Abstract
Bacteria are unicellular microorganism, which are found in nature quite often. They talk to each other using chemical signaling process (quorum sensing) and ion-channel mediated electrical signaling mechanism. Quorum sensing is a density dependent bacterial collective behaviour and/or cell-to -cell communication mechanism. This widespread bacterial behaviour is related with biofilm formation, gene expression, swarming, virulence and bioluminescence. In a recent realization (experimental and theoretical study), it was observed that bacteria can also talk to each other through the wave of potassium and an oscillatory dynamics was noticed in bacterial biofilms. In this present chapter, we present two different mathematical frameworks of bacterial communication system. The first model is based on the bacterial density dependent behaviour with up-regulation and down-regulation of the production of quorum sensing molecules. Second model, we introduce two different types of the bacterial communication process within a mathematical framework, which is also related to the biofilm formation. This mathematical framework combine quorum sensing mechanism as well as electrical signaling process. We discuss different spatiotemporal patterns and chaotic behaviour in this communication system. Moreover, it gives a significant and the fundamental role of noise in the complex biological conversation system. Finally we propose some open problem in the last section of this chapter, which are helpful for the future research of the bacterial communication system.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
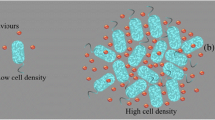
Nature is full of amazing organisms. Bacteria are one of them, which can exist everywhere in the world from the beginning of the life. It can be found from marine life to our everyday day life. The number of bacterial cells in adult human body is ten times the number of human cells. Bacterial cells are residing in host body and make a beneficial partnership between host and the guest, which is formally known as symbiosis. There is unity in diversity in the bacterial kingdom. Bacteria can fight together with their unique decision making technique. Now, we can make an analogy between bacterial and human behaviour. Human being can talk to each other using different languages. When we talk within a same community (e.g. Italian community, Deutsch community, English community), we use the same language. For example, Italian can talk to each other and understand Italian language. But when Italian is talking with English man/woman then he/she can use English language. Bacteria are also doing in a same way. Bacteria can talk to each other with chemical signaling molecules. They use same molecules for intra species communication (e.g. Vibrio fischeri use 3-Oxo-C6-HSL molecules for intra species conversation) and other type of signaling molecules for inter species communication. This bacterial communication process is known as quorum sensing (see Fig. 1).
What is the meaning of quorum sensing? Bacteria use very tiny biomolecules for there communication. These molecules are known as quorum sensing molecules (QSM) or autoinducers (AI). Bacteria are secreted out the QSM from the cell and the QSM is received by the other bacterium. When the threshold concentration of the quorum sensing molecules is achieved, then a coordinated change in bacterial behaviour is initiated. So, it is clear that bacteria sense by other cells present in their vicinity after attaining a certain threshold or quorum state [1,2,3,4,5,6,7,8,9].
Vibrio fischeri is a bioluminescent marine bacterium, where the quorum sensing mechanism was first observed [1]. This bacterium can be found in a free-living organism as well as a symbiont in the light-producing organ of an animal host, such as the Hawaiian bobtail squid. It was observed that Vibrio fischeri produced light in a batch culture, when the large numbers of bacterial cells were presented. In a free-living organism, Vibrio fischeri cannot produces light because of its low cell number densities. When the cell number densities are high enough then autoindusers become sufficient to induce transcription of genes (QS genes) that produce the enzyme luciferase, leading to bioluminescence [10,11,12]. Besides V.fischeri there are so many bacterium, which can also talk to each other using different types of signaling molecules (see Table 1).
Quorum sensing mechanism is attached with the biofilm formation. One can find biofilms in damp and wet environment and its play a significant role in a different infection. It is also associated to water treatment and remediation and many more [13]. From the point of view of medical science we can quote from the National Institutes of Health [14],
Biofilms are clinically important, accounting for over 80 percent of microbial infections in the body. Examples include: infections of oral soft tissues, teeth and dental implants; middle ear; gastrointestinal tract; urogenital tract; airway/lung tissue; eye; urinary tract prostheses; peritoneal membrane and peritoneal dialysis catheters, in-dwelling catheters for hemodialysis and for chronic administration of chemotherapeutic agents (Hickman catheters); cardiac implants such as pacemakers, prosthetic heart valves, ventricular assist devices, and synthetic vascular grafts and stents; prostheses, internal fixation devices, percutaneous sutures; and tracheal and ventilator tubing.
