Abstract
Shallow coastal ecosystems (SCEs) are generally recognized as not only significant organic carbon reservoirs but also as sources for CO2 emission to the atmosphere, thus posing a dilemma regarding their role in climate change mitigation measures. However, we argue that SCEs can act as sinks for atmospheric CO2 under a given set of biogeochemical and socioeconomic conditions. The key properties of SCEs that show net uptake of atmospheric CO2 are often characteristic of human-dominated systems, that is, high nutrient inputs from terrestrial systems, input of treated wastewater in which labile carbon has been mostly removed, and the presence of hypoxic waters. We propose a new perspective on the potential of human-dominated SCEs to contribute to climate change mitigation, both serving as carbon reservoirs and providing direct net uptake of atmospheric CO2, in light of human systems–ecosystem interactions. Namely, if we view the land and a SCE as an integrated system, with appropriate management of both wastewater treatment and SCE, we will be able to not only suppress CO2 release but also capture and store carbon.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Shallow Coastal Ecosystems (SCEs)
- Carbon Storage Rates
- Refractory Dissolved Organic Carbon (RDOC)
- Hypoxic Water Mass
- Blue Carbon
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
Recent research has demonstrated that the sediment of shallow coastal ecosystems (SCEs), such as mangroves, salt marshes, tidal flats, seagrass meadows, estuaries, and embayment, is important as a marine carbon reservoir (e.g., Nellemann et al. 2009; McLeod et al. 2011; Fourqurean et al. 2012; Duarte et al. 2013; Miyajima et al. 2015, 2017; Endo and Otani 2018; Inoue 2018; Miyajima and Hamaguchi 2018). Moreover, coastal ecosystems with high primary productivity, such as seagrass meadows, can serve as net sinks for atmospheric CO2 (i.e., total CO2 uptake minus total CO2 release is positive; Smith 1981; Tokoro et al. 2014).
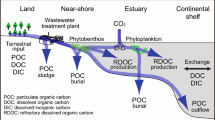
From the viewpoint of mitigating climate change, the net uptake of atmospheric CO2 through the exchange of CO2 at the air–water interface is a direct process, whereas the suppression of CO2 emission to the atmosphere by carbon storage in the marine ecosystem is an indirect process (Fig. 11.1). Although these are two completely different processes, both are effective for mitigating climate change. There is controversy as to which is more important, but ecosystems that show both net uptake of atmospheric CO2 and long-term storage of carbon are desirable.
Global carbon cycling. See Le Quéré et al. (2018) for atmosphere data (mean ± SD for 2007–2016), Nellemann et al. (2009) for the sedimentary accumulation rate, and IPCC (2013) for terrestrial input. SCE sediments accumulate 190 million tonnes of carbon (Tg C) every year, much faster than sediments in shelves and the open ocean
However, because ecosystems are dynamic natural systems characterized by complex fluctuations in biological communities and environmental conditions, atmospheric CO2 uptake and carbon storage do not occur at constant rates. The CO2 gas exchange at the air–water interface fluctuates through absorption and emission phases and the amount of carbon stored in the ecosystem increases and decreases over time (Tokoro et al. 2014, 2018). Therefore, in considering the effectiveness of ecosystem-based technology measures such as mitigation of climate change through the use of blue carbon ecosystems, setting a specified time and space of interest in advance is important to judge whether atmospheric CO2 is taken up or whether carbon is stored. As the temporal and spatial scales of the processes increase, the measure becomes more effective and more reliable.
The effect of human activity cannot be ignored at longer time scales. The geophysical setting of SCEs is often at the boundary between land and sea, making them socioeconomically important features. As a result, the carbon cycle of many SCEs has changed significantly over time due to the load of nutrients and organic matter (green carbon), freshwater use, and topographic modification (Bauer et al. 2013; Regnier et al. 2013). In particular, because nutrient loading and wastewater treatment have large impacts on the cycling of biogeochemical elements (e.g., carbon, nitrogen, and phosphorus) in the ecosystem (McIntyre et al. 2000), they may also have an impact on the uptake of atmospheric CO2 and carbon storage within the ecosystem.
