Abstract
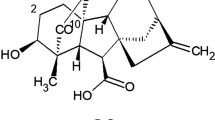
Gibberellins are growth hormones known to stimulate cell elongation and influence various developmental processes like stem elongation, seed germination, dormancy, flowering, sex expression, enzyme induction, and leaf and fruit senescence. Japanese scientists observed a common disease leading to excessive growth of rice plants. Eiichi Kurosawa (1926) investigated this bakanae (foolish seedling) disease in rice and found that tallness of diseased rice plants was induced by a chemical secreted by the fungus that had infected the plants. This chemical was isolated from the filtrates of the cultured fungus and was called gibberellin, after Gibberella fujikuroi (now renamed as Fusarium fujikuroi), the said fungus infecting rice plants. Kurosawa also noted that this active factor could promote the growth of maize, sesame, millet, and oat seedlings. In 1935, Yabuta and Hayashi successfully crystallized the fungal growth-inducing factor called gibberellin from the fungus Gibberella fujikuroi. All gibberellins are technically diterpene acids. They are either 19 or 20 carbon structures. A number of gibberellins are found in plants, of which only few are biologically active as hormones. The 19-carbon forms are, in general, biologically active gibberellins. Three most common biologically active gibberellins are GA1, GA3, and GA4. All other GAs serve either as active GAs or their degradation products (Fig. 17.1). In view of their acidic nature, gibberellins are also referred as gibberellic acids (GAs). GAs are named GA1 through GAn in order of discovery, and GA3 was the first GA to be structurally characterized. So far, 126 GAs have been identified in plants, fungi, and bacteria.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Gibberellins are growth hormones known to stimulate cell elongation and influence various developmental processes like stem elongation, seed germination, dormancy, flowering, sex expression, enzyme induction, and leaf and fruit senescence. Japanese scientists observed a common disease leading to excessive growth of rice plants. Eiichi Kurosawa (1926) investigated this bakanae (foolish seedling) disease in rice and found that tallness of diseased rice plants was induced by a chemical secreted by the fungus that had infected the plants. This chemical was isolated from the filtrates of the cultured fungus and was called gibberellin, after Gibberella fujikuroi (now renamed as Fusarium fujikuroi), the said fungus infecting rice plants. Kurosawa also noted that this active factor could promote the growth of maize, sesame, millet, and oat seedlings. In 1935, Yabuta and Hayashi successfully crystallized the fungal growth-inducing factor called gibberellin from the fungus Gibberella fujikuroi. All gibberellins are technically diterpene acids. They are either 19 or 20 carbon structures. A number of gibberellins are found in plants, of which only few are biologically active as hormones. The 19-carbon forms are, in general, biologically active gibberellins. Three most common biologically active gibberellins are GA1, GA3, and GA4. All other GAs serve either as active GAs or their degradation products (Fig. 17.1). In view of their acidic nature, gibberellins are also referred as gibberellic acids (GAs). GAs are named GA1 through GAn in order of discovery, and GA3 was the first GA to be structurally characterized. So far, 126 GAs have been identified in plants, fungi, and bacteria.
1 Biosynthesis
Gibberellins are tetracyclic diterpenoid acids and are synthesized from terpenoid pathway in plastids and subsequently modified in endoplasmic reticulum and cytosol into biologically active forms. Gibberellin biosynthetic pathway can be considered in three stages, each operating in different cellular compartments:
-
STAGE 1: Production of terpenoid precursors and ent-kaurene in plastids
Gibberellins are generally synthesized in plastids from glyceraldehyde-3-phosphate and pyruvate. In the endosperm of pumpkin seeds, isopentenyl diphosphate (IPP) is formed in the cytosol from mevalonic acid, which is itself derived from acetyl CoA. IPP isoprene units are added successively to produce intermediates of 10 carbons (geranyl diphosphate), 15 carbons (farnesyl diphosphate), or 20 carbons (geranylgeranyl diphosphate, GGPP). GGPP is the precursor for a number of terpenoid compounds, like carotenoids and many essential oils. After GGPP formation the pathway becomes specific for gibberellin biosynthesis. The cyclization reactions which convert GGPP to ent-kaurene represent the first step that is specific for gibberellin biosynthesis. The two enzymes which catalyze these reactions are localized in the proplastids of shoot meristem, and they are not localized in mature chloroplasts. Thus, green leaves lose their ability to synthesize gibberellins from IPP once their chloroplasts mature. Compounds such as AMO-1618, cycocel, and phosphon D are specific inhibitors of the first stage of gibberellin biosynthesis, and they are used to reduce plant height in agriculture.
