesis and auxin signaling involving expression of BRX (Mouchel et al. 2006). In lateral root development, again BRs and auxins shows synergistic roles since BRs plays a role in initiation only and auxins helps in both initiation as well as emergence of lateral root primordia (Casimiro et al. 2001; Bhalerao et al. 2002; Benkova et al. 2003; Bao et al. 2004).
On the other hand, antagonistic role of BR and auxin has also been reported in certain aspects. In Arabidopsis, external application of auxin adequately enhances the transcript levels of DWARF4 gene which induces BRX protein to increase BR biosynthesis endogenously. However, auxin can constrain the joining of BZR1 to DWARF4 promoter (Chung et al. 2011; Yoshimitsu et al. 2011). But, when the required amount of BR has been synthesized, then BR itself causes feedback inhibition of DWARF4 (Maharjan et al. 2011). Furthermore, for optimum root growth, transcription factor BZR1 is required and is constituted mainly by three factors viz. local BR catabolism, synthesis of auxin and signaling of BR. Here, BZR1 stimulates the genes that are expressed in transition-elongation zone, but suppress genes of the quiescent centre along with stem cells that surround it. But, auxins show reversible effect to BR on spatiotemporal gene expression (Chaiwanon and Wang 2015).
3 Brassinosteroids-Abscisic Acid
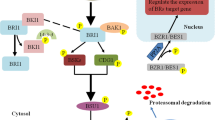
It is well acknowledged that ABA and BRs play antagonistic roles in plant growth and development. In plants, ABA inhibits seed germination and regulates seed dormancy during embryo maturation. While, BR boosts seed germination and post-germinative growth processes (Steber and McCourt 2001; Finkelstein et al. 2008; Hu and Yu 2014; Wang et al. 2018). However, physiological, biochemical and genetic studies conducted so far revealed that both BR and ABA jointly control the expression of nearly 100 genes but detailed molecular mechanism of whole crosstalk needs to be explored (Nemhauser et al. 2006; Zhang et al. 2009). Recent investigations reported physical interaction between BIN2 and ABI5 (ABSCISIC ACID-INSENSITIVE5; key ABA signaling component) where BIN2 positively controls ABA responses (Fig. 16.2). However, improper response of ABA was observed when mutant proteins were formed due to mutations on ABI5 for the BIN2 phosphorylation sites. Thereby, affirming that ABI5 is phosphorylated and stabilized by BIN2. On the other hand, when BR was applied, ABA mediated response was antagonized by controlling ABI5 by BIN2 (Hu and Yu 2014). In another study, AIB3 transcription was inhibited by the formation of transcriptional repressor complex such as BES1, TPL (TOPLESS) and HDA19 (HISTONE DEACETYLASE 19) that aids in histone deacetylation of ABI3 chromatin (Ryu et al. 2014). Furthermore, the binding of BZR1 to G-box of ABI5 promoter, suppresses the expression of ABI5 thereby increasing the sensitivity of plant to ABA. However, in the mutant bzr1-1Dthe sensitivity was reduced (Yang et al. 2016). Recently, in vitro mimicking of ABA signal transduction and RNA-sequencing analysis demonstrated that in order to control the phosphorylation of BES1, both ABI1 and ABI2 interacts as well as dephosphorylate BIN2. Analysis carried on revealed that ABA through ABA receptors promotes phosphorylation of BIN2 by suppressing ABI2. Moreover, ABA obstructs BR signaling by using primary signaling components of ABA along with its receptors and ABI2 (Wang et al. 2018).
Synergistic interactions between BR and ABA have been documented in mutant studies (Zhou et al. 2014). It was observed that both BR and ABA activated the generation ofH2O2, expression of RBOH1 (RESPIRATORY BURST OXIDASE HOMOLOG1), activity of NADPH oxidase and in conciliating heat and oxidative stress tolerance. In case of ABA-deficient mutant notabilis (not), BR enhances these responses while in BR synthesis mutant d^im, these were strong and lasted for longer time (Zhou et al. 2014).
4 Brassinosteroids-Gibberellins (GA)
In order to coordinate varied physiological processes including seed germination, stem elongation, hypocotyl elongation, expansion of leaf and hypocotyl, maturation of pollens, flowering, plant cell elongation, seedling growth etc., BRs interacts with GA (Ueguchi-Tanaka et al. 2007; Sun et al. 2010; Sun 2011; Li et al. 2012; Tong et al. 2014; Hu et al. 2017; Fig. 16.6). It is one of the best studied crosstalk between different hormones and BZR1/BES1 family plays an important role by interacting both via protein-DNA and protein-protein interactions (Vanstraelen and Benkova 2012; Li and He 2013; Li et al. 2018a, b).
In Arabidopsis and rice, during direct interaction BZR1/BES1 binds to the promoters of numerous GA metabolic genes and then controls their expression (Li et al. 2018a, b; Fig. 16.3). In Arabidopsis, to control the expression of GA biosynthetic gene GA20ox1 (GA 20-oxidase 1) both BZR1/BES1 joins to its non-E-box motif in a BR induced manner (Unterholzner et al. 2015). Alternately in rice, BZR1 promotes cell elongation by directly joining to GA20ox-2, GA30ox-2, GA2ox-3 promoters to enhance GA biosynthesis and repressing its inactivation (Tong et al. 2014). Direct interaction between BR-GA crosstalk have also been identified in numerous studies where BZR1/BES1 physically interacts with the master negative regulator of GA signaling, the DELLA proteins (Bai et al. 2012; Gallego-Bartolome et al. 2012; Li et al. 2012). In another investigation using ChIP (chromatin immune precipitation) study, of the five DELLA-encoding genes, four genes viz. RGA (REPRESSOR of GAI-3), GAI (GIBBERELLIC ACID INSENSITIVE), RGL1 and RGL3 were directly targeted by BZR1 thereby suggesting direct control of DELLA- encoding gene expression (Sun et al. 2010).