In comparison with the open ocean and shelves, SCEs are hotspots with a high rate of carbon accumulation to the sediment, although few measurements of CO2 exchange at the air–water interface have been conducted, highlighting SCEs as largely unexplored places (but see Borges et al. 2005; Cai 2011; Chen et al. 2013; Laruelle et al. 2013; Regnier et al. 2013; Akhand et al. 2018; Otani and Endo 2018; Tokoro et al. 2018; Watanabe and Nakamura 2018). SCEs are characterized by diverse biogeochemical cycles and biota. Their complexity reflects their position at the boundaries between air and water, water and sediment, and atmosphere and sediment, with very different physical properties (such those of fresh water and salt water) and with rapid exchange rates at the interfaces. Thus, the estimation of carbon stock and flow in SCEs is highly uncertain compared to that in other ecosystems. In this chapter, we discuss the potential for climate change mitigation by SCEs that have been strongly affected by human impacts for a long time.
11.2 CO2 Uptake and Carbon Storage in SCEs
11.2.1 Relationship Between Carbon Storage and CO2 Uptake
The essential functions required when considering mitigating climate change by utilizing blue carbon ecosystems are storing carbon (Miyajima and Hamaguchi 2018) and subsequently suppressing CO2 emission to the atmosphere, net direct atmospheric CO2 uptake (Tokoro et al. 2018), or both. However, SCEs are generally recognized to be net emitters of CO2 to the atmosphere (Borges and Abril 2011), due to the fact that the water generally contains a large amount of CO2 and organic green carbon inflowing from terrestrial sources (Laruelle et al. 2013; Regnier et al. 2013) (Fig. 11.2). Indeed, when summarized based on salinity, it is clear that those SCEs more influenced by fresh water and with lower salinity are greater sources of CO2 (Fig. 11.3).
Relationship between salinity and air–water CO2 exchange in SCEs. Those SCEs with lower salinity emit more CO2. (Modified from Chen et al. 2012)
In any case, if we look at this carbon flow from the viewpoint of climate change mitigation, we note that SCEs have a positive function of storing organic carbon and a negative function of being a net source of CO2 to the atmosphere. The dilemma of “storing carbon but emitting CO2” occurs because water intervenes between the sediment (the main pool of carbon) and the atmosphere. Similarly, this dilemma can also occur in inland waters such as lakes and rivers (Cole et al. 2007). In turn, in a forest or grassland ecosystem not interrupted by water, the same amount of carbon taken up from the atmosphere is stored in organisms and/or in the soil, assuming a closed system. Because of this, the dilemma has not been specifically discussed in terrestrial ecosystems. In reality, however, terrestrial ecosystems are also open systems, as large amounts of green carbon flow out of these ecosystems through rivers and into the sea (Fig. 11.1). Therefore, in forest and grassland ecosystems, the amount of net CO2 uptake is larger than the amount of organic carbon stored.
11.2.2 Carbon Storage in SCEs
Among the various processes that influence carbon storage in SCEs, the major ones supporting the high accumulation of organic carbon in the sediment include (1) large supplies of autochthonous organic matter (i.e., blue carbon formed within SCEs) and/or allochthonous organic matter (green carbon flowing in from terrestrial sources and/or blue carbon flowing from outside the SCE); (2) a large supply of mineral particles, which are the main component of the sediment (Sholkovitz 1976); and (3) aggregation of the mineral particles and organic matter to promote sedimentation (Kennedy et al. 2010; Zonneveld et al. 2010).
The seabed of SCEs where carbon is deposited is also dynamic. Due to external forcing by waves and currents, the sediment surface layer is disturbed, its thickness varies, and the seabed topography changes. For example, erosion at the sea bottom implies outflow of sedimentary mineral particles and carbon from the sediment surface. In turn, when the waves and currents near the seabed are calm, the sediment and carbon accumulation rates increase. Furthermore, in such calm physical conditions, fine sediment particles are more easily deposited and organic matter adsorbs to the fine particles, often resulting in the formation of muddy sediments where much carbon is stored.
Decomposition of organic matter becomes slower after deposition on the sea bottom. This is related to the anoxic environment of the sediment except for its vast surface layer (e.g., about the top several millimeters in a muddy sediment). Because terrestrial soil is aerobically decomposed by exposure to oxygen from the air, its decomposition proceeds on a scale of decades, whereas in the anaerobic environment of the seabed, organic matter is decomposed and mineralized over thousands of years (Chambers et al. 2001). This suppression of the decomposition rate promotes accumulation of organic matter at the seabed (Miyajima and Hamaguchi 2018).
