Abstract
Synthetic plastics have been very well recognized for the packaging industry. An evident inclination towards development of biodegradable Bioplastics based on biological material and, particularly for bulk packaging applications has been perceived as a strategy to overcome the dependency of packaging sector on fossil fuels and diminish the biospheric plastic waste burden. The packaging industry demands the raw material in quantity and quality both. Quality includes properties such as ample tensile strength, less brittleness, adequate gas, aroma, ultra-violet (UV) and water barrier properties as per the product to be packaged. The inherent hydrophobicity, biodegradability and enormous property range has branded the microbial poly (hydroxyalkanoates) (PHAs) as promising competitors of petro-plastics in the packaging market. This chapter addresses the basic characteristics and alluring advantages of PHAs as packaging materials. The major focus has been on discussing recent developments of PHAs in different material forms of specific relevance to packaging sector.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Polyhydroxyalkanoates
- Packaging
- Permeability
- Nanocomposites
- Multilayer films
- Paper coating
- Antimicrobial PHAs
14.1 Introduction
14.1.1 Plastics: “The Brighter Side”
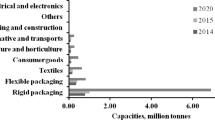
The term “plastic” existed as early as the 1600s. With the actual debut of synthetic plastics in the 1930s, global dependence on these materials has increased considerably over the years. Global production of plastics has gone up to 380 Mt (Plastics Europe 2016; Geyer et al. 2017). The synthetic or petroleum based plastic industry has rapidly developed catering to the myriad applications covering almost every sphere of human life, making them an indispensable part of our day to day life. Their widespread applications can be attributed to favorable mechanical, thermal and chemical properties, such as high strength, lightness, rigidity or flexibility, stability, durability, being molded into any shape, transparency or opacity, water resistance, resistance to majority of water-borne microorganisms and most importantly cost effectiveness (usually lower than 1€ kg−1) (Valentino et al. 2014; Colombo et al. 2016). It has been over a decade since the conventional materials viz. paper, jute, wood, metals, glass and ceramics have been replaced by the synthetic plastics in commercial sectors such as infrastructure, packaging, textile fibers, automotive, agriculture, electronics and medical appliances (North and Halden 2013). Their cost-effectiveness makes them an ideal material for small duration and single-use purposes viz. disposable medical devices and packaging. Packaging industry, the major player of the current era draws more than 40% of the global plastic production, of which almost 50% is employed as a food packaging material (Siddiqui and Pandey 2013; Rhim et al. 2013; Khosravi-Darani and Bucci 2015).
14.1.2 Plastics: “The Darker Side”
Of all the plastic products, packaging and carry bags, polyethylene terephthalate (PET) bottles and disposables are the main ubiquitous consumer item and sources of plastic waste on our planet. Due to their rapid increase in production and consumption, improper disposal, intrinsic non-biodegradability or very sluggish degradability, these commercial polymers have long been under environmental scrutiny (Pavani and Rajeswari 2014). As per the study of American Association for Advancement of Science (AAAS), by 2050 approximately 12,000 MMT of plastic waste will be discarded in natural environment. According to the Indian scenario, as estimated by Central Pollution Control Board (CPCB) approximately 5.6 MTPA of plastic waste is generated in our country amounting to 15,342 TPD (tonnes per day), of which approximately over 6137 tonnes of plastic waste remains uncollected and littered, either being land-filled or ending up in streams leading to land, ground water and marine water pollution (Central Pollution Control Board 2013).
Apart from the environmental pollution and waste management problems, an additional growing concern is utilization of limiting fossil feed stocks (petroleum and its allied components) for the production of these recalcitrant synthetic plastics. As a more promising approach, “the biobased – BIOPLASTICS” have emerged as apt sustainable alternatives worldwide (Anjum et al. 2016). The term Bioplastics is a broader term used for a variety of plastics. All biobased plastics are not biodegradable and all biodegradable plastics are not biobased (made from renewable resources). Amongst the diverse bioplastics, owing to their superior performance and consumer liking for sustainability, currently the market is visibly dominated by biobased and non-biodegradable plastics like biobased poly (ethylene), biobased poly (trimethylene terephthalate), biobased poly (ethylene) terephthalate, etc. (Gironi and Piemonte 2011; Chen 2014). However, development of biobased and biodegradable plastics such as polylactic acid (PLA), thermoplastic starch (TPS), cellulose based plastics and plastics from microbial fermentations, “The Polyhydroxyalkanoates (PHAs)” sounds a more environmentally ethical and sustainable solution to the conventional plastics (Saini 2017). The global production capacity of biobased polymers is likely to grow to nearly 7.85 million tonnes by 2020 (Aeschelmann and Carus 2015). The present day materials (PLAs, starch and cellulose based plastics) are short of the diverse material properties embraced by the PHAs (Chen 2014; Wang et al. 2014).
As the materials of choice, the presented chapter is focused on the primary properties of PHAs and a detail outline of their properties appropriate for the packaging applications. The major section covers recent research on the development of polyhydroxyalkanoates (PHAs) for packaging and lastly some case studies wherein PHAs have been used for packaging of varied food products.
14.2 The Microbial Polyhydroxyalkanoates (PHAs)
Polyhydroxyalkanoate(s) (PHA) are a family of natural thermoplastic and elastomeric biopolyesters synthesized and amassed by an extensive range of microbial genera as intracellular carbon/energy storage materials. The polymer formed is accumulated as distinct intracellular granules, number and size varying with producer strain (Keshavarz and Roy 2010; Tan et al. 2014). The current outlook has made known the microbial polyesters PHAs as one of the most scientifically innovative and favorite bioplastics for both mankind and the environment due to the complete green life cycle with 1) bio-based production (using renewable resources as carbon feedstocks); biosynthesis (involving microbes as producers); biospheric biocompatibility (eco-friendly disposal); inherent biodegradability (mineralization by natural microbial degraders to carbon dioxide and water) (Koller et al. 2013; Albuquerque and Malafaia 2018).
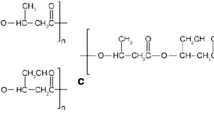
Figure 14.1 represents the common structure of polyhydroxyalkanoates. Structurally, PHAs have been classified into three classes namely, short chain (scl) PHAs having C4-C5, medium chain (mcl) PHAs (C6–C14), and long chain length (lcl) PHAs (>C14). This classification is derived from the substrate specificity of the PHA monomer polymerizing enzyme: PHA synthases, which accepts 3HA monomers of a specific carbon length. Additionally, based on the monomeric content of the polymer, PHAs can further be grouped as homo-polymers (either scl or mcl monomers) or copolymers (combination of different scl and/or mcl monomers) (Ali and Jamil 2016). Till date, a vast number of diverse PHAs, comprising over 150 distinct HA monomer units as constituents, has been isolated, making them the biggest group of natural biopolyesters. The most common monomer is 3-hydroxybutyrate (3HB) (Hazer and Steinbüchel 2007). A detailed compilation of various PHAs with varying length, functional groups, and straight or branched, saturated or unsaturated monomer side chains has been reviewed by Witholt and Kessler (1999). The structural diversity of PHAs is influenced by carbon substrate, medium composition, producer strain, PHA synthase specificity and the biosynthetic pathway (Steinbüchel and Lütke-Eversloh 2003; Mohapatra et al. 2017). Mechanical, physical, thermal properties and molecular weights are usually determined for assessing the PHA materials. PHA material properties such as mechanical, chemical, and thermal depend on their monomeric composition.
As represented in Table 14.1, the family is known to exhibit a variety of physico-chemical properties, which for some of the copolymers are quite comparable to the conventional plastics.
The properties range from stiff, brittle, high crystallinity [60–80%]; high melting (Tm) and glass transition temperatures (Tg) (for scl polymers) to flexibility and elasticity with lesser crystallinity of around 25%; reduced tensile strength and high elongation to break; low Tm (40–60 °C) and Tg below room temperature (−50 to −25 °C) (for mcl polymers). The properties of some typical PHAs are listed in Table 14.1 (Rai et al. 2011; Rudnik 2012; Bugnicourt et al. 2014; Koller 2014; Anjum et al. 2016; Możejko-Ciesielska and Kiewisz 2016; Wang and Chen 2017).
14.3 Industrial Advancements of PHAs
Efforts for commercial scale production of PHAs can be dated back to the late 1950s, Grace and Company. Further the 1970s petroleum crises had marked the dawn of pilot and industrial scale PHA production by various companies. The first industrialized and commercial PHA: P3HB was called as “The First Generation PHA”. Since then diverse polymers are being produced viz. poly (3-hydroxybutyrate-co-3-hydroxyvalerate): PHBV; PHBHHx; poly(3-hydroxybutyrate-co-4-hydroxybutyrate): P3HB4HB etc. Later in late 1990, ICI developed a PHA co-polymer Biopol, based shampoo bottles. Its other applications being packaging, woven medical patches, disposable knives and forks, disposable cups, nappy linings, surgical stitches, surgical pins, and disposable razors. As shown in Table 14.2, the present major players of the PHA commercial market are Metabolix Inc. (USA); Meridian Inc. (USA); Biomers (Germany); ADM (with Metabolix) (USA); Kaneka (with Procter and Gambles)(Japan); Tepha (USA), Biocycle (Brazil); Yikeman, Shandong and Tianjin Green Bio-Science (China) etc. Many Chinese companies also focused on large scale PHA production since the late 1990s.
