Abstract
With an ever aging population, identifying interventions that can alleviate age-related functional declines has become increasingly important. Dietary supplements have taken center stage based on various health claims and have become a multi-million dollar business. One such supplement is creatine, a major contributor to normal cellular physiology. Creatine, an energy source that can be endogenously synthesized or obtained through diet and supplement, is involved primarily in cellular metabolism via ATP replenishment. The goal of this chapter is to summarize how creatine and its associated enzyme, creatine kinase, act under normal physiological conditions, and how altered levels of either may lead to detrimental functional outcomes. Furthermore, we will focus on the effect of aging on the creatine system and how supplementation may affect the aging process and perhaps reverse it.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Creatine
- Creatine kinase
- Aging
- Supplementation
- Muscle function
- Motor and cognitive function
- Anti-aging intervention
Creatine
Aging is associated with declines in motor and cognitive functions. Humans have always been searching for a “fountain of youth”, and dietary supplements have been at the center of this search. One supplement that has garnered some support and is the focus of this chapter is creatine. Creatine, a derivative of the guanidinium cation involved in a variety of cellular functions, specifically those involved in cellular energy, was discovered by the French organic chemist Michel Eugene Chevreul (Fig. 6.1) (Chevreul 1835). Chevreul found creatine as an isolate from meat and derived the name from kreas, the Greek word for “meat.” In 1847, German chemist Justus von Liebig replicated Chevreul’s work and isolated crystalline creatine (Liebig 1847). Both scientists identified muscle as being the major reservoir for creatine.
Creatine is considered a non-essential nutrient that can be synthesized or obtained from a normal diet. The mammalian body produces the molecule in the liver, kidney, and pancreas with the amino acids arginine, glycine, and methionine, as the foundation for its biosynthesis (Brosnan et al. 2011). Arginine can be classified as a semi-essential or essential amino acid, where the distinction is dependent on the developmental stage of the subject. Pre-term infants are unable to synthesize arginine sufficiently due to paucity of arginine synthesis enzymes in the intestine, which can ultimately affect creatine concentrations. (Wu et al. 2004). Glycine is an amino acid that is considered essential in the human diet as the levels may vary in pre-term infants. This simple amino acid serves as a neurotransmitter for inhibitory chloride ion channels in the spinal cord (Graham et al. 1967), a co-agonist neurotransmitter that enhances the activity of excitatory glutamate channels in the brain (Henneberger et al. 2013), and as a precursor to creatine (Bloch and Schoenheimer 1941). Glycine is produced when serine hydroxymethyl transferase removes a methyl group from serine for placement on a tetrahydrofolate molecule. This reaction can occur in a variety of tissues and organisms as discussed in Hatefi et al. (1957). Furthermore, arginine and glycine come together and through the action of L-arginine:glycine amidinotransferase (AGAT), guanidinoacetic acid, an immediate precursor to creatine is formed and transported to the liver for further enzymatic reaction. In the liver, guanidinoacetic acid reacts with S-adenosylmethionine (product of methionine via a reaction by the methionine adenosyl transferase enzyme) to form creatine (Fig. 6.2) (Bera et al. 2008).
Diagram of creatine and creatine interaction in other biosynthetic pathways. Schematic used with permission from Bera et al. (2008)
From the liver, creatine is transported into the bloodstream and is distributed throughout the body. Up to 95% of creatine is stored in skeletal muscle, while the remaining 5% can be found in other tissues, including the brain, testes, kidneys, and liver (Walker 1979). Under normal conditions, creatine is synthesized constantly to meet the energy demands of cells, as it is rapidly used in ATP production. According to a study by Hoberman et al., the human body loses 2 g of creatine per day due to normal degradation (e.g., molecular and cellular breakdown) (Hoberman et al. 1948). To recover this loss each day, creatine must either be synthesized de novo or obtained from exogenous sources, such as diet or supplementation. Following creatine intake and absorption, it enters the cells via a cell specific ‘symporter’, the sodium- and chloride-dependent creatine transporter 1, a 635 amino acid protein encoded by the SLC6A8 gene (Nash et al. 1994). This protein is comprised of 12 transmembrane domains, with domains 1, 3, 6, and 8 contributing to the transport pathway. Creatine is transported into the cell along with chloride utilizing the sodium gradient. The transporter is part of the neurotransmitter sodium symporter family, which include the ɣ-aminobutyric acid, serotonin, norepinephrine, and dopamine transporters (Gozzo et al. 1993). The first image of the 3-dimensional protein structure was determined when a bacterial homologue to these proteins was used to form X-ray diffracting crystals to yield a high resolution data set. The Aquifex aeolicus leucine transporter, or LeuTAa, provided the crystallographic data to show the arrangement of the transmembrane domains as well as the active transport center of the protein (Yamashita et al. 2005).
Although creatine is endogenously synthesized, it can be obtained through exogenous means, such as a normal diet or supplementation. Beef and fish are abundant sources of creatine. One question that arises from the supplementation of the body’s own mechanism for obtaining creatine is how is exogenous creatine absorbed? Creatine may be absorbed within the gastrointestinal tract. While the exact mechanism of creatine absorption in the gut remains unclear, positive indication of the creatine transporter’s presence has been observed in the intestine, suggesting that dietary creatine supplementation may be absorbed here (Nash et al. 1994). Peral et al. (2002) narrowed down the location of these transporters to the small intestine, where active creatine transporters were found.
Movement of creatine into cells depends significantly on the expression of the creatine transporter. The creatine transporter was first cloned from rabbit brain and shown to be expressed in all tissues but the liver and intestine (Guimbal and Kilimann 1993). In this first study, the highest levels of creatine transporter mRNA were found in the heart, muscle, and kidney. Researchers cloned the human creatine transporter with similar organ expression (Gonzalez and Uhl 1994; Sora et al. 1994). It stands to reason that, to have successful absorption of creatine, there must be adequate levels of creatine transporter present. However, a study by Guerrero-Ontiveros and Wallimann suggested that the creatine transporter expression can be reduced by chronic intake of creatine (Guerrero-Ontiveros and Wallimann 1998). The investigators in the aforementioned study, administered dietary creatine (diet containing 4% creatine and 50 mM creatine in the water supply) for 3–6 months to rats and observed a reduction in creatine transporter expression in rat quadriceps muscles.
As a source of energy for cellular activities, creatine plays a vital role in early development and good health in humans. However, there are genetic disorders that can disrupt creatine synthesis, absorption, and distribution in the body. These are termed “creatine deficiency syndromes” and individuals afflicted by these syndromes have low cellular levels of creatine. Each of the deficiencies discussed below have a common outcome, but vary in the path to creatine deficiency. These deficiencies involved enzymes that lead to the synthesis of creatine at critical reactions or the protein transporting synthesized creatine into cells.
Creatine Deficiencies
Creatine Transporter Defect
As described above, the creatine transporter moves creatine into a cell against the creatine concentration gradient. This is achieved through co-transport with sodium and chloride ions. When working normally, the creatine transporter maintains creatine levels in cells, including brain and muscle tissue. The creatine transporter gene, SLC6A8 gene, is located on chromosome Xq28, at the tip of the X chromosome (Salomons et al. 2001). To understand the role of this gene, the investigators studied a young boy who was experiencing diminished maturity in speech and language abilities. Upon examination with magnetic resonance spectroscopy, it was determined that the levels of creatine in the child’s brain was undetectable. Furthermore, the patient’s creatine transporter gene had a single nucleotide substitution, a cytosine to thymine (Cecil et al. 2001). Ultimately, the expressed creatine transporter protein had 122 fewer amino acids than the wild type transporter that has 625 amino acids. In an effort to slow the progression of the disease and increase the creatine levels in the brain, the patient was placed on an oral creatine supplementation (creatine monohydrate in solution) regimen of 2.5 g creatine, administered three times a day. Nonetheless, the supplementation failed to increase the levels of creatine in brain and was therefore discontinued.
Arginine:Glycine Amidinotransferase Defect
There are several critical enzymatic steps in the pathway to creatine synthesis. One of these steps in this creatine pathway is the arginine: glycine amidinotransferase (AGAT). The AGAT enzyme reacts with arginine and glycine to form the products ornithine and guanidinoacetate, the immediate precursor to creatine. Patients with a deficiency in the AGAT enzyme presented with significantly low concentrations of creatine or phosphocreatine, as seen in the brain of female siblings (Item et al. 2001). These patients were observed to have reduced mental capacity (mental retardation) and low levels of guanidinoacetate in their urine. The AGAT enzyme in these patients had a single point mutation in the gene (mapped to chromosome 15q11.2), a substitution of guanine to adenosine, which introduced a stop codon in place of a tryptophan codon. This mutation resulted in expression of a gene that encodes a truncated (shortened) and non-functioning enzyme. The AGAT deficiency is recessive, requiring two copies of the mutant gene to exhibit the phenotype. The truncated AGAT protein was incapable of catalyzing arginine and glycine substrates to guanidinoacetate due to the absence of the enzyme’s active-site. The younger brother of the aforementioned sisters had the same mutation but had normal development by 18 months of age due to early intervention (Battini et al. 2006). In this case the newborn patient received creatine supplementation via two methods: the mother’s breast milk followed by direct supplementation. The authors point out that creatine supplementation of the mother’s milk was used due to a lack of toxicology data for direct creatine supplementation to a newborn patient. The mother’s diet was supplemented with creatine to achieve this goal, and this supplementation led to an intake range from 3 to 9 g of creatine per day. At 4 months of age, the patient received a direct supplementation of 100 mg/kg/day of creatine for the remainder of the time observed (age 18 months). During this time, plasma and urine creatine levels increased and remained elevated. Of note, the urine creatine levels of the patient were significantly higher than the normal control range (Battini et al. 2006). Following supplementation it was reported that, remarkably, the patient’s cognitive development remained normal throughout periodic observation until age 18 months.
