Abstract
This paper investigates the impact of partial replacement of Ordinary Portland Cement (OPC) with Metakaolin (MK) and Red Mud (RM) on reinforcement corrosion in presence of both chloride and sulfate ions. To achieve this objective, 36 slab specimens of size 300 × 300 × 52 mm were cast. First set of slabs had been cast with water to binder ratio 0.48, with the replacement of OPC by 5% of RM and 10% of MK one after the other. Similarly, the second set of slabs cast with water to binder ratio 0.51. Each set was admixed with 5% NaCl, 2% MgSO4, 5% NaCl + 2% MgSO4 by using weight of water. The corrosion behavior of reinforcing bars was monitored through half-cell potentials based on ASTM C876. To make sure the compressive strength of slabs, concrete cubes were tested. The numerous results, which indicate the effects of MK, RM, w/b ratios and concomitant presence of chloride and sulfate ions on reinforcement corrosion have been provided and compared the same. At higher w/b ratios (0.51), it was observed that the specimens with RM had lowest corrosion potentials as compared to OPC specimens and MK in the presence of chloride ions. Further in the presence of combined chloride and sulfate ions, corrosion potentials of MK and RM are less in comparison to the OPC concrete at higher water to binder ratios.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Corrosion of reinforced concrete structures is well-known durability problem in the construction industry throughout the world. The corrosion of reinforced concrete structures is a multimillion dollar problem in India and many other countries, specifically in environments containing chloride ions and sulfate ions from seawater or deicing salts. The durability issues can be caused by aggressive external agents specifically sulfate ions, chloride ions, and atmospheric carbon dioxide. The chloride ions are considered to be the primary motive of steel reinforcement corrosion within the concrete. Although it is known from previous studies that structures exposed to sulfate and chloride ions individually, causes the deterioration of concrete and the corrosion of steel rebar. However, the results of both ions present at a time in the concrete are ambiguous. The presence of sulfate ions may additionally have an effect on the chloride attack and likewise, the presence of chloride ions might also have an effect on the sulfate attack in concrete [1].
The resources of these ions into concrete are either the mix constituents (internal environment) or the penetration to the hardened concrete from the external environment. The materials used in the concrete construction such as coarse aggregates, fine aggregates and water can be infected with chloride and sulfate ions [2]. When seawater and sea-sand are used in the concrete, then it may get contaminated with both chlorides and sulfates. Maslehuddin et al. [3] observed that the concomitant presence of sulfate and chloride ions aggravates the corrosive attack on reinforcing steel. In order to improve the durability aspects of concrete, the use of supplementary cementitious materials (SCM) is widely accepted by the construction industry. Fly ash, GGBS, silica fume, etc., are most commonly used SCM as a cement substitute in concrete mixes. In the same line, mineral admixtures like Metakaolin (MK) and red mud (RM) may be used as corrosion inhibitors.
Metakaolin is a calcined product of the clay mineral kaolinite. The partial replacement of Ordinary Portland cement with MK produces refinement of the concrete pore structure and induces the formation of Friedel salt. These features make the Metakaolin as an effective agent in stopping the corrosion of reinforcement in chloride-rich environments [4]. Many researchers have concluded that the best replacement rate of MK was 10% by means of weight of ordinary Portland cement, that allows you to enhance the corrosion resistance and mechanical properties as well [5, 6].
Red mud is a by-product of the alumina from bauxite in the Bayer process, which involves reaction with NaOH at high temperature and pressure. The principle benefit of using the red mud as a corrosion inhibitor in reinforced concrete is because of its high alkalinity [7]. The presence of aluminates in the MK and RM will play an essential function in anchoring the chloride ions, which could in any other case be free and available to begin the corrosion process.
The present study describes the impact of partial replacement of OPC with Metakaolin (MK) and Red Mud (RM) on reinforcement corrosion in presence of both chloride and sulfate ions. To achieve this objective, specimens were cast with three different concrete mixes with two water–binder (w/b) ratios of 0.48, 0.51 and admixed with 5% NaCl, 2% MgSO4, 5% NaCl + 2% MgSO4 by weight of water to maintain the aggressive environment. The numerous test results, which indicate the effects of MK, RM, w/b ratios and concomitant presence of chloride and sulfate ions on reinforcement corrosion have been presented and as compared the same.
2 Experimental Procedure
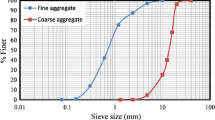
Ordinary Portland cement conforming to IS 12269-1999 was replaced by 10% of MK and 5% of RM. A total of three concrete mixes were prepared, out of which blend containing no mineral admixture was taken as the control mix. The fine aggregate used for the experimental works procured and conformed to grading Zone-III as according to IS 383-2002. The coarse aggregates of size 20 mm and 10 mm had been used in 60:40 proportions. The particle size distribution curves of fine aggregate and coarse aggregate are displayed in Fig. 1.