Metabolix Inc. PHA trade name Mirel, can be used to form injection molding, film, thermoforming, sheet products, paper coating, foam and nonwovens. Mirel can be used as an alternative to various forms of PEs, PPs and polystyrene (PS) (Chen 2009; Wang and Chen 2017; Masood 2017). Industrial PHAs have been employed for diverse applications viz. packaging and disposable products; biomaterials and drug carriers in medicine; chiral precursors for optically active compounds e.g., drugs; food additives; agricultural purposes (as mulch films, herbicides, insecticides, bacterial inoculants) and the most recent and upcoming biofuels (Fig. 14.2) (Anjum et al. 2016; Wang and Chen 2017).
However, their industrial manufacturing and applicability is not competitive owing to their elevated production costs. Research efforts for developing economic PHA production technologies via application of inexpensive carbon substrates, simplification of PHA recovery process, metabolic engineering, synthetic biology, and bioinformatics are underway. Over and above, use of these green and biodegradable PHAs for the production of wide variety of single use materials such as packaging and disposables would be biosphere favorable.
14.4 PHAs as Packaging Material
14.4.1 Criteria of Concern
To be applied as packaging’s for foods or other materials certain common requirements (Prasad and Kochhar 2014) need to be fulfilled such as:
-
1.
Proper wrapping and shielding from dust, moisture, dehydration, microbial and chemical contamination, UV-radiation and mechanical impairment.
-
2.
Preservation of sensory food characteristics.
-
3.
Use of food grade quality packaging material assisting in preservation of food purity.
-
4.
Maintenance of food stability under extreme storage conditions such as low temperature.
-
5.
Modified atmosphere (such as nitrogen atmosphere) maintenance in the product’s headspace particularly for storing ready to eat snack items e.g., Potato chips.
-
6.
During the material storage period, conditions favoring the biodegradation of packaging should be avoided whereas the same should be promoted or arise once the packaging is discarded.
14.4.2 Relevance in Packaging
PHAs should be alluring as packaging material due to certain key material and permeability properties discussed below (Fig. 14.3):
14.4.2.1 Flexible Material Properties
The PHAs, crystallinity, mechanical and thermal properties significantly influence the polymer processability and end application. The crystallinity of PHAs, which usually determines the brittleness of polymers, varies from 0% to 60% (highly crystalline to flexible polymers). The thermal properties such as Tg vary from −52 to 4 °C, a Tm above 177 °C is barely observed and thermodegradation temperature (Td) varies from 227 to 256 °C for PHAs. The occurrence of thermal degradation of PHAs nearer to their melting point is a critical parameter to be considered while opting for polymer processing methodology. The mechanical properties usually determined are Young’s modulus (defining the polymer stiffness); tensile strength (establishing the total amount of force required to pull a material before it breaks) and elongation at break (defining the stretchability of material before it breaks), respectively. The Young’s modulus ranges from 3.5 × 103 MPa (for stiffer scl-PHAs) to 0.008 MPa (for very ductile mcl-PHAs). For PHAs, the tensile strength generally ranges from 8.8 to 104 MPa. Elongation at break varies from 2% to 1000% (Chen 2010; Chanprateep 2010; Rai et al. 2011).
This diversity gives an advantage to user to choose an appropriate PHA suiting the target application. For instance, the most commonly biosynthesized and studied polymer, P3HB is water insoluble, moisture resistant, with low oxygen permeability; a high Tm more than 170 °C and a Tg of 4 °C. Its crystallinity, brittleness and low elasticity hinders with its injection molding making the wide applicability difficult (Wang and Chen 2017). Formation of P3HB copolyesters improvises its properties (Table 14.1). Such copolyesters are considered as potential candidates for food packaging application due to their high plasticity and accessibility towards melt extrusion, injection molding or thermoforming (Laycock et al. 2013). A higher HV content contributes to reduced PHB Tm (176–158 °C), brittleness and Young’s modulus making the resultant copolyester material more flexible (Thellen et al. 2008; Da Silva et al. 2005; Fu et al. 2014). The introduction of mcl monomers also greatly reduces PHB stiffness thereby improving its flexibility (Albuquerque and Malafaia 2018).
Additionally, formation of varied block and random copolymers, graft polymers and polymer blends leads to novel properties expanding PHA diversity and applicability in various fields. Thus, pliable degree of crystallinity and elasticity, accompanied with appropriate processing/molding methods, the PHAs can be processed to form products ranging from flexible wraps (via thermo-forming) to rigid storage boxes and containers (via injection molding) (Koller 2014).
14.4.2.2 Permeability
14.4.2.2.1 Water and Gas (Oxygen and Carbon-di-Oxide)
Comparable water vapor permeability values of PHB and its copolymer PHBV films to that of the petrochemical opponents such as (PET) and poly(vinyl chloride) (PVC) is another positive feature for considering these microbial polyesters for packaging applications (Miguel et al. 1997; Miguel and Iruin 1999a, b). Water insoluble PHAs also have the advantage of being non swelling and highly hydrophobic compared to starch, cellulose, chitosan and gluten. Evaluation of water vapor barrier properties of the packaging material is of major importance as physical or chemical deterioration of the packed food is related to their equilibrium moisture content, which plays an important role in maintaining or extending the shelf-life of packaged food. The water vapor barrier is quantified by the water vapor permeability coefficient (WVPC) (kg*m/(m2*s*Pa)) or the water vapor transfer rate (WVTR) (cc/m2·s or g/m2·day), signifying the water vapor amount permeating per unit area and time inside or out of a packaging (Koller 2014). The nature of the packaged food is considered while opting for packaging material (high or low water vapor barrier). Usually, avoiding desiccation is important while packing fresh food products, whereas for the bakery products least or no water permeability is preferred avoiding the packaged food from getting contaminated with fungal infections by molds.
The oxygen permeability value of a packaging material plays a decisive role in quality preservation of fresh products (e.g. meat, fruits, vegetables, salad, various ready to eat foods etc.). Oxidative degradation of packaged food may affect its color, flavor and microbial stability. The oxygen barrier is determined in terms of quantified by the oxygen permeability coefficients (OPCs) and/or oxygen transmission rate (OTR), defined as the steady state rate at which oxygen can permeate through a test polymeric material. It indicates the amount of oxygen permeating a packaging material via diffusion per unit of area and time (kg*m/(m2*s*Pa) or nmol ms−1GPa−1 etc.), at standardized conditions for temperature (23 °C) and humidity (often 50% relative humidity RH) (Siracusa 2012). An oxygen transmission rate below 2 nmol ms−1GPa−1; is desirable and such materials are often labeled as “Barrier polymers”. A polymer packaging with low oxygen permeability coefficient, maintains a very low oxygen pressure inside the packaging thereby retarding the oxidation and extending the shelf-life of the product.
The CO2 transport properties also play an important role in fresh vegetables packaging and the related bacteriostatic properties. The CO2 barrier is quantified as either CO2 permeability coefficients (CO2PC), representing the amount of CO2 permeating in packaging per unit area and time, denoted as kg m m−2 s−1 Pa−1 or as rate of CO2 transmission (CO2TR), expressed in cc m−2 s−1 (or g m−2 day−1) (Siracusa 2012).
Various literature reports have shown the water and oxygen permeability values covering a wide range for the different PHAs. For diverse PHB test samples, oxygen permeability and water vapor permeability has been in the range of 2–10 ml mm m−2 day−1 atm−1 and 1–5 g mm m−2 day−1, respectively. For copolyesters such as PHBV, water and oxygen permeabilities have varied from 1–3 g mm m−2 day−1 and 5–14 ml mm m−2 day−1 atm−1. The permeability values are reported to increase with increasing molar share of 3 HV in PHBV copolyesters. The water and oxygen permeabilities for other polymers observed were: polylactic acid (PLAs) (5–7 g mm m−2 day−1 and 15–25 ml mm m−2 day−1 atm−1), PCL (300 g mm m−2 day−1 and 20–200 ml mm m−2 day−1 atm−1), PVC (1–2 g mm m−2 day−1 and 2–8 ml mm m−2 day−1 atm−1), LDPE (0.5–2 g mm m−2 day−1 and 50–200 ml mm m−2 day−1 atm−1). In general, water and oxygen barrier properties of PHB and PHBV appear to be slightly better than those of PLAs and potentially competitive with that of various synthetic plastics (Miguel et al. 1997; Thellen et al. 2008; Sanchez-Garcia et al. 2008; Plackett and Siró 2011).