So, we can say that the biofilms are dangerous and it is associated with different infections, which is by itself a leading cause of death in all around the world. The development of biofilm can be characterized as a multistage process (see Fig. 2).
Schematic diagram of the bacterial biofilm formation (multistage process). (Adapted from Majumdar and Pal [15])
Neurophysiology is one of the active research field in brain research, where ion-channel mediated neuronal signaling process gives us structural configuration of different ion-channel and its fundamental insight of human brain. In a more recent study, bacterial ion-channels provide fundamental and significant role of the structural basis of this signaling mechanism [16]. Bacteria have different types of ion-channels. The experimentally observed bacterial ion-channels are as follows
-
Potassium ion-channel KcsA
-
Chloride channels
-
Calcium-gated potassium channels
-
Ionotropic glutamate receptor
-
Sodium channels
The above listed bacterial ion-channels are similar to those found in neuron. So one can think of investigating the bacterial ion-channels as a model. In this chapter, we are restricting ourself on potassium ion-channels (see Fig. 3). Now we are focused on some important experimental evidences of bacterial K + ion-channels and its structure and unique functional role in bacterial communication system and biofilms.
Recently, a great deal of effort has been devoted to understanding the unique function and structure of potassium ion channels of bacteria. G. M. Süel with his student and collaborators showed that potassium ion-channels conduct long-range electrical signals within Bacillus subtilis (gram positive bacteria) biofilm communities [17,18,19,20]. These waves form a positive feedback loop, in which a metabolic trigger induces release of intracellular potassium, which in turn depolarizes neighboring cells. This wave of depolarization coordinates metabolic states among cells in the interior and periphery of the biofilms (see Fig. 4).
It has been studied that the metabolic oscillation of bacterial membrane is triggered by nutrient limitation. Adherent communities of Bacillus subtilis form biofilms and grow in interval of cycles once the colony reaches threshold size of population. These cycles arise when the cells present in the biofilms rundown of glutamate due to consumption of high amount of amino acid by peripheral cells. Glutamate starvation in the interior cells reduces the production of ammonium ions, which is required by the peripheral cells. As a result, the cell growth diminishes drastically [15, 21]. These findings raise the question of whether such extracellular signals could extend beyond the biofilm, resulting in long-range interactions that could affect distant bacteria that are not part of the biofilm.
Next, they studied the attraction of motile cells, which was due to changes in extracellular potassium generated during biofilm oscillations using the microfluidic device. They demonstrated that changes in extracellular potassium gradients are sufficient to direct motile cell behavior. The role of the potassium ion channel in motile cell attraction is experimentally verified and it shows that the potassium ion channels in biofilm cells play very important role in generating the electrical signal that attracts motile cells. Moreover, it shows that attraction also depends on the membrane potential- mediated sensitivity of the motile cells to the potassium signals generated by the biofilm [15, 21].
Time-sharing is a strategy, which is tropically employed in engineering and technological systems where users take turns consuming recourses. So the different systems are competing with each other. B. subtilis biofilm communities are engaged in collective growth-rate oscillations due to glutamate starvation. These oscillations are driven by a spatially extended negative feedback loop, where growth of the biofilms result in glutamate stress within interior and this stress in turn interfaced with biofilm growth. It has been reported that these biofilm communities undergoing metabolic oscillations become coupled through electrical signals, which cause in synchronizing their growth dynamics. Also, it increases the competition by synchronizing demand for limited nutrients. They confirm that biofilms resolve this conflict by switching from in phase to anti- phase. Different biofilm communities take turns consuming nutrients. Thus distant biofilms can coordinate their behavior to resolve nutrient competition through time-sharing. This is a very intelligent and efficient strategy to share the limited resources [15, 21,22,23,24].
