Abstract
Liquid-based cytology (LBC) is currently an acceptable technique utilized by most cytology laboratories across the world. Two different techniques are approved, ThinPrep® (Hologic Co., Marlborough, MA, USA) employing methanol fixation and a filtration-based methodology and SurePath® (BD Diagnostics-TriPath, Burlington, NC, USA) employing ethanol fixation and a sedimentation-based methodology. While both methods have been proven to perform equally or better than conventional direct smears, they both induce their own set of artifacts as a result of the wet fixation, methodology used, and elimination of manual spreading. It is now acknowledged that pathologists need to be acquainted with LBC and how it differs from direct smears to gain enough experience before implementing it in their practice. This chapter reviewed the up-to-date literature on LBC and describes the morphologic presentation and diagnostic tips for the different thyroid lesions on both LBC techniques.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
Liquid-based cytology (LBC) was first introduced in the 1990s as a new methodology that creates a thin layer suitable for image analysis. The method soon became a very popular technique for cervical/vaginal smears due to the many advantages it offers over the conventional smears. Within years, the method became also a popular technique for many non-gynecological specimens. However, its utilization for fine needle aspirations particularly those with extracellular matrix such as thyroid and salivary glands remained controversial because of the effect of LBC on such matrix. Based on the literature in the last decade, LBC has gained more popularity even for such FNA samples, most likely due to the experience acquired over the last three decades in interpreting such specimens.
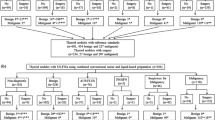
In order to properly interpret the literature, it is important to realize that different institutions applied the technique differently. In institutions that implement rapid on-site evaluation (ROSE), a small drop is used for the preparation of conventional smears (CS), and the needle is subsequently rinsed in the LBC collecting media from which the Prep is made; such sample will be referred to as split sample in the remaining text, i.e., CS plus LBC. In other institutions, the entire sample is rinsed in the LBC collecting media from which a random sample is seen on the Prep; such samples will be referred to as whole sample, i.e., LBC only or a split sample of LBC plus a cell block.
Two LBC methodologies are currently available and widely utilized in the cytology laboratories: ThinPrep® (Hologic Co. Marlborough, Ma., USA) and SurePath® (BD Diagnostics-TriPath, Burlington, NC, USA). While both methods result in a thin layer, they differ in their technique; the former is a filtration-based technique and the latter is a sedimentation-based technique.
13.1.1 Advantages of LBC
-
1.
The screening of LBC slides is much easier and more efficient:
-
(a)
The collection in the specialized media allows for immediate fixation and good cellular preservation. As a result, previous CS-related problems such as cellular crushing and air-drying artifact are eliminated.
-
(b)
The process eliminates obscuring elements such as blood, excessive inflammation, fibrin clots, etc.
-
(c)
The cells are evenly dispersed on the Prep within a well-defined circle (Fig. 13.1) and with minimal crowding and no manual artifacts.
-
(a)
-
2.
Decrease in number of slides needed to review per sample:
-
(a)
The slide is a random representation of the specimen.
-
(b)
Great advantage for countries in which regulations restrict the maximum number of slides screened per cytotechnologist per day. For non-gynecologic slides, the Prep is considered 0.5 slide against a maximum of 100/day in the USA.
-
(c)
Improve general laboratory efficiency.
-
(a)
-
3.
Decrease in the rate of nondiagnostic samples:
-
(a)
Better cellular preservation
-
(b)
Elimination of obscuring elements
-
(c)
Cellular concentration within a well-defined area
-
(a)
-
4.