A low and comparable CO2 permeability was reported for PHB with that of poly-vinylidene chloride (PVDC). Poley et al. (2005) reported CO2 diffusion coefficient value of 1 × 10−9 cm2 s−1 (at 25 °C) for PHB which was slightly higher than the determined value of 4.4–4.7 × 10−10 cm2 s−1 (at 30 °C) by Miguel et al. (1997). Miguel and Iruin (1999a) reported CO2 permeability values of PHB being similar to commodity thermoplastics unplasticized PVC and PET which are usually considered having relatively low CO2 permeability. Interestingly, at 4 atm (the CO2 pressure in carbonated beverages), PHB outperformed PET in the capture of CO2.
Recently, Follain et al. (2014), investigated the water and gas transport properties of commercial PHAs: PHB (Biomer P209 and P226) and P (3HB-co-3% 3HV) (TiaNan Enmat Y1000P) films. Higher water and gas permeability values were reported for the solvent cast films compared to the respective compressed counterparts. Enhanced polymer chain motions, microcavity formation and free volume between polymer chains favored permeability of gas molecules with solvent casting. Irrespective of the processing, PP209 film had maximum permeability and the least was of PY1000P film. Similar rankings were reported for diffusivity and solubility also. The CO2 and O2 permeability values for PP209 film (0.66, 12.3 and 1.95 Barrer), PP226 film (0.16, 3.04 and 0.49 Barrer) and PY1000P (0.25, 1.2 and 0.35 Barrer) was lower than LDPE (1.0, 28 and 6.9 Barrer) and comparable to PLA with L:D ratio of 96:4 (1.3, 10.2 and 3.3 Barrer) film, showing promising future of PHAs as ecological packaging.
14.4.2.2.2 Chemical
Weak or strong acidic nature of packaged food may cause hydrolytic reactions to the packaging material. PHAs are known to undergo acid-catalyzed hydrolytic degradation. Interaction between the chemical compounds and the polymers may affect the final mechanical properties of a polymer. Hence, assessment of biopolymer/s performance, suitability and stability with common food simulating solution as a function of time becomes imperative. Miguel et al. (1997) showed PHB films having high barrier properties for completely apolar (n-hexane, isopropyl ether and carbon tetrachloride) or very highly polar (methanol and water) solvents. Whereas, highest permeabilities were exhibited for solvents with moderately polar structures or polarities similar to PHB such as acetone, butyl acetate and toluene.
14.4.2.2.3 Flavoring Substances or Aroma
In many food packaging applications, enhanced barrier to loss of flavor during storage period is highly desirable, especially for beverages. 1-methyl-4-(1-methylethenyl)-cyclohexene [limonene] is usually used as a model aroma compound for testing the suitability of a material for packaging of flavorful foods. In this context, PHB was reported to show high barrier or lower permeability (0.088 * 10−kg m s−1 m−2 Pa-1) for limonene compared to PET (1.17 * 10−13 kg m s-1 m-2 Pa-1); whereas this value was significantly increased with PHBV copolyesters (Sanchez-Garcia et al. 2007).
14.4.3 Migration
Monomers and/or additives migration is of crucial concern in terms of food safety. Bucci et al. (2007) studied production of 500 mL (jar–cap set) PHB food packaging (Commercial grade: BIOCYCLE) through the injection process and also evaluated and compared its performance with polypropylene (PP) containers of the same shape. The authors evaluated the PHB component migration into a range of food simulants viz. distilled water, ethanol (15%), acetic acid (3%) and n-heptane at varied conditions. The overall migration rate was lesser than 8.0 mg/dm2 or 50 mg/kg (suggested limits), thus confirming the suitability of the polymer as a food packaging material. Authors based on migration evaluation indicated the applicability of such PHB containers for product storage under different conditions. Additionally, the light transmission analysis showed natural (zero % TE in 250–350 nm: UV range) and pigmented (Zero % TE in 250–750 nm: full barrier) PHB as better light barriers. Thus, recommending PHB as a packaging for foods such as soy oil and milk. Also, pigmentation and usage of a UV absorber additive could also be avoided with PHB. They also attempted for developing a better quality PHB material with fewer shortcomings and no odor. The biodegradation of the PHB packaging for up to 60 days indicated its immense applicability in packaging sector, circumventing the pollution and waste management issues of conventional plastics.
14.4.4 Biodegradation
The major challenge faced by the “Bioplastic based food packaging industry” is combining the sensorial maintenance along with an acceptable “shelf-life” of the product. Of utmost importance is, environmental conditions leading to packaging biodegradation must be evaded during the food product storage and only be present on subsequent discarding (Bucci et al. 2005).
The biodegradation of PHAs has been extensively studied under both aerobic and anaerobic conditions in varied environments such as soil and compost; fresh, marine and lake water; activated and anaerobic sludge; and laboratories (Goncalves et al. 2009; Correa et al. 2008; Shah et al. 2008). Microorganisms of various bacterial and fungal genera have been identified as potent PHA degraders, dominant being Pseudomonas, Bacillus, Streptomyces, Azotobacter, Penicillium, Cephalosporum, Paecilomyces, Trichoderma and Aspergillus (Shah et al. 2008; Correa et al. 2008; Lodhi et al. 2011; Plackett and Siró 2011). The biodegradation process involves biotic and abiotic hydrolysis followed by bioassimilation (Correa et al. 2008). Various degraders produce extracellular PHA depolymerases hydrolyzing the high molecular weight PHAs to CO2 and water aerobically (Khanna and Srivastava 2005). Factors influencing the PHA biodegradation rate are polymer characteristics (molar mass, monomeric composition, crystallinity, stereochemistry, amphiphilicity and chain mobility); environmental conditions; microorganism type and load (Khanna and Srivastava 2005). Usually, higher the polymer crystallinity and melting temperature, lower is the degradation rate. Degradation mechanisms are different under aerobic and anaerobic conditions. PHBV was observed to degrade more rapidly than PHB under aerobic conditions (Santos Rosa et al. 2004; Li et al. 2007); however, reverse was reported by Abou-Zeid et al. (2004). For, PHBV, faster degradation has been observed with increasing HV content (Renard et al. 2004). Degradation rate of PHA copolymers has also been represented as a function of monomer side chain i.e. maximum for PHB and least for PHBHHx (Li et al. 2007).
The variations in the permeability or barrier properties of PHAs could be attributed to polymer sample (varying structure, physical and thermal properties), film forming process, varied estimation techniques and equipments used and conditions at the time of measurements. Hence, evaluation of individual PHAs barrier properties for packaging of particular food types would be suggested. Combination of their barrier properties, biodegradability and microbial origin, conforms PHAs as promising substitutes of the long established petroleum plastics. Several research groups have been and are working on additional improvisation of PHAs properties for diverse packaging application. The forthcoming sections will be focused on recent research accomplished in this direction, highlighting the PHA based composites, nanocomposites, paper coatings, multilayer films, antimicrobial active packaging’s.
14.5 PHA Nanocomposites
PHA-composites are hybrid materials with fillers such as fiber, clay or particle, for modification of material properties. Usage of minimum one component in nanometer scales (nm, 10−9) defines the hybrids as Nanocomposites. Maiti et al. (2003) reported the fabrication of PHB/OMMT (organomodified montmorillonite) nanocomposites for the first time. The resultant nanocomposite had a improved storage modulus (>40%) and an intercalated morphology in contrast to pure PHB, with still maintaining its biodegradability.
The nanoscaled OMMT layers influence on PHBV crystallization was investigated by Wang et al. (2005). The small quantity addition of OMMT stimulated PHBV crystallization but reduced the chain mobility. Thus, though crystallization rate was increased, the relative crystallinity degree had decreased for the resulting PHBV/OMMT nanocomposites. The increasing OMMT content contributed to reduced Tm and enthalpy of fusion (ΔH m); expansion of the processing temperature range and lastly reduced biodegradability of nanocomposites in soil suspension. The stronger interaction of antimicrobial OMMT with PHBV had adversely affected the nanocomposites biodegradability. Therefore, small quantity addition of OMMT was recommended. Carli et al. (2011) investigated the influence of two types of nanoparticles: OMMT Cloisite® 30B (C-30B) and halloysite (HNT), on PHBV. The PHBV/C-30B nanocomposites had a partially exfoliated structure incontrast HNT dispersed uniformly in the PHBV matrix. The Tm had increased for PHBV/HNT nanocomposites. The Young’s modulus was also observed to increase. However, the enhancements for PHBV/C-30B nanocomposites were at the cost of the elongation at break and impact strength. Mohamed El-Hadi (2014) used octadecylamine and trimethyl stearyl ammonium (nanoclay A and B); ATBC and polyvinyl acetate (PVAc) plasticizers for reducing the PHB brittleness. Reduction in Tg from 5 to −13 °C was observed with plasticizer addition. With the nanoclay A addition the crystallization temperature and rate remained unaltered. In contrast nanoclay B addition led to enhanced crystallization rate and thermal stability. The nanoclay served as a nucleating agent resulting in increased nuclear density of the nanocomposites. Martínez-Sanz et al. (2014) developed PHBHV nanocomposites by incorporating bacterial cellulose nanowhiskers (BCNW). An improved water, oxygen and moisture permeability; and decline in Tm, enthalpy, rigidity and stiffness was observed with valerate incorporation in pure polymer. BCNW increased the thermal stability. Furthermore, a significant improvement in crystallinity, thermal stability and processability of PLA-PHB composites was reported with CNCs incorporation (Arrieta et al. 2014a, b, c). Incorporation of either pure or surfactant-modified CNCs was investigated. The ternary nanocomposite had improved oxygen barrier and stretch ability whereas reduced surface wettability UV-light transmission. The degradation of the nanocomposite was slowed due to PHB during composting experiments. Arrieta et al. (2015) worked on making an optically transparent PLA-PHB-ATBC-CNC flexible film, for food packaging with enhanced stretch ability, crystallization, and oxygen barrier; and slightly reduced ultra violet transmission. The nanocomposite film’s disintegration was assisted by plasticizer as well as and CNC in compost.
