Abstract
Sepsis is a common and main cause of morbidity and mortality in intensive care units and emergency departments. Recent evidence illustrated that patients who are suffering from sepsis undergo a prolonged immunosuppressive phase. As a consequence, many septic patients are at risk for secondary infection which is considered to be the major reason for the high mortality of this disease nowadays. In this paper, we discuss the clinical significance of secondary infection and its potential immune mechanisms. In addition, the conventional measures and novel immunomodulatory strategies are also summarized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Sepsis is an uncontrolled inflammatory response caused by infection. The estimated mortality of sepsis is 10–20%, which can reach up to 60% when shock is present. The large number of inflammatory mediators in sepsis contributes to shock and multiple organ dysfunction syndrome (MODS) in sepsis. Nevertheless, many recent studies have demonstrated that sepsis is associated with only a transient hyper-inflammatory phase. Subsequently, patients enter a prolonged immunosuppressive phase. As a result, patients who survive the acute phase of sepsis are at high risk of secondary nosocomial infection. The purpose of this paper is to discuss the current understanding of the clinical significance, mechanism, and treatment of secondary infection in sepsis.
12.1 Secondary Infection in Sepsis
During the past decades, the knowledge about the basic pathophysiologic processes of sepsis has been improved rapidly which contributes to the development of treatment strategies of sepsis. The first Surviving Sepsis Campaign (SSC) guideline for the management of severe sepsis and septic shock was published in 2004 and has been updated every 4 years. Due to these efforts, the outcomes of sepsis have been improved year by year. Recent evidence illustrated that the absolute mortality rate of patients with severe sepsis decreased from 35% in 2000 to 18.4% in 2012 [1]. A meta-analysis of 36 multicenter data also found that severe sepsis mortality decreased from 46.9% in 1991–1995 to 29% in 2006–2009 [2]. However, unlike other diseases, such as acute myocardial infarction and stroke, the mortality of sepsis still remains unacceptably high. More importantly, from 1997 to 2010, septic shock patients with hospital mortality decreased by only 9% [3]. Similar results were also reported by Goto et al. [4]. Until now, sepsis is still one of the main causes of death in clinical critically ill patients.
According to the recent evidence, about 70% of septic death occurred 3 days after admission. In a study of 476 septic patients, 62.7% of total deaths occurred in the late phase of the disease [5]. As the early-phase mortality fell significantly, the sustained high mortality from severe sepsis and septic shock may be due to increased mortality in the late phase of the disease [5]. It has been demonstrated that more than 80% of the deceased septic patients had signs of continuous infections and the rates of common opportunistic bacteria and fungi increased significantly in the late phase (>15 days) of severe sepsis and septic shock when compared with the early phase (<6 days) of the disease [6]. Additionally, 39% of septic shock patients who survived the early phase (<3 days) of the disease developed secondary nosocomial infection in the ICUs [5]. In addition to bacterial and fungi infection, a study observed that over 40% of septic patients had reactivation of latent herpes viruses [7]. Moreover, the risk of late death for septic shock patients with secondary infection was about 5.8 times higher than that for patients without [5]. Another retrospective study also found that septic shock patients who died more than 3 days after ICU admission were related to hospital-acquired complications, including secondary infections [8]. Recently, secondary infection has been considered as the major cause of death for patients with sepsis [9, 10].
12.2 Immune Dysfunction and Secondary Infection Post-sepsis
Our previous study found that age, the severity of the disease, invasive medical measures, and length of ICU hospital stay were associated with secondary infection in septic shock patients [5]. However, it is well known that pathogens cause disease, not only dependent on the pathogenicity of pathogens but also closely related to the immune function of the host. Evidence showed that patients admitted with severe sepsis were more susceptible to nosocomial infection than other ICU patients without sepsis and sepsis is an independent risk factor for nosocomial infection in ICU patients [11]. It has been recognized that the unique immune status of sepsis patients may influence their susceptibility to secondary infection.
12.2.1 Innate Immune Defects
12.2.1.1 Neutrophils
The innate immune system, also known as the non-specific immune system, is the first line of host defense against pathogenic organisms. The innate immune system consists of physical epithelial barriers, phagocytic cells, and circulating plasma molecules. Neutrophils comprise the largest number of the main innate immune cells in the body, and the majority of them remain housed in the bone marrow or immune centers. Together with macrophages, neutrophils constitute the professional phagocytes, and they have a primary role in protecting the host from pathogen invasion. Neutrophils kill or remove pathogenic microorganisms through the oxygen-dependent and the oxygen-independent mechanisms. In the oxygen-dependent process, killing was mediated by oxygen free radicals and other reactive oxygen species (ROS) generated by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex in response to pathogens. It has been reported that there was a significant reduction in the respiratory burst of BAL neutrophils from septic animals challenged with P. aeruginosa when compared to sham-treated mice [12]. Interestingly, similar to septic mice, the production of ROS in neutrophils in NADPH-deficient mice was also decreased, and an increased susceptibility to P. aeruginosa infection was also observed [12]. So, neutrophil deficiency may contribute to the secondary infection post-sepsis.