-
STAGE 2: Oxidation reactions on the ER leading to GA 12 and GA 53 biosynthesis
Kaurene is transported from the plastid to the ER and is oxidized to kaurenoic acid by kaurene oxidase, which is associated with the plastid envelope. Further conversions to GA12 take place on the ER. Paclobutrazol and other inhibitors of P450 monooxygenases specifically inhibit this stage of gibberellin biosynthesis before GA12-aldehyde formation, and they act as growth retardants. A methyl group on ent-kaurene is oxidized to a carboxylic acid. This is followed by contraction from six to five carbon rings to produce GA12-aldehyde, which is then oxidized to GA12. GA12 is the first gibberellin produced in this pathway. Many gibberellins in plants are also hydroxylated on carbon 13, which forms GA53 from GA12. All enzymes involved are monooxygenases which utilize cytochrome P450 in their reactions. These P450 utilizing monooxygenases are localized on the ER.
-
STAGE 3: All other gibberellins are formed from GA 12 or GA 53 in the cytosol
All subsequent steps in the pathway of GA biosynthesis are carried out by a group of soluble dioxygenases in the cytosol. These enzymes require 2-oxoglutarate and molecular oxygen as co-substrates and use Fe2+ and ascorbate as cofactors. Two basic chemical changes occur in most plants: (i) hydroxylation at carbon 13 (on ER) or carbon 3 or both and (ii) a successive oxidation at carbon 20. Final step of this oxidation process is the loss of carbon 20 as CO2. When these reactions involve gibberellins initially hydroxylated at C-13, the resulting gibberellin is GA20. This is then converted to the biologically active form, GA1, by hydroxylation of carbon 3. Inhibitors of the third stage of the gibberellin biosynthetic pathway interfere with enzymes that utilize 2-oxyglutarate as co-substrates (Fig. 17.2).
2 Modulation of Gibberellin Biosynthesis
Highest levels of gibberellins are found in immature seeds and developing fruits. Gibberellin biosynthetic enzymes are localized in young, actively growing buds, leaves, and upper internodes. In Arabidopsis, GA20ox is expressed primarily in the apical buds and young leaves which thus appear to be the principal sites of gibberellin biosynthesis. The gibberellins produced in shoots can be transported to the rest of the plant via the phloem stream. Gibberellins regulate their own metabolism by either switching on or inhibiting the transcription of the genes which encode enzymes of gibberellin biosynthesis and degradation. This is called as feedback or feedforward regulation. Application of gibberellin causes downregulation of biosynthetic gene GA20ox and elevation in the transcription of degradative gene GA2ox. Conversely, a mutation that lowers the level of active gibberellin in plants stimulates the transcription of the biosynthetic genes GA20ox and downregulates the degradative enzyme GA2ox.
Environmental factors, such as photoperiods and temperature, can alter the levels of active gibberellins by affecting gene transcription for specific steps in the biosynthetic pathway. Some seeds are known to germinate only in light, and in such cases, gibberellin application can stimulate germination in dark. The promotion of germination by light is due to increase in GA1 level, resulting from light-induced increase in the transcription of the gene for GA3ox, which converts GA20 to GA1. This effect shows red/far-red reversibility and is mediated by phytochrome. Spinach (a long day plant) plants maintain a rosette form in short days, and in this situation, the level of gibberellins hydroxylated at carbon 13 is relatively low. Interestingly, in response to increasing day length, plants begin to elongate, and the level of gibberellins starts to increase. In some plants, low temperature is a prerequisite for seed germination, and gibberellins can substitute for the cold treatment. Gibberellins can induce auxin biosynthesis and vice versa. In decapitated pea plants, reduced stem elongation is accompanied with reduced auxin and gibberellin content. Replacing the supply of auxin restores gibberellin levels.
3 Enzymes Involved in Gibberellin Metabolism
GA 20-oxidase: It catalyzes all the reactions involving the successive oxidation steps of C-20 between GA53 and GA20, including the removal of C-20 as CO2.
GA 3-oxidase: It functions as a 3β-hydroxylase and adds a hydroxyl group at C-3 position to form the active gibberellin, GA1.