Yu et al. (2014) prepared solvent casted PHBV bionanocomposites reinforcing with PHBV-grafted multi-walled carbon nanotubes (MWCNTs). The obtained nanocomposite films obtained (having 1–10 wt % PHBV-g-MWCNTs) were transparent in the visible wavelength range. The uniform distribution of the MWCNTs had improved the thermal stability, mechanical, barrier, and migration properties of PHBV. The nanocomposite film containing 7 wt % PHBV-g-MWCNTs had the tensile strength and Young’s modulus enhanced by 88% and 172% compared to the neat PHBV film. For the same nanocomposite film, the maximum decomposition temperature was improved by 22.3 °C. Thus overall, these nanocomposites had much broader melt-processing phase with diminished water uptake. Also, all nanocomposites migrations in polar as well as non-polar simulants, respectively were within the permissible limits.
A novel biodegradable and renewable PHBHV-Keratin composite material was successfully developed via melt compounding by Pardo-Ibáñez et al. (2014). The keratin additive was prepared from poultry feathers. The suitable and good dispersion for low additive loadings was accompanied by barely unmodified optical properties. Also, the composite with keratin (1 wt %) had significantly reduced water, limonene, and oxygen permeabilities. Also an increase of ca. 30% in the elastic modulus for the composite was achieved. This specific composition composite was proposed as a suitable candidate for the development of fully biodegradable renewable food packaging material wherein enhanced barrier properties are necessary. In contrast, composites with higher additive loading were suggested for specific packaging applications wherein transparency is not being considered and exchange of gases and/or water vapor is desirable.
Kiran et al. (2017) reported the development of a PHB: nanomelanin: glycerol polymer film, which was flexible, odorless, nontoxic having antimicrobial and antioxidant activities. Melanin, dark brown ubiquitous photosynthetic pigment has applications in medicine, fabrication of radio-protective materials, cosmetics and food packaging. Spherical nucleated nanomelanin (Nm) particles formed via sonication, proved as an effective antimicrobial. The Nm-PHB nanocomposite film was homogeneous, thermostable up to 281.87 °C, and strongly inhibiting Staphyloccoccus aureus. Thus, it could be utilized in food packaging sector, for protection against oxidation and bacterial contamination.
Incorporation of natural vermiculite and organoclay to PHBHV, for improving its properties was studied by Reis et al. (2016, 2017). The Scanning Electron Microscopy (SEM) and Optical Microscopy (OM) results showed organoclay vermiculite bionanocomposites to be more homogenous and suitable ones for food packaging. Also, the migration levels being within the permissible limits such materials could be used without compromising with any food security.
Different doses of gamma irradiation influence the physical and biodegradation properties of poly-3-hydroxybutyrate/sepiolite (PHB/SP) nanocomposites (Masood et al. 2018). Modification of sepiolite with vinyltriethoxy silane (VTES) enhanced its compatibility with PHB. The nanocomposite films showed a good network formation and enhanced thermal stability compared to the PHB. It also degraded faster under in-vitro and soil burial biodegradation studies. It suggests the application of these films as biodegradable food packaging material.
It can be concluded that a good amount of progress has already been achieved in terms of PHA-nanocomposites, and literature data shows the potential of such materials to compete with the properties of petro-based plastics, making them usable in the packaging industry. Still more in depth evaluation of every nanocomposites material and development of novel ones is needed to meet up the existing packaging industry requirements in all perspectives.
14.6 PHAs in Multilayer Films
Multilayer films comprise of hydrophilic and hydrocolloidal synthetic polymers sandwiched between the layers of better barrier properties possessing hydrophobic and biodegradable polymers. Limited literature is available on fabrication of PHA-based multilayer films. Fabra et al. (2013) developed multilayer films with electrospun zein nanofibers sandwiched between PHBV using compression molding and casting methodology. A multilayer film prepared by the compression molded multilayer film had better mechanical properties and water vapor barrier than the film prepared by the casting technique. However, the influence on the water vapour and limonene permeability was depended on zein content of the interlayers. Also, irrespective of the processing technique used zein nanostructure addition was reported to enhance the oxygen barrier properties of the multilayer films. Thellen et al. (2013) developed outer-PHAs and inner- polyvinyl alcohol (PVOH) multilayer films with coextrusion process. The peel strength of the multilayer improved by twofolds on grafting maleic anhydride to PHAs (reaction initiator was dicumyl peroxide). Significant degradation of unmodified and maleated PHAs was observed in contrast to the non biodegradable PVOH. Fabra et al. (2014a) reported significant improvement of the flexibility and oxygen barrier; and decreased transparency of PHB or PHBV mutlilayers with nanostructured zein interlayers addition. For the control multilayer PLA/PET structures, no influence of zein interlayer incorporation on the water and oxygen barrier was observed. Fabra et al. (2014b) also reported unaltered transparency, crystallinity, water and oxygen permeability of PHBV/zein multilayer films post storage. At 100% RH and over the 3 month storage period the Listeria monocytogenes growth was promoted.
14.7 PHAs as Paper and Cardboard Coating
Biodegradable packaging comprising of paper has also been well recognized, the only limitation being its hygroscopic nature. Paper based packaging’s are often coated with hydrophobic polymers such as PVC, poly(ethylene-co-vinyl alcohol), polyolefin and oriented PET, PLA and PHB (Rastogi and Samyn 2015) Such composites not only minimize disposal costs but also maintain the dimensional stability in wet environments. Very few research groups have evaluated PHAs as paperboard coatings. Both PHB and PHBV have been tested as paper and cardboard coating effectively resulting in significant lowering of the moisture absorption and water permeability. Bilayer films comprising of PHB and cellulose paper (solvent casted); PHB and cellulose cardboard (compression molded), respectively were developed by Cyras et al. (2007, 2009). Both studies reported improvement of the water barrier. The elastic modulus, tensile strength, and strain at break also improved with PHB layering in a concentration dependent manner. The study presented the PHB coatings as a better alternative for Tetra Paks. In another study, natural as well as artificially synthesized PHB and PHBV were applied for paper sizing and coatings. The pressing and heating of impregnated papers for a short duration resulted in thin PHB coating on their surface giving a better paper size. Also, PHB was found to be more suitable than PHBHV for paper sizing (Bourbonnais and Marchessault 2010).
Dagnon et al. (2010) described the first attempts producing scl-PHA-Kraft paper–composites. Post 8 week soil burial period uncoated Kraft paper had higher weight loss rate compared to the P(3HB-co-4HB) coated Kraft paper. Also P(3HB-co-4HB) biopolymer coating had significantly improved the thermo mechanical properties of Kraft paper. Such materials could be employed as liners for fabrication of fiberboard boxes intended for varied packaging applications wherein mechanical properties as well as environmental disposal is of prime concern. Rastogi and Samyn (2017) investigated the development of structured PHB microparticles (PHB-MP) and unstructured sub-micron particles (PHB-SP), respectively as hydrophobic coatings for packaging papers. Additionally, the carnauba plant wax was also used as a hydrophobizing additive. Phase-separation methodology was employed for making structured PHB-MP. Filter papers dipped in the particle suspension were additionally sized with the wax. Increase in the static contact angles from 105–122° to 129–144° corresponded to inherent hydrophobicity improvement of the PHB-MP-coated papers with wax addition. The maximum PHB and non-solvent concentrations favored enhancement of contact angles. For, unstructured PHB-SP, the particles prepared was mixed with aqueous NFC suspensions of varying 0–7 wt %, respectively. Static contact angles varied from 112 to 152° for filter papers dip-coated in PHB-SP/NFC suspensions and sized with wax solution. The progressive increase in hydrophobicity observed was governed more by NFC, which was responsible for entrapment of polymer PHB particles, thus assisting its anchorage to the paper fibers. An efficient improvement of the paper surface hydrophobicity was achieved coating with macro to nano-scale PHA particles, without compromising with the biodegradability and biocompatibility of the ecofriendly packaging material “paper”.