Easier to transfer samples from remote sites to the laboratory:
-
(a)
Practical for centralized or large commercial laboratories receiving specimens from numerous clinics across the country
-
(a)
13.1.1.1 Methodology [1]
13.1.1.1.1 ThinPrep® (TP)
The needle is rinsed in CytoLyt (20% buffered methanol-based solution). The specimen is centrifuged, and three to five drops of the pellet are transferred into PreservCyt (50% methanol-based fixative) and should be fixed for at least 20 min before insertion in the T2000 machine for preparing the ThinPrep. The processing technique involves three steps: (1) cell dispersion in which the cylinder with attached TranCyt filter is inserted in the vial and rotated to create a current within the fluid that disperses mucous and debris, (2) cell collection through a gentle vacuum created within the cylinder pulling the fluid through the filter and collecting the cells on the exterior surface of the filter, and (3) cell transfer by inverting the filter and pressing it against the slide, while a slight positive pressure introduced through the cylinder ensures adequate transfer of the cells. The slide is dropped in 95% alcohol and stained by the Papanicolaou method according to the laboratory protocol. The resulting Prep is a 20 mm circular area.
13.1.1.1.2 SurePath® (SP)
The needle is rinsed in CytoRich solution and subjected to centrifugation for 10 min at 600 g twice to concentrate and fix the cells. Pellet is transferred to a 12-mL tube containing 10-mL water and centrifuged at 600 g for 5 min. The supernatant is decanted and the tube is vortexed to homogenize the sample. The tube is loaded onto the PrepStain machine where the cells are allowed to settle at 1 g and stained with the Papanicolaou stain in the machine. The resulting Prep is a 13 mm circular area.
It should be noted that both methods were designed to produce fixed smears stained with the Papanicolaou technique and approved as such in the USA by the Food and drug Administration. Cochand-Priollet described a modification for TP to prepare May-Grünwald-Geimsa stain and reported good results [2].
13.2 Alterations Introduced by LBC [1, 3, 4]
Both techniques present new artifacts that require adequate training. However, recent studies proved that as pathologists gain experience with these new artifacts, the diagnostic accuracy improves, and performance on LBC is either equal or better than that based on CS.
13.2.1 ThinPrep®
-
1.
The presentation is close to a monolayer, and the cells are all seen in one plan of focus. This is attributed to the positive air pressure introduced during processing. This pressure also causes some flattening of cells and clusters.
-
2.
During processing the blood is hemolyzed leaving behind inflammatory cells and fibrin strands. Red blood cells (RBCs) may still be detected in significantly bloody samples.
-
3.
Methanol fixation results in noticeable cellular shrinkage.
-
4.
Because of the filtration process, many of the small cells such as lymphocytes, neutrophils, etc. are markedly decreased which may be problematic in the diagnosis of Hashimoto’s thyroiditis.
-
5.
Filtration also results in cellular concentration and consequently alters the relative ratio of cells to other components such as colloid, i.e., cannot rely on the relative number of cells to amount of colloid in distinguishing follicular neoplasms from benign nodules.
-
6.
The process alters the quality and quantity of colloid presented on the Prep. Watery colloid appears as thin transparent film, while hard colloid is broken down into smaller droplets.
-
7.
Large, complex fragments and papillae are broken down into smaller fragments.
-
8.
Nuclear features are well preserved, nucleoli are generally more prominent than those on CS. However, intranuclear pseudoinclusions (INI) tend to be markedly decreased as reported by several studies.
13.2.2 SurePath®
-
1.
As a result of cellular sedimentation with gravity and without applied pressure, the cells and clusters appear at different planes of focus.
-
2.
While RBCs, inflammatory cells, and colloid are decreased during sedimentation, they are still represented on the Prep in small numbers.
-
3.
Cells are well preserved and with minimal shrinkage.
-
4.
Large and complex papillary architecture are well preserved. Interestingly clusters and papillary groups tend to have a pronounced three dimensional (3-D) appearance as a result of wet fixations and lack of pressure during preparation. Such 3-D pattern could interfere with proper focusing and examination of such clusters. In this situation, it is best to examine smaller clusters in the background.
13.2.3 Nondiagnostic Rate (NDR)
A meta-analysis study evaluated whether LBC can substitute for CS. Based on the analysis of 24 studies, it was noted that average sample inadequacy was significantly lower in TP and SP compared to CS [5] (see Chap. 14). The nondiagnostic rate varies depending upon the protocol used by the individual institution. In institutions where ROSE is performed, the NDR for cases prepared with CS is lower than that for cases prepared by a whole sample TP [6, 7]. In institution where ROSE is not performed, the NDR was reported to be similar [8] or even lower in LBC [9]. Cochand-Priollet et al. [2] reported NDR of 22% for TP versus 10% for CS but attributed this high NDR to the use of split sample. A study on SP reported a NDR of 25% but attributed the higher than expected rate to the fact that 18% of their nodules were <15 mm [10].
