Abstract
Solubilization resembles emulsification in regard to how they are caused by surfactant self-assemblies. Here, the distinct difference is that solubilization is a state under thermodynamic equilibrium and thus is independent to the process applied, but on the other hand, emulsion is an unstable non-equilibrium state highly dependent on the preparation process. Systems called microemulsions require special attention because despite of the name, they are states in solubilization where swelled micelles are dispersed. The term solubilization is generally used for isotropic single-phase micelle solutions higher than the Kraft temperature, though there are recent trends to include lyotropic liquid crystals and gels.
The maximum solubilization by micelles for slightly soluble substances at certain temperature is quite important to measure. Therefore, this chapter deals with solubilization of volatile and/or nonvolatile substances by surfactant micellar solution.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Solubilization by micelles can generally be categorized as a phenomenon called sorption, and further divided as adsorption onto the micelle surface and absorption into the micelle core. Solubilization is increasing the solubility of relatively immiscible materials in solvents, in most cases water, by adding surfactant(s) to the system. Solubilization is a thermodynamically stable phenomenon of making single-phase solutions regardless of the process applied and one of the important properties of surfactants such as emulsification or dispersibility. The term solubilization is usually used for isotropic single-phase micelle solutions higher than the Kraft temperature, though there are recent trends to include lyotropic liquid crystals and gels. Therefore, solubilization could be regarded as a property of surfactant solutions to increase the solubility of organic materials. There is a similar phenomenon called hydrotropy, which can be observed when adding alcohol or acetone to water. This is distinguished from solubilization due to the lack of micelles or self-assemblies. Solubilization also resembles emulsification in regard that both self-assemblies are caused by surfactant. Here, distinct differences are that solubilization is a state under a thermodynamic equilibrium and is independent to the process applied, whereas emulsion is an unstable non-equilibrium state highly dependent on the preparation process. Systems called microemulsions require special attention because despite of the name, they are states in solubilization where swelled micelle is dispersed.
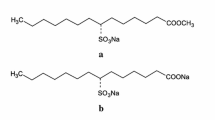
There are four types of solubilizations by micelles (a)–(d) as shown in Fig. 11.1, based on the position of solubilizate in the micelle. For (a), hydrophobic materials such as aliphatic or aromatic hydrocarbons are solubilized in the micelle core at the hydrocarbon tails of surfactant, (b) is cases when fatty alcohol, fatty acids, and fatty amines are solubilized in between the surfactant hydrocarbons nearby the micelle surface called palisade, (c) is when polar organic materials and dyes with rather large molecules are solubilized at the micelle surface, and (d) is cases where water soluble materials with affinity to surfactant hydrophilic moiety are solubilized at the surface hydrosphere of surfactant. The approximate solubilization capacity is generally recognized as (d) > (b) > (a) > (c). Micelles swell when materials are solubilized and are more noticeable for substances with polar groups. Solubilization by reversed micelles occurs in polar materials and water.
11.2 Solubilization for Nonvolatile Materials
The amount of solubilized nonvolatile dyes can be measured by optical absorption at the maximum wavelength of the dye molecule. Solubilization of molecules without absorption can be measured by the opacity of the solution at around 700 nm. The actual method is measuring the absorption of the varied dye or material concentration solubilized in micelles with a fixed surfactant concentration. The determined absorption values are plotted against the dye or material concentration, which linearly increases with concentration and then deviates from the line as shown in Fig. 11.2. The concentration when the deviation starts is the maximum solubilization (Smax) of the micelle at the given surfactant concentration. Next, Smax are plotted to the surfactant concentration as shown in Fig. 11.3. The solubilization capacity can be obtained from the slope of the line, and the intercept point corresponds to the cmc of the surfactant.
11.3 What to Look Out for
The Smax of solubilizates will be different depending on the method applied, i.e., either batch or continuous. The batch method consists of multiple surfactant solutions with same concentration. Dyes are added to each surfactant solution in incremental amounts to each solution in the beakers. In the continuous method, small amounts of dye are added to solubilize successively like titration. As a result, the Smax of the continuous method is larger than the batch method.
11.4 Solubilization of Volatile Chemicals Such as Fragrances
11.4.1 Determination of Solubilization Equilibrium Constant
The solubilization equilibrium constant (K: L/mol) represents the partition constant of volatile materials in between micelles and the bulk at the equilibrium. K is a ratio of the mole fraction of the solubilized volatile in the micelle (X org) and in the bulk (C org) as shown in Eq. 11.1.
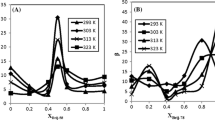
A larger K value indicates more solubilization of volatile in the micelle, and the dimension of K is (M−1). Generally, the Hansen-Millar equation [1] expresses a linear relationship between the activity coefficient, in this case the solubilization constant K and molarity X org [2]. This is true for the volatile molecule when X org is lower than 0.3. However, when X org is higher than 0.3–0.6, a better relationship is known as shown in Eq. 11.2 [3,4,5,6]:
where K 0 is extrapolated K at X = 0 and B is a constant value. The activity coefficient of volatile in the micelle is shown as Eq. 11.3. This empirical relationship is well accepted for many volatile chemicals solubilized in micelles [3,4,5].
where \( A=\frac{1}{K_0{C}_{\mathrm{org}}^0} \)
11.5 Static Headspace Method [7, 8]
The static headspace method, or headspace gas chromatography, is a method to evaluate the solubilization of volatiles in solutions by measuring the partial vapor pressure of the volatile in the gas phase. Under an equilibrium condition of gas and liquid phase, the partial vapor pressure of the target molecule will increase with increasing the concentration of the molecule in the liquid so the partial vapor pressure corresponds to the concentration in the solution.