GA 2-oxidase: It inactivates GA1 by catalyzing the addition of a hydroxyl group to C-20.
4 Gibberellin Metabolism
Although active gibberellins exist in free forms, a variety of gibberellin glycosides are formed by a covalent linkage between gibberellin molecule and a sugar. These gibberellin conjugates are particularly prevalent in some seeds. The conjugating sugar is usually glucose and may be attached to the gibberellin molecule via a carboxyl group forming a gibberellin glycoside or via a hydroxyl group forming a gibberellin glycosyl ether. Glycosylation of gibberellins represents a mode of their inactivation.
5 Physiological Roles of Gibberellins
5.1 Internode Elongation
Evidence for the role of gibberellins in stem elongation comes from the study of rosette plants. A rosette is an extreme case of dwarfism in which the absence of internode elongation results in closely spaced leaves. The failure of internode elongation may result from genetic mutation or it may be environmentally induced. Exogenous application of gibberellin is known to promote internodal elongation in a wide range of species, a phenomenon known as bolting (Fig. 17.3). This leads to decrease in stem thickness and leaf size and pale green coloration of leaves. Some plants assume a rosette form in short days and undergo shoot elongation in long days. GA3 results in bolting in plants kept in short days, and normal bolting is regulated by endogenous gibberellin. Thus, the target of gibberellin action is intercalary meristem (meristem near the base of the internode). Deepwater rice shows pronounced effect of gibberellin on internode elongation so that its foliage may remain above water in the field. Striking growth rates of as much as 25 cm per day have been observed in rice plants under flooding conditions. Partial submergence of rice plants is believed to reduce partial pressure of O2 which triggers biosynthesis of ethylene precursor ACC. ACC transported to the aerial parts get converted to ethylene. Ethylene, in turn, reduces the level of ABA which acts as an antagonist to GA. Submerged rice tissue thus becomes more responsive (enhanced sensitivity) to endogenous GA, resulting in marked elongation of internodes. Gibberellins also regulate the transition from juvenile to adult phase of plant growth. Numerous perennials do not flower till they reach a certain stage of maturity. Till then they are said to be juvenile. In English ivy (Hedera helix), gibberellin application can cause reversion from mature to juvenile state, and juvenile conifers can be induced to enter the reproductive phase by the application of nonpolar gibberellins, like GA4 + GA7. Thus, gibberellins can regulate juvenility in both directions depending on species (i.e., mature to juvenile or vice versa).
5.2 Floral Initiation and Sex Determination
Gibberellins can substitute for long day or cold requirement for flowering in many plants. Thus, it is a component of flowering stimulus. In plants where flowers are unisexual, sex determination is regulated genetically as well as by environment and nutritional status. For example, in maize plants, staminate flowers are observed in tassel. Exposure to short days and cool nights increases endogenous gibberellin levels and causes feminization of tassel flowers. On the other hand, in dicots, like cucumber and spinach, gibberellins have an opposite effect, i.e., they promote formation of staminate flowers.
5.3 Seed Germination
Light requirement of seeds for germination can be overcome by treatment with gibberellins. This might be either through weakening of growth-restricting endosperm layer surrounding the embryo or due to mobilization of food reserves stored in the endosperm.
5.4 Fruit Production
Gibberellins are sprayed to increase the stalk length of seedless grapes. Because of shortness of individual stalks, bunches are too compact and the growth of the berries is restricted. GA3 spray promotes elongation of fruit and also increases its sweetness. Gibberellins also delay senescence in citrus fruits. This allows fruits to be left on the tree for a longer period to extend their shelf life.
5.5 Stimulation of Cell Elongation and Cell Division
Gibberellins increase both cell elongation and cell division. Mitotic activity increases markedly in the subapical region of the meristem of the rosette of long-day plants after treatment with gibberellins. Unlike auxins, gibberellins enhance cell wall extensibility without acidification. The elongation rate is influenced by both cell wall extensibility and the osmotically driven rate of water uptake. Gibberellins cause an increase in both mechanical extensibility of the cell walls and stress relaxation of the walls of living cells. There is evidence that the enzyme xyloglucan endotransglycosylase (XET) is involved in gibberellin-promoted cell wall extension. The function of XET may be to facilitate the penetration of expansins into the cellulose microfibrils of the cell wall.