Vegetated SCEs such as mangroves, salt marshes, and seagrass meadows have among the fastest rates of carbon storage to their sediments, with average values ranging from 138 to 226 g C/m2/year (range: 18–1713 g C/m2/year); the rates are at least 1000 times greater than that in the open ocean (0.018 g C/m2/year) (Nellemann et al. 2009; McLeod et al. 2011). This difference cannot be explained solely by the difference in the production rate of blue carbon (SCE: 1044–2784 g C/m2/year, open ocean: 120 g C/m2/year; Gattuso et al. 1998). Rather, the existence of vegetation slows water currents and promotes the trapping and sedimentation of suspended particulate organic matter, causing an increase in the carbon accumulation rate (Hendriks et al. 2007; Kennedy et al. 2010).
Other factors also influence the carbon storage rate in SCEs, as explained in Chap. 2 (Miyajima and Hamaguchi 2018). These include chemical factors such as the quality (e.g., whether it is easy or difficult to decompose) of organic matter supplied and degradation enzyme activity; geophysical factors such as temperature, water depth, and the grain size and surface area of the sediment (Miyajima et al. 2017); and biological factors such as bioturbation (Zonneveld et al. 2010; Koho et al. 2013).
11.2.3 Requirements for a SCE Becoming a Net Sink of Atmospheric CO2
As we explained, the carbon storage rate in aquatic ecosystems is not equal to the net atmospheric carbon uptake rate because these are open systems in which water intervenes between the sediment and atmosphere. In addition, the inorganic–organic conversion in the water column is complex. As a result, the amount of material exchanged at the air–water interface and that exchanged at the water–sediment interface generally do not balance.
Gas exchange between the atmosphere and the ocean occurs at the air–water interface. If the concentration of CO2 in seawater is lower than that in air, then atmospheric CO2 will be absorbed into the sea (Wanninkhof 1992). Currently, the atmospheric CO2 concentration fluctuates from about 350 to 450 ppm; in turn, the CO2 concentration in SCE surface waters ranges from about 20 ppm to more than 3000 ppm. Thus, the actual gas exchange rate and direction of the flux (i.e., whether the ecosystem is a sink or a source of CO2) are dependent on the CO2 concentration in the surface water.
The CO2 concentration in the surface water becomes undersaturated and atmospheric CO2 is taken up (1) if the CO2 concentration in the influent water from outside the target area is lower than that of the atmosphere, or (2) if the concentration decreases below the atmospheric concentration due to the occurrence of processes lowering the CO2 concentration in the surface water. Rivers are major CO2 influents from outside SCEs, and their CO2 concentrations are high. The partial pressure of CO2 in more than 95% of global inland waters is higher than that in the air, with a median value of about 3100 μatm (Raymond et al. 2013). Thus, in order for the surface water of SCEs to be undersaturated, it is necessary to have a process that lowers the CO2 concentration in surface water. As explained in Chap. 6, such processes include decreasing temperature, increasing total alkalinity (Ca2+ and NO3 − concentrations are the main ions that determine total alkalinity), and net uptake of CO2 by organisms (Tokoro et al. 2018).
The environmental conditions that result in the uptake of CO2 are not continuous in the natural world. In reality, as environmental conditions such as light, temperature, and salinity change from moment to moment, cycles of uptake and emission occur frequently (Tokoro et al. 2014, 2018). In other words, it is critical to take a long-term view of the balance of uptake and emission as we discuss an ecosystem contributing to the mitigation of climate change. The requirements for SCEs serving as long-term net sinks are conceptualized in Fig. 11.4. Overall, the system can also be viewed as a process of transporting carbon like a pump; that is, the carbon transport is unidirectional from the viewpoint of the long-term balance. This type of pump is well-known in the field of marine science: “biological pump” in the open ocean (Longhurst and Harrison 1989) and “continental shelf pump” in shelves (Tsunogai et al. 1999).