14.8 Antibacterial Active PHAs for Packaging
An innovative way to protect minimally processed and/or “ready to eat” products are the use of packaging with antimicrobial properties. Recently, the inclusion of antimicrobial additives in food packaging materials has drawn significant attention (Appendini and Hotchkiss 2002). Research on functionalization and rendering of PHAs with antimicrobial activity supports and encourages their development for food packaging applications.
Effective antimicrobial efficacy against diverse foodborne pathogens, food spoilage bacteria, and fungi was observed using eugenol incorporated PHB films with and without combination of crude bacteriocin (pediocin) (Narayanan et al. 2013). The additive eugenol had reduced the melting point, crystallinity and tensile strength; but enhanced the flexibility of the resultant polymer film. The study demonstrated PHB as a suitable bio-based packaging material. Ramachandran et al. (2013) conferred antimicrobial activity to PHB films by incorporation of natural antimicrobial additive, Clitoria ternatea seed ethanolic extract. The resultant films showed appreciable antimicrobial action against various multi drug resistance (MDR) human bacterial pathogens. Xavier et al. (2015) studied the antimicrobial efficacy of vanillin (4-hydroxy-3-methoxybenzaldehyde) incorporated PHB films against various food-contaminating bacterial and fungal pathogens. Enhanced vanillin migration into the hydrophobic milieu demonstrated their relevance for fat containing foods. Because of reduced maximum thermal decomposition temperature and tensile strength of the antimicrobial films, the authors suggested such vanillin containing PHB films as secondary films over the primary food wrappings. Fan et al. (2015) reported preparation of antimicrobial PHB fibrous membrane by incorporation of the antimicrobial agent N-halamine, poly [5,5-dimethyl-3-(3′-triethoxysilylpropyl) hydantoin] (PSPH) in PHB material using electrospinning technique. The PHB and PHB/PSPH fibrous membranes had a fairly uniform morphology. Also, additive N-halamine had a small influence on the electrospun PHB film’s thermal properties. Chlorine bleach exposure conferred the membranes to be biocidal. The chlorinated PHB/PSPH samples displayed excellent antimicrobial activity against S.aureus and E. coli O157:H7 within a very short contact time of 30 min, respectively. Such PHB-based antimicrobial fibrous membranes could have great potential for food packaging applications.
Nanocomposite systems employing nanoparticles (metals like Cu, Ag, and Au, respectively); nanomaterials (metal oxides such as ZnO, TiO2 and MgO, respectively); and carbon nanotubes have also been drawing focus for the development effective antimicrobial packaging materials.
Due to higher surface to volume ratio, the nanosized agents possess better antimicrobial activities compared to their micro- or macroscale counterparts. ZnO nanoparticles are being foreseen as future inexpensive and reliable food packaging solution. Díez-Pascual and Díez-Vicente (2014a) reported the evaluation of ZnO-reinforced PHBV biodegradable nanocomposites as suitable food packaging material by assessing their morphological, material and antibacterial properties. The nanocomposites displayed uniform distribution of nanoparticles without addition of any coupling agents. Raised crystallization temperature and crystallinity index of the matrix with decreasing crystallite size were observed. The nanocomposites also displayed rise in thermal stability; superior mechanical properties such as improved stiffness, strength, toughness, and glass transition temperature; and improved barrier properties such as reduced water uptake, oxygen and water vapor permeability compared to the neat biopolymer. The PHBV/ZnO films also demonstrated significant antibacterial action against human pathogenic bacteria E.coli and S.aureus. Additionally, the overall migration levels of the nanocomposites were reported to be well below the limits as per current food packaging material norms. Antibacterial efficiency and barrier properties of the nanocomposite system were being influenced by factors viz. size, distribution and interaction of nanoparticles with the polymer. The same research group (Díez-Pascual and Díez-Vicente 2014b) also described PHB-ZnO bionanocomposites. The nanoparticle addition led to increase in the crystallization temperature, crystallinity degree, thermal stability and also improved varied mechanical properties. Decreased water uptake and superior barrier properties (gas and vapour) were also exhibited by nanocomposites compared to pure PHB. The antibacterial activity against gram-positive as well as gram-negative microorganisms enhanced with increasing concentration of ZnO. The migration was reported to decrease in food simulants at higher nanoparticle concentration. The authors proposed such sustainable nanomaterials for use as beverage and food containers and also as disposable articles or wrap films.
Kwiecień et al. (2014) reported oligo (3-hydroxybutyrate) (OHB) conjugates with preservatives sorbic acid and benzoic acid. Synthesis of such conjugates was achieved by anionic ring-opening oligomerization of racemic β-butyrolactone initiated by the sodium salt of selected preservatives. Electrospray ionization multistage mass spectrometry (ESI-MSn) structural characterization confirmed the covalent bonding of unaltered preservative molecular structures to OHB. As the preservative molecules in the conjugates were covalently linked to the oligomer chains; limited migration of the antimicrobial additives was assumed. Such preservative-OHB conjugates could potentially be applied as coating of the food packaging surface.
Castro-Mayorga et al. (2016) developed an antimicrobial PHA-Ag nanoparticle multilayer system. Significant reduction of Salmonella enterica population was attained even at very low (0.002–0.0005 wt %) silver concentration. The study demonstrated an innovative antimicrobial material for efficient prevention of microbial contamination in food packaging’s and contact surfaces.
Salama et al. (2017) investigated the antimicrobial activity of chitosan biguanidine hydrochloride ChG and PHB based graft copolymers (ChG-grafted PHB). The grafts were prepared via condensation reaction between the carboxylic groups of PHB and the amino groups of ChG. The chitosan derivatives showed excellent antimicrobial activity against the test bacterial and fungal strains used, implying their usage as efficient biomedical material. Such derivates could also be evaluated for food spoilage and food borne pathogens thus finding applications of such graft polymeric materials in the packaging sector.
14.9 Application of PHAs in Food Packaging: Demonstration
Very limited studies have done realistic demonstration of using PHAs for packaging of food products, to quote a few:
Kantola and Helén (2001) were the first to evaluate quality changes in organic tomatoes stored in Biopol@ [copolymer poly(HB-co-HV)] -coated paperboard trays and other biodegradable and LDPE packages, respectively at 11 ± 1 °C and 75–85% RH for 3 weeks. The biodegradable packaging materials tested were: Mater-Bi@ type ZF03U (corn starch based); Biopol@ [PHBHV]; cellophane (regenerated cellulose). The varied packagings tested were (1) perforated Mater bag; (2) a Biopol-coated paperboard tray additionally wrapped with a perforated Mater bag, (3) PLA coated paperboard tray additionally wrapped with a perforated Mater bag, and lastly (4) perforated cellophane bag. Post 3 weeks storage period 1.7% weight loss was observed for tomatoes in LDPE-bags whereas >2.5% was for the biodegradable materials’ bags. Tomatoes sensory qualities were almost independent of the package type but were significantly influenced by the storage duration.
The study of Haugaard et al. (2003) showed the potential of PHB and PLA packaging cups to be as effective as the conventional HDPE for an orange juice (acidic foods) and dressing (fatty food) simulant storage under fluorescent light or darkness at 4 °C for 10 weeks. The quality changes measured for the orange juice simulant were colour and ascorbic acid degradation; for dressing simulant were colour, peroxide value (POV), development of secondary lipid oxidation products development and α–tocopherols degradation. Due to analogous quality variations observed for both the simulants packed in PHB and PLA and HDPE, respectively; PHB and PLA both could be expected to be used for other commercial juices, acidic beverage/s and fatty food/s packaging. Additionally, the study also revealed that the quality changes were primarily light induced and were being governed by light permeability differences of the packages used.
Bucci et al. (2005) studied and compared the use of injection molded P3HB food packaging with PP (polypropylene) using varied dimensional and mechanical tests. The P3HB was reported as a rigid material than PP with 50% lesser deformation value. Though the PHB performance was inferior to PP at normal freezing and refrigeration conditions, it performed better at higher temperatures. The study stressed on the necessity of designing special molds and/or optimization of injection conditions and processing temperature for PHB. The sensorial investigations with the foods (margarine, mayonnaise and cream cheese) tested indicated no notable difference (p < 0.05), displaying PHB performance being equally good to conventional plastic PP for storage of lipophilic products.
Muizniece-Brasava and Dukalska (2006), reported plasticized PHB (plasticizers: dioctylsebacate, bisoflex) material suitable for packaging of sour cream (dairy product) with inconsiderable quality changes in comparison to the conventional dairy packaging material such as lean pouches, PE (with light protective graphite layer) and PS cups, respectively.
