Abstract
Over the past 30 years, geosynthetic clay liners (GCLs) have been demonstrated to be a valuable barrier technology in numerous environmental containment applications. However, past research has also shown that the hydraulic conductivity (k) of GCLs is sensitive to the electrolyte concentration of the permeant liquid. At low effective stresses, a sufficiently high electrolyte concentration has the potential to result in a multiple order of magnitude higher k than the same GCL exhibits to water. To expand the boundaries of GCL functionality, recent research has focused on the use of enhanced (e.g., polymer modified) bentonites (EBs) for improving the resilience of bentonites, and GCLs, subjected to high concentration electrolyte solutions. These EBs have frequently incorporated anionic polymers (e.g. sodium polyacrylate) into the bentonite using a plethora of methods, (e.g. dry mixing, wet mixing, in-situ polymerization.) However, existing standard indicator tests (swell index, fluid loss, modified Atterberg limits) for GCL k may not always be effective for EB-GCLs. A rapid fluid indicator test (FIT) for screening high k versus low k of EB-GCLs and unmodified sodium bentonites, is proposed. Preliminary tests were conducted on granular sodium bentonite and an EB (bentonite polymer composite). Results of the FIT were compared with the swell index and k for these materials over a range of CaCl2 solutions. The results support that a passing FIT generally correlates to low k for the same fluid under low stress conditions.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Geosynthetic clay liners (GCLs) are used in environmental containment systems due to the low hydraulic conductivity (k) of sodium bentonite (NaB) to water [1, 2]. Unfortunately, NaB may not maintain low k when exposed to inorganic waste leachates with high concentrations of dissolved ions, particularly at low effective stresses [1, 2]. Enhanced bentonites (EBs), a composite material composed of NaB blended with polymers or high molecular weight organic molecules, are a potential solution to the instability of natural bentonite in many containment applications [3,4,5]. Enhanced bentonites have shown improved performance (lower k, lower rates of diffusion, greater membrane efficiency) relative to unamended NaB when exposed to high concentration inorganic aqueous solutions, but the mechanisms underlying these improvements are largely unknown [5].
The k of GCLs containing NaB is controlled primarily by the swelling of NaB component [1, 2, 5]. The test used to measure the swelling capability of a NaB in a specific solution is the swell index (SI) defined by ASTM 5890 [6]. The SI is used to screen the k of GCLs when both tests are conducted with the same solution [2, 7]. However, swelling (and Atterberg limits or fluid loss) do not universally correlate to the k of EB-GCLs [5]. For example, the relationship between k and SI is shown for a NaB and an EB-GCL (bentonite polymer composite, BPC) in Fig. 1. For NaB, a low SI correlates with increased k (>1 × 10−10 m/s). However, the opposite trend was observed for BPC, where the lowest SI correlates to lower k (≪1 × 10−11 m/s). These data provide an illustration of how the SI test may not be an effective indicator test for EB-GCLs.
The purpose of this study was to develop a rapid and simple indicator test for GCL k in various aqueous inorganic solutions that is effective for both GCLs containing unmodified bentonites and EBs. A new method is described and compared to k and SI in 5, 20, 50, 200, and 500 mM calcium chloride (CaCl2) solutions.
2 Materials
The NaB used in this study, Volclay CG-50, was obtained from the Colloid Environmental Technologies Company (CETCO) of Hoffman Estates, IL, USA, and is the same NaB used in Bentomat GCLs as well as other studies [5]. Bentonite-polymer composite is prepared by free radical initiated additional polymerization acrylic acid within a bentonite slurry to create a covalent crosslinked polymer network. Properties of NaB and BPC (cation exchange capacity, mineralogical composition, polymer content) are reported in [5].
Solutions of 5, 20, 50, 200, and 500 mM CaCl2 were used in this study. The electrical conductivities of these solutions were 1.22 mS/m (5 mM CaCl2), 4.50 mS/m (20 mM CaCl2), 10.4 mS/m (50 mM CaCl2), 38.5 mS/m (200 mM CaCl2), and 86.5 mS/m (500 mM CaCl2). The pH of these solutions were near neutral (5.7–6.9). Solutions were prepared by dissolving calcium chloride di-hydrate (CaCl2 + 2H2O) in deionized water, and were stored in collapsible carboys with no headspace.