Investigations pertaining to cell expansion during photomorphogenesis revealed synergistic role of BR and GA simultaneously through the occurrence of BR-activated BZR1 and GA-inactivated DELLA transcription regulators. In the study, it was found that BR signaling is essential for GA promoted cell elongation. On the contrary, GA-deficient dwarf phenotype can be suppressed by BR or active BZR1 (Gallego-Bartolome et al. 2012). Also, in both in vitro and in vivo studies, direct interaction of DELLA with BZR1 was seen leading to the inhibition in recognizing environmental signals necessary for elongation of cell and etiolation of seedling (Bai et al. 2012; Gallego-Bartolome et al. 2012; Li and He 2013). Similar investigations for strong GA response due to presence of active BZR1 protein have also been carried on which reported that the expression of GA20ox was responsive to exogenous BR, thereby, demonstrating synergistic effects of BRs and GA (Stewart Lilley et al. 2013).
Antagonistic role of BRs and GA have also been reported in rice root immunity during root oomycete, Pythium graminicola infection. It was observed that the pathogen used BRs as virulent factors thereby controlling BR machinery in rice to inflict symptoms of disease (Nakashita et al. 2003; Bajguz and Hayat 2009). Furthermore, the above immunosuppressive effect of BRs was explained due to opposite GA crosstalk by increasing the stability of rice DELLA protein OsSLR1 (SLENDER RICE1) which acts as an important regulator of resistance for P. graminicola in rice (Li and He 2013). In another study, it was observed that the expression of OsSLR1 can be enhanced both by pathogen infection as well as by exogenous treatment of BR. Thus, these studies suggested that BRs may constrict the GAs regulated defense responses in rice by interfering in GA signaling (De Vleesschauwer et al. 2012).
5 Brassinosteroids-Jasmonic Acid (JA)
Brassinosteroids are found to promote rice plants’ susceptibility to Brown Plant Hopper (BPH) infestation by modulating the Jasmonic acid (JA) pathway (Pan et al. 2018). It was found that BR pathway was inhibited by BPH whereas JA pathway was found activated. qRT-PCR exhibited that decrease in BZR1 (BRASSINAZOLERESISTANT 1) – a BR signaling component and BRI – 1 (BR insensitive 1) -a BR receptor was observed post BPH infection (24 h). Also, for the genes (D2 and D11) related to biosynthesis of BR, similar expressions have been recorded (Feng et al. 2016). Like other phytohormones, JA also plays a role in providing defense to the plants against insects (Aljbory and Chen 2018), so investigation of genes related to such defensive ways was also done. It was found that after 24 h of BPH infection, expression of OsMYC2, OsLOX1 and OsAOS2 was found enhanced unexpectedly in rice plants which were also treated with BL. Induction of these JA related genes was observed in BR overproducing plants whereas their suppression was observed in the BR deficient plants post BPH infestation (Pan et al. 2018; Fig. 16.4).
Hormonal crosstalk of BRs with JA also plays an important role in the developmental processes of plant and its stress responses as reported by Ren et al. (2009), Campos et al. (2009), Yang et al. (2011), Kim et al. (2018) and Per et al. (2018). Nahar et al. (2013) also, expressed antagonistic interaction between BR and JA in O. sativa. It was revealed that OsDWARF and OsD11 (BR biosynthetic genes) were negatively regulated by JA in the roots of O. sativa and on the other hand, JA biosynthesis was also affected negatively where OsAOS2 expression was found down regulated. Therefore, BR biosynthesis was found suppressed by JA in a mutually antagonistic manner. Similarly, DWARF4 expression was also found negatively regulated in CoI1-dependent manner in Arabidopsis, where again BR was found to inhibit root inhibition and JA – dependent gene induction (Ren et al. 2009; Kim et al. 2011, 2013; Fig. 16.4).
Another study was conducted by He et al. (2017) regarding JA and BRs interaction where suppression in BR mediated Rice Black Streaked Dwarf Virus (RBSDV) infection was observed by the treatment of JA in rice plants. Application of Brassinazole or Methyl Jasmonate to the infected plants through foliar spray significantly reduced RBSDV infection whereas, it increased when treated with epibrassinolide. This BR mediated susceptibility and JA mediated resistance was demonstrated by using mutants- coi1-13 and Go. Efficient suppression in the expression of BR genes due to methyl jasmonate application was related to OsCO/1 (JA coreceptor) (Fig. 16.4).