In section “Mathematical Modeling of Quorum Sensing”, we discuss a one mathematical model of the quorum sensing system of bacteria (V. fischeri) proposed by Ward et al. in 2001 [25] and in section “Mathematical Model of Electro-Chemical Bacterial Communication System” we emphasis the recent mathematical framework of bacterial two types of communication (chemical and electrical communication) which is proposed in [26] by Majumdar and Roy. In the final section “Open Problems”, we shorted out some important and significant question for the future research in the context of bacterial communication.
Mathematical Modeling of Quorum Sensing
The cell number density of the bacterial population regulates quorum sensing mechanism or cell-to-cell communication system. J.P. Ward, J.R. King, A. J. Koerber, P. Williams, J. M. Croft and R. E. Sockett proposed the very interesting and useful mathematical model of quorum sensing in 2001 [25]. In this mathematical approach a system of ordinary differential equation is used to explain the cell growth and quorum sensing molecules production in a well-mixed population of cells.
In case of quorum sensing process of the V.fischeri, quorum sensing molecules (QSM) binds with the appropriate protein to form a complex, then this complex can bind to the lux-box part of the quorum sensing (QS) gene region of the chromosome and the binding of lux-box induces activation of the QS genes from a down-regulated state to an up-regulated state [25].
Model Assumptions
This mathematical model is based on some primary and compatible assumptions as follows (see Fig. 5)
-
The bacterial population consists of up-regulated (density N u, viewed as the number of cells per unit volume) and down-regulated (with density N d) sub-population of cells, corresponding to bacteria with a complex-bound or empty lux-box respectively.
-
The quorum sensing molecules are produced by up-regulated and down-regulated cells, at the rate k u and k d respectively with k d ≪ k u.
-
Down-regulated cells are up-regulated by quorum sensing molecules, with the rate constant a.
-
Concentration A is changing.
-
Down-regulated occurs spontaneously, due to breakdown of lux-box bound QSM-QSP complex at the rate β.
-
Quorum sensing molecules can be broken down by the medium, and hence lost to the system, at the rate λ.
-
Cell division of one down-regulated cells produces two down-regulated cells.
-
Cell division of up-regulated cells produces on average γ up-regulated and (2 − γ) down-regulated cells (where 0 ≤ γ ≤ 2) assuming that only a population of replicated chromosomes contain occupied lux-boxes. We anticipate that γ ≈ 1, which indicates that division of one up-regulated cell produces one up-regulated and one down-regulated cell.
-
Cell division rate of up-regulated and down-regulated cells are equal, being determined by the parameter r, where the doubling rate is ln(2)/r at low densities.
Illustration of the down-regulation and up-regulation of the cells. (Adapted from [10])
Model
Now we can write the dynamical system as follows (based on the above assumptions) [25]
The above dynamical system has order three with nonlinearity. F(.) is consider as a dimensionless bacterial growth function and F(0) = 0. The total density of the bacterial cells are quantify as N T = N d + N u. Now we can add the Eqs. (1) and (2) we have,
We can further assume that bacterial cell growth as a logistic growth with carrying capacity K. So we get, \( F\left({N}_T\right)=1-\frac{N_T}{K} \) and we can simplify this with an additional assumption that F(N T) is continuous with a single positive zero N T = K, where F ′(0) > 0 and F ′(K) < 0, thus we have a stable and unstable steady state at N T = K and N T = 0 respectively.
Now we can focus on the function G(A). As per the [25], one can describe the process of QSM-QSP complex formation and lux-box binding, as the units of which being QSM concentration. Here we can consider the function G(A) = A is linear. One can also use G(A) = A/(1 + k a A) at high concentration of QSM.