12.2.1.2 Monocytes and Macrophages
It has been observed that, similar to the phenomenon of endotoxin tolerance, the cytokine production capacity of monocytes and macrophages from septic mice and humans in response to LPS was severely reduced [13]. This phenomenon is a crucial pathophysiological adaptation to regulate overexuberant inflammation. On the other hand, it also contributes to immunosuppression after sepsis. Previous evidence illustrated that the levels of human leukocyte antigen (HLA)-DR on macrophages from sepsis were significantly decreased and were associated with the outcomes as well as the incidence of secondary infection [14, 15]. Additionally, the macrophages from septic patients secrete high levels of IL-10 which plays an important role in inhibiting the activity of Th1 cells, macrophages, as well as NK cells. As a result, the susceptibility of host to secondary infection in sepsis was increased [14, 15]. In addition, the expression of IL-1 receptor-associated kinase (IRAK)-M in macrophages was upregulated in sepsis, and the expression of the Toll-like receptor (TLR)-4 was reduced, which contribute to the decreased expression of pro-inflammatory cytokine and phagocytic function of macrophages [16]. The restored pathogen clearance ability of macrophages and increased survival rate of septic animals were observed after inhibiting IRAK-M expression [16].
12.2.1.3 Dendritic Cells
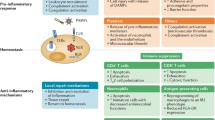
Dendritic cells (DCs), originating from CD34+ hematopoietic stem cells, are one of the most powerful types of antigen-presenting cells (APCs). Mature DCs express major histocompatibility complex (MHC) molecules, co-stimulatory molecules (CD40, CD80, CD83, and CD86), and adhesion molecules and migrate to lymphoid organs and induce specific immune response of T cells [17]. A large number of previous studies have confirmed that the number of DCs in sepsis was significantly decreased and the percentage of mature DCs also trended toward reduced levels [17]. In addition, the ability of DCs to secrete pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-12, and IL-1β is reduced in sepsis, whereas the production of anti-inflammatory cytokines including IL-10 and transforming growth factor (TGF)-β was significantly increased [17, 18]. IL-2 is a key molecule for T-cell proliferation and survival, and the ability of septic DC cells to induce proliferation and IL-2 production of T cells were reduced. Adoptive transfer of DCs from normal mice protected mice from secondary P. aeruginosa infection after sepsis, but the DCs from septic mice did not reduce the susceptibility of sepsis mice to secondary infection, suggesting that the decreased number and impaired function of DCs may be the crucial reasons of secondary infection in sepsis [19] (Fig. 12.1).
12.2.2 Adaptive Immune Response Dysfunction
12.2.2.1 CD4+T Lymphocytes
Cytokines and chemokines secreted by CD4+T cells are crucial for the activation of macrophages and neutrophils. Additionally, the interactions between T cells and B cells are essential for the production of neutralizing antibodies to pathogens. Evidence have been illustrated that the proliferation of CD4+T cells in response to polyclonal and antigen-specific stimulation was reduced [20]. A shift from Th1 to Th2 and increased apoptosis of T cells were also observed in sepsis [20]. Clinical studies illustrated that the number of circulating T cells was significantly decreased in septic patients and was positively associated with the illness severity and mortality [20]. In addition, surviving CD4+T cells exhibited lower capability to secrete cytokines in response to LPS. Our previous studies found that mitofusin-2, a mitochondrial membrane protein that participates in mitochondrial fusion in mammalian cells, plays an important role in regulating CD4+T cell immune function and apoptosis through Ca2+-NFAT signaling pathway [21]. Recently, it has been found that increased levels of programmed cell death protein 1 (PD-1), cytotoxic T lymphocyte-associated antigen (CTLA)-4, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and B and T lymphocyte attenuator (BTLA) in CD4+T cells might contribute to the defects of immune function of the cells in sepsis and the decrease in resistance to infectious pathogens in host survival [22, 23].