Thermomechanically treated (TMT) bars of diameter 10 mm are used in the slab specimens. The preparation and pre-conditioning of steel specimens were done according to the guidelines given in ASTM G 109. The w/c ratio, cement content, fine aggregate content, and coarse aggregate content with three types of mixes are presented in Table 1.
2.1 Specimens for Compressive Strength
Concrete cubes of size 150 × 150 × 150 mm were prepared with three concrete mixes such as OPC, replacing the OPC with 10% MK and 5% RM as per IS 10269-2009 by varying the cement contents 354, 333 m3/kg for water–binder ratios 0.48 and 0.51. All the cube specimens have been allowed to curing for 28 days. Compressive strength test was performed at 28 days for all the mixes.
2.2 Specimens for Half-Cell Potential Test
A total of 36 slab specimens had been cast for half-cell potentials measurements. Slab specimens of size 300 × 300 × 52 mm were prepared with all the three concrete mixtures for water–binder ratios 0.48 and 0.51 in conjunction with centrally embedded reinforcement steel as shown in the Fig. 2. In addition, slab specimens were admixed with 5% NaCl, 2% MgSO4, 5% NaCl + 2% MgSO4 by means of weight of water to hold the aggressive environment. The concentration of admixed salts and admixed ions are presented in Table 2. All the specimens had been allowed to curing for 28 days. Half-cell potential measurements have been taken at 60 days.
3 Results and Discussions
3.1 Compressive Strength
Compressive strength testing was performed on concrete cubes at 28 days through compression testing machine (CTM). At the test age, the specimens are taken out of the curing tank and kept outside for 10 min. Then each specimen is tested on the CTM by placing them perpendicular to the casting position. The test results are plotted for compressive strength versus water to binding ratios as shown in Fig. 3 and compressive strength values are presented in Table 3.
From Fig. 3, for all the w/b ratios, the strength of concrete with MK and RM were higher than that of OPC without a mineral admixture. This is because, as it is well recognized that the conversion of Ca(OH)2 into C-S-H gel because of the presence of reactive silica in MK and RM. This offers the additional strength to the concrete. A concrete mixture containing the 5% RM shows the little bit higher strength values both in case of w/b ratios 0.48 and 0.51. This is presumably due to the improved pozzolanic activity due to the addition of RM. It was also observed that change in water to binder ratio has no significant effect on the 28-day strength of concrete containing MK and RM. Pozzolanic reaction continues for a long time, hence it would be predicted the change in compressive strength with respect to w/b ratio for 56 or 90 days.
3.2 Half-Cell Potential Values
The most sensible manner to decide the severity of corrosion in reinforcement steel is to measure its corrosion potential. The corrosion potential Ecorr is measured as a potential difference (or voltage) against a reference electrode based on the ASTM C876. The half-cell potentials were measured on all the slab specimens at the age of 60 days from the day of preparation. The potential measurements were made by using a high-impedance voltmeter.
In this test saturated calomel electrode was used as reference electrode. The reference electrode is connected to the negative end of a voltmeter and the positive end of the voltmeter is connected to the reinforcement steel. To hold the proper electrical contact with the reference electrode, the conducting sponge was wetted with soap solution and placed on the surface of slab specimen. Then the corrosion potentials were taken at five intervals on the slab specimen along the length of steel bar. Depending on the measured half-cell corrosion potential value, the probability of active corrosion is determined. If the potential between SCE and the working electrode (reinforcing steel) was more positive than −125 mV, it is considered as a passive state for steel or less than 10% probability of corrosion. While values were in between −275 and −125 indicates the uncertain state of corrosion. If the potential values were more negative than −275, it indicates active state of corrosion or greater than 90% probability of corrosion.
The half-cell potentials of steel bar embedded in different concrete mixtures exposed to internal chlorides, sulfates and composite chloride and sulfate ions, at w/b ratios 0.48 and 0.51 are shown in Fig. 4a, b. From Fig. 3a, b , it is observed that the potential values of OPC in the presence of chloride ions were more negative at higher w/b (0.51) ratios compared to lower w/b (0.48) ratio. This may be attributed to the truth that, the higher w/c ratio affords enough internal moisture after which reducing the electrical resistivity of concrete for favoring the corrosion process [8]. It results in the more electronegative potential values at higher w/b ratio. In the presence of chloride ions, it was observed that concrete with 10% MK and 5% RM giving more negative potential values at 0.48 w/b ratio as compared to 0.51 w/b ratios. This can be due to the high surface area of MK and RM call for extra water for hydration [9]. Concrete with 10% MK and 5% RM showing lower potential values in the presence of chloride ions corresponding to OPC, at higher w/b ratio 0.51. While contrary conduct was observed at lower w/b ratio 0.48. This indicates that chloride binding is more at higher w/b ratios. This phenomenon might be because of greater porosity and permeability of the higher w/b ratios allowing the greater access of chloride ions to the reactive alumina present in the MK and RM.