Discussion About Quorum Sensing Model
A bacterial quorum sensing mechanism is describing through this above mention model. Model is focused on the activity and the production of a single QSM and its subsequent effects on the bacterial population. The assumption of the model is quit natural and very much effective to describing growth and production within a batch culture. The solution of the model predicts that in quorum sensing mechanism there is a switching behaviour, which is also observed in the experiment. One can dimensionless the mathematical model and perform the linear stability analysis, steady state analysis and asymptotic analysis (see detail in [25]). From the point of view of stability analysis of the model, we can say that the stable solution and general solution of the model is compatible with the real solution, but it is not clear (from the analysis) what happens after the quorum is achieved [10].
Perspectives of the Model
This is a very simple model for the quorum sensing mechanism, where a single quorum sensing molecule and two different bacterial states are considered. As a matter of facts, the biological reality is not so simple. We can consider this mathematical framework as a first step towards more complex modeling approach. One can extend this investigation by introducing new important parameters with this model. The study of quorum sensing using this mathematical model gives us valuable insight into bacterial chemical communication system. We can implement this quantitative understanding for future research in medical science.
Mathematical Model of Electro-Chemical Bacterial Communication System
The densely packed bacterial populations develop a coordinated motion on the scales length (10–100 μm) in comparison to the size of a each single bacterium of the order 3 μm when the bacterial cell density reaches a sufficiently high value. Let us assume that the collective behavior of the densely packed bacteria inside the biofilm is similar to the behavior of the dense granular system. The dense granular system usually behaves like a fluid, which is quite different from the ordinary fluid. The finite size of the bacteria indicates the existence of an intermediate length scale, which leads us to introduce a source of fluctuation, which is quite different than thermodynamic fluctuation. This new type of fluctuation can be considered as a non-local noise. The swimming induced stresses on the bacteria that can change the local arrangement of bacteria induce stress fluctuations. This stress fluctuation can lead to shear motion and hence is called non-local. Thus, two different type of noise are present in the bacterial communication system and dominance of one over the other depends on the force \( \overline{F}=\frac{f}{\rho g} \) which is applied to the complex biological system where f be the volume density of the forcing and g is the acceleration due to gravity [27].
Viscosity and Non-local Theory
Let us consider the state space (ρ, v) of one component fluid, where ρ be the density and v be the velocity of the fluid. The stress tensor and/or the pressure term are the only constitutive quantity in this framework. We consider the higher order derivatives of the basic variable (density and velocity) to extend theory of usual hydrodynamics to weakly non-local hydrodynamics. Without loss of generality, the balance of mass and momentum can be expressed as
and
Here P is the pressure and ϑ be the force density. This is formally known as Cauchy momentum equation. Now, we can extend this framework by considering the state space spanned by (ρ, ∇ρ, v, ∇v, ∇2 ρ).
One can show that there exists a scalar valued function ϕ v or non-local potential such that [26, 28]
where ϕ v is the course- grained potential or kinematic viscosity potential and σij be shear tensor.
One can calculate the viscosity potential from the entropy density function
The non-local potential can be written as
where ν is kinematic viscosity and \( \nu =\frac{\mu }{\rho } \) (μ is dynamical viscosity of the fluid). We define a kinematic velocity as \( {u}_k=\frac{\nu }{2}\nabla \ln \rho \) [26], which is depends upon the cell density. Here we introduce the kinematic velocity in order to relate to a kind of fluctuations due to the existence of finite length scale associated to granular nature of the fluid. Finally (after some algebraic calculation), we get a general expression as
where ∇η = − ν∇2(∆u k) and ∆u k = u − u k.
The above Eq. (10) is known as noisy Burgers equation. We emphasize that the non-local hydrodynamical model (based on Ginzburg-Landau framework) can explain the quorum sensing phenomena in a consistent way. This noise gives rise to kinematic viscosity, which helps to understand the metastable states for quorum sensing.