Guanidinoacetate Methyltransferase (GAMT) Deficiency
Guanidinoacetate methytransferase (GAMT), also known as S-adenosyl-L-methionine:N-guanidinoacetate methyltransferase, takes a methyl group from S-adenosyl-L-methionine and adds it to guanidinoacetate. From this reaction, guanidinoacetate becomes the key reactant in the synthesis of creatine. Should the GAMT enzyme not function properly, no creatine is synthesized and guanidinoacetate accumulates within the body. This enzymatic dysfunction was reported in a patient who had low creatine levels and an extrapyramidal movement disorder (Stockler et al. 1994). Despite a normal birth, the 5 year old patient could not perform normal basic functions like roll over, sit, and swallow. Upon study with phosphorous magnetic resonance spectroscopy and proton spectroscopy, the patient had no detectable levels of creatine or phosphocreatine. Arginine supplementation failed to yield improvement in function, but creatine supplementation at 400 mg/kg/d in the absence of arginine resulted in an improvement in the male patient’s condition. The patient was “more alert, followed with his eyes, grasped and moved small toys…and began to turn over and crawl” (Stockler et al. 1994).
GAMT enzyme malfunction has been associated with mutations within the gene. The gene was first cloned in 1995 (Isbrandt and von Figura 1995) and later found to map with human chromosome 19p 13.3 (Chae et al. 1998). The patient exhibited seizures, low muscle tone, and developmental delay. Similarly to the male patient described in the previous paragraph, the GAMT deficiency was also observed in an unrelated female patient exhibiting seizures, low muscle tone, and developmental delay (Stockler et al. 1996). Liver samples were taken from the previously described male and female patients with GAMT deficiency and subjected to DNA sequencing. The male patient’s GAMT gene DNA sequence showed mutations. There were four identified transcripts of the GAMT enzyme in the male patient: an insertion of 13 nucleotides following position 309 in the GAMT gene, a guanine to adenosine mutation at position 327 followed by an insertion of 44 nucleotides, a transcript that combined these two mutations/insertions, and deletion of 146 nucleotides following position 181. These combinations of transcripts reflect the results of splice variants of the same mutant gene. The father was heterozygous with copies of the wild type and position 327 guanine to adenosine and 44 nucleotide insertion alleles while the mother was heterozygous for the wild type and the insertion of 13 nucleotides following position 309 alleles. The patient’s brother was heterozygous with the wild type and the position 309 insertion of 13 nucleotides mutant alleles. The unrelated female patient was homozygous with the position 327 G to A mutation with insertion of 44 nucleotides in the GAMT gene along with the insertion of the 146 nucleotide deletion. As compared to the unrelated male patient, the female patient was homozygous this mutant gene. Further analysis showed that the 327 G to A mutation leads to the deletion of 146 nucleotides following position 181.
In 2014, Stockler-Ipsiroglu et al. reported on the collected data for 48 patients with GAMT deficiency (Stockler-Ipsiroglu et al. 2014). Of the 48 patients, 30 patients had increased cerebral creatine levels following creatine supplementation (ranging from 300–800 mg/kg). The effects of creatine supplementation appeared to reduce symptoms associated with epilepsy, movement disorders, and increased brain creatine levels. While creatine supplementation alone failed to reverse intellectual disabilities, combinatorial therapy, such as including L-ornithine with creatine supplementation, showed reductions of guanidinoacetate and improvements in one patient, in the areas of social skills and attention. Interestingly, those patients that had mutant GAMT gene who began treatment at prenatal, 1 week, 3 weeks, and 9 months of age were observed to be either normal (prenatal, 1 week, and 3 week old subjects) or borderline (9 month old) in measures of developmental delay and intellectual disability. Each of these patients were treated with creatine supplementation, a high-dose of ornithine, and a low protein diet. After months of treatment, three of these patients remained normal while the 9 month old was near normal. One could characterize these as prophylactic creatine supplementation, as the more severe deficits had not been observed in these patients at the time of the study. This may indicate that early and consistent creatine intervention is needed to avoid developmental delay. However, patients who began treatment at age 10 and 11 months showed mild and moderate symptoms of the deficiency. Based on the available data, an early intervention has potential in maintaining near normal behavior in patients with GAMT deficiency.
Creatine Supplementation in Healthy Young Individuals
Dietary supplementation was projected to be a $14 billion industry in 2000 (Zeisel 1999). Consumer surveys on dietary supplementation revealed that most individuals take supplements to either improve or maintain their health (Bailey et al. 2013). Dietary supplements consist of vitamins, minerals, and other over the counter products that may not face scrutiny for efficacy and safety from the Food and Drug Administration. In spite of a lack of regulation, patients continue to complement their diets with these supplements, and they are often consumed without the guidance of their primary care physician.
Creatine supplementation has shown therapeutic efficacy in cases of creatine deficiency, as described above in the case of patients with low to no detectable creatine levels. While replacing creatine reversed some of the effects of movement disorders and slowed the decline in cognitive abilities, it did not affect established pathologies. Creatine supplementation maintained the current condition of the patient at the time of treatment onset and served as a prophylactic, or preventative, intervention before pathology began. As is the case of many prophylactic interventions, the efficacy of the treatment depends on when the intervention is administered and how long it is maintained.
The aforementioned case studies focused on genetic deficiencies that benefit from creatine supplementation and may suggest to lay individuals that creatine supplementation may be of benefit to them as well. The nutritional supplement industry touts the benefits of creatine supplementation and based on these claims, one would consider that supplementation of a nutrient like creatine could have added benefit in healthy individuals. Creatine is a popular supplement for athletes, but has also generated some interest amongst non-athletes.
Typically, the human body will produce 2 g of creatine per day, either from ingestion of food or synthesis in the body. This is added to the normal amount of creatine found in the body (approximately 120 g) (Walker 1979). Creatine supplementation is common in the realm of athletics, as they use it as a source of energy to replenish what is lost in working muscles. In a review that focused on the use of creatine in sports, the authors found that there were a variety of dosing regimens for creatine (Butts et al. 2018). In athletes, one regimen suggested the use of loading doses and maintenance doses. For example, an athlete may take 20 g per day in the loading phase then switch to a lower dose during the maintenance phase, in order to keep creatine levels elevated (Mesa et al. 2002). The length of the loading phase varies between studies. Creatine loading in men, 20 g per day of creatine for 6 days followed by 2 g per day of creatine in the maintenance phase was reported as the rapid way to load creatine in muscle. However, a daily dose of 3 g of creatine was sufficient to achieve the same elevated levels of creatine as the 20 g loading dose/2 g maintenance dose protocol (Hultman et al. 1996). An optimized proposed protocol for oral creatine supplementation suggested that the loading phase consist of 2 days, where 5 g of creatine is ingested four time each day (a total of 20 g creatine per day) and be followed by maintenance doses of 3–5 g creatine during the maintenance phase (Mesa et al. 2002). Related to athletic performance, Havenetidis reviewed research on the use of creatine in the military (Havenetidis 2016). Within these studies, approximately 27% of supplement users consumed creatine. Surprisingly, creatine failed to provide significant enhancement of performance in the majority of the studies investigated. However, creatine did show effectiveness as a supplement in anaerobic tasks, such as muscle strength and power lifting (Law et al. 2009). As pointed out by Havenetidis, anaerobic exercise depends on creatine phosphate stores in the skeletal muscle for energy, not on typical aerobic energy sources. Thus, a study that focuses on an aerobic-dependent energy source may not be the appropriate measure of efficacy of creatine. Furthermore, creatine has been used as a supplement for a variety of conditions, mostly based on anecdotal evidence of efficacy. For some disorders, there are published research studies that suggest creatine is helpful in treating symptoms of the disease. Creatine has shown positive effects in studies of osteoarthritis (Neves et al. 2011), muscular dystrophy (Nabuurs et al. 2013), and fibromyalgia (Leader et al. 2009; Alves et al. 2013). A recent report evaluated creatine as an anti-nociceptive compound in an animal model of thermal and inflammatory pain (Izurieta-Munoz et al. 2017). In this study, creatine supplementation decreased nociceptive behaviors in response to inflammation and supports an anti-nociception activity with the use of creatine.