Levkane et al. (2008) proposed the use of plasticized PHB and PLA packaging films for Sous vide (under vaccum) thermal food treatment of meat and mayonnaise salad samples. At temperatures not more than +63 ± 0.5 °C, consistency of salads was reported to be maintained with reduced total microbial colony count. Influence of pasteurization regime and packaging material for maintaining the meat and mayonnaise salad samples packed in vacuum using conventional, plasticized PHB and PLA packaging films, respectively was focus of their study. Hermida et al. (2008) reported the aptness of PHB for gamma radiation (used for sterilization of food or packaging materials), with no significant decline in PHB properties.
14.10 Conclusion
The presented chapter has demonstrated that PHA biopolyesters very well meet most criteria for developing them as efficient packaging materials for both short-shelf life as well as long-shelf life products. The diversity of barrier properties provides an opportunity to choose from a range of PHA materials based on the type of food or product to be packaged. Ranging from high-quality copolymers to novel composites and multilayered materials with functionalized PHA moieties opens the door for innovative and high-tech, packaging devices with tailored target application in the food sector. Their inherent biodegradability offers a clear advantage making it a superior choice over the existing array of bioplastics. Based on the PHA material type proposed, there is a need for periodic critical evaluation accessing the functionality of these bio-based packaging materials in order to certify them for market launch as sole substitutes for conventional packaging materials. Upcoming research is focused on identification of new PHA compatible antimicrobial compounds with broad spectrum activity and low toxicity for the development of PHAs as active antimicrobial packaging materials. A thoughtful consideration towards migration evaluation of various nano-technology based additives from the PHA films and development of multilayer films is needed for appreciable penetration of these polymer in the packaging sector.
14.11 Opinion
The wonderful family of Polyhydroxyalkanoates (PHAs) may have bright opportunities in the packaging sector if their physical performance and prices both become competitive to synthetic polymers. Global research and development efforts making use of inexpensive substrates for PHA production and simple PHA recovery methods has assisted and is still constantly being worked on worldwide for achieving their competitive production prices. There is a constant need of robust and super PHA producing strains selection, feedstock evaluation, and development of competent fermentation regimes and processing technologies for addressing product quality, aptness, and sustainability and cost issues of PHAs. A close collaboration amongst academia, industries, and government could support these excellent candidates in reaching from packaging production units to the common man.
References
Abou-Zeid DM, Müller RJ, Deckwer WD (2004) Biodegradation of aliphatic homopolyesters and aliphatic± aromatic copolyesters by anaerobic microorganisms. Biomacromolecules 5:1687–1697. https://doi.org/10.1021/bm0499334
Aeschelmann F, Carus M (2015) Polymers in the world: capacities, production, and applications – status quo and trends toward 2020. Industrial Biotechnology (Short report by Nova Institute, Germany on full market study)
Albuquerque PBS, Malafaia CB (2018) Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int J Biol Macromol 107:615–625. https://doi.org/10.1016/j.ijbiomac.2017.09.026
Ali I, Jamil N (2016) Polyhydroxyalkanoates: current applications in the medical field. Front Biol 11:19–27. https://doi.org/10.1007/s11515-016-1389-z
Anjum A, Zuber M, Zia KM, Noreen A, Anjum MN, Tabasum S (2016) Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int J Biol Macromol 89:161–174. https://doi.org/10.1016/j.ijbiomac.2016.04.069
Appendini P, Hotchkiss JH (2002) Review of antimicrobial food packaging. Innov Food Sci Emerg Technol 3:113–126. https://doi.org/10.1016/S1466-8564(02)00012-7
Arrieta MP, Fortunati E, Dominici F, Rayón E, López J, Kenn JM (2014a) Multifunctional PLA-PHB/cellulose nanocrystal films: processing, structural and thermal properties. Carbohydr Polym 107:16–24. https://doi.org/10.1016/j.carbpol.2014.02.044
Arrieta MP, López J, Hernández A, Rayón E (2014b) Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eur Polym J 50:255–270. https://doi.org/10.1016/j.eurpolymj.2013.11.009
Arrieta MP, López J, Rayón E, Jiménez A (2014c) Disintegrability under composting conditions of plasticized PLA-PHB blends. Polym Degrad Stab 108:307–318. https://doi.org/10.1016/j.polymdegradstab.2014.01.034
Arrieta MP, Fortunati E, Dominici F, López J, Kenn JM (2015) Bionanocomposite films based on plasticized PLA-PHB/cellulose nanocrystal blends. Carbohydr Polym 121:265–275. https://doi.org/10.1016/j.carbpol.2014.12.056
Bourbonnais R, Marchessault RH (2010) Application of polyhydroxyalkanoate granules for sizing of paper. Biomacromolecules 11:989–993. https://doi.org/10.1021/bm9014667
Bucci TZ, Tavares LBB, Sell I (2005) PHB packaging for the storage of food products. Polym Test 24:564–571. https://doi.org/10.1016/j.polymertesting.2005.02.008
Bucci DZ, Tavares LBB, Sell I (2007) Biodegradation and physical evaluation of PHB packaging. Polym Test 26:908–915. https://doi.org/10.1016/j.polymertesting.2007.06.013
Bugnicourt E, Cinelli P, Lazzeri A, Alvarez V (2014) Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. Polym Lett 8:791–808. https://doi.org/10.3144/expresspolymlett.2014.82
Carli LN, Crespo JS, Mauler RS (2011) PHBV nanocomposites based on organomodified montmorillonite and halloysite: the effect of clay type on the morphology and thermal and mechanical properties. Compos Part A 42:1601–1608. https://doi.org/10.1016/j.compositesa.2011.07.007
Castro-Mayorga JL, Fabra MJ, Cabedo L, Lagaron JM (2016) On the use of the electrospinning coating technique to produce antimicrobial polyhydroxyalkanoate materials containing in situ-stabilized silver nanoparticles. Nanomaterials 7. https://doi.org/10.3390/nano7010004
Central Pollution Control Board (2013) Overview of plastic waste management. Annual report (2011–12) on implementation of PWM
Chanprateep S (2010) Current trends in biodegradable polyhydroxyalkanoates. J Biosci Bioeng 110:621–632. https://doi.org/10.1016/j.jbiosc.2010.07.014
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio-and materials industry. Chem Soc Rev 38:2434–2446. https://doi.org/10.1039/b812677c
Chen GQ (2010) Plastics completely synthesized by bacteria: polyhydroxyalkanoates. In: Chen GQ (ed) Plastics from bacteria: natural functions and applications, microbiology monographs. Springer, Berlin, pp 17–37. https://doi.org/10.1007/978-3-642-03287_5_2
Chen YJ (2014) Bioplastics and their role in achieving global sustainability. J Chem Pharm Res 6:226–231
Colombo B, Pepè Sciarria T, Reis M, Scaglia B, Adani F (2016) Polyhydroxyalkanoates (PHAs) production from fermented cheese whey by using a mixed microbial culture. Bioresour Technol 218:692–699. https://doi.org/10.1016/j.biortech.2016.07.024
Correa MCS, Rezende ML, Rosa DS, Agnelli JAM, Nascente PAP (2008) Surface composition and morphology of poly(3-hydroxybutyrate) exposed to biodegradation. Polym Test 27:447–452. https://doi.org/10.1016/j.polymertesting.2008.01.007
Cyras VP, Commisso SM, Mauri AN, Vázquez A (2007) Biodegradable double-layer films based on biological resources: polyhydroxybutyrate and cellulose. J Appl Polym Sci 106:749–756. https://doi.org/10.1002/app.26663
Cyras VP, Soledad CM, Analía V (2009) Biocomposites based on renewable resource: acetylated and nonacetylated cellulose cardboard coated with polyhydroxybutyrate. Polymer 50:6274–6280. https://doi.org/10.1016/j.polymer.2009.10.065
Da Silva MG, Vargas H, Poley LH, Rodriguez RS, Baptista GB (2005) Structural impact of hydroxyvalerate in polyhydroxyalkanoates (PHAscl) dense film monitored by XPS and photothermal methods. J Braz Chem Soc 16:790–795. https://doi.org/10.1590/S0103-50532005000500017
Dagnon KL, Thellen C, Ratto JA, D’Souza NA (2010) Physical and thermal analysis of the degradation of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) coated paper in a constructed soil medium. J Polym Environ 18:510–522. https://doi.org/10.1007/s10924-010-0231-y