Two studies evaluated whether a second LBC slide could improve adequacy. The first [11] concluded that it would not be beneficial, while the second reported that a second slide reverted 16/39 cases to adequate [12]. The discrepant results are likely due to the use of split sample (TP and cell block) in the former versus whole sample in the latter. One study evaluated the utility of gross visual assessment of the needle rinse in CytoRich Red® and reported that the NDR was more frequent in the visually inadequate group (38.1%) when compared to the group with adequate visual assessment (10.5%) [13].
13.2.3.1 Diagnostic Accuracy
A meta-analysis study of 24 studies evaluating whether LBC can substitute for CS reported that specificity and sensitivity by sROC were similar or slightly superior for LBC versus CS. Various cytomorphological changes by each method were reported, and it was concluded that a learning curve is essential for adapting to such alterations [5]. Geer et al. compared the performance of SP versus frozen section (FS) [10]. SP had a sensitivity of 77% (29% for FS), specificity of 81% (100% for FS), and diagnostic accuracy of 80% (90% for FS). The authors concluded that supplementary FS analysis should be limited to cases suspicious for papillary carcinoma. Stamataki et al. reported 87.8% sensitivity, 99.5% specificity, and overall accuracy of 97.5% for TP [14].
A report by the College of American Pathologists (CAP) Interlaboratory Comparison Program that evaluated the difference in responses for unknown cases based on CS versus LBC for a period of 9 years (2001–2009) was published in 2013 [15]. The cases were comprised of 94% CS and 6% LBC (both TP and SP). Responses were discrepant in 14.9% for CS versus 5.9% for LBC for the negative category. Benign goiters were misdiagnosed as follicular neoplasm (FN) in 7.8% of CS versus 1.3% in LBC. For the malignant category, the discrepant responses were 4.8% for CS and 7.2% for LBC.
13.3 Cytomorphology Features by LBC [1,2,3,4, 16]
13.3.1 Cystic Lesions
The Prep contains various numbers of macrophages occasionally laden with hemosiderin. Ciliated cells from thyroglossal duct cysts are easily recognized. The background is usually clean (Fig. 13.2). In case of hemorrhagic cysts, the blood is mostly hemolyzed or filtered on TP although ghosts of RBCs may be seen in cases with excess blood. On SP, the RBCs are adequately represented on the slide.
13.3.2 Benign Nodule
13.3.2.1 Chronic Lymphocytic Thyroiditis (CLT)/Hashimoto’s Thyroiditis (HT)
The Prep usually contains scattered small- to medium-sized Hürthle cells in a background of variable number of scattered lymphocytes frequently not in the same field if viewed at high magnifications (Fig. 13.3a). Earlier literature reported CLT as a pitfall either missing some cases or overcalling others on TP [2, 17,18,19]. More recent literature on TP reported better results likely because of the experience gained with the technique [14, 20]. The pitfall can be attributed to several factors:
-
1.
The number of lymphocytes on the Prep may be reduced relative to those seen on CS.
-
2.
In bloody aspirates, peripheral blood cells remaining after the blood hemolysis may be seen on the Prep causing erroneous interpretation as CLT.
Diagnostic Clues:
-
1.
Look for the lymphocytes infiltrating the Hürthle cell clusters (see Chap. 36).
-
2.
Lymphocytes may pool in small groups away from the epithelial clusters or become entrapped by fibrin (Fig. 13.3b).
-
3.
Look for lymphocytes at the periphery of the ring where small cells tend to be more readily seen.
Lymphocytes are usually adequately represented on SP (Fig. 13.4a, b).