This mathematical framework indicates a comprehensive view of an internal structure of the complex biological communication system and viscosity is the property which makes the bacterial cells stick together into clusters predicted by Zeldovich approximation, just mimicking gravitational effect on the smaller scales [27]. This approximation describes the general structure of this nonlinear biological phenomenon. It is to be mentioned that the origin of viscosity is traced back to the weakly non-local effects in the internal structure of the system. One of the present authors (SR) along with Llinas [27] showed that kinematic viscosity plays a vital role in forming the metastable states of the bacteria responsible for quorum sensing. Moreover, bacteria in biofilm form various types of patterns. Now we study the formation of patterns in Biofilms and the role of kinematic viscosity.
Kwak Transformation and Reaction- Diffusion Systems
The quorum sensing system is modeled by noisy Burger equation (Eq. 10). We can rewrite the Eq. (11) as
with \( h(x)=\frac{\nabla \eta }{\nu^2} \) . By using Kwak transformation \( J(u)=\left(u,,{u}_x,,-\frac{1}{2}{u}^2\right) \) we can obtain a new system as
The above Eqs. (12, 13, and 14) is a reaction- diffusion system, which gives the mathematical framework for the pattern formation.
Pattern Formations and Viscosity
In this multicellular system bacterial cells form different patterns based on chemical gradients of QSM signal that is synthesized by quorum sensing bacterial cells. The above theoretical analysis reveals that parameters like kinematic viscosity (associated to non-local noise) play most significant roles to form patterns over space and time. Furthermore, the mathematical approach is able to predict how the system behaves if we change the initial values. We emphasize that these are crucial physical parameters (kinematic viscosity and noise) of the system. It should be noted that the regulatory behaviors mentioned above are nontrivial consequence of the model. In our system, we observed that the quorum takes place in a certain range of kinematic viscosity [0.01, 0.32]m 2/s which is considered as very small viscosity of the fluid (see detail in [29, 30]). We also use different numerical scheme and initial data to show the quorum sensing system behaviour. The behaviour changes with the initial data and system forms different wave patterns.
Electrical Communications and Non-linear Schrödinger Equation
The recent findings suggest that bacteria communicate through electrical signaling using waves associated to Potassium ions. One of the present authors (SR) along with Rodolfo Llinas showed that Potassium ions follow non-linear Schrödinger equation [31]. This equation can be written in the following form:
where ψ is the wave function of Potassium ion. Now one arrives Complex Ginzburg-Landau equation by adding perturbation to the above non-linear Schrödinger equation following Melinkov approach.
Here ψ(x, t) is a complex field and ε > 0 while h = h(Ξ) and g = g(Ξ) are real analytic functions over [0, inf).
This non-linear Schrödinger equation is valid at the level of ion channel where the noise associated to opening and closing of the ion channel predominates. On the other hand the perturbation due to non-local noise becomes predominant at the cellular level. At the cellular level, the non-thermal fluctuation arises due to the presence of finite size of the cell or grain of the granular medium. This fluctuation gives rise to the perturbation on non-linear Schrödinger equation and we get generalized Complex Ginzburg-Landau (GL) equation. This Complex GL equation is used for the description of cellular communication through the chemical molecules and also needed to understand the generation of various patterns in biofilms.
Discussion
Following the above approach we state that the generalized complex Ginzburg-Landau equation is able to explain the quorum sensing phenomena as well as the electrical communication mediated by bacterial ion- channels. This mathematical framework gives us different type of phase transition, spatiotemporal pattern in the complex biological system.
Now we simulate one-dimensional complex Ginzburg-Landau equation in large domain with periodic boundary condition using pseudo- spectral method. This is in general a stiff problem in dynamics of this communication process evolving over both fast and slow timescales. Here, simulation is carried out by exponential time-stepping methods.
This approach captures near threshold behaviour of the quorum sensing system. Patterns are changing over space and time continuously and we notice an oscillation. This oscillation is triggered by nutrient limitation. Specifically, interior and peripheral cells compete for glutamate and as a result biofilm growth halts periodically. We call this phenomenon as cooperative and completion in bacterial communities. This oscillation increases when the bacterial community exceeds certain colony size. If we change the initial condition the patterns are also changing. Quorum sensing mechanism can be initiated periodically, when the number cell destiny reach a certain threshold. This chemical communication process completely depend on nonlocal noise, a range of kinematic viscosity and density values because they are inter related quantities and follow the equation ∇η = − ν∇2(∆u k), ∆u k = u − u k, \( {u}_k=\frac{\upnu}{2}\nabla \ln \rho \), \( \nu =\frac{\mu }{\rho } \).