Synergistic relationship of JA with BRs was also observed in enhancing the tolerance in plants against abiotic stress. In rice plants under stress, Kitanaga et al. (2006) found improvement in the jasmonic acid level due to BR. Effect of brassinazole was also found on JA level by Peng et al. (2011) where they observed anthocyanins accumulated due to JA hindrance in Arabidopsis. When effect of brassinosteroid was low, the transcript level of JA initiated signaling gene and JA biosynthesis quality genes were found down regulated but when focus of BR was high, both the transcript level of JA signaling as well as biosynthesis gene were found up regulated (Peng et al. 2011). Moreover, exogenously applied JA down regulated OSBRI1 and OsDWF4 (BR signaling and biosynthesis genes) thereby exhibiting counter communication in between JA and BR in roots of rice plants (Nahar et al. 2013).
6 Brassinosteroids-Ethylene
Brassinosteroids are found interacting with ethylene antagonistically (Banerjee and Roychoudhary 2018). Ethylene is reported to play a role in gravitropic reorientations in seedlings and in fruit ripening where such gravitropic reorientations were observed during desiccation stress (Vandenbussche et al. 2013) whereas, BRs shows negative regulation of shoot gravitropism. Buer et al. (2006) also reported the BR- ethylene antagonistic relation during root gravitropic responses and found the suppressive effect of ethylene and promotion by BRs. BRs and ethylene were also found in an antagonistic relationship in terms of regulation of AOX (alternative oxidase) activity in Carica papaya during fruit ripening (Mazorra et al. 2013). The activity of antioxidative oxidase is in response to the changes in the phytohormone-mediated signals, electron transport chain, metabolites which are associated with respiration (respiratory metabolites) and reactive oxygen species (Vanlerberghe 2013). Moreover, ethylene signaling is also regulated by RAVL1 via activating the EIL1 in rice where RAVL1 is an upstream component of brassinosteroid signaling and biosynthesis (Zhu et al. 2018; Fig. 16.5).
Effect of overproduction of ethylene by using eto1-1 (ethylene over producer 1) on other plant hormones has been also investigated (Li et al. 2018b). Hormonal contents (for various hormones) and transcript level of their associated biosynthetic genes were determine in wild type (WT) plants and 10 days old Arabidopsis eto1-1 mutant and then comparative analysis was made between these two. Overproduction of ethylene didn’t affect JA level which was found to be due to the unaltered expression of allene oxide synthase (a rate limiting JA biosynthetic gene) (Li et al. 2018a, b).
Interaction of ethylene and BRs was also observed by Zhu et al. (2016) in tomato fruits under salt stress. This interaction was mediated by H2O2, as ROS scavenger when applied, underwent significant blocking of ethylene production induced by brassinosteroids. So, due to reduction in ethylene production by using 1-MCP, the reversion in tolerance (BR-induced) to salt stress was observed thereby indicating the downstream action of ethylene to exhibit tolerance against salt stress (Banerjee and Roychoudhary 2018).
7 Brassinosteroids-Salicylic Acid (SA)
BR plays a significant role in plant response to both biotic and abiotic stress and at the same time SA shows a remedial effect during abiotic (salinity) stress (Ahmad et al. 2017) (Fig. 16.6). Studies have shown that crosstalk between BR and SA exist via non-expressor of pathogenesis-related genes 1 (NPR1); which regulates SA mediated genes involved in plant defence (Ohri et al. 2015). NPR1 is a redox- sensitive protein which is also an important component of EBR- mediated increase in salt tolerance and thermotolerance. NPR1 bring about this stress tolerance by controlling BZR1 and BIN2; which are important components of BR signaling (Divi et al. 2010). Further it has been found that NPR1 protein is not required for induction of PR-1 (PATHOGENESIS-RELATED1) gene expression mediated by EBR. This shows that BR can show anti-stress activity independently also (Divi et al. 2010). Earlier studies on tobacco plant has shown that BR increases the resistance against Oidium sp. (the fungal pathogen), Pseudomonas syringae pv. Tabaci (the bacterial pathogen) and tobacco mosaic virus (the viral pathogen) independent of SA (Nakashita et al. 2003). Similar studies on rice plant have shown that BR increases resisitance against Xanthomonas oryzae (the bacterial pathogen) and Magnaporthe grisea (the fungal pathogen) (Nakashita et al. 2003). Earlier it was thought that the Plant innate immunity was positively regulated by BR. But some studies have shown that Pythium graminicola uses BR as virulence factor and exploits BR machinery of rice plant to cause disease, which shows a negative crosstalk between BR and SA (De Vleesschauwer et al. 2012). Moreover studies have shown that suppression of SA defence responses mediated by BR occur downstream of SA biosynthesis and upstream of OsWRKY45 and NPR1 gene (De Vleesschauwer et al. 2012). SA induces the expression of Transcription factor OsWRKY45 which plays an important role in plant stress response (Huangfu et al. 2016). Studies on Brassica juncea L. seedlings has revealed that lead (Pb) toxicity is reduced by a collective effect of salicylic acid and 24-epibrassinolide, thereby advocating for modulating various metabolites (Kohli et al. 2018).