Creatine Kinase
History
In 1927, Eggleton and Eggleton described the existence of an extremely labile phosphate compound in the muscle fiber, which was later isolated and characterized as creatine phosphoric acid (Fiske and Subbarow 1925). In 1936, further experimentation by Karl Lohmann established that hydrolysis of creatine phosphoric acid in an adenylic system was reversible; this reaction, as discussed in the next section, is now known as ‘high energy transfer reaction’ and plays a major role in ATP production and energy consumption both in mitochondria and cytosol (Rapoport 1978). During the investigation of the role of creatine kinase (CK), Banga and Askonas described the role of CK in two enzyme systems; one system catalyzed the hydrolysis of ATP and the other transferred phosphate from creatine phosphate (Cr~P) to adenosine-diphosphate (ADP) (Banga et al. 1939; Askonas 1951). However, it was only in 1954 that Kuby and colleagues were able to isolate and partially crystalize the enzyme CK from rabbit muscle (Kuby et al. 1954). Over the years the use of serum CK, as an indicator of muscle degradation, started gaining popularity, and advances led to reports of the existence of different isoenzymes. It was initially suggested by Dance (1962) that this enzyme exists as a dimer and that the isoenzymes can be separated upon electrophoresis (Dawson et al. 1965; Dawson and Fine 1967). As described in detail below, CK exists in three different types of isoenzymes; namely brain and muscle (cardiac and skeletal) which have homologous dimers and other tissues that have heterologous dimers consisting of both muscle and brain (Eppenberger 1994). In 1986, Perrymen and colleagues isolated a full length cDNA for human muscle (M) creatine kinase (MCK) and further, following the expansion of molecular genetics, significant advances were made in understanding the structure and location of specific genes for the enzyme. As a result, the location of MCK was narrowed down to human chromosome 19 and mapped to 19q13.2-q13.3 (Nigro et al. 1987; Qin et al. 1998; Perryman et al. 1986).
Structure, Function, and Role in Biology
Evolutionarily, creatine kinase is a 40 kDa polypeptide structure, which consists of some highly conserved and some variable parts. The highly conserved sites of the framework of CK are responsible for basic functions like substrate binding while the variable sites are responsible for isoenzymes or species specific functions. The highly conserved part of this enzyme is retained across all species and all isoforms (Muhlebach et al. 1994). Creatine kinase is a member of the phosphagen kinase family of guanidine kinases, and the primary role of these enzymes is to assist in ATP hydrolysis (Muhlebach et al. 1994). It was originally considered predominantly to be a vertebrate phosphagen, but it was subsequently discovered that this enzyme is also present in invertebrates like sponges, mollusca, and arthropoda (Ellington 2000; Suzuki and Furukohri 1994). To be functionally active, CK needs to form dimers to create a protein structure of ~ 84 KDa units. Creatine kinase is typically present in tissues that demand a high level of energy like brain and muscle. Most vertebrate animals also have a tissue and compartment specific isoenzyme of CK (Schlattner et al. 2006; Schlegel et al. 1988). The type of CK found in the cytosol is composed of two polypeptide subunits of 42 kDa units each. These subunits can either be B (brain type) or M (muscle type) and these subunits come together to form dimers that are present in three different isoenzymes: CK- BB (brain), CK-MM (skeletal muscle) and CK-MB (cardiac muscle). Another form of CK is localized in between the cristae and intermembrane space of mitochondria (Mt-CK). The two different isoenzymes of Mt-CK are ubiquitous and sarcomeric. The ubiquitous form is expressed in brain, smooth muscle and sperm while the sarcomeric form is expressed in striated muscle found in cardiac and skeletal muscle. To form a functional entity, the Mt-CK complex is expressed as an octameric structure (Schlegel et al. 1988; Schlattner et al. 2006; Liu et al. 2010).
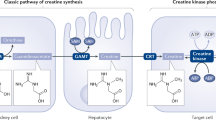
To fulfill demands of cellular bioenergetics, the interplay between the cytosolic and mitochondrial isoenzymes of CK is essential. Cytosolic Creatine (Cr) is reversibly converted to a phosphorylated form phosphocreatine (PCr) which regulates the concentration of ATP in the cell; both cytosolic and mitochondrial. These two isoenzymes are connected by a shuttle or circuit PCr/Cr (Fig. 6.3). The compartmentalization of these isoenzymes and shuttling of PCr into the cytosol helps phosphocreatine (PCr) generated in the mitochondria to be shuttled to the cytoplasm, and be further utilized to regenerate ATP in the cytoplasm. This regeneration of ATP takes place in the presence of ATPases present in the cytoplasm during muscle contraction or in kidneys during sodium retention. Models depicting the function of CK system explain that Cr/PCr act as a ‘temporal’ and ‘spatial’ energy buffering system. According to these models, Cr/PCr shuttle system has five main functions: (1) acts as a temporal energy buffer; (2) acts as a spatial energy buffer; (3) prevents rise in intracellular free ADP by seizing the free ADP present in the cytoplasm and converting it to ATP, thus regulating the net pool of adenine nucleotide in the cell (Iyengar et al. 1982; Iyengar 1984); (4) acts as a proton buffering system by preventing acidification of the tissues both at cellular or global levels during ATP hydrolysis, and; (5) maintains a low ATP/ADP ratio in the mitochondria while maintaining a high ATP/ADP ratio in the cytosol, which is important to stimulate oxidative phosphorylation in the mitochondria and ATP consumption in the cytosol (Wallimann et al. 1989; Eppenberger 1994).
Creatine/Phosphocreatine shuttle system to maintain cellular energy homeostasis; based on (Schlattner et al. 2006). The schematic depicts compartment specific CK (mitochondrial CK-mtCK and cytosol CK-cyCK). (1)-Decrease ATP/ADP in the mitochondria (2)-Increase ATP/ADP ratio in the cytosol (3)-Increase in ATPases activity to consume ATP in the cytosol (4)-Cr/PCr shuttle system to maintain ATP/ADP ratios globally both in mitochondria and cytosol
Distribution in Tissues
The ratio of the subunits of CK found in the cytosol varies according to the tissue. Skeletal muscle typically contains 98% MM and 2% MB and cardiac muscle contains 70–80% MM and 20–30% MB, while the brain predominantly has only the BB type of the isoenzyme (Schlattner et al. 2006; Wallimann and Hemmer 1994). High amounts of energy is utilized in the brain and with only small amount of energy stores like glucose, glycogen from aerobic respiration, the organ has to rely on the Cr/PCr shuttle system for its energy source (Norwood et al. 1983). CK from brain mitochondria is very specific and different from the isoenzyme found in the heart mitochondria (Schlegel et al. 1988; Wyss et al. 2000). Adult human brain also contains MM-CK and is restricted to specific regions of the brain (Hamburg et al. 1990). There are huge regional differences in the activity of CK within the different brain regions. In the cerebellar cortex, higher levels of CK and PCr were measured in the molecular layer than in the white matter (Maker et al. 1973). Further, the activity of CK was higher in the cerebellum, striatum and pyramidal cells when compared to whole brain (Manos et al. 1991). Creatine kinase is not limited to neuronal cells but is also present in glial cells, astrocytes and oligodendrocytes (Manos et al. 1991; Yoshimine et al. 1983). In these cells, CK function is coupled with myelin synthesis, transport and assembly and therefore is postulated to play a significant role in certain neurodegenerative diseases (David et al. 1998).
The MM-CK muscle specific CK is able to interact with M-band (myomesin) of sarcomere and functionally is coupled with a variety of ATPase pumps present at the sarcoplasmic reticulum and regulates various ATP dependent functions (Saks et al. 1978). The myofibrils in the heart also contain specific MM-CK and Mt-CK isoenzymes which are localized in the sarcoplasmic reticulum, plasma membrane, myofilaments, mitochondria and glycolytic complexes of cardiac muscles (Ventura-Clapier et al. 1987). In smooth muscles all types of CK isoenzymes were detected (BB, MB and MM), however, BB-CK and Mt-CK are the main isoforms that were identified in smooth muscle fibers and may perform specific functions associated with that cell (Clark 1994). CK is also present in cells like spermatozoa, retinal cells, pancreas, placenta, thyroid, thymus, brush-border of the intestine, cartilage and bone cells (Wallimann and Hemmer 1994). The tissue specific distribution of the CK enzyme in different cellular organelles further suggest that the presence of Cr/PCr shuttle system is able to fulfill the intermittent and/or high energy demands of a cell.