12.2.2.2 Regulatory T Cells
Regulatory T cells (Tregs), as one of the T-cell subsets, can be classified into natural Tregs (nTregs), adaptive/induced Tregs (iTregs), type 1 Tregs (Tr1), T helper 3 (Th3), and CD8+Tregs. Forkhead/winged helix transcription factor p3 (Foxp3) is principally found within the CD4+CD25+Treg cell population and plays an important role in the development and functionality of these cells [24]. Tregs are crucial for modulating the immune responses in tumor immunity, transplantation tolerance, as well as infectious diseases. The suppressive function of Tregs is dependent on cell-cell contact mechanism and immunosuppressive cytokines [24]. Cytotoxic T lymphocyte antigen (CTLA)-4 and glucocorticoid-induced tumor necrosis factor receptor (GITR) mediate the function of cell contact-dependent suppression of Tregs [24]. CD25 on the surface of Tregs has high capacity to capture IL-2 which contributes to the suppressive function of the cells. Additionally, transforming growth factor (TGF)-β and interleukin (IL)-10 secreted by Tregs also play a role in immune depression [24].
Clinical studies found that the proportion of CD4+CD25+Treg in the peripheral blood of patients with sepsis is significantly increased [25]. Further studies illustrated that the absolute number of CD4+CD25+Treg cells did not increase significantly, but its immunosuppressive activity was enhanced [26, 27]. The expression of CTLA-4 and Foxp3 and the levels of IL-10 and TGF-β in Treg cells were significantly higher than those in the non-sepsis group [26, 27]. In addition, in patients with sepsis, the immunosuppressive function of Tregs is higher in the death group when compared with that in the survival group [26, 27]. Evidence illustrated DEREG mice depleted of Foxp3+Treg cells before secondary infection with P. aeruginosa 7 days post-CLP had no effects on the course of secondary infection as well as cytokine levels [28]. Nevertheless, our study found partial depletion of CD25+Tregs by PC61 treatment 3 days post-CLP also enhanced host immune responses against secondary acute P. aeruginosa and improved the outcomes of the disease (unpublished data). The difference in the results might be explained on the basis of experimental techniques including septic models and the depletion strategies of Tregs. The exact role of Treg in secondary infection post-sepsis remains to be investigated.
12.2.2.3 B Lymphocytes
B lymphocytes arise from hemopoietic stem cells in the bone marrow which are characterized by their ability to differentiate into immunoglobulin secreting plasma cells. In addition to their role in humoral immunity, B cells have an important role in regulating immune homeostasis by their antigen-presenting and cytokine-producing capabilities [29]. Although there are some controversies, it is widely accepted that B cells act as effective antigen-presenting cells which are required for the initiation of T-cell immune responses during infection [29, 30]. Additionally, the bacterial products, such as LPS and CpG DNA, can cause the activation and cytokine production of B cells through Toll-like receptors (TLR) both in vivo and in vitro [31]. Recently, regulatory B cells, a phenotypically distinct subset of B cells, have been recognized as a crucial regulator of T-cell-mediated inflammatory responses through the production of IL-10 [32].
Previously study found that septic mice with adaptive immune system defects, including B cell apoptosis, display diminished survival. Nevertheless, the role of B cells in sepsis was not well examined. Rauch et al. [33] found that mice lacking B-cell-derived GM-CSF are unable to clear bacteria and succumb to infection. Kelly-Scumpia et al. [34] found that B-cell-deficient, but not α/β T-cell-deficient, mice display decreased inflammatory cytokine and chemokine production and increased mortality after sepsis. Clinical study has demonstrated that patients with septic shock suffer from a severe retraction of circulating B lymphocytes [35]. An exhausted B-cell phenotype has been associated with a CD19+CD5+CD27−CD21−/low “tissue-like memory” B-cell subset in human immunodeficiency virus (HIV)-infected patients. Recently, it was reported that the fraction of CD21-exhausted B cells in sepsis patients was higher than that in healthy subjects [36]. Moreover, the IgM production was impaired in elderly septic patients, and this phenomenon was associated with increased susceptibility to secondary infection post-sepsis, as elderly septic patients showed a higher rate of Candida albicans and higher sputum colony counts than adult septic patients [36]. So, the defects of B lymphocyte function may be associated with immunosuppression and secondary infection in patients with sepsis.
12.3 Therapeutic Strategies for Secondary Infection
12.3.1 Conventional Strategies
According to the criteria of the Centers for Disease Control (CDC), there are four main types of nosocomial infections including central line-associated bloodstream infections (CLABSI), surgical site infection (SSI), catheter-associated urinary tract infection (CAUTI), and ventilator-associated pneumonia (VAP). Prevention and control guidelines have been developed to reduce the incidence of these infections. The measures recommended by these guidelines, including the standard and transmission-based precautions as well as strategies focused on specific nosocomial infections, also can be applied to prevent and control secondary infection after sepsis [37]. It should be noted that secondary infection after sepsis has its own characteristics. Evidence illustrated that pneumonia was the most frequent secondary infection in septic shock patients and the most frequently isolated microorganism was Acinetobacter baumannii [5]. Additionally, as mentioned above, age, the SOFA score, length of stay in the ICU, and endotracheal intubation were observed to be associated with secondary infection post-sepsis [5]. So, improving the care of these patients may be useful for reducing the incidence of secondary infections after sepsis.
