Abstract
Pro-angiogenic factors such as bFGF, VEGF, and PDGF play a significant role in the invasion, metastasis, and neovascularization of hepatocellular carcinoma (HCC) cells. The expression levels of VEGFR, FGFR, and PDGFR along with the expression of their respective ligands are elevated in HCC. Increased expression levels of bFGF, not acidic FGF, are observed in HCC patients showing capsular infiltration of tumorous cells. Overexpressed VEGF and VEGFR are correlated to progression, angiogenesis, metastasis, tumor recurrence, and poor prognosis in HCC patients. Overexpressed levels of PDGF are associated with an increase in the metastatic potential of HCC. In this chapter, I will discuss VEGF and PDGF roles in metastatic properties of HCC.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Today, primary liver cancer, emanating in the liver, is the sixth most diagnosed cancer [1]. Furthermore, hepatocellular carcinoma (HCC), a primary liver malignancy, was reported to be the third cause of cancer-related deaths in 2012. However, HCC incidence and mortality rates vary vastly around the world [2]. The dominant risk factor for HCC is cirrhosis due to chronic hepatitis B or hepatitis C. Other risk factors include, but are not limited to, age, having a body mass index higher than 30, diabetes mellitus, and related nonalcoholic fatty liver disease [3]. Like many cancers, the best approach to treating HCC is its prevention.
However, if cancer does develop, HCC is known to be a highly vascularized cancer. The process of developing new blood vessels from pre-existing vessels known as angiogenesis is thought to contribute to HCC’s development and progression. VEGF (vascular endothelial growth factor), characterized in angiogenesis, levels have been shown to be helpful in diagnosing and monitoring patients with HCC ([4].
9.2 VEGF Overview
There are a total of nine proteins in the immediate VEGF family [5]. VEGFs are highly conserved in all vertebrate species; for example, VEGF-A has been identified in zebrafish, frogs, birds, and mammals [6]. This high level of conservation strongly suggests that VEGF, especially its isoform VEGF-A, may be involved with an important role in biological processes.
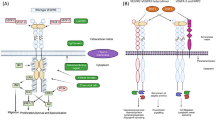
In fact, VEGF and its receptors are known for their vital role as regulators of angiogenesis and their involvement in vascular permeability. Currently, nine family proteins have been identified, but due to alternative splicing, many isoforms exist; for example, VEGF-A undergoes alternative splicing leading to nine different subtypes. Interestingly, it is thought that each different VEGF isoform plays a distinct role in vascular and arterial development. For example, VEGF-A has been shown to interact with VEGFR-1 and VEGFR-2; VEGF-1 is characterized more so in pathological conditions such as cancer, ischemia, and inflammation, while VEGFR-2 is involved in endothelial growth and survival signals, but both acting as tyrosine kinase receptors (Fig. 9.1) [7].
VEGF family members transduce their signal intracellularly via a membrane-bound tyrosine kinase receptor. VEGF-A and VEGF-B share a stronger affinity to receptors VEGFR-1; in addition, VEGF-A, VEGF-C, VEGF-D, and VEGF-E are capable of binding and activating VEGFR-2, while VEGF-C and VEGF-D bind preferentially to VEGFR-3 [8]. The activation of VEGF receptors is essential for angiogenesis.
Many of the VEGF family members are regulated by hypoxia-inducible factor (HIF) [9]; hypoxia initiates expression of many growth factors including VEGF and other angiogenetic factors. In liver cancer, HIF-1α is highly expressed at levels significantly higher than levels in normal liver tissues [6]. Other metabolic regulators and transcription factors include E-twenty-six growth factor and reactive oxygen species which regulate the expression of VEGF family of ligands and its receptors [10, 11].
9.3 PDGF Overview
Platelet-derived growth factor (PDGF) is a key area of research in cancer development and progression. An abundance of PDGFR activity may increase tumor growth. It has been shown that throughout the progression of HCC in combination with epithelial-mesenchymal transition, levels of PDGF-A, PDGFRα, and PDGFRβ were both increased (Fig. 9.1) [12].
First, it is important to understand PDGF normal structure and function. PDGF is a dimeric molecule that has disulfide-bonded A and B polypeptide chains. The chains can homo- and heterodimerize. Their cellular effects are mediated by binding to their tyrosine kinase receptors known as the alpha-receptor (PDGFRα) and the beta-receptor (PDGFR β) [13]. The family isoforms are known to stimulate growth, survival, and motility in many cell types and play an important role in adult tissue homeostasis [13].