Factors Affecting Level of Creatine Kinase
According to the standard established by the International Federation of Clinical Chemistry (IFCC) the upper reference level for CK for males is 171 U/I and for females is 145 U/I (Schumann and Klauke 2003). The sex difference in CK levels could be attributed to higher proportion of muscle mass in males (Neal et al. 2009). Similarly, creatine kinase levels in the plasma can be altered by a myriad of factors that include race, age, physical activity, medications, minor injuries and diseased states. Medications like statins are known to cause muscle damage, and therefore as an indicator of muscle damage, it is common practice by physicians to monitor CK levels in patients on statins. Recent reports from large number of clinical studies conducted to study the effects of statins on different age groups and ethnicity has led to a better understanding of differences in creatine kinase levels (Neal et al. 2009). Creatine kinase levels in African Americans in both sexes were significantly higher in comparison to other races such as Caucasians, Hispanics and South Asians (Neal et al. 2009). However, CK levels among non-African Americans were similar. A post-mortem study in black and white males, comparing CK levels in tissues from different organs such as cerebrum, cerebellum, heart, renal artery and skeletal muscle reported a 70% increase in CK in African American males (Brewster et al. 2012). The results from recent studies highlighting the racial differences in CK levels are in concurrence with the some studies conducted 3–4 decades ago (Meltzer and Holy 1974; Wong et al. 1983; Black et al. 1986). Therefore, the National Muscle Expert Association has recommended ‘race-ethnicity-and-sex’- specific values for upper limits of normal (ULN) for diagnoses of abnormal CK levels (Thompson et al. 2006).
Age is another factor that has an impact on CK levels. In newborns, levels of CK are reported to be 10 times higher than in adults (Gilboa and Swanson 1976). Gilboa and Swanson reported a high increase in CK activity in the first 4 days following delivery and pointed that the increase in activity could be explained by increased physical stress endured by the fetus during birth. Further, these levels returned to normal levels by 6–10 weeks post birth (Gilboa and Swanson 1976; Zellweger and Antonik 1975; Rudolph and Gross 1966). A study in females revealed changes in CK levels throughout their life; the CK levels increased dramatically during pre-puberty, pregnancy, and postmenopause (Bundey et al. 1979; Fukutake and Hattori 2001).
Exercise is also a major factor affecting CK levels. In males who trained for distance running, CK levels were elevated to 168 ± 15 U/L during training and these levels were significantly lower when the training was reduced (Houmard et al. 1990). Therefore, it can be concluded that low to moderate exercise causes an increase in level of serum CK; however, these levels are back to baseline levels within 7–9 days once training is discontinued. During mild to moderate exercise the body is able to facilitate the repair of the damaged muscle tissue, to regulate the metabolic disruption of cellular components in muscle and ultimately restore normal serum CK levels (Totsuka et al. 2002). However, excessive physical exertion like running marathons, can cause skeletal muscle damage and in some extreme cases, in untrained individuals could lead to a condition called as rhabdomyolysis (Morandi et al. 2006). This disease is characterized by symptoms such as muscle pain, soreness, increased weakness, and darkened urine. During this state, lysis of skeletal muscle cells releases intracellular toxins into the systemic circulation and if left untreated can result in kidney damage (Morandi et al. 2006). Creatine kinase levels are highly sensitive to muscle injury and can therefore be used as a tool to diagnose muscle damage. Serum CK levels during rhadomyolysis can raise up to 300 × 106 U/L (Efstratiadis et al. 2007). However, the exact mechanism resulting in such an increase in CK levels is poorly understood. Direct muscle injuries include injuries occurring from natural disasters like, earthquakes or car and industrial accidents. Such individuals undergo severe pathophysiological changes due to extreme trauma and require dialysis to prevent rhabdomyolysis-induced renal failure (Criddle 2003; Vanholder et al. 2000). In patients with metabolic-endocrine disorders like hypokalemia, hyponatremia, hypophosphatemia, and hypothyroidism, the permeability of the sarcolemma can be altered distorting the function of sarcomere resulting in rhabdomyolysis (Vanholder et al. 2000; Shiber and Mattu 2002). Similarly, drugs like statins, fenofibrates, antiretrovirals, angiotensin-II receptor antagonist, immunosuppressants, and hydroxychloroquine result in significant muscle damage and may also contribute to rhabdomyolysis (Jamal et al. 2004; Warren et al. 2002). Patients who suffer from genetic disorder like Duchenne and Becker muscular dystrophies suffer from rapid progressive degeneration of the muscle fibers beginning at 3–5 years of age due to faulty dystrophin gene. These individuals are susceptible to complications like malignant hyperthermia as a result of anesthesia used during surgery. Acute rhabdomyolysis is one of the key contributing factors for malignant hyperthermia and eventual death of these patients (Morris 1997; Hayes et al. 2008). Creatine kinase is also increased in patients suffering from certain autoimmune disease like polymyositis and dermatomyositis (Thakur et al. 1996; Galarraga et al. 2003). Therefore, it can be concluded that early and continuous monitoring of serum CK can be used as a clinical tool to detect onset and severity of muscle disorders.
Aging and Creatine
Oxidative Capacity and Aging: Levels of Creatine
In a normal 70 kg human, the total creatine reserve is 120 g, with 2 g/day being produced from endogenous sources (Walker 1979). The levels of creatine and the activity of creatine kinase seem to decrease as a function of age in both animals and humans.
Skeletal muscle changes with age are associated with diminished capacity to accomplish daily tasks. Reports are inconsistent regarding whether these functional changes are also associated with decreases in oxidative capacity of the muscles. Mitochondrial enzymes and respiratory rates of muscles seem to be decreased with age in humans (Short et al. 2005), and oxidative capacity, measured by in vivo phosphorus magnetic resonance spectroscopy (MRS), has been shown to be lower in gastronecmius (McCully et al. 1993) and vastus lateralis (Conley et al. 2000) in older individuals than in young ones. However, other laboratories reported conserved levels of oxidative metabolism with age in humans when matching for physical activity (Rasmussen et al. 2003). This was supported by studies using phosphocreatine (PCr) rate of recovery post-contraction as a MRS measure of oxidative capacity showing no differences between young and old subjects (Lanza et al. 2005). The inconsistency of outcomes may be due to a variety of factors, including the level of activity of an individual, and muscle differences. A study by Larsen et al. tackled this potential issue and determined that the differences in oxidative capacity, measured by PCr recovery from contraction using magnetic resonance spectroscopy, were sex- and muscle- dependent and that physical activity influenced the outcome (Larsen et al. 2012).
Using volumetric proton magnetic resonance spectroscopic imaging to map brain metabolites, it was determined that creatine was higher in gray matter than in white matter, and that its levels were higher in old individuals than in young ones (Pfefferbaum et al. 1999). Increased levels of creatine signify a decrease in PCr levels, and thereby a decrease in overall oxidative metabolism. Furthermore, in post-mortem samples of the frontal and occipital regions, creatine kinase activity was decreased in old controls vs. young controls (Smith et al. 1991). Interestingly, in neuronal cultures from hippocampus, Aksenova et al. reported a gradual increase with “age” in creatine kinase levels associated with a decreased enzymatic activity, thereby suggesting an accumulation of inactive creatine kinase molecules (Aksenova et al. 1999). In gerbils, the activity of CK was found to be decreased with aging in the brain, along with other markers of oxidative stress (Carney et al. 1994).
The creatine/phosphocreatine (Cr/PCr) system is important for metabolism and therefore plays a vital role in the normal cellular function, in particular high energy demand cells such as muscle and brain cells. Depletion of creatine has been associated with brain disorders including cognitive and motor impairments, and language/speech issues (van de Kamp et al. 2014; Joncquel-Chevalier Curt et al. 2015). A study of the cerebral creatine deficiency syndrome-2 mice, which have a mutation in the creatine transporter, reported impairments of short- and long-term memory along with other markers of brain dysfunction (Baroncelli et al. 2016). Another model of creatine deficiency, creatine transporter knockout mice, have shown severe deficits in cognitive function in males (Skelton et al. 2011), and relatively mild deficits in the females (Hautman et al. 2014).
Overall, cell, rodent and human studies indicated a derangement of the creatine, phosphocreatine and creatine kinase system at least in muscle and brain tissues associated with aging. Furthermore, creatine depletion has been shown to lead to phenotypes of motor and cognitive impairments, and motor and cognitive dysfunctions are hallmarks of the aging process. Overall, these studies suggest that supplementation with creatine has the potential to reverse functional declines associated with aging (and to some extent age-associated diseases).