12.3.2 Immunomodulatory Therapy
12.3.2.1 Experimental Studies
12.3.2.1.1 Cytokines and Cytokine-Targeted Therapies
Cytokines are crucial regulators of the immune response to infections, and imbalance in inflammatory network has been found to be associated with the death of septic patients. Previous studies suggest that pro-inflammatory cytokines, such as IL-6, IL-1β, and TNF-α, contributed to tissue damage and organ failure of sepsis [38]. Nevertheless, the failure of several anti-inflammatory clinical trials in sepsis has led researchers to re-recognize the function of pro-inflammatory cytokines. In fact, increased mortality was observed in TNF–/–LT–/– knockout mice with infection [39]. So, pro-inflammatory cytokines may be also important for protecting host from infections. In contrast to pro-inflammatory cytokines, anti-inflammatory cytokines, such as IL-4 and IL-10, play a crucial role in inhibiting inflammation and contribute to immunosuppression in sepsis. Song et al. [40] found that IL-4-deficient mice that underwent CLP were resistant to secondary pulmonary P. aeruginosa infection which is characterized by better bacterial clearance and improved survival. Neutralization of TNF-α could reverse the enhanced protection against secondary infection in septic IL-4 KO mice, indicating the crucial role of TNF-α in this process [40]. Additionally, IL-10 and IL-27 were also observed to be associated with immunosuppression in sepsis, and neutralization of IL-10 or IL-27 could reverse sepsis-induced dysfunction of macrophages and improve both survival and clearance of bacteria from the lungs of septic mice infected with P. aeruginosa [41, 42].
IL-7, a 25-kDa glycoprotein produced by the bone marrow and thymic stromal cells, plays an important role in regulating T- and B-cell development and function [43,44,45]. Through its binding to a receptor (IL-7R), IL-7 can increase the levels of B-cell lymphoma 2 (BCL2) and the numbers of circulating CD4+ and CD8+T cells [46]. IL-7 also improves the ability of T cells to move to sites of infection by increasing the cell adhesion molecule expression. IL-7 treatment restores immunity and improves survival in a viral model of lymphocytic choriomeningitis [47]. Previous studies have illustrated that rhIL-7 treatment inhibited the apoptosis of T lymphocyte by upregulating Bcl-2 expression and decreased the mortality of septic animals [48]. Additionally, in septic mice followed by P. aeruginosa and fungal infection, treatment with rhIL-7 could improve survival effectively [49]. More importantly, IL-7 treatment does not induce a hyper-inflammatory response. IL-7 is currently undergoing numerous clinical trials, including in patients with septic shock (ClinicalTrials.gov identification # NCT02640807, # NCT02797431).
In addition to IL-7, IL-15 is also under consideration for treating sepsis. Studies have shown that IL-15 could inhibit the apoptosis of NK cells, DC cells, and CD8+T cell in sepsis [50]. In mice with peritonitis or P. aeruginosa pneumonia, IL-15 improved T lymphocyte survival, enhanced the capacity of cell to secrete cytokines, and ultimately decreased the mortality of the animals [50]. However, IL-15 did not improve the capacity of mice to clear bacteria [50]. Additionally, it should be noted that IL-15 superagonist has potential hepatotoxicity and its clinical safety remains scarce [51,52,53].
12.3.2.1.2 Immune Checkpoint Therapies
Immune checkpoints are molecules in the immune system that are crucial for maintaining self-tolerance. The CTLA-4 and PD-1 immune checkpoints are negative regulators of the immune function of T cells [54,55,56]. PD-1, a newly discovered co-stimulatory receptor, belongs to the CD28 superfamily of receptors [54,55,56]. PD-L1 and PD-L2 are the main ligands of PD-1. PD-L1 is widely expressed on dendritic cells, macrophages, and activated T lymphocytes and B lymphocytes [54,55,56]. Increased levels of PD-L1 on T cells and monocytes were observed in septic patients [57,58,59]. Studies also illustrated that PD-L1 can be used as a predictive and prognostic marker for sepsis. Blocking PD-L1 can reduce TNF-α and anti-CD3/anti-CD28 antibody-induced CD4+T and CD8+T cell apoptosis and can promote the expression of IL-6, TNF-α, and IFN-γ in monocytes and lymphocytes [58,59,60]. CTLA-4, as an analogue of CD28, has a higher binding activity to B7.1 and B7.2 [54]. Nevertheless, binding of CTLA-4 to B7 does not produce a simulator signal. So, it has been proposed that CTLA-4 dampens the activation of T cells by outcompeting CD28 in binding B7. In addition, evidence also illustrated that CTLA-4 binding to B7 on T cells also actively delivers inhibitory signals to the T cells, blocks TCR activating, and then inhibits T-cell proliferation [54]. Animal studies found that blocking PD1/PD-L1 and CTLA-4 improved the outcomes of sepsis or sepsis with secondary fungal infection [23]. Recently, nivolumab and pembrolizumab, both antibodies against PD-1, received FDA approval for the treatment of patients with unresectable or metastatic melanoma as well as metastatic squamous and nonsquamous non-small cell lung cancer (NSCLC) [61]. However, the clinical evidence of immune checkpoint therapies in sepsis are still lacking and need further investigation.