8 Brassinosteroids-Polyamines (PAs)
Brassinosteroids play an important role in stem elongation and polyamines are associated with ageing and diseases (Fig. 16.7). It has been established that Brassinosteroids signaling or biosynthesis pathways are not affected by Polyamines (Anwar et al. 2015). A crosstalk between BR and PA is at its beginning stage. But a co-application of both has shown better results in copper stress tolerance and nodulation. Studies have shown that an exogenous application of EBr and Spd can enhance Cu tolerance in radish. Their collective application reduces the Cu uptake which can be associated with down regulation of genes like RsCOPT2 (6.9-fold) and RsCOPT1 (220-fold) (Choudhary et al. 2012). RsHMA5 is another gene involved in Cu assimilation (Andres-Colas et al. 2006). It has been found that a combined application of EBr and Spd decreased the expression of RsHMA5 by 3.9-folds where as it increased the expression of RsCCH1 genes by 1.8-folds (Choudhary et al. 2012). Studies have also shown that 24-epibrassinolide (EBL) and polyamines (PAs) play an important role in the regulation of nodule formation in plants. In 2016, Lopez-Gomez et al. has reported that in response to EBL treatment to the roots there is an increase in the level of PAs in shoot which collectively suppresses the nodule formation in rhizobium-legumes. Another example of EBL and PAs crosstalk is found in growth of plants under stress. Under salt stress, EBL increases the level of spermine (Spm) which further restores growth (Lopez-Gomez et al. 2016). These studies suggest a great potential of crosstalk between BR and PAs which requires further studies to establish the modulation of the expression of various genes encoding PA enzymes and its effects on other phytohormones. Studies have also revealed that in rice, phytochelatin synthesis may be influenced by polyamines under Cd stress (Pal et al. 2017).
9 Brassinosteroids-Strigolactones (SL)
Strigolactone is a terpenoidphytohormone that plays a significant role in suppression of shoot branching (Fig. 16.8). A crosstalk between BR and SLs revolves around a common transcription factor BES1. BES1 (bri1-EMS-suppressor 1) is a positive regulator of BR signaling pathway (Yin et al. 2002). MAX2 is a key component of SL signaling which interacts with BES1 and regulates SL-responsive gene expression. Moreover, AtD14, a putative receptor of SLs degrades the transcription factor BES1. Removal of BES1 from max2-1 mutant results in suppression of branching phenotype. This shows that both BR and SLs regulate BES1 distinctly in order to control some specific developmental processes related to shoot branching (Wang et al. 2013). Formation of nodules in leguminous plants is another example where both BR and SL show positive interaction. Studies have shown that BR has a positive role in nodule formation in pea plant (Ferguson et al. 2005). Similarly, SL has also shown a positive result in development of nodules in pea plants (Soto et al. 2010; Foo and Davies 2011; Liu et al. 2013). It shows a crosstalk between BR and SL in nodule formation which is genetically controlled by AON (autoregulation of nodulation) pathway. But studies on mutant pea plants have shown that BR and SL plays a key role in nodule formation but act independent of AON pathway (Foo et al. 2014).
10 Conclusion
Brassinosteroid acts as a powerful plant growth regulator due to its involvement in various functions. The wide range of functions is accredited to its manifold targets and complex regulatory mechanisms. Serious and rigorous global efforts are being carried out in understanding the complexity of the hormonal crosstalk of BRs with other phytohormones. The pace of BR research is accelerating rapidly, and with the proliferation of cloned genes and advances in micro-chemical techniques, the range of experimental approaches in understanding BR action continues to expand. Hormonal crosstalk of BRs with other phytohormones showed growth promoting effects as well as inhibitory effects (Fig. 16.9). Although there is vast knowledge of BRs but there are unravelling interactions with these phytohormones will add new dimension to BR research in future.
References
Ahammed, G. J., Zhou, Y. H., Xia, X. J., Mao, W. H., Shi, K., & Yu, J. Q. (2013). Brassinosteroid regulates secondary metabolism in tomato towards enhanced tolerance to phenanthrene. Biologia Plantarum, 57, 154–158.
Ahammed, G. J., Xia, X. J., Li, X., Shi, K., Yu, J. Q., & Zhou, Y. H. (2014). Role of brassinosteroid in plant adaptation to abiotic stresses and its interpay with other hormones. Current Protein and Peptide Science, 16, 462–473.
Ahmad, F., Singh, A., & Kamal, A. (2017). Ameliorative effect of salicylic acid in salinity stressed Pisum sativum by improving growth parameters, activating photosynthesis and enhancing antioxidant defense system. Bioscience Biotechnology Research Communications, 10, 481–490.
Ahmad, F., Singh, A., & Kamal, A. (2018). Crosstalk of brassinosteroids with other phytohormones under various abiotic stresses. Journal of Applied Biology and Biotechnology, 6, 56–62.
Aljbory, Z., & Chen, M. S. (2018). Indirect plant defense against insect herbivores: A review. Insect Sci, 25, 2–23.
Andres-Colas, N., Sancenon, V., Rodriguez-Navarro, S., Mayo, S., Thiele, D. J., Ecker, J. R., Puig, S., & Penarrubia, L. (2006). The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. The Plant Journal, 45, 225–236.
Anwar, R., Mattoo, A. K., & Handa, A. K. (2015). Polyamine interactions with plant hormones: Crosstalk at several levels. Polyamines (pp. 267–302). Tokyo: Springer.
Bai, M. Y., Shang, J. X., Oh, E., Fan, M., Bai, Y., Zentella, R., Sun, T. P., & Wang, Z. Y. (2012). Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nature Cell Biology, 14, 810–817.
Bajguz, A. (2010). An enhancing effect of exogenous brassinolide on the growth and antioxidant activity in Chlorella vulgaris cultures under heavy metals stress. Environmental and Experimental Botany, 68, 175–179.