Creatine Supplementation, Diseases and Aging
Creatine and Diseases
Creatine is an ergogenic compound that is attractive to athletes due to its propensity to increase ATP formation (Hall and Trojian 2013) and the very few unwanted side effects associated with its intake (Juhn and Tarnopolsky 1998). Its supplementation leads to increase PCr which is a critical form of energy storage needed for high intensity exercise. Creatine supplementation has been extensively studied in young individuals and in association with exercise (Bemben and Lamont 2005). With the emphasis on the effect of creatine on metabolism and its other properties, the focus of creatine supplementation shifted from athletes to a plethora of diseases; diseases involving neuromuscular disorders and/or mitochondrial dysfunction such as Parkinson’s and Huntington’s diseases and others (Smith et al. 2014). Furthermore, creatine is found to be relatively safe, can penetrate the blood-brain barrier, and has shown some efficacy in animal models, thus making it a good candidate as a neuroprotective agent. A randomized double-blind study determined that creatine could not be rejected as futile for therapy of Parkinsonism, and could be considered for Phase III trials for Parkinson’s Disease (Investigators 2006). A thorough review by Persky and Brazeau provided mechanisms of actions and a summary of the effects of creatine supplementation in animal and human studies for pathological conditions such as heart disease, musculoskeletal disorders, depression associated with stroke, gyrate atrophy, and nephrotoxicity (Persky and Brazeau 2001). Other studies have proposed that creatine could benefit patients with fibromyalgia (Leader et al. 2009), and could reduce morbidity and mortality associated with high-risk pregnancies (Dickinson et al. 2014). Another nice review by Klopstock et al. reported the outcomes of creatine supplementation in rodent and human studies of neurodegenerative diseases such as Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis, and reflected that while creatine seemed promising in mice studies, it did not translate well in human clinical trials. However, it is noteworthy that there has not been many published reports of creatine supplementation in humans, and that perhaps the mice models used are to be questioned as to how well they reflect human conditions (Klopstock et al. 2011).
Creatine Supplementation and Aging
Aging studies of creatine supplementation are sparse; however some parallel to successful intervention such as caloric restriction can be drawn making it a worthy compound to alleviate the negative effects of aging. It has been shown to have anti-apoptotic (O’Gorman et al. 1997) and antioxidative properties (Lawler et al. 2002), two capacities that should help reverse the effects of age.
Sarcopenia, an age-related loss of muscle mass and function, and its associated bone loss results in frailty and increased risk of fall, leading to decreased independence, increased social isolation and depression, and possibly death. Finding ways to reduce or reverse age-related declines in function are of the utmost importance to improve survival and quality of life as we age. Creatine is a particularly attractive supplement especially in the elderly due to its safety potential with few unwanted side effects. However, caution must be advised, as long-term studies of creatine supplementation are lacking, and there is a potential risk of side effects that is increased especially in individuals with kidney diseases/issues (which is a likely scenario in older individuals). After only 5 days of creatine intake, middle-aged individuals had a higher PCr availability and PCr resynthesis than younger subjects, and increased time to exhaustion to the same degree in both age groups (Smith et al. 1998). A meta-analysis revealed that creatine supplementation associated with resistance training increased muscle mass, and improved upper body strength to a further extent that training alone (Candow et al. 2014). Several studies have determined that intake of creatine have anabolic (muscle mass, strength, volume training) and anti-catabolic (protein catabolism, oxidation) effects (Dalbo et al. 2009). On the other hand, other studies have reported a lack of effect on sarcopenia, in this case in a mouse model of accelerated aging (Derave et al. 2005). Older, healthy and normally active men and women responded positively to creatine intake for 7 days and exhibited improvements in muscle strength and performance on physical tasks (Gotshalk et al. 2002, 2008). In 70 year old males, creatine supplementation along with strength training improved leg strength, endurance and power as well as lean tissue mass (Chrusch et al. 2001). In a group of 75 ± 6 year old men and women, creatine supplementation for 14 days resulted in increased upper body strength and increased fatigue threshold (Stout et al. 2007). More recently, a review and meta-analysis by Chilibeck et al. revealed that creatine supplementation had beneficial effect in old participants during resistance training, with increased lean tissue mass and, muscular strength (Chilibeck et al. 2017). Creatine may also have beneficial effect on bone health via direct or indirect (byproduct of improved muscle health) actions, associated with improved bone health in older males (Chilibeck et al. 2005). In in-vivo work on osteoblast-like cells, creatine’s presence increased mineralization and metabolic activity supporting potential beneficial effect of creatine on bone repair (Gerber et al. 2005).
Studies of creatine supplementation have been well documented in neuromuscular and neurodegenerative diseases (Wallimann et al. 2011), however studies in healthy individuals are less common. The neuroprotective effects of creatine have been hypothesized to be due to its antioxidative, antiapopototic and bioenergetics properties (Wallimann et al. 2011). Aging is associated with redox and bienergetic dysregulation, therefore creatine could be a useful supplement to reverse effects of aging on overall health and more specifically brain health. In healthy mice, creatine supplementation increased their median life span, and improved healthspan, along with measures of oxidative stress and neuroprotection (Bender et al. 2008). In a human study, creatine intake for 2 weeks was reported to improve memory on most tasks, however the study had low power (McMorris et al. 2007). In the aging rodent a study of creatine supplementation by Bender et al. (2008) found that some biomarkers such as DNA oxidation, age pigment lipofuscin, and BDNF (Brain-derived neurotrophic factor) followed the same trends as observed with caloric restriction (Sohal and Weindruch 1996; Duan et al. 2001). While these effects were relatively small, they amounted to an overall antiaging effect of creatine which warrants more studies. However, it is noteworthy that clinical trials of antiaging interventions are seldomly done and funding is extremely difficult to gain.
Is Creatine a Viable Option to Alleviate Age–Related Dysfunction?
Creatine is a safe and inexpensive supplement that has shown to have numerous benefits in the athletic population. However, studies of the effects of creatine in the elderly population remain sparse, and the outcomes of these studies are equivocal and are relatively small (see review from Dalbo et al. for details of studies). As for any antiaging interventions, there are many factors to consider including early vs. late life implementation, the duration and the age and sex of the subjects, all of which may influence the outcome. Prolonged exposure to high creatine concentration may also lead to down-regulation of the creatine transporter, but would need to be studied to determine whether on/off supplementation can lead to further benefits compared to continuous supplementation. While some age-related adaptations may decrease the response of older individuals to creatine supplementation, there remains strong support for creatine enhancing muscular performance in short intervention times (5–7 days). The US Society for Sarcopenia, Cachexia and Wasting Disease has reviewed the literature and made recommendations on supplements (Morley 2015). They suggest that short-term creatine may be of benefit along with exercise for sarcopenia, however long-term studies for this and other conditions are needed. Effects on neurodegenerative diseases remain to be seen in clinical trials, and consistent effects on age-associated declines need to be further studied.
References
Aksenova MV, Aksenov MY, Markesbery WR, Butterfield DA (1999) Aging in a dish: age-dependent changes of neuronal survival, protein oxidation, and creatine kinase BB expression in long-term hippocampal cell culture. J Neurosci Res 58(2):308–317