Spatiotemporal Disordered Regimes
In this bacterial communication system, we find some particular spatiotemporal patterns, when we are simulating our mathematical model in the following form
where, x ∈ [0, L] and \( \overline{\beta}>0 \). Our model have plane wave solution as \( \psi ={a}_k{e}^{i\left( kx+{\omega}_kt\right)} \) with \( {a}_k^2=\frac{\left(1-{k}^2\right)}{\overline{\beta}} \) and \( {\omega}_k=\frac{1}{\overline{\beta}}-\left(\overline{\alpha}+\frac{1}{\overline{\beta}}\right){k}^2 \). These plane wave solutions are linearly stable with the condition \( \overline{\alpha}<\overline{\beta} \), and \( {k}^2<{k}_{Eckhaus}^2 \). On the other hand all solutions are unstable for \( \overline{\alpha}>\overline{\beta} \) which is formally known as Bejamin-Feir line.
Here, the oscillatory state of the bacterial commutation system undergone a Hopf bifurcation and it is considerable importance of a spatially extended non-equilibrium communication system. Two different limiting cases arise one is dissipative (\( \overline{\alpha}=0 \)and \( \overline{\beta} \) tends to infinity) and other one is dispersive (\( \overline{\alpha} \) tends to infinity and \( \overline{\beta}=0 \)). Dispersive case is equivalent to the integrable nonlinear Schrödinger equation. As a matter of fact, away from the intricacy of the bifurcation diagrams at small sizes (L < 50), there exists a large-size limit beyond which chaos becomes extensive and can be characterized by intensive quantities independent of system size, boundary conditions, and, to a large extent, initial conditions [26, 28]. Moreover, one can showed that the Lyapunov dimension is proportional to the system size L [26, 28]. We observed different disordered phase and spatiotemporal chaos, which play an important role for the statistical analysis of the disordered phases.
We observe a strongly disordered phase (see Fig. 6) above the BF line. This phenomenon is known as defect turbulence, which is characterized by a quasi exponential decay of the space-time correlation functions. It is very strong spatiotemporal chaotic phenomena in communication system. It indicates that the pulses of ∣ψ∣ grow under the effect of dispersion term. The self focusing is stopped by the action of dissipation, breaking the pulse. Turbulence in the region is characterized by defects (points in space-time where ψ = 0). Pulses are the relevant objects to consider when approaching the nonlinear Schrödinger limit [26, 28, 32].
On the other hand, a weakly disordered regime is observed which we can call a phase turbulence (see Fig. 7). It can be defined by the absence of space-time defects. In this case ψ never reaches zero and the total phase is conserved. This is a form of chaotic behaviour, but the chaos is very weak in this regime. It indicates diffusive or sub-diffusive modes and describes the phase dynamics near the BF line.
Below the BF line another spatiotemporal disorder regime has been noticed (see Fig. 8). This regime is spatiotemporal intermittency regime, which consist of space-time regions of stable plane waves separated by localized objects evolving and interacting in a complex manner. This K + waves constitute the passive absorbing state while the localized objects carry the spatiotemporal disorder. Here the defects don’t appear spontaneously, the localized object carrying the disorder produces them. This localized structure is completely depending on the bacterial coordinated motion inside the biofilm.