5.6 Regulation of Transcription of Cell Cycle Kinases
Growth rate of the internodes of deepwater rice dramatically increases in response to submergence, and this is due to enhanced rate of cell divisions in the intercalary meristem. In these plants, gibberellins activate the cell division cycle first during the transition phase from G1 to S phase, leading to an increase in mitotic activity. This is followed by regulation of transition from G2 to M phase. To achieve this, gibberellins induce the expression of genes for several cyclin-dependent protein kinases (CDKs). Three types of gibberellin response mutants are useful in the identification of genes involved in the gibberellin signaling pathway involved in stem growth: (i) gibberellin-insensitive dwarfs (e.g., gai-1), (ii) gibberellin deficiency reversion mutants (e.g., rga), and (iii) constitutive gibberellin responders (slender mutants, e.g., spy). GAI and RGA are nuclear transcription factors responsible for the repression of growth. In the presence of gibberellins, these transcription factors are degraded. Mutant gai-1 and the related wheat dwarfing gene mutant rht have lost the ability to respond to gibberellins. SPY encodes a glycosyl transferase that is a member of a signal transduction chain prior to GAI/RGA. Whenever a mutation interferes with the repressor function of any of these, the plants grow tall.
6 Mode of Action
Gibberellin signal transduction with reference to stimulation of α-amylase synthesis in cereal aleurone layers has been extensively worked out to understand mode of GA action (Fig. 17.4). Cereal grains are composed of three main parts, viz., diploid embryo, triploid endosperm, and fused testa called pericarp (morphologically fused seed coat and fruit wall). Embryo has a specialized absorptive organ, the scutellum, which functions in absorbing the solubilized food reserves from the endosperm and transmits them to the growing embryo. Endosperm is composed of a central starchy component surrounded by aleurone layer. Cells of aleurone layer contain protein storage vacuoles (PSVs), also called protein bodies. During seed germination, food stored in the endosperm is broken down and transported to the growing embryo by the activity of α and β amylases. Main function of aleurone layer is the synthesis and release of hydrolytic enzymes. Cells of aleurone layer undergo programmed cell death thereafter.
When de-embryonated half seeds of barley are kept close to excised embryo, they trigger the release of α-amylase, and starch is digested. GA3 is known to substitute for embryo in stimulating starch degradation. It exerts its effect by enhancing the transcription of α-amylase mRNA. This is evident from the observation that GA3-stimulated α-amylase production is blocked by the inhibitors of transcription and translation such as actinomycin D and cycloheximide. Radioactive isotope-labeling studies have demonstrated that the stimulation of α-amylase activity by gibberellin involves de novo synthesis of the enzyme from amino acids rather than activation of preexisting enzymes. In another experiment, the α-amylase gene tagged to the promoter sequence of GUS reporter gene gives blue color in the presence of an artificial substrate, upon gene expression. When such a chimeric gene is introduced into aleurone protoplasts, the blue color intensity is stimulated by gibberellins. In addition to α-amylase, proteolytic enzymes (proteases), β-amylase, and other starch-degrading enzymes are also involved in mobilizing the endosperm reserves. The stimulation of α-amylase gene expression by gibberellins is mediated by a special transcription factor which binds to the promoter region of α-amylase gene. Certain regulatory DNA sequences (gibberellin response elements) have been identified in the promoters that are involved in binding the protein. The sequence of gibberellin response element in the α-amylase gene promoter has been identified to be similar to that of the binding site for MγB transcription factors which regulate phytochrome responses. Synthesis of GA-MγB mRNA in the aleurone cells increases within 3 h of GA3 application. This happens to be several hours before the increase in α-amylase mRNA level. Protein synthesis inhibitors have no effect on MγB mRNA production. Thus, GA-MγB is a primary response gene or early gene. α- amylase gene is a secondary response gene or late gene , as its transcription is blocked by protein synthesis inhibitor, like cycloheximide (CHI) (Fig. 17.5).
GA signaling is mediated through the DELLA protein (nuclear protein) which functions as negative regulators of growth. GA is perceived by a soluble receptor protein GID1 (GA-insensitive dwarf 1). Binding of GA with GID1 proteins enhances its interaction with DELLA which in turn enhances its interaction with F-box protein (SLY1). This leads to ubiquitination of DELLA protein followed by its degradation by 26S proteasome pathway. Removal of DELLA proteins facilitates the transcription of GA-regulated genes (Fig. 17.6).