Alves CR, Santiago BM, Lima FR, Otaduy MC, Calich AL, Tritto AC, de Sa Pinto AL, Roschel H, Leite CC, Benatti FB, Bonfa E, Gualano B (2013) Creatine supplementation in fibromyalgia: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res 65(9):1449–1459. https://doi.org/10.1002/acr.22020
Askonas BA (1951) Effect of thyroxine on creatinephosphokinase activity. Nature 167(4258):933–934
Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT (2013) Why US adults use dietary supplements. JAMA Intern Med 173(5):355–361. https://doi.org/10.1001/jamainternmed.2013.2299
Banga I, Ochoa S, Peters RA (1939) Pyruvate oxidation in brain: some dialysable components of the pyruvate oxidation system. Biochem J 33(12):1980–1996
Baroncelli L, Molinaro A, Cacciante F, Alessandri MG, Napoli D, Putignano E, Tola J, Leuzzi V, Cioni G, Pizzorusso T (2016) A mouse model for creatine transporter deficiency reveals early onset cognitive impairment and neuropathology associated with brain aging. Hum Mol Genet 25(19):4186–4200. https://doi.org/10.1093/hmg/ddw252
Battini R, Alessandri MG, Leuzzi V, Moro F, Tosetti M, Bianchi MC, Cioni G (2006) Arginine:glycine amidinotransferase (AGAT) deficiency in a newborn: early treatment can prevent phenotypic expression of the disease. J Pediatr 148(6):828–830. https://doi.org/10.1016/j.jpeds.2006.01.043
Bemben MG, Lamont HS (2005) Creatine supplementation and exercise performance: recent findings. Sports Med 35(2):107–125
Bender A, Beckers J, Schneider I, Holter SM, Haack T, Ruthsatz T, Vogt-Weisenhorn DM, Becker L, Genius J, Rujescu D, Irmler M, Mijalski T, Mader M, Quintanilla-Martinez L, Fuchs H, Gailus-Durner V, de Angelis MH, Wurst W, Schmidt J, Klopstock T (2008) Creatine improves health and survival of mice. Neurobiol Aging 29(9):1404–1411. https://doi.org/10.1016/j.neurobiolaging.2007.03.001
Bera S, Wallimann T, Ray S, Ray M (2008) Enzymes of creatine biosynthesis, arginine and methionine metabolism in normal and malignant cells. FEBS J 275(23):5899–5909. https://doi.org/10.1111/j.1742-4658.2008.06718.x
Black HR, Quallich H, Gareleck CB (1986) Racial differences in serum creatine kinase levels. Am J Med 81(3):479–487
Bloch K, Schoenheimer R (1941) The biological precursors of creatine. J Biol Chem 138(1):167–194
Brewster LM, Coronel CM, Sluiter W, Clark JF, van Montfrans GA (2012) Ethnic differences in tissue creatine kinase activity: an observational study. PLoS One 7(3):e32471. https://doi.org/10.1371/journal.pone.0032471
Brosnan JT, da Silva RP, Brosnan ME (2011) The metabolic burden of creatine synthesis. Amino Acids 40(5):1325–1331. https://doi.org/10.1007/s00726-011-0853-y
Bundey S, Crawley JM, Edwards JH, Westhead RA (1979) Serum creatine kinase levels in pubertal, mature, pregnant, and postmenopausal women. J Med Genet 16(2):117–121
Butts J, Jacobs B, Silvis M (2018) Creatine use in sports. Sports Health 10(1):31–34. https://doi.org/10.1177/1941738117737248
Candow DG, Chilibeck PD, Forbes SC (2014) Creatine supplementation and aging musculoskeletal health. Endocrine 45(3):354–361. https://doi.org/10.1007/s12020-013-0070-4
Carney JM, Smith CD, Carney AM, Butterfield DA (1994) Aging- and oxygen-induced modifications in brain biochemistry and behavior. Ann N Y Acad Sci 738:44–53
Cecil KM, Salomons GS, Ball WS Jr, Wong B, Chuck G, Verhoeven NM, Jakobs C, Degrauw TJ (2001) Irreversible brain creatine deficiency with elevated serum and urine creatine: a creatine transporter defect? Ann Neurol 49(3):401–404
Chae YJ, Chung CE, Kim BJ, Lee MH, Lee H (1998) The gene encoding guanidinoacetate methyltransferase (GAMT) maps to human chromosome 19 at band p13.3 and to mouse chromosome 10. Genomics 49(1):162–164. https://doi.org/10.1006/geno.1998.5236
Chevreul E (1835) Sur la composition chimique du bouillon de viandes. J Pharm Sci Access 21:231–242
Chilibeck PD, Chrusch MJ, Chad KE, Shawn Davison K, Burke DG (2005) Creatine monohydrate and resistance training increase bone mineral content and density in older men. J Nutr Health Aging 9(5):352–353
Chilibeck PD, Kaviani M, Candow DG, Zello GA (2017) Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J Sports Med 8:213–226. https://doi.org/10.2147/OAJSM.S123529
Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG (2001) Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc 33(12):2111–2117
Clark JF (1994) The creatine kinase system in smooth muscle. Mol Cell Biochem 133-134:221–232
Conley KE, Jubrias SA, Esselman PC (2000) Oxidative capacity and ageing in human muscle. J Physiol 526(Pt 1):203–210
Criddle LM (2003) Rhabdomyolysis. Pathophysiology, recognition, and management. Crit Care Nurse 23(6):14–22 24–16, 28 passim; quiz 31–12
Dalbo VJ, Roberts MD, Lockwood CM, Tucker PS, Kreider RB, Kerksick CM (2009) The effects of age on skeletal muscle and the phosphocreatine energy system: can creatine supplementation help older adults. Dyn Med 8:6. https://doi.org/10.1186/1476-5918-8-6
Dance N (1962) Comparison of creatine phosphotransferase from rabbit and brown-hare muscle. Biochem J 84:114–115
David S, Shoemaker M, Haley BE (1998) Abnormal properties of creatine kinase in Alzheimer’s disease brain: correlation of reduced enzyme activity and active site photolabeling with aberrant cytosol-membrane partitioning. Brain Res Mol Brain Res 54(2):276–287
Dawson DM, Fine I (1967) Creatine kinase in human tissues. Arch Neurol 16(2):175–180. https://doi.org/10.1001/archneur.1967.00470200063005
Dawson DM, Eppenberger HM, Kaplan NO (1965) Creatine kinase: evidence for a dimeric structure. Biochem Biophys Res Commun 21(4):346–353. https://doi.org/10.1016/0006-291X(65)90200-7
Derave W, Eijnde BO, Ramaekers M, Hespel P (2005) No effects of lifelong creatine supplementation on sarcopenia in senescence-accelerated mice (SAMP8). Am J Physiol Endocrinol Metab 289(2):E272–E277. https://doi.org/10.1152/ajpendo.00039.2005
Dickinson H, Ellery S, Ireland Z, Larosa D, Snow R, Walker DW (2014) Creatine supplementation during pregnancy: summary of experimental studies suggesting a treatment to improve fetal and neonatal morbidity and reduce mortality in high-risk human pregnancy. BMC Pregnancy Childbirth 14:150. https://doi.org/10.1186/1471-2393-14-150
Duan W, Guo Z, Mattson MP (2001) Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem 76(2):619–626
Efstratiadis G, Voulgaridou A, Nikiforou D, Kyventidis A, Kourkouni E, Vergoulas G (2007) Rhabdomyolysis updated. Hippokratia 11(3):129–137
Ellington WR (2000) A dimeric creatine kinase from a sponge: implications in terms of phosphagen kinase evolution. Comp Biochem Physiol B Biochem Mol Biol 126(1):1–7
Eppenberger HM (1994) A brief summary of the history of the detection of creatine kinase isoenzymes. In: Saks VA, Ventura-Clapier R (eds) Cellular bioenergetics: role of coupled Creatine kinases. Springer, Boston, MA, pp 9–11. https://doi.org/10.1007/978-1-4615-2612-4_2
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66(2):375–400
Fukutake T, Hattori T (2001) Normalization of creatine kinase level during pregnancy in idiopathic hyperCKemia. Clin Neurol Neurosurg 103(3):168–170
Galarraga B, Sinclair D, Fahie-Wilson MN, McCrae FC, Hull RG, Ledingham JM (2003) A rare but important cause for a raised serum creatine kinase concentration: two case reports and a literature review. Rheumatology 42(1):186–188
Gerber I, Ap Gwynn I, Alini M, Wallimann T (2005) Stimulatory effects of creatine on metabolic activity, differentiation and mineralization of primary osteoblast-like cells in monolayer and micromass cell cultures. Eur Cell Mater 10:8–22
Gilboa N, Swanson JR (1976) Serum creatine phosphokinase in normal newborns. Arch Dis Child 51(4):283–285
Gonzalez AM, Uhl GR (1994) ‘Choline/orphan V8-2-1/creatine transporter’ mRNA is expressed in nervous, renal and gastrointestinal systems. Brain Res Mol Brain Res 23(3):266–270
Gotshalk LA, Volek JS, Staron RS, Denegar CR, Hagerman FC, Kraemer WJ (2002) Creatine supplementation improves muscular performance in older men. Med Sci Sports Exerc 34(3):537–543
Gotshalk LA, Kraemer WJ, Mendonca MA, Vingren JL, Kenny AM, Spiering BA, Hatfield DL, Fragala MS, Volek JS (2008) Creatine supplementation improves muscular performance in older women. Eur J Appl Physiol 102(2):223–231. https://doi.org/10.1007/s00421-007-0580-y
Gozzo ML, Avolio A, Forni F, Agnes S, Colacicco L, Barbaresi G, Castagneto M (1993) Enzymatic determinations in acute rejection after liver transplantation: preliminary report on necrosis index. Clin Chim Acta 214(2):175–184
Graham LT Jr, Shank RP, Werman R, Aprison MH (1967) Distribution of some synaptic transmitter suspects in cat spinal cord: glutamic acid, aspartic acid, gamma-aminobutyric acid, glycine and glutamine. J Neurochem 14(4):465–472