Hashimoto’s thyroiditis prepared by SurePath. (a) Several groups of Hürthle cells surrounded by numerous lymphocytes. Notice the 3-D appearance rendering the cells in different planes of focus (Papanicolaou, ×200). (b) Higher magnification of a well-spread area showing well visualized Hürthle cell groups and surrounding lymphocytes (Papanicolaou, ×400)
13.3.3 Benign Follicular Nodule/Nodular Goiter (BFN)
The follicular cells are seen as small well-spaced honeycomb sheets or small clusters in a background of macrophages in cases with cystic change (Fig. 13.5a). Colloid is easily recognized on SP Preps (Fig. 13.5b), while it tends to be decreased on TP and appear mostly as small droplets. When watery colloid is abundant, it tends to appear as small thin cellophane-like transparent fragments on TP (Fig. 13.5c).
Benign follicular nodule. (a) ThinPrep showing a flat sheet of well-organized follicular cells. Thick colloid appears as small- to medium-sized droplets. Few white blood cells are seen representing residual cells from hemolyzed peripheral blood (Papanicolaou, ×400). (b) Both the well-organized follicular groups and colloid are represented on SurePath with more 3-D appearance (Papanicolaou, ×200). (c) Macrophages and watery colloid appearing on ThinPrep as transparent and occasionally folded sheets. Fibrin is represented as more granular fragments next to the colloid (Papanicolaou, ×400)
Rossi et al. initially reported significant increase of follicular neoplasm (FN) from 16.6% to 23.3%. The authors explained that the false-positive diagnoses were a result of mistaking BFN for FN likely due to the different appearance of colloid [21]. In the author’s experience, we encountered such overcall in the earlier years as a result of increased follicular cellularity relative to the amount of colloid. However, a later study by Kim et al. [22] reported an increase of BFN rate on TP versus TP combined with CS from 51.4% to 57%, likely due to better cellular preservation, elimination of obscuring material and gaining more experience with TP.
13.3.4 Neoplasm/Suspicious for Follicular Neoplasm (FN/SFN)
The Preps are usually cellular and contain numerous microfollicles, singly or within small clusters, syncytial sheets, and minimal colloid (Fig. 13.6a–d). Suzuki et al. evaluated features helpful in distinguishing BFN from FN on SP [23]. They concluded that the incidences of intercellular spaces, distinct outer margin, and cytoplasmic process were significantly higher in BFN. Elongated microfollicles were seen in 55.4% of FN and 10.5% of BFN. No difference was found between follicular adenoma and carcinoma (see Chap. 45).
Follicular cell neoplasm. (a) Cellular ThinPrep containing syncytial sheets and microfollicles with little or no colloid in the background (Papanicolaou, 600×). (b) Microfollicles as seen in a different area of the ThinPrep (Papanicolaou, 600×). (c) Cellular SurePath Prep containing sheets and microfollicles at low magnification (Papanicolaou, 100×). (d) Microfollicles exhibiting 3-D appearance in a SurePath Prep (Papanicolaou, 600×). (c, d) (Courtesy of Paul Wakely, M.D., Ohio State University, Columbus, Ohio)
Kim et al. reported that while the rate of follicular lesions of undetermined significance was equal in TP plus CS versus CS alone, FN and SFN decreased from 1.2% to 0.3% [22].
13.3.5 Neoplasm/Suspicious for Hürthle Cell Neoplasm (HCN)
LBC suffers from the same diagnostic pitfalls encountered in HC-rich lesions as described in other chapters [2, 19] (see Chaps. 46 and 47). Preps of HCN are cellular and consist predominantly of small clusters and single cells. Larger syncytial sheets may occasionally be present particularly in carcinoma cases. The cells are widely spaced by the abundant granular cytoplasm. The nuclei are enlarged with prominent nucleoli. Colloid is very scant if present. In the author’s experience, the cytoplasm may appear somewhat vacuolated rather than granular on TP particularly in the cell block sections (Fig. 13.7a–d).