In the presence of sulfate ions, corrosion potential values for all the concrete mixtures were found below −126 mV (vs SCE) at w/b ratio 0.48. This corresponds to lower chance of occurrence of steel reinforcement corrosion. It was additionally found that concrete with 10% MK and 5% RM giving more negative potentials values at higher w/b ratio in the presence of sulfate ions. This is probably due to the formation of greater quantity of gypsum and ettringite at higher w/b ratio.
In the presence of composite chloride and sulfate ions, it was observed that control concrete mix (OPC) showing lower corrosion potential values corresponding to other two mixtures (MK and RM), at lower w/b ratios. But, at the higher w/b ratio 0.51 specimens made with MK and RM exhibited lower corrosion potential values in comparison to specimens made with OPC. It was also observed that regardless of w/b ratios, control concrete mixture exhibited lower corrosion potential values in the presence of composite chloride and sulfate ions corresponding to chloride environment alone. In combined chloride and sulfate environment, sulfate ions decreased the chloride impact on reinforcement corrosion. The identical form of phenomenon was observed in Bulu Pradhan [1] investigation on Corrosion behavior of steel reinforcement in concrete exposed to composite chloride–sulfate environment.
4 Conclusions
From the findings of the present work, the following conclusions can be drawn:
-
1.
Replacement of OPC with 10% MK and 5% RM shows high compressive strength at 28 days as compared to OPC specimens with no mineral admixture.
-
2.
Concrete with 5% RM gives little bit more strength corresponding to OPC specimens and 10% MK.
-
3.
Corrosion resistance of OPC concrete decreased with increase in w/b ratio in the presence of chloride ions.
-
4.
At higher w/b ratio, concrete with 10% MK and 5% RM exhibited lower corrosion potentials as compared to control mix (OPC) in the presence of chloride ions. Whereas opposite behavior was observed at lower w/b ratio.
-
5.
The replacement with OPC with 10% MK and 5% RM increased the risk of corrosion in the presence of sulfate ions at higher w/b ratios.
-
6.
The corrosion potentials and corrosion density values are less in combined chloride and sulfate environment as compared to only chloride environment.
-
7.
Concrete with 10% MK and 5% RM performed far better in corrosion resistance corresponding to OPC at higher water to binder ratio except in sulfate environment.
References
Pradhan, B. (2014). Corrosion behavior of steel reinforcement in concrete exposed to composite chloride-sulfate environment. Construction and Building Materials, 72, 398–410.
Santhanam, M., Cohen, M., & Olek, J. (2006). Differentiating seawater and groundwater sulfate attack in Portland cement mortars. Cement and Concrete Research, 36(12), 2132–2137.
Maslehuddin, M., Rasheeduzzafar, R., Al-Amoudi, O. S. B., & Al-Mana, A. I. (1995). Concrete durability in a very aggressive environment. American Concrete Institute, SP, 92(3), 191–211.
Issn, O. (2018). The role of metakaolin in the protection of concrete against the deleterious action of chlorides. Revista IBRACON de Estruturas e Materiais, pp. 1–27.
Kim, H. S., Lee, S. H., & Moon, H. Y. (2007). Strength properties and durability aspects of high strength concrete using Korean metakaolin. Construction and Building Materials, 21(6), 1229–1237.
udovít Krajči, L., Mojumdar, S. C., Janotka, I., Puertas, F., Palacios, M., & Kuliffayová, M. (2015). Performance of composites with metakaolin-blended cements. Journal of Thermal Analysis and Calorimetry, 119(2), 851–863.
Díaz, B., Joiret, S., Keddam, M., Nóvoa, X. R., Pérez, M. C., & Takenouti, H. (2004). Passivity of iron in red mud’s water solutions. Electrochimica Acta, 49(17–18), 3039–3048.
Rocha, F. C., Medeiros-JUNIOR, R. A., Helene, P., Medeiros M. H. F. (2017). Corrosion potential: Influence of moisture, water-cement ratio, chloride content and concrete cover. Revista IBRACON de Estruturas e Materials, 10(4), pp. 864–885.
Wang, P., & Liu, D. Y. (2012). Physical and chemical properties of sintering red mud and bayer red mud and the implications for beneficial utilization. Materials (Basel), 5(10), 1800–1810.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Sudheer, S., Raghu Babu, U., Kondraivendhan, B. (2019). Influence of Metakaolin and Red Mud Blended Cement on Reinforcement Corrosion in Presence of Chloride and Sulfate Ions. In: Das, B., Neithalath, N. (eds) Sustainable Construction and Building Materials. Lecture Notes in Civil Engineering , vol 25. Springer, Singapore. https://doi.org/10.1007/978-981-13-3317-0_64
Download citation
DOI: https://doi.org/10.1007/978-981-13-3317-0_64
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3316-3
Online ISBN: 978-981-13-3317-0
eBook Packages: EngineeringEngineering (R0)