Guerrero-Ontiveros ML, Wallimann T (1998) Creatine supplementation in health and disease. Effects of chronic creatine ingestion in vivo: down-regulation of the expression of creatine transporter isoforms in skeletal muscle. Mol Cell Biochem 184(1–2):427–437
Guimbal C, Kilimann MW (1993) A Na(+)-dependent creatine transporter in rabbit brain, muscle, heart, and kidney. cDNA cloning and functional expression. J Biol Chem 268(12):8418–8421
Hall M, Trojian TH (2013) Creatine supplementation. Curr Sports Med Rep 12(4):240–244. https://doi.org/10.1249/JSR.0b013e31829cdff2
Hamburg RJ, Friedman DL, Olson EN, Ma TS, Cortez MD, Goodman C, Puleo PR, Perryman MB (1990) Muscle creatine kinase isoenzyme expression in adult human brain. J Biol Chem 265(11):6403–6409
Hatefi Y, Huennekens FM, Kay LD (1957) Manometric assay and cofactor requirements for serine hydroxymethylase. J Biol Chem 224(1):435–444
Hautman ER, Kokenge AN, Udobi KC, Williams MT, Vorhees CV, Skelton MR (2014) Female mice heterozygous for creatine transporter deficiency show moderate cognitive deficits. J Inherit Metab Dis 37(1):63–68. https://doi.org/10.1007/s10545-013-9619-x
Havenetidis K (2016) The use of creatine supplements in the military. J R Army Med Corps 162(4):242–248. https://doi.org/10.1136/jramc-2014-000400
Hayes J, Veyckemans F, Bissonnette B (2008) Duchenne muscular dystrophy: an old anesthesia problem revisited. Paediatr Anaesth 18(2):100–106. https://doi.org/10.1111/j.1460-9592.2007.02302.x
Henneberger C, Bard L, King C, Jennings A, Rusakov DA (2013) NMDA receptor activation: two targets for two co-agonists. Neurochem Res 38(6):1156–1162. https://doi.org/10.1007/s11064-013-0987-2
Hoberman HD, Sims EA, Peters JH (1948) Creatine and creatinine metabolism in the normal male adult studied with the aid of isotopic nitrogen. J Biol Chem 172(1):45–58
Houmard JA, Costill DL, Mitchell JB, Park SH, Fink WJ, Burns JM (1990) Testosterone, cortisol, and creatine kinase levels in male distance runners during reduced training. Int J Sports Med 11(1):41–45. https://doi.org/10.1055/s-2007-1024760
Hultman E, Soderlund K, Timmons JA, Cederblad G, Greenhaff PL (1996) Muscle creatine loading in men. J Appl Physiol 81(1):232–237. https://doi.org/10.1152/jappl.1996.81.1.232 (1985)
Investigators NN-P (2006) A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 66(5):664–671. https://doi.org/10.1212/01.wnl.0000201252.57661.e1
Isbrandt D, von Figura K (1995) Cloning and sequence analysis of human guanidinoacetate N-methyltransferase cDNA. Biochim Biophys Acta 1264(3):265–267
Item CB, Stockler-Ipsiroglu S, Stromberger C, Muhl A, Alessandri MG, Bianchi MC, Tosetti M, Fornai F, Cioni G (2001) Arginine:glycine amidinotransferase deficiency: the third inborn error of creatine metabolism in humans. Am J Hum Genet 69(5):1127–1133. https://doi.org/10.1086/323765
Iyengar MR (1984) Creatine kinase as an intracellular regulator. J Muscle Res Cell Motil 5(5):527–534
Iyengar MR, Fluellen CE, Iyengar C (1982) Creatine kinase from the bovine myometrium: purification and characterization. J Muscle Res Cell Motil 3(2):231–246
Izurieta-Munoz H, Gonzales EB, Sumien N (2017) Effects of creatine supplementation on nociception in young male and female mice. Pharmacol Rep 70:316–321. https://doi.org/10.1016/j.pharep.2017.11.002
Jamal SM, Eisenberg MJ, Christopoulos S (2004) Rhabdomyolysis associated with hydroxymethylglutaryl-coenzyme a reductase inhibitors. Am Heart J 147(6):956–965. https://doi.org/10.1016/j.ahj.2003.12.037
Joncquel-Chevalier Curt M, Voicu PM, Fontaine M, Dessein AF, Porchet N, Mention-Mulliez K, Dobbelaere D, Soto-Ares G, Cheillan D, Vamecq J (2015) Creatine biosynthesis and transport in health and disease. Biochimie 119:146–165. https://doi.org/10.1016/j.biochi.2015.10.022
Juhn MS, Tarnopolsky M (1998) Oral creatine supplementation and athletic performance: a critical review. Clin J Sport Med 8(4):286–297
Liebig J (1847) Researches on the chemistry of food, edited from the author’s manuscript by William Gregory. Taylor and Walton, London
Klopstock T, Elstner M, Bender A (2011) Creatine in mouse models of neurodegeneration and aging. Amino Acids 40(5):1297–1303. https://doi.org/10.1007/s00726-011-0850-1
Kuby SA, Noda L, Lardy HA (1954) Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem 209(1):191–201
Lanza IR, Befroy DE, Kent-Braun JA (2005) Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol 99(5):1736–1744. https://doi.org/10.1152/japplphysiol.00566.2005 (1985)
Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA (2012) Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab 37(1):88–99. https://doi.org/10.1139/h11-135
Law YL, Ong WS, Gillianyap TL, Lim SC, Von Chia E (2009) Effects of two and five days of creatine loading on muscular strength and anaerobic power in trained athletes. J Strength Cond Res 23(3):906–914. https://doi.org/10.1519/JSC.0b013e3181a06c59
Lawler JM, Barnes WS, Wu G, Song W, Demaree S (2002) Direct antioxidant properties of creatine. Biochem Biophys Res Commun 290(1):47–52. https://doi.org/10.1006/bbrc.2001.6164
Leader A, Amital D, Rubinow A, Amital H (2009) An open-label study adding creatine monohydrate to ongoing medical regimens in patients with the fibromyalgia syndrome. Ann N Y Acad Sci 1173:829–836. https://doi.org/10.1111/j.1749-6632.2009.04811.x
Liu CY, Lai YC, Wu YC, Tzeng CH, Lee SD (2010) Macroenzyme creatine kinase in the era of modern laboratory medicine. J Chin Med Assoc 73(1):35–39
Maker HS, Lehrer GM, Silides DJ, Weiss C (1973) Regional changes in cerebellar creatine phosphate metabolism during late maturation. Exp Neurol 38(2):295–300. https://doi.org/10.1016/0014-4886(73)90153-2
Manos P, Bryan GK, Edmond J (1991) Creatine kinase activity in postnatal rat brain development and in cultured neurons, astrocytes, and oligodendrocytes. J Neurochem 56(6):2101–2107
McCully KK, Fielding RA, Evans WJ, Leigh JS Jr, Posner JD (1993) Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol 75(2):813–819. https://doi.org/10.1152/jappl.1993.75.2.813 (1985)
McMorris T, Mielcarz G, Harris RC, Swain JP, Howard A (2007) Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14(5):517–528. https://doi.org/10.1080/13825580600788100
Meltzer HY, Holy PA (1974) Black-white differences in serum creatine phosphokinase (CPK) activity. Clin Chim Acta 54(2):215–224
Mesa JL, Ruiz JR, Gonzalez-Gross MM, Gutierrez Sainz A, Castillo Garzon MJ (2002) Oral creatine supplementation and skeletal muscle metabolism in physical exercise. Sports Med 32(14):903–944
Morandi L, Angelini C, Prelle A, Pini A, Grassi B, Bernardi G, Politano L, Bruno C, De Grandis D, Cudia P, Citterio A (2006) High plasma creatine kinase: review of the literature and proposal for a diagnostic algorithm. Neurol Sci 27(5):303–311. https://doi.org/10.1007/s10072-006-0701-0
Morley JE (2015) Nutritional supplementation and sarcopenia: the evidence grows. J Am Med Dir Assoc 16(9):717–719. https://doi.org/10.1016/j.jamda.2015.06.001
Morris P (1997) Duchenne muscular dystrophy: a challenge for the anaesthetist. Paediatr Anaesth 7(1):1–4
Muhlebach SM, Gross M, Wirz T, Wallimann T, Perriard JC, Wyss M (1994) Sequence homology and structure predictions of the creatine kinase isoenzymes. Mol Cell Biochem 133-134:245–262
Nabuurs CI, Choe CU, Veltien A, Kan HE, van Loon LJ, Rodenburg RJ, Matschke J, Wieringa B, Kemp GJ, Isbrandt D, Heerschap A (2013) Disturbed energy metabolism and muscular dystrophy caused by pure creatine deficiency are reversible by creatine intake. J Physiol 591(2):571–592. https://doi.org/10.1113/jphysiol.2012.241760
Nash SR, Giros B, Kingsmore SF, Rochelle JM, Suter ST, Gregor P, Seldin MF, Caron MG (1994) Cloning, pharmacological characterization, and genomic localization of the human creatine transporter. Receptors Channels 2(2):165–174
Neal RC, Ferdinand KC, Ycas J, Miller E (2009) Relationship of ethnic origin, gender, and age to blood creatine kinase levels. Am J Med 122(1):73–78. https://doi.org/10.1016/j.amjmed.2008.08.033
Neves M Jr, Gualano B, Roschel H, Fuller R, Benatti FB, Pinto AL, Lima FR, Pereira RM, Lancha AH Jr, Bonfa E (2011) Beneficial effect of creatine supplementation in knee osteoarthritis. Med Sci Sports Exerc 43(8):1538–1543. https://doi.org/10.1249/MSS.0b013e3182118592
Nigro JM, Schweinfest CW, Rajkovic A, Pavlovic J, Jamal S, Dottin RP, Hart JT, Kamarck ME, Rae PM, Carty MD et al (1987) cDNA cloning and mapping of the human creatine kinase M gene to 19q13. Am J Hum Genet 40(2):115–125
Norwood WI, Ingwall JS, Norwood CR, Fossel ET (1983) Developmental changes of creatine kinase metabolism in rat brain. Am J Phys 244(3):C205–C210