Hürthle cell neoplasm. (a) Cellular ThinPrep containing syncytial sheets and background single Hürthle cells (Papanicolaou, 200×). (b) High magnification of the Hürthle cells with abundant cytoplasm. Notice the finely vacuolated rather than granular cytoplasm, an artifact occasionally encountered on TP (Papanicolaou, 600×). (c) Cellular SurePath showing clusters and single Hürthle cells (Papanicolaou, 200×). (d) High magnification showing the abundant granular cytoplasm (Papanicolaou, 1000×). (c, d) (Courtesy of Paul Wakely, M.D., Ohio State University, Columbus Ohio)
13.3.6 Papillary Carcinoma (PTC)
The Preps are generally cellular, and the presentation will vary depending on the type of LBC used (Figs. 13.8 and 13.9) (see Chap. 15). The complex papillae are usually well preserved and present as 3-D groups on SP. Examining the cells is easier at the periphery of these complex 3-D groups to evaluate the nuclear features. INI tend to be seen more readily on SP than TP cases although generally reduced in both compared to CS based on the author’s experience, personal communication, and reported literature. Psammoma bodies and bubble gum colloid are preserved. On TP, the complex papillae are usually broken down and present as small fingerlike projections, clusters, or sheets of crowded cells. The nuclear features while present are usually subtle compared to CS, and INI are decreased if seen at all. Psammoma bodies are preserved. Bubble gum colloid appears as very thick fragments.
Papillary carcinoma, ThinPrep. (a) Branching papillary structure, notice the simple configuration (Papanicolaou, 100×). (b) Sticky colloid is well preserved (Papanicolaou, 400×). (c) A rare example pulled out bubble gum colloid (Papanicolaou, 200×). (d) High magnification showing oval nuclei, nuclear molding and faint groves, powdery chromatin and an intranuclear pseudo-inclusion (Papanicolaou, 1000×). (e) Nuclear irregularity can be a very helpful feature in identifying papillary carcinoma (Papanicolaou, 600×)
Papillary carcinoma, SurePath. (a) Complex papillary structure with 3-D configuration (Papanicolaou, 100×). (b) High magnification showing oval nuclei, molding, groves and pale chromatin (Papanicolaou, 1000×). (c) Papillary cluster with well-preserved psammoma body (Papanicolaou, 600×) (Courtesy of Michael Henry, M.D. Mayo Clinic, Rochester, Minnesota)
Zhang et al. statistically analyzed 40 cases of PTC and 17 other lesions prepared by TP for 10 characteristics [24]. They found that high cellularity and the presence of papillary clusters or large sheets, powdery chromatin, nuclear grooves, nuclear molding, and small nuclei were significant though none of these features could predict PTC by itself. Not surprising, INI had the highest predictive power. They recorded detecting many INI in 12/40 cases and rare INI in 18/40 cases. A similar study evaluated 161 PTC, 55 BFN, and 21 FN cases prepared by SP versus CS [23]. They reported a specificity of 97.4% for convoluted nuclei and 96.1% for perinuclear halos. Nuclear grooves and inclusions were equally seen. Chromatin was finely granular but not clear. Tall cell and hobnail variants were easier to recognize on SP. Another study investigated the cytological features of the tall cell variant on SP and concluded that the tall cells were easier to detect on SP than on CS where tall cells exceeded 50% of cells on the SP in 4/5 cases (see Chap. 27). Cells were easier to detect when singly scattered, and when present in clusters, cells were easier to find at the margins of the cohesive groups. The nuclear features were well preserved on both preparations; however, the cytoplasm was better preserved on SP where tall cells were seen as elongated, columnar cells with abundant cytoplasm and sharply distinct cytoplasmic borders. INI appeared as small and multiple, imparting “soap-bubble” appearance [25]. A case report of hobnail variant of PTC by TP described a cellular Prep containing 3-D clusters surrounded by surface follicular cells with peculiar centrifugal placement of the nuclei and caterpillar-shaped central psammoma bodies. Nuclear groves, dispersed chromatin, tiny nucleoli, and irregular nuclear membranes were noted. However, there were no INI or mitosis [26] (see Chap. 30).
Generally all studies reported good diagnostic accuracy for LBC with some cases misinterpreted as BFN [2, 15, 18, 27]. The under-calling of PTC can be attributed to:
-
1.
More subtle nuclear features such as nuclear molding and grooves.
-
2.
INI may not be detected or relatively decreased.
-
3.
Papillary fragments are broken down on TP and may not be recognized.