Conclusion
It is clear from the above analysis that the bacterial communication at cellular level i.e. through chemical signaling the non-local noise and hence the kinematic viscosity plays significant role in understanding the quorum sensing of the bacteria in biofilm. Again the patterns in biofilm are generated for small range of values of kinematic viscosity. We use the non-local hydrodynamics as described by complex Ginzburg-Landau equation, which explain both quorum sensing and pattern formations in biofilm. Since it depends on certain range of kinematic viscosity this can be verified experimentally in the laboratory. The experimental observations clearly indicate that bacteria communicate also through electrical signaling. We show that the same complex Ginzburg-Landau equation describes the propagation of potassium ionic waves under certain condition. This particular complex Ginzburg-Landau equation can be recanted as Non-Linear Schrödinger equation. This is valid at the level of ion channels. As soon as we go up to the next level i.e.at the cellular level, the non-local noise perturbs this equation and we arrive at the generalized complex Ginzburg-Landau equation. This non-local noise or perturbation is negligible at the level of ion channel. So we have a single framework, which can explain both types of communications in a comprehensive manner. It is yet to be understood the significance of two paradigms i.e. one classical description at the level of chemical communication and one quantum paradigm at the level of electrical signaling for the same system. It will be studied in the subsequent works.
Open Problems
In this section we have listed some open problems in the research field of bacterial communication as follows
-
What is the exact role of noise in the bacterial communication system?
-
What is the origin of noise in the bacterial communication system?
-
Does noise driven oscillation takes place in this context?
-
Is there any role of quantum noise?
-
What is the meaning of quantum quorum sensing?
-
Is it possible that bacteria have any intelligence?
-
What information is processing by the talking bacteria?
-
How bacteria process the information through electrical communication?
-
What are the significant parameters in cell communication?
-
Is there any kind of condensation (Bose-Einstein like condensation) at room temperature?
-
What is the underling theory of this biological communication process?
-
How can we design the new device to detect the microbial infection?
References
Nealson, K. H., Platt, T., & Hastings, J. W. (1970). Cellular control of the synthesis and activity of the bacterial luminescent system. Journal of Bacteriology, 104, 313–322.
Gray, K. M., Passador, L., Iglewski, B. H., & Greenberg, E. P. (1994). Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. Journal of Bacteriology, 176, 3076–3080.
Fuqua, C., Winans, S. C., & Greenberg, E. P. (1996). Census and consensus in bacterialecosystems: The LuxRLuxI family of quorum-sensing transcriptional regulators. Annual Review of Microbiology, 50, 727–751.
Shapiro, J. A. (1998). Thinking about bacterial populations as multicellular organisms. Annual Review of Microbiology, 52, 81–104.
Miller, M. B., & Bassler, B. L. (2001). Quorum sensing in bacteria. Annual Review of Microbiology, 55(1), 165–199.
Shapiro, J. A. (2007). Bacteria are small but not stupid: Cognition, natural genetic engineering and socio-bacteriology. Studies in History and Philosophy of Science Part C. Studies in History and Philosophy of Biological and Biomedical Sciences, 38(4), 807–819.
Williams, P., Winzer, K., Chan, W. C., & Camara, M. (2007). Look who’s talking: Communication and quorum sensing in the bacterial world. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 362(1483), 1119–1134.
Majumdar, S., & Mondal, S. (2016). Conversation game: Talking bacteria. Journal Cell Communication Signal, 10(4), 331–335.
Majumdar, S., & Pal, S. (2016). Quorum sensing: A quantum perspective. Journal of Cell Communication and Signaling, 10(3), 173–175.
Majumdar, S., Datta, S., & Roy, S. (2012). Mathematical modelling of quorum sensing and bioluminescence in bacteria. International Journal of Advance and Applied Science, 1(3), 139–146.
Majumdar, S. (2013). Hydrodynamic conditions of quorum sensing in bacteria. International Journal of Biochemistry and Biophysics, 2(1), 1–4.
Majumdar, S. (2016). Math behind quorum sensing. Society for Industrial and Applied Mathematics (SIAM).
Klapper, I., & Dockery, J. (2010). Mathematical description of microbial biofilms. SIAM Review, 52(2), 221–265.
National Institutes of Health. (2007). Immunology of biofilms (R01). http://grants.nih.gov/grants/guide/pa-files/PA-07-288.html.
Majumdar, S., & Pal, S. (2017). Cross-species communication in bacterial world. Journal Cell Communication and Signaling, 11(2), 187–190.