B and T lymphocyte attenuator (BTLA) is a co-inhibitory receptor which is expressed on B and T lymphocytes, macrophages, dendritic cells, as well as NK cells [62]. The ligand of BTLA is herpes virus entry mediators (HVEM) that belong to tumor necrosis factor receptor (TNFR) family [62]. BTLA has potential role in inhibiting CD4+T cell and B-cell function and diminishing pro-survival signaling in CD4+T cells [62]. Clinical studies have shown that the levels of BTLA on peripheral blood CD4+T cells in patients with sepsis are significantly higher than that in patients without sepsis [63]. Additionally, in patients admitted with SIRS, BTLA could serve as a biomarker of hospital infection [63]. However, another clinical study found that the number of BTLA+CD4+T cells in patients with sepsis was significantly lower than that of healthy volunteers at 24 hours after hospitalization and it was lower in dead patients when compared with survivors [64]. The different results may be due to the different stages of the disease. In addition to BTLA, V-domain immunoglobulin suppressor of T-cell activation (VISTA), T-cell immunoglobulin- and mucin-domain-containing molecules (Tim), and lymphocyte activation gene-3 (LAG-3) also have potential role in modulating host immune function and are expected to be new immune therapy targets of sepsis [65,66,67].
12.3.2.1.3 Other Strategies
Due to the advances in the understanding of immune pathophysiology of sepsis, many immunomodulatory drugs or agents have been discovered and used in preclinical studies. Caspase inhibitors and TAT-conjugated anti-apoptotic Bcl-2-like peptides have been proved to inhibit immune cell apoptosis in septic animals [68]. The immunomodulatory effects of Xuebijing and Astragalus polysaccharide, as traditional Chinese medicines, have been illustrated by some studies [69, 70]. Ethyl pyruvate (EP) is a simple ramification derived from the pyruvic acid and has been shown to be an experimental therapeutic on immune dysfunction. Our recent study have found that EP treatment protected septic mice from secondary P. aeruginosa pneumonia and the protective effects of EP may via decreasing lung IL-10 and plasma HMGB1 expression, inhibiting the function of Tregs and relieving the apoptosis of splenic immune cells [71] (Table 12.1).
12.3.2.2 Clinical Trials
12.3.2.2.1 IFN-γ
IFN-γ is a member of the type II IFN family. Although mice deficient of IFN-γ and its receptor (IFN-γR) are resistant to LPS-induced toxicity, previous studies showed that IFN-γ is also crucial for an effective host response to variety of pathogens including virus and bacteria [72, 73]. Additionally, evidence also illustrated that INF-γ receptor mutations are associated with the increased host susceptibility to infections [72, 74]. Moreover, an important study found that, in septic patients with low monocytic HLA-DR expression, IFN-γ treatment restored the deficient HLA-DR expression and in vitro LPS-induced TNF secretion [75]. Recovery of monocyte function resulted in clearance of sepsis in eight of nine patients [75]. In addition, in severe trauma patients with less than 30% HLA-DR expression on alveolar macrophages, about 50% of patients had elevated levels of HLA-DR in macrophages after INF-γ inhalation, and the incidence of hospital-acquired pneumonia in these patients was significantly reduced [76]. Although this study included only 21 patients, the results suggested the potential value of INF-γ in preventing secondary infection trauma and sepsis [76]. Recently, a phase III clinical trial investigating the role of IFN-γ in sepsis-induced immune suppression and secondary infection is ongoing (ClinicalTrials.gov identification # NCT01649921).
12.3.2.2.2 G-CSF and GM-CSF
The colony-stimulating factors (CSFs) comprise a group of cytokines including granulocyte colony-stimulating factor (G-CSF) and granulocyte/macrophage colony-stimulating factor (GM-CSF). As other CSFs, G-CSF and GM-CSF are crucial for the hematopoiesis of blood cells, the maintenance of homeostasis, and immune competence [77]. In the past few decades, there are many trials investigating the effect of G-CSF and GM-CSF as potential adjunctive immunomodulatory agents in patients with sepsis. It has found that G-CSF and GM-CSF treatment reduced the duration of mechanical ventilation and hospital stay in severe sepsis and septic shock patients with low HLA-DR levels [78]. In non-traumatic abdominal infection patients, GM-CSF could reduce the complications of infection and the length of hospital stay [79]. In addition, GM-CSF treatment has been shown to reduce the incidence of nosocomial infection in children with MODS [80]. However, recent meta-analysis found that G-CSF and GM-CSF failed to reduce the overall mortality of sepsis patients [81]. Because most trails included in the meta-analysis did not stratify study patients according to their immunological state and the effect of G-CSF and GM-CSF on the function of immune system was not reported, the exact effect of G-CSF or GM-CSF therapy in sepsis requires further evaluation.