Bajguz, A., & Hayat, S. (2009). Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiology and Biochemistry, 47, 1–8.
Banerjee, A., & Roychoudhury, A. (2018). Interactions of brassinosteroids with major phytohormones: Antagonistic effects. Journal of Plant Growth Regulation, 37(4), 1025–1032. https://doi.org/10.1007/s00344-018-9828-5.
Bao, F., Shen, J., Brady, S. R., Muday, G. K., Asami, T., & Yang, Z. (2004). Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiology, 134, 1624–1631.
Bari, R., & Jones, J. D. G. (2009). Role of plant hormones in plant defence responses. Plant Molecular Biology, 69, 473–488.
Benkova, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertova, D., Jurgens, G., & Friml, J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell, 115, 591–602.
Bhalerao, R. P., Eklof, J., Ljung, K., Marchant, A., Bennett, M., & Sandberg, G. (2002). Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. The Plant Journal, 29, 325–332.
Buer, C. S., Sukumar, P., & Muday, G. K. (2006). Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiology, 140, 1384–1396.
Campos, M. L., DeAlmeida, M., Rossi, M. L., Martinelli, A. P., Junior, C. G., Figueira, A., Rampelotti-Ferreira, F. T., Vendramim, J. D., Benedito, V. A., & Peres, L. E. (2009). Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. Journal of Experimental Botany, 60, 4347–4361.
Casimiro, I., Marchant, A., Bhalerao, R. P., Beeckman, T., Dhooge, S., Swarup, R., Graham, N., Inzé, D., Sandberg, G., Casero, P. J., & Bennett, M. (2001). Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell, 13, 843–852.
Chaiwanon, J., & Wang, Z. Y. (2015). Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Current Biology, 25, 1031–1042.
Choudhary, S. P., Oral, H. V., Bhardwaj, R., Yu, J. Q., & Tran, L. S. P. (2012). Interaction of brassinosteroids and polyamines enhances copper stress tolerance in Raphanus sativus. Journal of Experimental Botany, 63, 5659–5675.
Chung, Y., Maharjan, P. M., Lee, O., Fujioka, S., Jang, S., Kim, B., Takatsuto, S., Tsujimoto, M., Kim, H., Cho, H., Hwang, I., & Choe, S. (2011). Auxin stimulates DWARF4 expression and brassinosteroid biosynthesis in Arabidopsis. The Plant Journal, 66, 564–578.
Çoban, O., & Göktürk Baydar, N. (2016). Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (Mentha piperita L.) under salt stress. Industrial Crops and Products, 86, 251–258.
De Vleesschauwer, D., Buyten, V. E., Satoh, K., Balidion, J., Mauleon, R., Choi, I. I.-R., Vera-Cruz, C., Kikuchi, S., & Hofte, M. (2012). Brassinosteroids antagonize gibberellin– and salicylate-mediated root immunity in rice. Plant Physiology, 158, 1833–1846.
Divi, U. K., Rahman, T., & Krishna, P. (2010). Brassinosteroid mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biology, 10, 151.
Feng, Z., Wu, C., Wang, C., Roh, J., Zhang, L., Chen, J., Zhang, S., Zhang, H., Yang, C., Hu, J., You, X., Liu, X., Yang, X., Guo, X., Zhang, X., Wu, F., Terzaghi, W., Kim, S.-K., Jiang, L., & Wan, J. (2016). SLG controls grain size and leaf angle by modulating brassinosteroid homeostasis in rice. Journal of Experimental Botany, 67, 4241–4253.
Ferguson, B. J., Ross, J. J., & Reid, J. B. (2005). Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiology, 138, 2396–2405.
Finkelstein, R., Reeves, W., Ariizumi, T., & Steber, C. (2008). Molecular aspects of seed dormancy. Annual Review of Plant Biology, 59, 387–415.
Foo, E., & Davies, N. W. (2011). Strigolactones promote nodulation in pea. Planta, 234, 1073–1081.
Foo, E., Ferguson, B. J., & Reid, J. B. (2014). The potential roles of strigolactones and brassinosteroids in the autoregulation of nodulation pathway. Annals of Botany, 113, 1037–1045.
Gallego-Bartolome, J., Minguet, E. G., Grau-Enguix, F., Abbas, M., Locascio, A., Thomas, S. G., Alabadi, D., & Blazquez, M. A. (2012). Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proceedings of the National Academy of Sciences, 109, 13446–13451.
Hao, J., Yin, Y., & Fei, S.-Z. (2013). Brassinosteroid signaling network: Implications on yield and stress tolerance. Plant Cell Reports, 32, 1017–1030.
He, Y., Zhang, H., Sun, Z., Li, J., Hong, G., Zhu, Q., Zhou, X., MacFarlane, S., Yan, F., & Chen, J. (2017). Jasmonic acid-mediated defense suppresses brassinosteroid-mediated susceptibility to rice black streaked dwarf virus infection in rice. The New Phytologist, 214, 388–399.
Hu, Y., & Yu, D. (2014). BRASSINOSTEROID INSENSITIVE2 interacts with ABSCISIC ACID INSENSITIVE5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in Arabidopsis. Plant Cell, 26, 4394–4408.