PDGF signaling is evident in epithelial cancers; the signaling leads to stromal recruitment which possibly helps initiate epithelial-mesenchymal transition and, as a result, increases tumor growth, angiogenesis, invasion, and metastasis [14].
9.4 VEGF and HCC
Meta-analysis, conducted by Zhan et al., studied VEGF levels and its possible effect on the prognostic significance in patients with HCC. Their data suggests that above normal levels of VEGF was associated with poor overall survival in HCC patients [15]. Using an enzyme immunoassay, plasma VEGF levels in varying stages were analyzed and measured. The later stages (Stage IVB) had levels of VEGF measured as high as 103.1 ± 123.2 pg/ml [16]. Strengthening this finding, Jinno et al. findings show similar data that advanced metastasis in patients with HCC has increasingly higher levels of VEGF compared to patients at earlier stages.
In another study, VEGF-A and its receptor VEGFR-1 had significant higher levels in HCC patients compared to controls (p < 0.001). However, in serum there was no significant difference in measured levels of VEGF-C and its receptor VEGFR-2 [17]. These findings support that different VEGF members have different biological roles and may help in targeting therapies to specific ligands and their receptors mediating their effects. This targeting of VEGF-A and VEGFR-1 may prove to be beneficial.
9.5 PDGF and HCC
In a study using HCC whole-cell lysates, the majority of HCC tissues measured PDGFRα levels contained a large (sevenfold) increase compared to their controls. Higher levels in PDGFRβ were only characterized in 6 of 22 tumors but were higher in samples associated with cirrhosis [18]. PDGFRα is essential in the development in several tissues, proliferation, morphogenesis, angiogenesis, and epithelial-mesenchymal interactions [19]. It has been previously established that PDGFRα is associated with malignant proliferation, progression, and angiogenesis.
Using an in vivo assay using hepatoma cells, overexpression of PDGFRα led to high tumorigenic potential; these samples also included increased microvessel density compared to the controls [20].
When injury occurs or vascular damage presents, thrombosis occurs. Platelets become activated, adhered to the injured area, aggregate together, and secrete platelet granules. These granules can contain several factors including both VEGF and PDGF (and others). Since both these molecules are elevated in HCC patients, it suggests that platelets may play a significant role in tumor development and metastasis [21]. The role of platelets and their granules has been investigated and characterized in many types of cancer. Furthermore, targeting VEGF and PDGF and their respective receptors may be useful in treating patients with HCC and better patient prognosis.
9.6 Current Treatments for HCC
The process of angiogenesis is essential for cancer development and its metastasis. Since VEGF is critical for angiogenesis to occur, VEGF-targeted agents have already been developed as potential treatments for patients with HCC.
One agent developed for cancer treatment use was sorafenib. Sorafenib is an orally active multikinase inhibitor. The function of the drug is to block many important cellular factors involved in tumor cell proliferation and angiogenesis and has been found to increase the rate of apoptosis [22].
Sorafenib, a multikinase inhibitor, prevents the serine-threonine kinase activity of Raf-1 and B-Raf and the receptor tyrosine kinase activities of VEGFR-1, VEGFR-2, VEGFR-3, and PDGFR-β (Fig. 9.1) [22, 23]. Utilizing mouse xenografts, it has been shown that administering sorafenib reduces angiogenesis and increases cancerous cell apoptosis [24]. Furthermore, in a phase III trial called Sorafenib Hepatocarcinoma Assessment Randomized Protocol (SHARP; ClinicalTrials.gov number, NCT00105443), administering sorafenib increased median survival and the time to progression by 3 months in patients with HCC [25]. In fact, as of 2017, sorafenib is currently the sole systemic agent approved in the United States for HCC treatment. As with all the treatments, there are downsides. Not only is the drug costly; it has been shown to have considerable drug-related symptoms for little benefit [26]. Also some patients exhibit resistance or intolerance to sorafenib.
Another drug, lenvatinib, is a different but very similar inhibitor to sorafenib. Lenvatinib specifically targets VEGFR, FGFR, PDGFR-β, RET, and KIT [27]. A recent 2017 study investigated sorafenib versus lenvatinib as the first recommended therapy for unresectable HCC. The study concluded that lenvatinib showed noninferiority in overall patient survival and had improvements in secondary end points, for example, in time to progression [27]. Sorafenib being the only approved drug treatment for HCC patients needs to change. As trials continue with lenvatinib and other potential treatments, hopefully the prognosis improves for patients with HCC.