Díez-Pascual AM, Díez-Vicente AL (2014a) Poly(3-hydroxybutyrate)/ZnO bionanocomposites with improved mechanical, barrier and antibacterial properties. Int J Mol Sci 15:10950–10973. https://doi.org/10.3390/ijms150610950
Díez-Pascual AM, Díez-Vicente AL (2014b) ZnO-reinforced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bionanocomposites with antimicrobial function for food packaging. ACS Appl Mater Interfaces 6:9822–9834. https://doi.org/10.1021/am502261e
Fabra MJ, Lopez-Rubio A, Lagaron JM (2013) High barrier polyhydroxyalcanoate food packaging film by means of nanostructured electrospun interlayers of zein. Food Hydrocoll 32:106–114. https://doi.org/10.1016/j.foodhyd.2012.12.007
Fabra MJ, Lopez-Rubio A, Lagaron JM (2014a) Nanostructured interlayers of zein to improve the barrier properties of high barrier polyhydroxyalkanoates and other polyesters. J Food Eng 127:1–9. https://doi.org/10.1016/j.jfoodeng.2013.11.022
Fabra MJ, Sanchez G, Lopez-Rubio A, Lagaron JM (2014b) Microbiological and ageing performance of polyhydroxyalkanoate-based multilayer structures of interest in food packaging. LWT-Food Sci Technol 59:760–767. https://doi.org/10.1016/j.lwt.2014.07.021
Fan X, Jiang Q, Sun Z, Li G, Ren X, Liang J, Huang TS (2015) Preparation and characterization of electrospun antimicrobial fibrous membranes based on polyhydroxybutyrate (PHB). Fibers Polym 16:1751–1758. https://doi.org/10.1007/s12221-015-5108-1
Follain N, Chappey C, Dargent E, Chivrac F, Crétois R, Marais S (2014) Structure and barrier properties of biodegradable polyhydroxyalkanoate films. J Phys Chem C 118:6165–6177. https://doi.org/10.1021/jp408150k
Fu XZ, Tan D, Aibaidula G, Wu Q, Chen JC, Chen GQ (2014) Development of Halomonas TD01 as a host for open production of chemicals. Metab Eng 23:78–91. https://doi.org/10.1016/j.ymben.2014.02.006
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:e1700782. https://doi.org/10.1126/sciadv.1700782
Gironi F, Piemonte V (2011) Bioplastics and petroleum-based plastics: strengths and weaknesses. Energy Sources A: Recover Utilization Environ Eff 33:1949–1959. https://doi.org/10.1080/15567030903436830
Goncalves SPC, Martins-Franchetti SM, Chinaglia DL (2009) Biodegradation of the films of PP, PHBV and its blend in soil. J Polym Environ 17:280–285. https://doi.org/10.1007/s10924-009-0150-y
Haugaard V, Danielsen B, Bertelsen G (2003) Impact of polylactate and poly(hydroxybutyrate) on food quality. Eur Food Res Technol 216:233–240. https://doi.org/10.1007/s00217-002-0651-6
Hazer B, Steinbüchel A (2007) Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl Microbiol Biotechnol 74:1–12. https://doi.org/10.1007/s00253-006-0732-8
Hermida EB, Mega VI, Yashchuk O, Fernández V, Eisenberg P, Miyazaki SS (2008) Gamma irradiation effects on mechanical and thermal properties and biodegradation of poly(3-hydroxybutyrate) based films. Macromol Symp 263:102–113. https://doi.org/10.1002/masy.200850313
Kantola M, Helén H (2001) Quality changes in organic tomatoes packaged in biodegradable plastic films. J Food Qual 24:167–176. https://doi.org/10.1111/j.1745-4557.2001.tb00599.x
Keshavarz T, Roy I (2010) Polyhydroxyalkanoates: bioplastics with a green agenda. Curr Opin Microbiol 13:321–326. https://doi.org/10.1016/j.mib.2010.02.006
Khanna S, Srivastava A (2005) Recent advances in microbial polyhydroxyalkanoates. Process Biochem 40:607–619. https://doi.org/10.1016/j.procbio.2004.01.053
Khosravi-Darani K, Bucci DZ (2015) Application of poly (hydroxyalkanoate) in food packaging: improvements by nanotechnology. Chem Biochem Eng Q 29(2):275–285. https://doi.org/10.15255/CABEQ.2014.2260
Kiran GS, Jackson SA, Priyadharsini S, Dobson ADW, Selvin J (2017) Synthesis of Nm-PHB (nanomelanin-polyhydroxy butyrate) nanocomposite film and its protective effect against biofilmforming multi drug resistant Staphylococcus aureus. Sci Rep 7:9167. https://doi.org/10.1038/s41598-017-08816-y
Koller M (2014) Poly(hydroxyalkanoates) for food packaging: application and attempts towards implementation. Appl Food Biotechnol 1:3–15
Koller M, Salerno A, Muhr A, Reiterer A, Braunegg G (2013) Polyhydroxyalkanoates: biodegrad-able polymers and plastics from renewable resources. Mater Technol 47:5–12
Kwiecień I, Adamus G, Bartkowiak A, Kowalczuk M (2014) Synthesis and structural characterization at the molecular level of oligo(3-hydroxybutyrate) conjugates with antimicrobial agents designed for food packaging materials. Des Monomers Polym 17:311–321. https://doi.org/10.1080/15685551.2013.840505
Laycock B, Halley P, Pratt S, Werker A, Lanta P (2013) The chemomechanical properties of microbial polyhydroxyalkanoates. Prog Polym Sci 38:536–583. https://doi.org/10.1016/j.progpolymsci.2012.06.003
Levkane V, Muizniece-Brasava S, Dukalska L (2008) Pasteurization effect to quality of salad with meat and mayonnaise. Foodbalt:69–73
Li Z, Lin H, Ishii N, Chen GQ, Inoue Y (2007) Study of enzymatic degradation of microbial copolyesters consisting of 3-hydroxybutyrate and medium-chain-length 3-hydroxyalkanotes. Polym Degrad Stab 92:1708–1714. https://doi.org/10.1016/j.polymdegradstab.2007.06.001.
Lodhi AF, Hasan F, Shah Z, Hameed A, Faisal S, Shah AA (2011) Optimization of culture conditions for the production of poly(3-hydroxybutyrate) depolymerase from newly isolated Aspergillus fumigatus from soil. Pak J Bot 43:1361–1372
Maiti P, Batt CA, Emmanuel G (2003) Renewable plastics: synthesis and properties of PHB nanocomposites. Polym Mater Sci Eng 88:58–59
Martínez-Sanz M, Villano M, Oliveira C, Albuquerque MG, Majone M, Reis M, Lopez-Rubio A, Lagaron JM (2014) Characterization of polyhydroxyalkanoates synthesized from microbial mixed cultures and of their nanobiocomposites with bacterial cellulose nanowhiskers. New Biotechnol 31:364–376. https://doi.org/10.1016/j.nbt.2013.06.003
Masood F (2017) Polyhydroxyalkanoates in the food packaging industry. In Oprea AE, Grumezescu AM (eds) Nanotechnology applications in food. Academic/Elsevier, London, pp 153–177. ISBN:978-0-12-811942-6
Masood F, Aziz M, Haider H, Shakil O, Yasin T, Hameed A (2018) Biodegradation of gamma irradiated poly-3-hydroxybutyrate/sepiolite nanocomposites. Int Biodeterior Biodegrad 126:1–9. https://doi.org/10.1016/j.ibiod.2017.09.012
Miguel O, Iruin JJ (1999a) Evaluation of the transport properties of poly(3-hydroxybutyrate) and its 3-hydroxyvalerate copolymers for packaging applications. Macromol Symp 144:427–438. https://doi.org/10.1002/masy.19991440140
Miguel O, Iruin JJ (1999b) Water transport properties in poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymers. J Appl Polym Sci 73:455–468. https://doi.org/10.1002/(SICI)1097-4628(19990725)73:4<455::AID-APP1>3.0.CO;2-Y
Miguel O, Fernandez-Berridi MJ, Iruin JJ (1997) Survey on transport properties of liquids, vapors, and gases in biodegradable poly(3-hydroxybutyrate) (PHB). J Appl Polym Sci 64:1849–1859. https://doi.org/10.1002/(SICI)1097-4628(19970531)64:9<1849::AID-APP22>3.0.CO;2-R
Mohamed El-Hadi A (2014) Investigation of the effect of nano-clay type on the non-isothermal crystallization kinetics and morphology of poly(3(R)-hydroxybutyrate) PHB/clay nanocomposites. Polym Bull 71:1449–1470. https://doi.org/10.1007/s00289-014-1135-0
Mohapatra S, Maity S, Dash HR, Das S, Pattnaik S, Rath CC, Samantaray D (2017) Bacillus and biopolymer: prospects and challenges. Biochem Biophys Rep 12:206–213. https://doi.org/10.1016/j.bbrep.2017.10.001
Możejko-Ciesielska J, Kiewisz R (2016) Bacterial polyhydroxyalkanoates: still fabulous? Microbiol Res. https://doi.org/10.1016/j.micres.2016.07.010
Muizniece-Brasava S, Dukalska L (2006) Impact of biodegradable PHB packaging composite materials on dairy product quality. In: Proceedings of the Latvia University of Agriculture LLU Raksti 16:79–87
Narayanan A, Neera M, Ramana KV (2013) Synergized antimicrobial activity of eugenol incorporated polyhydroxybutyrate films against food spoilage microorganisms in conjunction with pediocin. Appl Biochem Biotechnol 170:1379–1388. https://doi.org/10.1007/s12010-013-0267-2
North EJ, Halden RU (2013) Plastics and environmental health: the road ahead. Rev Environ Health 28:1–8. https://doi.org/10.1515/reveh-2012-0030.