3 Methods
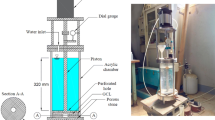
The fluid indicator test (FIT) apparatus is a pseudo-rigid wall permeameter as shown in Fig. 2. A hollow acrylic cylinder (outside diameter, Do = 63.5 mm) contains sandwiched layers of mesh aluminum screens, filter paper, and NaB or EB forming a pseudo-GCL (with filter paper in leu of geotextiles; specimen diameter, D = 38.1 mm). The cylinder base is fitted with 6.35-mm outside diameter tubing that is used to supply or receive water for hydration and flow. A second hollow acrylic cylinder (outside diameter, D = 38.1 mm; inside diameter, Di = 25.4) sits above the pseudo GCL and within the outer acrylic cylinder. The 38.1-mm diameter cylinder is filled with the selected liquid in both the hydration and testing phases. Weights are added to the top of the inner acrylic cylinder to apply a total normal stress of 6 kPa to the pseudo GCL. A flexible membrane and O-rings surround the connection between the two cylinders.
The FIT is run in two stages, a hydration stage (Fig. 3a), and a flow stage (Fig. 3b). The FIT apparatus (Fig. 2) is placed on a stand with height, HS, of 225 mm, fitted with a hole for the 6.35-mm outside diameter tubing. The tubing is connected to a vertical burette at a height, HB of 400 mm above the lab floor. To control the flow of solution, a valve is included near the base of the burette. After the pseudo-GCL is assembled within the FIT apparatus, and weight(s) are placed atop the apparatus yielding a applied normal stress of 6 kPa, a small amount (<10 mL) of solution is added to burette to removal of air bubbles from the tubing. During this step, the bentonite should not be allowed to contact the solution (i.e., the bentonite should not be allowed to hydrate). Once all air is removed from the tubing, solution is added to the burette, hydrating the bentonite from the bottom up until the system reaches equilibrium at a solution height, Hw of 1.0 m above the lab floor. The system is then left to hydrate for 24 h while the bentonite hydrates under the 6 kPa total normal stress.
After 24 h hydration period, the valve is closed, and the burette is moved to the lab floor (–HB). The acrylic cylinder above the pseudo-GCL is filled with solution through a hole in the weights (~100 mL total volume, Hf = 18 cm). To initiate flow, the valve is opened and the volumetric flow through the pseudo-GCL is measured for a period of 5 min. The fluid retention (FR) is calculated by subtracting the flow through the pseudo-GCL from 100 mL. If FR = 100 mL, the specimen passes the FIT and is anticipated to potentially have a low k to the testing solution under low effective stress conditions. If the FR < 100 mL the specimen fails the FIT, and is antipated to potentially have a high k to the testing solution under low effective strsess conditions.
4 Results
The results of the FIT test for the NaB and BPC are shown in Fig. 4 and summarized in Table 1. These results are compared with the k and SI for the same material in the same solution [5]. The FIT test generally captures hydraulic compatibility (low or high k to a given solution) of both NaB and EB. Two outliers are evident in Fig. 4. The NaB tested with 50 mM CaCl2 solution produced a passing result for the FIT but when hydrated and permeated with the same solution produced a high k. The reason for this outlier is unknown and is under investigation. The BPC with 500 mM CaCl2 solution also produced an anomaly with a failing FIT but a low k. This outlier is explained by the polymer (sodium polyacrylic acid) behavior in the 500 mM CaCl2 solution. Polymer in BPC acts to clog the pores of the GCL [5] via a tacky polymer hydrogel within the bentonite matrix. When hydrated with 500 mM CaCl2, the network of polymer within BPC interconnects and tightens resulting in shrinkage of the specimen away from the sidewalls, producing sidewall leakage upon execution of the FIT test. Sidewall leakage was proven for this experimental combination by dying the specimen during the FIT as shown in Fig. 5. Preliminary tests of BPC with 500 mM CaCl2 under a higher total stress (20 kPa) produced a passing FIT, correlating to the low measured k of this material-permeant solution combination. The higher load enabled maintenance BPC edge seal preventing sidewall leakage.
Photographs of polymer effects on FIT: (a) preferential flow of BPC with 500 mM CaCl2; (b) visible dispersion of polymer in inflow column of BPC tested with 50 mM CaCl2 (left) versus no visible dispersion of BPC tested in 200 mM CaCl2 (right); (c) crosslinked polymer on inflow side of BPC tested with 200 mM CaCl2; (d) polymer eluted into outflow line of BPC tested in 200 mM CaCl2.
5 Discussion
The FIT results shown in Fig. 4 illustrate the potential use of this method as an indicator for high or low k of NaBs and EBs with potential permeant solutions. For comparison SI as an indicator test, an empirical equation for k based on SI from the literature [7] was used calculate the expected a predicted k(kc) from SI
where kc is in cm/s, and a, b, and c are fitting parameters equal to −0.31, 8.69 mL/2 g solid, and 3.09 × 10−9 cm/s, respectively, and SI is the measured swell index in mL/2 g.