12.3.2.2.3 Thymosin Alpha1
Thymosin alpha1 (Tα1) is a thymus-derived immunomodulatory peptide which acts as an endogenous regulator of immune systems [82]. It has reported that Tα1 played a unique role in maintaining the balance of pro- and anti-inflammatory cytokine production [82, 83]. Tα1 has been widely used in clinical trials for the treatment of disease associated with immune dysfunction [84, 85]. The efficiency of Tα1 in treating chronic B and C hepatitis as well as some types of cancers has been proved [84, 86]. Previous animal study found that Tα1 could improve the survival of septic mice [87]. Clinical trial also found that Tα1 treatment increased the mHLA-DR levels and decreased the mortality in septic patients [88]. As Tα1 has shown immune-enhanced effects in both animal and clinical studies, the effect of it on secondary infection due to immunosuppression in sepsis deserves further attention.
12.4 Conclusions
Due to the advance in early goal-directed therapy and new antibiotic and adjunct strategies, more and more septic patients survive the phase of acute circulation failure and organ dysfunction and enter a prolonged immunosuppressive state which is characterized by the defects of both innate and adaptive immune responses. Secondary infection is a clinical manifestation of immune dysfunction in sepsis and contributes to poor outcomes of the patients. Recently, in addition to conventional strategies, the effect of immunomodulatory therapy in preventing secondary infection after sepsis has been proved by both preclinical studies and few clinical trials. These efforts may help to reduce the incidence of secondary infection in sepsis and further reduce the mortality of the disease.
References
Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–16.
Stevenson EK, Rubenstein AR, Radin GT, et al. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42(3):625–31.
Walkey AJ, Wiener RS, Lindenauer PK. Utilization patterns and outcomes associated with central venous catheter in septic shock: a population-based study. Crit Care Med. 2013;41(6):1450–7.
Goto T, Yoshida K, Tsugawa Y, Filbin MR, Camargo CA Jr, Hasegawa K. Mortality trends in U.S. adults with septic shock, 2005–2011: a serial cross-sectional analysis of nationally-representative data. BMC Infect Dis. 2016;16:294.
Zhao GJ, Li D, Zhao Q, Song JX, et al. Incidence, risk factors and impact on outcomes of secondary infection in patients with septic shock: an 8-year retrospective study. Sci Rep. 2016;6:38361.
Otto GP, Sossdorf M, Claus RA, Rödel J, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011;15(4):R183.
Walton AH, Muenzer JT, Rasche D, et al. Reactivation of multiple viruses in patients with sepsis. PLoS One. 2014;9(2):e98819.
Daviaud F, Grimaldi D, Dechartres A, et al. Timing and causes of death in septic shock. Ann Intensive Care. 2015;5(1):16.
Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126(1):23–31.
Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–8.
León C, Ruiz-Santana S, Saavedra P, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34(3):730–7.
Delano MJ, Thayer T, Gabrilovich S, et al. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186(1):195–202.
López-Collazo E, del Fresno C. Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Crit Care. 2013;17(6):242.
Lekkou A, Karakantza M, Mouzaki A, et al. Cytokine production and monocyte HLA-DR expression as predictors of outcome for patients with community-acquired severe infections. Clin Diagn Lab Immunol. 2004;11(1):161–7.
Lukaszewicz AC, Grienay M, Resche-Rigon M, et al. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med. 2009;37(10):2746–52.
Deng JC, Cheng G, Newstead MW, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116(9):2532–42.
Fan X, Liu Z, Jin H, Yan J, Liang HP. Alterations of dendritic cells in sepsis: featured role in immunoparalysis. Biomed Res Int. 2015;2015:903720.
Luan YY, Dong N, Xie M, et al. The significance and regulatory mechanisms of innate immune cells in the development of sepsis. J Interf Cytokine Res. 2014;34(1):2–15.
Pène F, Zuber B, Courtine E, Rousseau C, et al. Dendritic cells modulate lung response to Pseudomonas aeruginosa in a murine model of sepsis-induced immune dysfunction. J Immunol. 2008;181(12):8513–20.
Cabrera-Perez J, Condotta SA, Badovinac VP, et al. Impact of sepsis on CD4 T cell immunity. J Leukoc Biol. 2014;96(5):767–77.