There are two types of the headspace method, dynamic and static. With the dynamic headspace method, gases with a target volatile are evaporated through a looped apparatus and then absorbed onto the absorption resin or bed for a fixed time period. Then the absorbed molecules are released by various methods and are quantitatively analyzed by gas chromatography. Kinetic measurement of the rate of evaporation is possible with this dynamic method.
The static method consists of the following processes. First, aqueous solutions or solids with volatile materials are put into sealed container to hold still until they reach the equilibrium under a constant temperature, and then the gas at the headspace of the container is analyzed by gas chromatography. This static headspace method is commonly applied to the analysis of volatile materials in foods and fragrances. To apply this method, solubilized oily materials in the specimen must be volatile, and two continuous partition equilibriums exist in the system as shown in Fig. 11.4. One is a solubilization equilibrium of volatile molecules between the micelle and bulk solution, and the other is a gas (headspace) and liquid partition equilibrium of the molecule.
The partial vapor pressure of volatiles (solubilizates) in the headspace gas is decreased when the solubilized amount of volatile in the micelle is increased as these two equilibriums are interrelated to each other. The partial vapor pressure of the volatile molecule is equivalent to the molar amount of the molecule in the headspace. Therefore, the solubilization amount at each volatile concentration in the solution or the maximum solubilization (Smax) can be indirectly measured by determining the partial vapor pressure of the volatile in the headspace.
11.6 Experiment Procedure for the Static Headspace Method
Sealed vials with solutions of fixed surfactant concentration and varied amounts of volatiles (solubilizates) are set to reach equilibrium. The headspace gases of each vial are sampled by gas-tight syringes and analyzed by gas chromatography. This process when below or above the maximum solubilization (Smax) is shown in Fig. 11.5, and the relationship between the partial vapor pressure (P/P 0) and Smax is shown in Fig. 11.6. In order to correlate the solubilization equilibrium of volatiles between micelles and bulk solutions to the gas-liquid equilibrium, the activity coefficient of volatiles in the aqueous solutions must be determined. Additionally, the solubilization capacity can be determined from the slope of the line by plotting the Smax at different surfactant concentrations against the surfactant concentration.
11.7 An Alternative Method to Determine the Solubilization Equilibrium Constant (K)
The following is a short note of the semi-equilibrium dialysis (SED) method for determining K [3, 4, 9], in which details can be found in the original papers referred. Solubilized solutions are placed in the retentate of the dialysis cell while pure water is set into the permeate, which are separated by a cellulose membrane with pores to cut molecules over 6000 Dalton off the dialysis. After 24 h at 30 °C, the concentrations of the surfactants and volatile solubilizates in the permeate part of the cell are analyzed. K can be determined with Eq. 11.4
where X is mole fraction of the solubilizate (fragrance) in the micelle, C is the concentration of the solubilizate (fragrance) in the bulk solution, X bulk is its mole fraction in the bulk, Cm is the concentration of the solubilizate (fragrance) in the micelle, and Csf is concentration of surfactant in solution.
References
R.S. Hansen, F.A. Millar, J. Phys. Chem. 58, 193 (1954)
G.A. Smith, S.D. Christian, E.E. Tucker, J.F. Scamehorn, J. Solut. Chem. 15, 519 (1986)
B.H. Lee, S.D. Christian, E.E. Tucker, J.F. Scamehorn, Langmuir 6, 230 (1990)
M. Abe, K. Mizuguchi, Y. Kondo, K. Ogino, H. Uchiyama, J.F. Scamehorn, E.E. Tucker, S.D. Christian, J. Colloid Interface Sci. 160, 16 (1993)
Y. Kondo, M. Abe, K. Ogino, H. Uchiyama, J.F. Scamehorn, E.E. Tucker, S.D. Christian, Langmuir 9, 899 (1993)
H. Uchiyama, S.D. Christian, J.F. Scamehorn, M. Abe, K. Ogino, Langmuir 7, 95 (1991)
T. Shikata, Y. Sakaiguchi, H. Urakami, A. Tamura, H. Hirata, J. Colloid Interface Sci. 133, 288 (1989)
M.E. Morgan, H. Uchiyama, S.D. Christian, E.E. Tucker, J.F. Scamehorn, Langmuir 10, 2170 (1992)
Y. Kondo, K. Mizuguchi, Y. Tokuoka, H. Uchiyama, K. Kamogawa, M. Abe, J. Jpn Soc. Colour Mater 68, 271 (1995)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Abe, M. (2019). Solubilization by Micelles. In: Abe, M. (eds) Measurement Techniques and Practices of Colloid and Interface Phenomena. Springer, Singapore. https://doi.org/10.1007/978-981-13-5931-6_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-5931-6_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5930-9
Online ISBN: 978-981-13-5931-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)