Hu, S., Wang, C., Sanchez, D. L., Lipka, A. E., Liu, P., Yin, Y., Blanco, M., & Lubberstedt, T. (2017). Gibberellins promote brassinosteroids action and both increase heterosis for plant height in maize (Zea mays L.). Frontiers in Plant Science, 8, 1039.
Huangfu, J., Li, J., Li, R., Ye, M., Kuai, P., Zhang, T., & Lou, Y. (2016). The transcription factor OsWRKY45 negatively modulates the resistance of rice to the brown plant hopper Nilaparvata lugens. International Journal of Molecular Sciences, 17, 697.
Kim, T. W., Guan, S., Burlingame, A. L., & Wang, Z. Y. (2011). The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Molecular Cell, 43, 561–571.
Kim, T. W., Michniewicz, M., Bergmann, D. C., & Wang, Z. Y. (2012). Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature, 482, 419.
Kim, B., Fujioka, S., Kwon, M., Jeon, J., & Choe, S. (2013). Arabidopsis brassinosteroid-overproducing gulliver3-D/dwarf4-D mutants exhibit altered responses to jasmonic acid and pathogen. Plant Cell Reports, 32, 1139–1149.
Kim, J., Dotson, B., Rey, C., Lindsey, J., Bleecker, A. B., Binder, B. M., & Patterson, S. E. (2018). New clothes for the jasmonic acid receptor COI1: Delayed abscission, meristem arrest and apical dominance. PLoS One, 8, e60505.
Kitanaga, Y., Jian, C., Hasegawa, M., Yazaki, J., Kishimoto, N., Kikuchi, S., Nakamura, H., Ichikawa, H., Asami, T., Yoshida, S., Yamaguchi, I., & Suzuki, Y. (2006). Sequential regulation of gibberellin, brassinosteroid, and jasmonic acid biosynthesis occurs in rice coleoptiles to control the transcript levels of anti-microbial thionin genes. Bioscience, Biotechnology, and Biochemistry, 70, 2410–2419.
Kohli, S. K., Handa, N., Sharma, A., Gautam, V., Arora, S., Bhardwaj, R., Nasser, M., Alyemeni, Wijaya, L., & Ahmad, P. (2018). Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma, 255, 11–24.
Li, Q.-F., & He, J.-X. (2013). Mechanisms of signaling crosstalk between brassinosteroids and gibberellins. Digestive Plant Signal Behavior, 8, e24686.
Li, Q.-F., Wang, C., Jiang, L., Li, S., Sun, S. S., & He, J.-X. (2012). An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Science Signaling, 5, ra72.
Li, X., Ahammed, G. J., Li, Z.-X., Zhang, L., Wei, J.-P., Shen, C., Yan, P., Zhang, L.-P., & Han, W.-Y. (2016). Brassinosteroids improve quality of summer tea (Camellia sinensis L.) by balancing biosynthesis of polyphenols and amino acids. Frontiers in Plant Science, 7, 1–9.
Li, X., Zhang, L., Ahammed, G. J., Li, Z.-X., Wei, J.-P., Shen, C., Yan, P., Zhang, L.-P., & Han, W.-Y. (2017). Nitric oxide mediates brassinosteroid-induced flavonoid biosynthesis in Camellia sinensis L. Journal of Plant Physiology, 214, 145–151.
Li, Q.-F., Lu, J., Yu, J. W., Zhang, C.-Q., He, J.-X., & Liu, Q.-Q. (2018a). The brassinosteroid-regulated transcription factors BZR1/BES1 function as a coordinator in multisignal-regulated plant growth. Biochimica et Biophysica Acta-Gene Regulatory Mechanisms, 1861, 561–571.
Li, W., Nishiyama, R., Watanabe, Y., Ha, C. V., Kojima, M., An, P., Tian, L., Tian, C., Sakakibara, H., & Tran, L.-S. P. (2018b). Effects of overproduced ethylene on the contents of other phytohormones and expression of their key biosynthetic genes. Plant Physiology and Biochemistry, 128, 170–177.
Liu, J., Lovisolo, C., Schubert, A., & Cardinale, F. (2013). Signaling role of strigolactones at the interface between plants, (micro) organisms, and a changing environment. Journal of Plant Interactions, 8, 17–33.
López-Gómez, M., Castellanos, J. H., Lluch, C., & Herrera-Cervera, J. A. (2016). 24-Epibrassinolide ameliorates salt stress effects in the symbiosis Medicago truncatula-Sinorhizobium meliloti and regulates the nodulation in cross-talk with polyamines. Plant Physiology and Biochemistry, 108, 212–221.
Maharjan, P. M., Schulz, B., & Choe, S. (2011). BIN2/DWF12 antagonistically transduces brassinosteroid and auxin signals in the roots of Arabidopsis. Journal of Plant Biology, 54, 126–134.
Mazorra, L. M., Oliveira, M. G., Souza, A. F., da Silva, W. B., dos Santos, G. M., da Silva, L. R. A., da Silva, M. G., Bartoli, C. G., & de Oliveira, G. (2013). Involvement of brassinosteroids and ethylene in the control of mitochondrial electron transport chain in postharvest papaya fruit. Theoretical and Experimental Plant Physiology, 25, 203–212.
Mouchel, C. F., Osmont, K. S., & Hardtke, C. S. (2006). BRX mediates feedback between brassinosteroid levels and auxin signaling in root growth. Nature, 443, 458–461.