Relatively new imaging equipment and radiofrequency ablation (RFA) instruments have changed treatments available. For example, the use of RFA may be an alternative to surgical resection. Its benefits are numerous including it is minimally invasive, it is easy to operate, and the procedure is repeatable. It also has been seen to increase immunity and reduce the levels of VEGF in serum [28].
9.7 Conclusion
For cancer to thrive, it needs to create its own blood supply. This process of angiogenesis and the expression of VEGF are critical for tumor development. There has been a plethora of evidence showing that VEGF and PDGF serum levels, especially the ligands and receptors, are overexpressed in HCC and are highly characterized and present. We conclude that these still remain important targets for future treatments in patients with HCC. As lenvatinib trials continue, drugs continue to be developed and other treatment methods utilized; hopefully HCC will no longer have such a high prevalence of cancer-related deaths.
References
Ananthakrishnan A, Gogineni V, Saeian K (2006) Epidemiology of primary and secondary liver cancers. Semin Interv Radiol 23(1):47–63
Gelband H et al (2015) Liver cancer. In: Gelband H et al (eds) Cancer: disease control priorities, vol 3, 3rd edn. World Bank, Washington, DC
Mittal S, El-Serag HB (2013) Epidemiology of HCC: consider the population. J Clin Gastroenterol 47(0):S2–S6
Kaseb AO et al (2009) Vascular endothelial growth factor in the management of hepatocellular carcinoma. Cancer 115(21):4895–4906
Dormer A, Beck G (2005) Evolutionary analysis of human vascular endothelial growth factor, angiopoietin, and tyrosine endothelial kinase involved in angiogenesis and immunity. In Silico Biol 5(3):323–339
Holmes DI, Zachary I (2005) The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol 6(2):209
Takahashi H, Shibuya M (2005) The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 109(3):227–241
Sullivan LA, Brekken RA (2010) The VEGF family in cancer and antibody-based strategies for their inhibition. MAbs 2(2):165–175
Germain S et al (2010) Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Curr Opin Hematol 17(3):245–251
Randi AM et al (2009) Regulation of angiogenesis by ETS transcription factors. Biochem Soc Trans 37(Pt 6):1248–1253
Ushio-Fukai M, Nakamura Y (2008) Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett 266(1):37–52
Gotzmann J et al (2006) A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene 25(22):3170–3185
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79(4):1283–1316
Andrae J, Gallini R, Betsholtz C (2008) Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22(10):1276–1312
Zhan P, Qian Q, Yu LK (2013) Serum VEGF level is associated with the outcome of patients with hepatocellular carcinoma: a meta-analysis. Hepatobiliary Surg Nutr 2(4):209–215
Jinno K et al (1998) Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol 33(3):376–382
Kemik O et al (2010) Circulating levels of VEGF family and their receptors in hepatocellular carcinoma. Bratisl Lek Listy 111(9):485–488
Stock P et al (2007) Platelet-derived growth factor receptor-alpha: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther 6(7):1932–1941
Liu L et al (2002) Platelet-derived growth factor receptor alpha (pdgfr-alpha) gene in zebrafish embryonic development. Mech Dev 116(1–2):227–230
Wei T et al (2014) Overexpression of platelet-derived growth factor receptor alpha promotes tumor progression and indicates poor prognosis in hepatocellular carcinoma. Oncotarget 5(21):10307–10317
Gay LJ, Felding-Habermann B (2011) Contribution of platelets to tumour metastasis. Nat Rev Cancer 11(2):123–134
Chang YS et al (2007) Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol 59(5):561–574
Wilhelm SM et al (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–7109
Liu L et al (2006) Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 66(24):11851–11858
Llovet JM et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Sanoff HK et al (2016) Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist 21(9):1113–1120
Cheng AL, Fin R, Qin S et al (2017) A phase III trial of lenvatinib (LEN) vs sorafenib (SOR) in first-line treatment of patients with unresectable hepatocellular carcinoma (REFLECT study). J Clin Oncol 35(Suppl) abstr:4001
Guan Q et al (2015) Correlation between vascular endothelial growth factor levels and prognosis of hepatocellular carcinoma patients receiving radiofrequency ablation. Biotechnol Biotechnol Equip 29(1):119–123
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bastien, A.J. (2018). VEGFR and PDGFR: Their Targeting in Liver Cancer. In: Nagaraju, G. (eds) Role of Tyrosine Kinases in Gastrointestinal Malignancies. Springer, Singapore. https://doi.org/10.1007/978-981-13-1486-5_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-1486-5_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1485-8
Online ISBN: 978-981-13-1486-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)