Zhao GJ, Yao YM, Lu ZQ, et al. Up-regulation of mitofusin-2 protects CD4+ T cells from HMGB1-mediated immune dysfunction partly through Ca(2+)-NFAT signaling pathway. Cytokine. 2012;59(1):79–85.
Arens C, Bajwa SA, Koch C, et al. Sepsis-induced long-term immune paralysis—results of a descriptive, explorative study. Crit Care. 2016;20:93.
Chang KC, Burnham CA, Compton SM, et al. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17(3):R85.
Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70(4):326–36.
Monneret G, Debard AL, Venet F, et al. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31(7):2068–71.
Cavassani KA, Carson WF 4th, Moreira AP, Wen H, Schaller MA, Ishii M, Lindell DM, Dou Y, Lukacs NW, Keshamouni VG, Hogaboam CM, Kunkel SL. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010;115(22):4403–11.
Huang LF, Yao YM, Dong N, Yu Y, He LX, Sheng ZY. Association between regulatory T cell activity and sepsis and outcome of severely burned patients: a prospective, observational study. Crit Care. 2010;14(1):R3.
Tatura R, Zeschnigk M, Hansen W, et al. Relevance of Foxp3+ regulatory T cells for early and late phases of murine sepsis. Immunology. 2015;146(1):144–56.
LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570–80.
Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9.
Browne EP. Regulation of B-cell responses by toll-like receptors. Immunology. 2012;136(4):370–9.
Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–12.
Rauch PJ, Chudnovskiy A, Robbins CS, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335(6068):597–601.
Kelly-Scumpia KM, Scumpia PO, Weinstein JS, et al. B cells enhance early innate immune responses during bacterial sepsis. J Exp Med. 2011;208(8):1673–82.
Monserrat J, de Pablo R, Diaz-Martín D, et al. Early alterations of B cells in patients with septic shock. Crit Care. 2013;17(3):R105.
Suzuki K, Inoue S, Kametani Y, et al. Reduced Immunocompetent B cells and increased secondary infection in elderly patients with severe Sepsis. Shock. 2016;46(3):270–8.
Mehta Y, Gupta A, Todi S, et al. Guidelines for prevention of hospital acquired infections. Indian J Crit Care Med. 2014;18(3):149–63.
Chaudhry H, Zhou J, Zhong Y, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013;27(6):669–84.
Netea MG, van Tits LJ, Curfs JH, et al. Increased susceptibility of TNF-alpha lymphotoxin-alpha double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans. J Immunol. 1999;163(3):1498–505.
Song Z, Zhang J, Zhang X, et al. Interleukin 4 deficiency reverses development of secondary Pseudomonas aeruginosa pneumonia during sepsis-associated immunosuppression. J Infect Dis. 2015;211(10):1616–27.
Steinhauser ML, Hogaboam CM, Kunkel SL, et al. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162(1):392–9.
Cao J, Xu F, Lin S, et al. IL-27 controls sepsis-induced impairment of lung antibacterial host defence. Thorax. 2014;69(10):926–37.
Namen AE, Lupton S, Hjerrild K, et al. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333(6173):571–3.
Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104(28):11730–5.
Corfe SA, Paige CJ. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin Immunol. 2012;24(3):198–208.
Sheikh V, Porter BO, DerSimonian R, Kovacs SB, et al. Administration of interleukin-7 increases CD4 T cells in idiopathic CD4 lymphocytopenia. Blood. 2016;127(8):977–88.
Audigé A, Hofer U, Dittmer U, et al. Evaluation of the immunomodulatory and antiviral effects of the cytokine combination IFN-α and IL-7 in the lymphocytic choriomeningitis virus and friend retrovirus mouse infection models. Viral Immunol. 2011;24(5):375–85.
Unsinger J, McGlynn M, Kasten KR, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184(7):3768–79.
Shindo Y, Fuchs AG, Davis CG, et al. Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia. J Leukoc Biol. 2017;101(2):543–54.
Inoue S, Unsinger J, Davis CG, et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184(3):1401–9.
Waldmann TA, Lugli E, Roederer M, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117(18):4787–95.
Wege AK, Weber F, Kroemer A, et al. IL-15 enhances the anti-tumor activity of trastuzumab against breast cancer cells but causes fatal side effects in humanized tumor mice (HTM). Oncotarget. 2017;8(2):2731–44.
Guo Y, Luan L, Rabacal W, et al. IL-15 superagonist-mediated immunotoxicity: role of NK cells and IFN-γ. J Immunol. 2015;195(5):2353–64.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106.
Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 pathway blockade: combinations in the clinic. Front Oncol. 2015;4:385.
Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–53.