-
4.
In some cases, PTC present as sheets that appear under low magnification as honeycomb sheets although at higher magnification, these sheets are more crowded than normal [27].
Diagnostic clues:
-
1.
Even in the cases with honeycomb-like sheets, the Preps are usually very high in cellularity.
-
2.
On high magnification, the nuclear membrane is frequently wrinkled, and there is evident nuclear crowding within the sheets.
-
3.
The nuclear chromatin is pale and finely granular.
13.3.7 Medullary and Anaplastic Carcinomas
These carcinomas are seldom reported in published studies and are usually lumped together under carcinomas. In general these are obviously malignant and correctly diagnosed. Malle et al. reported nine anaplastic carcinomas, all correctly diagnosed by TP [19] (see Chap. 50). In our experience, the epithelioid type presents mainly as giant cells with high degree of pleomorphism, bizarre nuclei, and numerous intracytoplasmic neutrophils (Fig. 13.10).
Only two cases of medullary carcinoma were reported [2, 4]. Based on the author’s experience, these aspirates can have variable cellularity. The cells are plasmacytoid with hyperchromatic nuclei. Amyloid may be present in variable amounts and manifests as small thick wax-like fragments (see Chaps. 39 and 40). Calcitonin along with thyroglobulin as a negative control is pivotal in establishing the diagnosis (Fig. 13.11).
Medullary carcinoma, ThinPrep. (a) Clusters and single cells with rounded and elongated eccentric nuclei imparting a plasmacytoid appearance (Papanicolaou, 1000×). (b) Amyloid is well preserved and seen as a waxy material frequently associated with the cells or loose in the background (Papanicolaou, 600×)
References
Michael CW, McConnel J, Pecott J, Afify AM, Al-Khafaji B. Comparison of ThinPrep and TriPath PREP liquid-based preparations in nongynecologic specimens: a pilot study. Diagn Cytopathol. 2001;25(3):177–84.
Cochand-Priollet B, Prat JJ, Polivka M, et al. Thyroid fine needle aspiration: the morphological features on ThinPrep slide preparations. Eighty cases with histological control. Cytopathology. 2003;14(6):343–9.
Hoda RS. Non-gynecologic cytology on liquid-based preparations: a morphologic review of facts and artifacts. Diagn Cytopathol. 2007;35(10):621–34.
Michael CW, Hunter B. Interpretation of fine-needle aspirates processed by the ThinPrep technique: cytologic artifacts and diagnostic pitfalls. Diagn Cytopathol. 2000;23(1):6–13.
Chong Y, Ji SJ, Kang CS, Lee EJ. Can liquid-based preparation substitute for conventional smear in thyroid fine-needle aspiration? A systematic review based on meta-analysis. Endocr Connect. 2017;6(8):817–29.
Afify AM, Liu J, Al-Khafaji BM. Cytologic artifacts and pitfalls of thyroid fine-needle aspiration using ThinPrep: a comparative retrospective review. Cancer. 2001;93(3):179–86.
Nagarajan N, Schneider EB, Ali SZ, Zeiger MA, Olson MT. How do liquid-based preparations of thyroid fine-needle aspiration compare with conventional smears? An analysis of 5475 specimens. Thyroid. 2015;25(3):308–13.
Fadda G, Rossi ED. Liquid-based cytology in fine-needle aspiration biopsies of the thyroid gland. Acta Cytol. 2011;55(5):389–400.
Chang H, Lee E, Lee H, Choi J, Kim A, Kim BH. Comparison of diagnostic values of thyroid aspiration samples using liquid-based preparation and conventional smear: one-year experience in a single institution. APMIS. 2013;121(2):139–45.
Geers C, Bourgain C. Liquid-based FNAC of the thyroid: a 4-year survey with SurePath. Cancer Cytopathol. 2011;119(1):58–67.
Hasteh F, Pang Y, Pu R, Michael CW. Do we need more than one ThinPrep to obtain adequate cellularity in fine needle aspiration? Diagn Cytopathol. 2007;35(11):740–3.