Pardo-Ibáñez P, Lopez-Rubio A, Martínez-Sanz M, Cabedo L, Lagaron JM (2014) Keratin–polyhydroxyalkanoate melt-compounded composites with improved barrier properties of interest in food packaging applications. J Appl Polym Sci 131:39947/1–39947/10. https://doi.org/10.1002/app.39947
Pavani P, Rajeswari TR (2014) Impact of plastics on environmental pollution. National Seminar on impact of toxic metals, minerals and solvents leading to environmental pollution. J Chem Pharm Sci 3:87–93
Plackett D, Siró I (2011) Polyhydroxyalkanoates (PHAs) for food packaging. In: Lagarón JM (ed) Multifunctional and nanoreinforced polymers for food packaging. Woodhead Publishing, Cambridge, pp 498–526. https://doi.org/10.1533/9780857092786.4.498
Plastics Europe (2016) Plastics-the facts 2016: an analysis of European plastics production, demand and waste data
Poley LH, Siqueira APL, Da Silva MG, Sanchez R, Prioli R, Mansanares AM, Vargas H (2005) Photothermal methods and atomic force microscopy images applied to the study of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3- hydroxyvalerate) dense membranes. J Appl Polym Sci 97:1491–1497. https://doi.org/10.1002/app.21891
Prasad P, Kochhar A (2014) Active packaging in food industry: a review. IOSR J Environ Sci Toxicol Food Technol (IOSR-JESTFT) 8:01–07
Rai R, Keshavarz T, Roether J, Boccaccini AR, Roy I (2011) Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater Sci Eng R 72:29–47. https://doi.org/10.1016/j.mser.2010.11.002
Ramachandran D, Latha RR, Gowri GR (2013) Incorporation of clitoria ternatea seed extract into bioplastic sheets having induced plasticity and their antimicrobial activity against multi-drug resistant clinical pathogens. Int J Pharm Pharm Sci 5:543–550
Rastogi VK, Samyn P (2015) Bio-based coatings for paper applications. Coatings 5:887–930
Rastogi VK, Samyn P (2017) Synthesis of polyhydroxybutyrate particles with micro-to-nanosized structures and application as protective coating for packaging papers. Nanomaterials 7:5. https://doi.org/10.3390/nano7010005
Reis DCC, Lemos-Morais AC, Carvalho LH, Alves TS, Barbosa R (2016) Assessment of the morphology and interaction of PHBV/clay bionanocomposites: uses as food packaging. Macromol Symp 367:113–118. https://doi.org/10.1002/masy.201500143
Reis DCC, Oliveira TA, Carvalho LH, Alves TS, Barbosa (2017) The influence of natural clay and organoclay vermiculite on the formation process of bionanocomposites with poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Matéria (Rio J) 22:1186. https://doi.org/10.1590/S1517-707620170004.0220
Renard E, Walls M, Guérin P, Langlois V (2004) Hydrolytic degradation of blends of polyhydroxyalkanoates and functionalized polyhydroxyalkanoates. Polym Degrad Stab 85:779–787. https://doi.org/10.1016/j.polymdegradstab.2003.11.019
Rhim JW, Park HM, Ha CS (2013) Progress in polymer science bio-nanocomposites for food packaging applications. Prog Polym Sci 38:1629–1652. https://doi.org/10.1016/j.progpolymsci.2013.05.008
Rudnik E (2012) Compostable polymer properties and packaging applications In: Ebnesajjad S (ed) Plastic films in food packaging. William Andrew. 2013, pp 217–248. ISBN:978-1-4557-3112-1
Saini RD (2017) Biodegradable polymers. Int J Appl Chem 13:179–196
Salama HE, Saad GR, Sabaa MW (2017) Synthesis, characterization and antimicrobial activity of biguanidinylated chitosan-g-poly[(R)-3-hydroxybutyrate]. Int J Biol Macromol 101:438–447. https://doi.org/10.1016/j.ijbiomac.2017.03.075
Sanchez-Garcia MD, Gimenez E, Lagaron JM (2007) Novel PET nanocomposites of interest in food packaging applications and comparative barrier performance with biopolyester nanocomposites. J Plas Film Sheet 23:133–148. https://doi.org/10.1177/8756087907083590
Sanchez-Garcia MD, Gimenez E, Lagaroân JM (2008) Morphology and barrier properties of nanobiocomposites of poly(3-hydroxybutyrate) and layered silicates. J Appl Polym Sci 108:2787–2801. https://doi.org/10.1002/app.27622
Santos Rosa D, Calil MR, Guedes CGF, Rodrigues TC (2004) Biodegradability of thermally aged PHB, PHB-V, and PCL in soil compostage. J Environ Polym Degr 12:239–245. https://doi.org/10.1007/s10924-004-8151-3
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26:246–265. https://doi.org/10.1016/j.biotechadv.2007.12.005
Siddiqui J, Pandey G (2013) A review of plastic waste management strategies. Int Res J Environ Sci 2:84–88
Siracusa V (2012) Food packaging permeability behavior: a report. Int J Polym Sci 2012:302029., 11 pages. https://doi.org/10.1155/2012/302029
Steinbüchel A, Lütke-Eversloh T (2003) Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem Eng J 16:81–96. https://doi.org/10.1016/S1369-703X(03)00036-6
Tan GYA, Chen CL, Li L, Ge L, Wang L, Razaad IMN, Li Y, Zhao L, Mo Y, Wang JY (2014) Start a research on biopolymer polyhydroxyalkanoate (PHA): a review. Polymer 6:706–754. https://doi.org/10.3390/polym6030706
Thellen C, Coyne M, Froio D, Auerbach M, Wirsen C, Ratto JA (2008) A processing, characterization and marine biodegradation study of melt-extruded polyhydroxyalkanoate (PHA) films. J Polym Environ 16:1–11. https://doi.org/10.1007/s10924-008-0079-6
Thellen C, Cheney S, Ratto JA (2013) Melt processing and characterization of polyvinyl alcohol and polyhydroxyalkanoate multilayer films. J Appl Polym Sci 127:2314–2324. https://doi.org/10.1002/app.37850
Valentino F, Beccari M, Fraraccio S, Zanaroli G, Majone M (2014) Feed frequency in a sequencing batch reactor strongly affects the production of polyhydroxyalkanoates (PHAs) from volatile fatty acids. New Biotechnol 31:264–275. https://doi.org/10.1016/j.nbt.2013.10.006
Wang Y, Chen GQ (2017) Polyhydroxyalkanoates: sustainability, production, and industrialization. In Tang C, Ryu CY (eds) Sustainable polymers from biomass. Wiley-VCH Verlag GmbH & Co. KGaA, pp 11–33
Wang S, Song C, Chen G, Guo T, Liu J, Zhang B, Takeuchi S (2005) Characteristics and biodegradation properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/organophilic montmorillonite (PHBV/OMMT) nanocomposites. Polym Degrad Stab 87:69–76. https://doi.org/10.1016/j.polymdegradstab.2004.07.008
Wang Y, Yin J, Chen GQ (2014) Polyhydroxyalkanoates, challenges and opportunities. Curr Opin Biotechnol 30:59–65. https://doi.org/10.1016/j.copbio.2014.06.001
Witholt B, Kessler B (1999) Perspectives of medium chain length poly(hydroxyalkanoates), a versatile set of bacterial bioplastics. Curr Opin Biotechnol 10:279–285. https://doi.org/10.1016/s0958-1669(99)80049-4
Xavier JR, Babusha ST, George J, Ramana KV (2015) Material properties and antimicrobial activity of Polyhydroxybutyrate (PHB) films incorporated with vanillin. Appl Biochem Biotechnol 176:1498–1510. https://doi.org/10.1007/s12010-015-1660-9
Yu HY, Qin ZY, Binsun YXG, Yao JM (2014) Reinforcement of transparent poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by incorporation of functionalized carbon nanotubes as a novel bionanocomposite for food packaging. Compos Sci Technol 94:96–104. https://doi.org/10.1016/j.compscitech.2014.01.018
Acknowledgements
The authors wish to thank Council of Scientific and Industrial Research (CSIR), New Delhi, India, for the Senior Research Fellowship. School of Sciences, Jain University is also thankfully acknowledged for providing the necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Israni, N., Shivakumar, S. (2019). Polyhydroxyalkanoates in Packaging. In: Kalia, V. (eds) Biotechnological Applications of Polyhydroxyalkanoates. Springer, Singapore. https://doi.org/10.1007/978-981-13-3759-8_14
Download citation
DOI: https://doi.org/10.1007/978-981-13-3759-8_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3758-1
Online ISBN: 978-981-13-3759-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)