Shao R, Fang Y, Yu H, et al. Monocyte programmed death ligand-1 expression after 3-4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study. Crit Care. 2016;20(1):124.
Zhang Y, Li J, Lou J, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15(1):R70.
Chang K, Svabek C, Vazquez-Guillamet C, et al. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care. 2014;18(1):R3.
Zhang Y, Zhou Y, Lou J, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14(6):R220.
Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4:48.
Murphy KM, Nelson CA, Sedý JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6(9):671–81.
Shubin NJ, Monaghan SF, Heffernan DS, et al. B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections in ICU patients. Crit Care. 2013;17(6):R276.
Shao R, Li CS, Fang Y, et al. Low B and T lymphocyte attenuator expression on CD4+ T cells in the early stage of sepsis is associated with the severity and mortality of septic patients: a prospective cohort study. Crit Care. 2015;19:308.
Ren F, Li J, Jiang X, et al. Plasma soluble Tim-3 emerges as an inhibitor in sepsis: sepsis contrary to membrane Tim-3 on monocytes. Tissue Antigens. 2015;86(5):325–32.
Nowak EC, Lines JL, Varn FS, et al. Immunoregulatory functions of VISTA. Immunol Rev. 2017;276(1):66–79.
Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004.
Hotchkiss RS, McConnell KW, Bullok K, et al. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176(9):5471–7.
Liu YC, Yao FH, Chai YF, et al. Xuebijing injection promotes M2 polarization of macrophages and improves survival rate in septic mice. Evid Based Complement Alternat Med. 2015;2015:352642.
Liu QY, Yao YM, Yu Y, et al. Astragalus polysaccharides attenuate postburn sepsis via inhibiting negative immunoregulation of CD4+CD25(high) T cells. PLoS One. 2011;6(6):e19811.
Chen W, Lian J, Ye JJ, et al. Ethyl pyruvate reverses development of Pseudomonas aeruginosa pneumonia during sepsis-induced immunosuppression. Int Immunopharmacol. 2017;52:61–9.
Car BD, Eng VM, Schnyder B, et al. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179(5):1437–44.
Romero CR, Herzig DS, Etogo A, et al. The role of interferon-γ in the pathogenesis of acute intra-abdominal sepsis. J Leukoc Biol. 2010;88(4):725–35.
Jouanguy E, Altare F, Lamhamedi S, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guérin infection. N Engl J Med. 1996;335(26):1956–61.
Döcke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3(6):678–81.
Nakos G, Malamou-Mitsi VD, Lachana A, et al. Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med. 2002;30(7):1488–94.
Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28(5):509–54.
Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180(7):640–8.
Orozco H, Arch J, Medina-Franco H, et al. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis: a randomized, double-blind, placebo-controlled clinical trial. Arch Surg. 2006;141(2):150–3.
Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37(3):525–32.
Bo L, Wang F, Zhu J, et al. Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: a meta-analysis. Crit Care. 2011;15(1):R58.
Romani L, Bistoni F, Montagnoli C, et al. Thymosin alpha1: an endogenous regulator of inflammation, immunity, and tolerance. Ann N Y Acad Sci. 2007;1112:326–38.
Romani L, Bistoni F, Perruccio K, et al. Thymosin alpha1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood. 2006;108(7):2265–74.
You J, Zhuang L, Cheng HY, et al. Efficacy of thymosin alpha-1 and interferon alpha in treatment of chronic viral hepatitis B: a randomized controlled study. World J Gastroenterol. 2006;12(41):6715–21.
Wang X, Li W, Niu C, et al. Thymosin alpha 1 is associated with improved cellular immunity and reduced infection rate in severe acute pancreatitis patients in a double-blind randomized control study. Inflammation. 2011;34(3):198–202.
Garaci E, Pica F, Rasi G, et al. Thymosin alpha 1 in the treatment of cancer: from basic research to clinical application. Int J Immunopharmacol. 2000;22(12):1067–76.
Wan J, Shan Y, Shan H, et al. Thymosin-alpha1 promotes the apoptosis of regulatory T cells and survival rate in septic mice. Front Biosci (Landmark Ed). 2011;16:3004–13.
Wu J, Zhou L, Liu J, et al. The efficacy of thymosin alpha 1 for severe sepsis (ETASS): a multicenter, single-blind, randomized and controlled trial. Crit Care. 2013;17(1):R8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lu, Zq. (2019). Secondary Infection in Sepsis: Clinical Significance, Immune Mechanism, and Therapy Strategies. In: Fu, X., Liu, L. (eds) Severe Trauma and Sepsis. Springer, Singapore. https://doi.org/10.1007/978-981-13-3353-8_12
Download citation
DOI: https://doi.org/10.1007/978-981-13-3353-8_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3352-1
Online ISBN: 978-981-13-3353-8
eBook Packages: MedicineMedicine (R0)