Doyle, D. A., Cabral, M. J., Pfuetzner, A. R., Kuo, A., Gulbis, M. J., Cohen, L. S., Chait, T. B., & MacKinnon, R. (1998). The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science, 280(5360), 69–77.
Prindle, A., Jintao, L., Munehiro, A., San, L., Jordi, G.-O., & Süel, G. M. (2015). Ion channels enable electrical communication in bacterial communities. Nature, 527(7576), 59–63.
Beagle, S. D., & Lockles, S. W. (2015). Electrical signaling goes bacterial. Nature, 527, 44.
Liu, J., Arthur, P., Jacqueline, H., Maral, G.-S., Munehiro, A., Lee Dong-yeon, D., San, L., Jordi, G.-O., & Süel, G. M. (2015). Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature, 523(7562), 550–554.
Humphries, J., Xiong, L., Liu, J., Prindle, A., Yuan, F., Arjes, H. A., Tsimring, L., & Süel, G. M. (2017). Species-independent attraction to biofilms through electrical signaling. Cell, 168(1), 200–209.
Majumdar, S., & Pal, S. (2017). Bacterial intelligence: Imitation games, time-sharing and long- range quantum coherence. Journal Cell Communication and Signaling, 11(3), 281–284.
Liu, J., Corral, M. R., Prindle, A., Dong-yeon, L. D., Larkin, J., Sagarra, G. M., Ojalvo, G. J., & Süel, M. G. (2017). Coupling between distant biofilms and emergence of nutrient time-sharing. Science, 356(6338), 638–642.
Majumdar, S.(2017). Experimental evidence of coherence in bacterial communication system. Science (eLetter).
Majumdar, S., & Roy, S. (2017). The role of coherence in bacterial communication. bioRxiv. https://doi.org/10.1101/119503.
Ward, P. J., King, R. J., Koerber, J. A., Williams, P., Croft, M. J., & Sockett, E. R. (2001). Mathematical modelling of quorum sensing in bacteria. IMA Journal of Mathematics Applied in Medicine and Biology, 18(3), 263–292.
Majumdar, S., & Roy, S. (2018). Relevance of quantum mechanics in bacterial communication. Neuro Quantology, 16(3), 1–6.
Roy, S., & Llinas, R. (2016). Non-local hydrodynamics of swimming bacteria and self-activated process., BIOMAT 2015 Proceedings of the International Symposium on Mathematical and Computational Biology. World Scientific 153–165.
Majumdar, S., & Roy, S. (2017). Spatiotemporal patterns and chaos in non-equilibrium bacterial communication. 17th International symposium on mathematical and computational biology, at Institute of Numerical Mathematics, Russian Academy of Sciences, Moscow, Russia.
Majumdar, S., Roy, S., & Llinas, R. (2017). Bacterial conversations and pattern formation. bioRxiv. https://doi.org/10.1101/098053.
Majumdar, S. (2016). Mathematical model of talking bacteria. Workshop on industrial and applied mathematics. Hamburg: University of Hamburg.
Roy, S., & Llinas, R. (2009). Relevance of quantum mechanics on some aspects of ion channel function. Comptes Rendus Biologies, 332(6), 517–522.
Majumdar, S. (2017). Bacterial communication: classical and quantum aspects. AquaDiva Recruitment Symposium, Friedrich-Schiller-Universitat Jena, Germany.
Acknowledgement
One of the authors (SR) greatly acknowledges Homi Bhabha Council, Mumbai for the grant under which the work has been done.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Majumdar, S., Roy, S. (2018). Mathematical Model of Quorum Sensing and Biofilm. In: Pallaval Veera Bramhachari (eds) Implication of Quorum Sensing System in Biofilm Formation and Virulence. Springer, Singapore. https://doi.org/10.1007/978-981-13-2429-1_24
Download citation
DOI: https://doi.org/10.1007/978-981-13-2429-1_24
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2428-4
Online ISBN: 978-981-13-2429-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)