Rossi ED, Morassi F, Santeusanio G, Zannoni GF, Fadda G. Thyroid fine needle aspiration cytology processed by ThinPrep: an additional slide decreased the number of inadequate results. Cytopathology. 2010;21(2):97–102.
Moon WJ, Baek JH, Choi JW, et al. The Value of Gross Visual Assessment of Specimen Adequacy for Liquid-Based Cytology during Ultrasound-Guided, Fine-Needle Aspiration of Thyroid Nodules. Endocr Pract. 2015;21(11):1219–26.
Stamataki M, Anninos D, Brountzos E, et al. The role of liquid-based cytology in the investigation of thyroid lesions. Cytopathology. 2008;19(1):11–8.
Fischer AH, Clayton AC, Bentz JS, et al. Performance differences between conventional smears and liquid-based preparations of thyroid fine-needle aspiration samples: analysis of 47,076 responses in the College of American Pathologists Interlaboratory Comparison Program in Non-Gynecologic Cytology. Arch Pathol Lab Med. 2013;137(1):26–31.
Ljung BM. Thyroid fine-needle aspiration: smears versus liquid-based preparations. Cancer. 2008;114(3):144–8.
Biscotti CV, Hollow JA, Toddy SM, Easley KA. ThinPrep versus conventional smear cytologic preparations in the analysis of thyroid fine-needle aspiration specimens. Am J Clin Pathol. 1995;104(2):150–3.
Frost AR, Sidawy MK, Ferfelli M, et al. Utility of thin-layer preparations in thyroid fine-needle aspiration: diagnostic accuracy, cytomorphology, and optimal sample preparation. Cancer. 1998;84(1):17–25.
Malle D, Valeri RM, Pazaitou-Panajiotou K, Kiziridou A, Vainas I, Destouni C. Use of a thin-layer technique in thyroid fine needle aspiration. Acta Cytol. 2006;50(1):23–7.
Rossi ED, Zannoni GF, Lombardi CP, et al. Morphological and immunocytochemical diagnosis of thyroiditis: comparison between conventional and liquid-based cytology. Diagn Cytopathol. 2012;40(5):404–9.
Rossi ED, Raffaelli M, Zannoni GF, et al. Diagnostic efficacy of conventional as compared to liquid-based cytology in thyroid lesions: evaluation of 10,360 fine needle aspiration cytology cases. Acta Cytol. 2009;53(6):659–66.
Kim SY, Kim EK, Moon HJ, et al. Combined use of conventional smear and liquid-based preparation versus conventional smear for thyroid fine-needle aspiration. Endocrine. 2016;53(1):157–65.
Suzuki A, Hirokawa M, Higuchi M, et al. Cytological characteristics of papillary thyroid carcinoma on LBC specimens, compared with conventional specimens. Diagn Cytopathol. 2015;43(2):108–13.
Zhang Y, Fraser JL, Wang HH. Morphologic predictors of papillary carcinoma on fine-needle aspiration of thyroid with ThinPrep preparations. Diagn Cytopathol. 2001;24(6):378–83.
Lee SH, Jung CK, Bae JS, Jung SL, Choi YJ, Kang CS. Liquid-based cytology improves preoperative diagnostic accuracy of the tall cell variant of papillary thyroid carcinoma. Diagn Cytopathol. 2014;42(1):11–7.
Schwock J, Desai G, Devon KM, Mete O, Dube V. Hobnail-variant of papillary thyroid carcinoma in liquid-based cytology. Diagn Cytopathol. 2015;43(12):990–2.
Michael CW, Pang Y, Pu RT, Hasteh F, Griffith KA. Cellular adequacy for thyroid aspirates prepared by ThinPrep: how many cells are needed? Diagn Cytopathol. 2007;35(12):792–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Michael, C.W. (2019). Liquid-Based Cytology Technique for Thyroid Cytology. In: Kakudo, K. (eds) Thyroid FNA Cytology. Springer, Singapore. https://doi.org/10.1007/978-981-13-1897-9_13
Download citation
DOI: https://doi.org/10.1007/978-981-13-1897-9_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1896-2
Online ISBN: 978-981-13-1897-9
eBook Packages: MedicineMedicine (R0)