Nahar, K., Kyndt, T., Hause, B., Höfte, M., & Gheysen, G. (2013). Brassinosteroids suppress rice defense against root-knot nematodes through antagonism with the jasmonate pathway. Molecular Plant-Microbe Interactions, 26, 106–115.
Nakashita, H., Yasuda, M., Nitta, T., Asami, T., Fujioka, S., Arai, Y., Sekimata, K., Takatsuto, S., Yamaguchi, I., & Yoshida, S. (2003). Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. The Plant Journal, 33, 887–898.
Nemhauser, J. L., Hong, F., & Chory, J. (2006). Different plant hormones regulate similar processes through largely non overlapping transcriptional responses. Cell, 126, 467–475.
Oh, E., Zhu, J. Y., Bai, M. Y., Arenhart, R. A., Sun, Y., & Wang, Z. Y. (2014). Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife, 3, e03031.
Ohri, P., Bhardwaj, R., Bali, S., Kaur, R., Jasrotia, S., Khajuria, A., & Parihar, R. D. (2015). The common molecular players in plant hormone crosstalk and signaling. Current Protein & Peptide Science, 16, 369–388.
Pál Magda, C. G., Szalai, G., Oláh, T., Khalil, R., Yordanova, R., Gell, G., Birinyi, Z., Németh, E., & Janda, T. (2017). Polyamines may influence phytochelatin synthesis during Cd stress in rice. Journal of Hazardous Materials, 340, 272–280.
Pan, G., Liu, Y., Ji, L., Zhang, X., He, J., Huang, J., Qiu, Z., Liu, D., Sun, Z., Xu, T., Liu, L., Wang, C., Jiang, L., Cheng, X., & Wan, J. (2018). Brassinosteroids mediate susceptibility to brown planthopper by integrating with the salicylic acid and jasmonic acid pathways in rice. Journal of Experimental Botany, 69, 4433–4442.
Peng, Z., Han, C., Yuan, L., Zhang, K., Huang, H., & Ren, C. (2011). Brassinosteroid enhances jasmonate-induced anthocyanin accumulation in Arabidopsis seedlings. Journal of Integrative Plant Biology, 53, 632–640.
Per, T. S., Khan, M. I. R., Anjum, N. A., Masood, A., Hussain, S. J., & Khan, N. A. (2018). Jasmonates in plants under abiotic stresses: Crosstalk with other phytohormones matters. Environmental and Experimental Botany, 145, 104–120.
Ren, C., Han, W., Peng, Y., Huang, Z., Peng, X., Xiong, Q., Zhu, B., Gao, D., & Xie, A. (2009). Leaky mutation in DWARF4 reveals an antagonistic role of brassinosteroid in the inhibition of root growth by jasmonate in Arabidopsis. Plant Physiology, 151, 1412–1420.
Ryu, H., Cho, H., Bae, W., & Hwang, I. (2014). Control of early seedling development by BES1/TPL/HDA19-mediated epigenetic regulation of ABI3. Nature Communications, 5, 4138.
Saini, S., Sharma, I., Kaur, N., & Pati, P. K. (2013). Auxin: A master regulator in plant root development. Plant Cell Reports, 32, 741–757.
Saini, S., Sharma, I., & Pati, P. K. (2015). Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Frontiers in Plant Science, 6, 950.
Sharma, I., Bhardwaj, R., & Pati, P. K. (2015). Exogenous application of 28- Homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. Journal of Plant Growth Regulation, 34, 509–518.
Soto, M. J., Fernandez-Aparicio, M., Castellanos-Morales, V., Garcia-Garrido, J. A., Delgado, M. J., & Vierheilig, H. (2010). First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biology and Biochemistry, 42, 383–385.
Sreeramulu, S., Mostizky, Y., Sunitha, S., Shani, E., Nahum, H., Salomon, D., Hayun, L. B., Gruetter, C., Rauh, D., Ori, N., & Sessa, G. (2013). BSKs are partially redundant positive regulators of brassinosteroid signaling in Arabidopsis. The Plant Journal, 74, 905–919.
Steber, C. M., & McCourt, P. (2001). A role for brassinosteroids in germination in Arabidopsis. Plant Physiology, 125, 763–769.
Stewart Lilley, J. L., Gan, Y., Graham, I. A., & Nemhauser, J. L. (2013). The effect of DELLAs on growth change with developmental stage and brassinosteroid levels. The Plant Journal, 76, 165–173.
Sun, T. P. (2011). The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Current Biology, 21, R338–R345.
Sun, Y., Fan, X. Y., Cao, D. M., Tang, W., He, K., Zhu, J. Y., He, J.-X., Bai, M.-Y., Zhu, S., Oh, E., Patil, S., Kim, T.-W., Ji, H., Wong, W. H., Rhee, S. Y., & Wang, Z.-Y. (2010). Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Developmental Cell, 19, 765–777.
Tanaka, K., Asami, T., Yoshida, S., Nakamura, Y., Matsuo, T., & Okamoto, S. (2005). Brassinosteroid homeostasis in Arabidopsis is ensured by feedback expressions of multiple genes involved in its metabolism. Plant Physiology, 138, 1117–1125.
Tong, H., Xiao, Y., Liu, D., Gao, S., Liu, L., Yin, Y., Jin, Y., Qian, Q., & Chu, C. (2014). Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell, 26, 4376–4393.