O’Gorman E, Beutner G, Dolder M, Koretsky AP, Brdiczka D, Wallimann T (1997) The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett 414(2):253–257
Peral MJ, Garcia-Delgado M, Calonge ML, Duran JM, De La Horra MC, Wallimann T, Speer O, Ilundain A (2002) Human, rat and chicken small intestinal Na+ − cl- -creatine transporter: functional, molecular characterization and localization. J Physiol 545. (Pt 1:133–144
Perryman MB, Kerner SA, Bohlmeyer TJ, Roberts R (1986) Isolation and sequence analysis of a full-length cDNA for human M creatine kinase. Biochem Biophys Res Commun 140(3):981–989. https://doi.org/10.1016/0006-291X(86)90732-1
Persky AM, Brazeau GA (2001) Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev 53(2):161–176
Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO (1999) In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med 41(2):276–284
Qin W, Khuchua Z, Cheng J, Boero J, Payne RM, Strauss AW (1998) Molecular characterization of the creatine kinases and some historical perspectives. Mol Cell Biochem 184(1–2):153–167
Rapoport S (1978) 51 to 49 years ago: Lohmann and ATP. Trends Biochem Sci 3(3):163. https://doi.org/10.1016/S0968-0004(78)90392-4
Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN (2003) Experimental evidence against the mitochondrial theory of aging. A study of isolated human skeletal muscle mitochondria. Exp Gerontol 38(8):877–886
Rudolph N, Gross RT (1966) Creatine phosphokinase activity in serum of newborn infants as an indicator of fetal trauma during birth. Pediatrics 38(6):1039–1046
Saks VA, Rosenshtraukh LV, Smirnov VN, Chazov EI (1978) Role of creatine phosphokinase in cellular function and metabolism. Can J Physiol Pharmacol 56(5):691–706
Salomons GS, van Dooren SJ, Verhoeven NM, Cecil KM, Ball WS, Degrauw TJ, Jakobs C (2001) X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet 68(6):1497–1500. https://doi.org/10.1086/320595
Schlattner U, Tokarska-Schlattner M, Wallimann T (2006) Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta 1762(2):164–180. https://doi.org/10.1016/j.bbadis.2005.09.004
Schlegel J, Wyss M, Schurch U, Schnyder T, Quest A, Wegmann G, Eppenberger HM, Wallimann T (1988) Mitochondrial creatine kinase from cardiac muscle and brain are two distinct isoenzymes but both form octameric molecules. J Biol Chem 263(32):16963–16969
Schumann G, Klauke R (2003) New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: preliminary upper reference limits obtained in hospitalized subjects. Clin Chim Acta 327(1–2):69–79
Shiber JR, Mattu A (2002) Serum phosphate abnormalities in the emergency department. J Emerg Med 23(4):395–400
Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 102(15):5618–5623. https://doi.org/10.1073/pnas.0501559102
Skelton MR, Schaefer TL, Graham DL, Degrauw TJ, Clark JF, Williams MT, Vorhees CV (2011) Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency. PLoS One 6(1):e16187. https://doi.org/10.1371/journal.pone.0016187
Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR (1991) Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A 88(23):10540–10543
Smith SA, Montain SJ, Matott RP, Zientara GP, Jolesz FA, Fielding RA (1998) Creatine supplementation and age influence muscle metabolism during exercise. J Appl Physiol 85(4):1349–1356. https://doi.org/10.1152/jappl.1998.85.4.1349 (1985)
Smith RN, Agharkar AS, Gonzales EB (2014) A review of creatine supplementation in age-related diseases: more than a supplement for athletes. F1000Res 3:222. https://doi.org/10.12688/f1000research.5218.1
Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273:59–63
Sora I, Richman J, Santoro G, Wei H, Wang Y, Vanderah T, Horvath R, Nguyen M, Waite S, Roeske WR et al (1994) The cloning and expression of a human creatine transporter. Biochem Biophys Res Commun 204(1):419–427
Stockler S, Holzbach U, Hanefeld F, Marquardt I, Helms G, Requart M, Hanicke W, Frahm J (1994) Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res 36(3):409–413. https://doi.org/10.1203/00006450-199409000-00023
Stockler S, Isbrandt D, Hanefeld F, Schmidt B, von Figura K (1996) Guanidinoacetate methyltransferase deficiency: the first inborn error of creatine metabolism in man. Am J Hum Genet 58(5):914–922
Stockler-Ipsiroglu S, van Karnebeek C, Longo N, Korenke GC, Mercimek-Mahmutoglu S, Marquart I, Barshop B, Grolik C, Schlune A, Angle B, Araujo HC, Coskun T, Diogo L, Geraghty M, Haliloglu G, Konstantopoulou V, Leuzzi V, Levtova A, Mackenzie J, Maranda B, Mhanni AA, Mitchell G, Morris A, Newlove T, Renaud D, Scaglia F, Valayannopoulos V, van Spronsen FJ, Verbruggen KT, Yuskiv N, Nyhan W, Schulze A (2014) Guanidinoacetate methyltransferase (GAMT) deficiency: outcomes in 48 individuals and recommendations for diagnosis, treatment and monitoring. Mol Genet Metab 111(1):16–25. https://doi.org/10.1016/j.ymgme.2013.10.018
Stout JR, Sue Graves B, Cramer JT, Goldstein ER, Costa PB, Smith AE, Walter AA (2007) Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64–86 years). J Nutr Health Aging 11(6):459–464
Suzuki T, Furukohri T (1994) Evolution of phosphagen kinase. Primary structure of glycocyamine kinase and arginine kinase from invertebrates. J Mol Biol 237(3):353–357. https://doi.org/10.1006/jmbi.1994.1237
Thakur V, Desalvo J, McGrath H Jr, Weed S, Garcia C (1996) Case report: polymyositis-induced myoglobinuric acute renal failure. Am J Med Sci 312(2):85–87
Thompson PD, Clarkson PM, Rosenson RS, National Lipid Association Statin Safety Task Force Muscle Safety Expert Panel (2006) An assessment of statin safety by muscle experts. Am J Cardiol 97(8A):69C–76C. https://doi.org/10.1016/j.amjcard.2005.12.013
Totsuka M, Nakaji S, Suzuki K, Sugawara K, Sato K (2002) Break point of serum creatine kinase release after endurance exercise. J Appl Physiol 93(4):1280–1286. https://doi.org/10.1152/japplphysiol.01270.2001 (1985)
van de Kamp JM, Mancini GM, Salomons GS (2014) X-linked creatine transporter deficiency: clinical aspects and pathophysiology. J Inherit Metab Dis 37(5):715–733. https://doi.org/10.1007/s10545-014-9713-8
Vanholder R, Sever MS, Erek E, Lameire N (2000) Rhabdomyolysis. J Am Soc Nephrol 11(8):1553–1561
Ventura-Clapier R, Saks VA, Vassort G, Lauer C, Elizarova GV (1987) Reversible MM-creatine kinase binding to cardiac myofibrils. Am J Phys 253(3 Pt 1):C444–C455. https://doi.org/10.1152/ajpcell.1987.253.3.C444
Walker JB (1979) Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol 50:177–242
Wallimann T, Hemmer W (1994) Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem 133-134:193–220
Wallimann T, Schnyder T, Schlegel J, Wyss M, Wegmann G, Rossi AM, Hemmer W, Eppenberger HM, Quest AF (1989) Subcellular compartmentation of creatine kinase isoenzymes, regulation of CK and octameric structure of mitochondrial CK: important aspects of the phosphoryl-creatine circuit. Prog Clin Biol Res 315:159–176
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40(5):1271–1296. https://doi.org/10.1007/s00726-011-0877-3
Warren JD, Blumbergs PC, Thompson PD (2002) Rhabdomyolysis: a review. Muscle Nerve 25(3):332–347
Wong ET, Cobb C, Umehara MK, Wolff GA, Haywood LJ, Greenberg T, Shaw ST Jr (1983) Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am J Clin Pathol 79(5):582–586
Wu G, Jaeger LA, Bazer FW, Rhoads JM (2004) Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem 15(8):442–451. https://doi.org/10.1016/j.jnutbio.2003.11.010
Wyss JM, Chambless BD, Kadish I, van Groen T (2000) Age-related decline in water maze learning and memory in rats: strain differences. Neurobiol Aging 21(5):671–681
Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E (2005) Crystal structure of a bacterial homologue of Na+/cl--dependent neurotransmitter transporters. Nature 437(7056):215–223. https://doi.org/10.1038/nature03978
Yoshimine T, Morimoto K, Homburger HA, Yanagihara T (1983) Immunohistochemical localization of creatine kinase BB-isoenzyme in human brain: comparison with tubulin and astroprotein. Brain Res 265(1):101–108
Zeisel SH (1999) Regulation of “nutraceuticals”. Science 285(5435):1853–1855
Zellweger H, Antonik A (1975) Newborn screening for Duchenne muscular dystrophy. Pediatrics 55(1):30–34
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sumien, N., Shetty, R.A., Gonzales, E.B. (2018). Creatine, Creatine Kinase, and Aging. In: Harris, J., Korolchuk, V. (eds) Biochemistry and Cell Biology of Ageing: Part I Biomedical Science. Subcellular Biochemistry, vol 90. Springer, Singapore. https://doi.org/10.1007/978-981-13-2835-0_6
Download citation
DOI: https://doi.org/10.1007/978-981-13-2835-0_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2834-3
Online ISBN: 978-981-13-2835-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)