Measured k, km, are compared to those calculated using Eq. 1, kc, in Fig. 6. The data plotted shows agreement between kc and km when SI is above 14 mL/2 g. This swell index is close to the lower limit (15 mL/2 g) that [7] noted was related to low k. A SI of 14 mL/2 g is also noted by [8] as a minimum SI associated with low k at low effective stress. The poor correlation zone shown in Fig. 6 illustrates that Eq. 1 predicts a k that is > two orders of magnitude lower than km for NaB specimens with a SI < 14 mL/2 g. Equation 1 also predicts a kc > one order of magnitude higher for BPC specimens with a SI < 14 mL/2 g.
Unlike the SI test, the FIT generally matched low and high k of the materials tested. The FIT test was also qualitatively useful in enabling observations of BPC polymer behavior. As shown in Fig. 5b–d, polymer elution was visible in the testing and breakdown of the FIT with CaCl2 solutions. In relatively dilute solutions (≤50 mM CaCl2), the polymer was dispersed into solution upon filling the acrylic inflow cylinder (Fig. 5b). In 200 mM CaCl2, the creation of solid polymer precipitates was visible (Fig. 5c). As shown previously in Fig. 5, the BPC FIT tested in 500 mM CaCl2 caused catastrophically cross-linked polymer chains within the pore matrix such that no dispersion or elution occurred. The photographs shown in Fig. 5 illustrate the potential utility of visual observations of EB hydration in different aqueous solutions for an indicator of polymer migration, and behavior. Efforts to standardize quantification of mobilized polymer are ongoing.
6 Conclusions and Recommendations
A fluid indicator test (FIT) was developed as a rapid indicator test to predict the hydraulic conductivity (k) of geosynthetic clay liners (GCLs), and GCLs containing enhanced bentonites (EBs). Results of the FIT illustrate the pass/fail criterion developed provided a better indication of high and low k for bentonite-polymer composite (BPC) than the swell index (SI) test. The FIT also allowed for qualitative observations of polymer behavior (e.g. elution and crosslinking) that were not observed upon inspection of the SI test. The FIT requires further testing to prove if the pass/fail criterion can accurately predict the high or low measured k of other EBs produced with different polymers and with other material preparation techniques. Additional study is also needed to with more complex aqueous inorganic solutions that may be more representative of field solutions.
References
Ruhl JL, Daniel DE (1997) Geosynthetic clay liners permeated with chemical solutions and leachates. J Geotech Geoenviron Eng ASCE 123(4):369–381
Shackelford C, Benson C, Katsumi T, Edil T, Lin L (1000) Evaluating the hydraulic conductivity of GCLs permeated with non-standard liquids. Geotext Geomembr 18(2–3):133–161
Onikata M, Kondo M, Kamon M (1996) Development and characterization of a multiswellable bentonite. In: Kamon M (ed) Environmental geotechnics. A A Balkema, Rotterdam, pp 587–590
Di Emidio G (2010) Hydraulic and chemico-osmotic performance of polymer treated clays. Ghent University, Faculty of Engineering, Ghent
Scalia J, Benson C, Bohnhoff G, Edil T, Shackelford C (2014) Long-term hydraulic conductivity of a bentonite-polymer composite permeated with aggressive inorganic solutions. J Geotech Geoenviron Eng ASCE 140(3):04013025-1–04013025-13
ASTM D5890 (2018) Standard test method for swell index of clay mineral component of geosynthetic clay liner. ASTM International, West Conshohocken
Katsumi T, Ishimori H, Ogawa A, Yoshikawa K, Hanamoto K, Fukagawa R (2007) Hydraulic conductivity of nonprehydrated geosynthetic clay liners permeated with inorganic solutions and waste leachates. Soils Found 47(1):79–96
Scalia J, Benson C, Finnegan M (2018) Alternate procedures for swell index testing of granular bentonite from GCLs. Geotech Test J Rev (2018)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Norris, A., Scalia, J., Shackelford, C. (2019). Fluid Indicator Test (FIT) for Screening the Hydraulic Conductivity of Enhanced Bentonites to Inorganic Aqueous Solutions. In: Zhan, L., Chen, Y., Bouazza, A. (eds) Proceedings of the 8th International Congress on Environmental Geotechnics Volume 2. ICEG 2018. Environmental Science and Engineering(). Springer, Singapore. https://doi.org/10.1007/978-981-13-2224-2_55
Download citation
DOI: https://doi.org/10.1007/978-981-13-2224-2_55
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2223-5
Online ISBN: 978-981-13-2224-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)