Ueguchi-Tanaka, M., Nakajima, M., Motoyuki, A., & Matsuoka, M. (2007). Gibberellin receptor and its role in gibberellin signaling in plants. Annual Review of Plant Biology, 58, 183–198.
Unterholzner, S. J., Rozhon, W., & Papacek, M. (2015). Brassinosteroids are master regulators of gibberellin biosynthesis in Arabidopsis. Plant Cell, 27, 2261–2272.
Vandenbussche, F., Callebert, P., Zadnikova, P., Benkova, E., & Van Der Straeten, D. (2013). Brassinosteroid control of shoot gravitropism interacts with ethylene and depends on auxin signaling components. American Journal of Botany, 100, 215–225.
Vanlerberghe, G. C. (2013). Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. International Journal of Molecular Sciences, 14, 6805–6847.
Vanstraelen, M., & Benkova, E. (2012). Hormonal interactions in the regulation of plant development. Annual Review of Cell and Developmental Biology, 28, 463–487.
Vert, G., Walcher, C. L., Chory, J., & Nemhauser, J. L. (2008). Integration of auxin and brassinosteroid pathways by Auxin response factor2. Proceedings of the National Academy of Sciences, 105, 9829–9834.
Vukašinović, N., & Russinova, E. (2018). BRexit: Possible brassinosteroid export and transport routes. Trends in Plant Science, 23, 285–292.
Wang, Y., Sun, S., Zhu, W., Jia, K., Yang, H., & Wang, X. (2013). Strigolactone/MAX2-induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Developmental Cell, 27, 681–688.
Wang, H., Tang, J., Liu, J., Hu, J., Liu, J., Chen, Y., Cai, Z., & Wang, X. (2018). Abscisic acid signaling inhibits brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2. Molecular Plant, 11, 315–325.
Yang, C. J., Zhang, C., Lu, Y. N., Jin, J. Q., & Wang, X. L. (2011). The mechanisms of brassinosteroids’ action: From signal transduction to plant development. Molecular Plant, 4, 588–600.
Yang, X., Bai, Y., Shang, J., Xin, R., & Tang, W. (2016). The antagonistic regulation of abscisic acid-inhibited root growth by brassinosteroids is partially mediated via direct suppression of ABSCISIC ACID INSENSITIVE 5 expression by BRASSINAZOLE RESISTANT 1. Plant, Cell & Environment, 39, 1994–2003.
Ye, H., Li, L., & Yin, Y. (2011). Recent advances in the regulation of brassinosteroid signaling and biosynthesis pathways. Journal of Integrative Plant Biology, 53, 455–468.
Yin, Y., Wang, Z. Y., Mora-Garcia, S., Li, J., Yoshida, S., Asami, T., & Chory, J. (2002). BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell, 109, 181–191.
Yoshimitsu, Y., Tanaka, K., Fukuda, W., Asami, T., Yoshida, S., Hayashi, K., Kamiya, Y., Jikumaru, Y., Shigeta, T., Nakamura, Y., & Matsuo, T. (2011). Transcription of DWARF4 plays a crucial role in auxin regulated root elongation in addition to brassinosteroid homeostasis in Arabidopsis thaliana. PLoS One, 6, e23851.
Youn, J. H., Kim, M. K., Kim, E. J., Son, S. H., Lee, J. E., Jang, M. S., Kim, T. W., & Kim, S. K. (2016). ARF7 increases the endogenous contents of castasterone through suppression of BAS1 expression in Arabidopsis thaliana. Phytochemistry, 122, 34–44.
Youn, J. H., Kim, T. W., Joo, S. H., Son, S. H., Roh, J., Kim, S., Kim, T. W., & Kim, S. K. (2018). Function and molecular regulation of DWARF1 as a C-24 reductase in brassinosteroid biosynthesis in Arabidopsis. Journal of Experimental Botany, 69, 1873–1886.
Zhang, S., Cai, Z., & Wang, X. (2009). The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proceedings of the National Academy of Sciences, 106, 4543–4548.
Zhou, J., Wang, J., Li, X., Xia, X.-J., Zhou, Y.-H., Shi, K., Chen, Z., & Yu, J.-Q. (2014). H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. Journal of Experimental Botany, 65, 4371–4383.
Zhu, T., Deng, X., Zhou, X., Zhu, L., Zou, L., Li, P., Zhang, D., & Lin, H. (2016). Ethylene and hydrogen peroxide are involved in brassinosteroids-induced salt tolerance in tomato. Scientific Reports, 6, 35392.
Zhu, X. F., Yuan, D., Zhang, C., Li, T. Y., & Xuan, Y. H. (2018). RAVL1, an upstream component of brassinosteroid signaling and biosynthesis, regulates ethylene signaling via activation of EIL1 in rice. Plant Biotechnology Journal, 16, 1399–1401.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ohri, P. et al. (2019). Emerging Trends on Crosstalk of BRS with Other Phytohormones. In: Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A. (eds) Brassinosteroids: Plant Growth and Development. Springer, Singapore. https://doi.org/10.1007/978-981-13-6058-9_16
Download citation
DOI: https://doi.org/10.1007/978-981-13-6058-9_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6057-2
Online ISBN: 978-981-13-6058-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)