Summary
-
Gibberellins are growth hormones known to stimulate cell elongation and influence various developmental processes like stem elongation, seed germination, dormancy, flowering, sex expression, enzyme induction, and leaf and fruit senescence.
-
Gibberellins are tetracyclic diterpenoids made up of four isoprenoid units. They are either 19 or 20 carbon structures. The 19-carbon forms are, in general, biologically active gibberellins. Three most common biologically active gibberellins are GA1, GA3, and GA4.
-
Gibberellin biosynthetic pathway can be considered in three stages, each operating in different cellular compartments. The first step involves production of terpenoid precursors and ent-kaurene in plastids followed by oxidation reactions on the ER leading to GA12 and GA53 biosynthesis. All other gibberellins are formed from GA12 or GA53 in the cytosol.
-
Compounds such as AMO-1618, cycocel, and phosphon D are specific inhibitors of the first stage of gibberellin biosynthesis, and they are used to reduce plant height in agriculture.
-
A variety of gibberellin glycosides are formed by a covalent linkage between gibberellin molecule and a sugar (usually glucose). Glycosylation of gibberellins represents a mode of their inactivation.
-
GA3 spray promotes elongation of fruit and also increases its sweetness. Gibberellins also delay senescence in citrus fruits.
-
Gibberellins induce the expression of genes for several cyclin-dependent protein kinases (CDKs) thereby activating the cell division cycle ultimately leading to an increase in growth rate of internodes of deepwater rice. Gibberellins also enhance cell wall extensibility without acidification, unlike auxin.
-
GA3 is known to substitute for embryo in stimulating starch degradation. It exerts its effect by enhancing the transcription of α-amylase mRNA.
-
The stimulation of α-amylase gene expression by gibberellins is mediated by a special transcription factor which binds to the promoter region of α-amylase gene. Synthesis of GA-MγB mRNA in the aleurone cells increases within 3 h of GA3 application.
-
GA signaling is mediated through the DELLA protein (nuclear protein) which functions as negative regulators of growth. Binding of GA with GID1 receptor proteins enhances its interaction with DELLA which in turn promotes its interaction with F-box protein (SLY1). This leads to ubiquitination of DELLA protein followed by its degradation by 26S proteasome pathway. Removal of DELLA proteins facilitates the transcription of GA-regulated genes.
Suggested Further Reading
Gao X, Zhang Y, He Z, Fu X (2017) Gibberellins. In: Li J, Li C, Smith SM (eds) Hormone metabolism and signaling in plants. Academic Press, San Diego, pp 39–76
Author information
Authors and Affiliations
Multiple-Choice Questions
Multiple-Choice Questions
-
1.
The specialized absorptive organ in cereals which functions in absorbing the solubilized food reserves from the endosperm is known as:
-
(a)
Scutellum
-
(b)
Pericarp
-
(c)
Plumule
-
(d)
Coleoptile
-
(a)
-
2.
Gibberellin was first isolated from which of the following fungus:
-
(a)
Gibberella fujikuroi
-
(b)
Gibberella acuminate
-
(c)
Gibberella africana
-
(d)
Gibberella gaditjirrii
-
(a)
-
3.
Gibberellins are chemical derivatives of:
-
(a)
Phenols
-
(b)
Terpenoids
-
(c)
Alkaloids
-
(d)
None of the above
-
(a)
-
4.
Which of the following is not an inhibitor of gibberellin biosynthetic pathway?
-
(a)
Phosphon D
-
(b)
Cycocel
-
(c)
Maleic hydrazide
-
(d)
AMO-1618
-
(a)
-
5.
Functions of gibberellic acid involve:
-
(a)
Internode elongation
-
(b)
Seed germination
-
(c)
Stimulation of cell elongation and division
-
(d)
All of the above
-
(a)
-
6.
Which of the following was the first GA to be structurally characterized?
-
(a)
GA1
-
(b)
GA3
-
(c)
GA12
-
(d)
GA4
-
(a)
Answers
1. a | 2. a | 3. b | 4. c | 5. d | 6. b |
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kalra, G., Bhatla, S.C. (2018). Gibberellins. In: Plant Physiology, Development and Metabolism. Springer, Singapore. https://doi.org/10.1007/978-981-13-2023-1_17
Download citation
DOI: https://doi.org/10.1007/978-981-13-2023-1_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2022-4
Online ISBN: 978-981-13-2023-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)