Conceptualized diagram of carbon flow contributing to net uptake of atmospheric CO2 in SCEs. We assume that net uptake of atmospheric CO2 occurs only when there is a unidirectional carbon flow (pump) over a long period of time, resulting in the water CO2 concentration being lower than the atmospheric CO2 concentration. The net uptake of atmospheric CO2 occurs when (1) CO2 emission is suppressed by generation of refractory dissolved organic carbon (RDOC); (2) precipitation and burial of particulate organic carbon (POC); and (3) transportation of POC and DOC to the deep sea. Although the wastewater treatment plant that removes POC (sludge) indirectly contributes to lowering the CO2 concentration in the SCE, the treatment plant functions as a CO2 emitter because organic matter in the wastewater is decomposed and CO2 is generated in the open aeration tank. (Modified from Kuwae et al. 2016)
Among the various unidirectional pumps, those particularly important for the net uptake of atmospheric CO2 are (1) formation of refractory dissolved organic carbon (RDOC); (2) particulate organic carbon (POC) being conveyed to the sea bottom and stored in the sediment (Miyajima and Hamaguchi 2018); and (3) POC and dissolved organic carbon (DOC) export (Sugimatsu et al. 2015; Abo et al. 2018). Although the components of RDOC and the reasons for the refractory properties are still not fully understood, Arrieta et al. (2015) proposed that RDOC has a molecular or physical structure (unspecified) that is difficult for bacteria to use, or its concentration is too low to be available for bacteria. Because these three pumps tend to not flow in reverse (i.e., the opposite process is weak), they function to suppress both the decomposition of organic matter and the emission of CO2 to the atmosphere over a long period.
11.2.4 Carbon Storage in Water and Organisms
Of the three requirements for a net long-term CO2 sink (Fig. 11.4), the storage of RDOC in water is the least understood (Jiao et al. 2014). The organic carbon pool is not limited to sediments and organisms but can be in the water column as well if only the DOC is refractory. DOC accounts for 28% (246 Tg C/year) of the total green carbon flowing from rivers to oceans on the global scale (Cai 2011). Thus, how much DOC decomposes in microbial and photochemical reactions (Moran et al. 2000), how much DOC remains as refractory dissolved green carbon (Kubo et al. 2015), and how much refractory dissolved blue carbon is newly formed at the site can be major factors determining the amount of CO2 uptake and emission.
Among them, new formation of refractory dissolved blue carbon is particularly unclear. Phytoplankton, bacteria, macrophytes (seagrasses and seaweeds), and corals are organisms responsible for the formation of refractory dissolved blue carbon (Ogawa et al. 2001; Wada et al. 2008; Kragh and Søndergaard 2009; Lønborg et al. 2009; Tanaka et al. 2011a, b). From a technical perspective, however, it is extremely difficult to quantify refractory dissolved blue carbon separately from refractory dissolved green carbon because the concentration of DOC is low and salt in seawater acts as an inhibitor in the chemical analysis.
The sequence of (1) uptake of CO2 by macrophytes and phytoplankton, (2) production of their body (POC) and mucus (DOC), and (3) transportation and sinking of POC and DOC in the deep ocean is also an important mechanism for carbon storage. Even if POC and DOC get decomposed in the deep ocean and become CO2, the transport to the surface and return to the atmosphere occur over geological time scales. According to recent reports, the global estimate of POC and DOC derived from seagrasses transported from SCEs to the deep ocean is about 24 Tg C/year (Duarte and Krause-Jensen 2017) and that derived from kelps is around 36–279 Tg C/year (Krause-Jensen and Duarte 2016). However, the variability in these amounts and the factors controlling their transport are still unknown, leading to high uncertainty in the estimates.
11.3 Hypothesis that Human-Impacted SCEs Act As a Net CO2 Sink
Strong human impacts can result in changes to the carbon cycle (McIntyre et al. 2000). In particular, nutrient load, wastewater treatment, and freshwater use will increase with increasing human and livestock populations and farmland area. As a result of human impacts, the cycling of green carbon and blue carbon related to climate change mitigation, such as CO2 exchange between the atmosphere and water and carbon storage in SCEs, is affected (Table 11.1).
Kuwae et al. (2016) hypothesized that some characteristics of SCEs subject to human impacts actually strengthen the carbon cycling structure that supports the net uptake of atmospheric CO2 (net CO2 sink; Fig. 11.5). This idea is likely to be controversial, because urban coastal waters are seen as places where eutrophication progresses and a large amount of CO2 is emitted by decomposition of organic matter. In this section, we explain why a human-impacted SCE functions as a sink of atmospheric CO2 from a mechanistic perspective and provide empirical evidence from previous studies.
Conceptual diagram illustrating how human-impacted SCEs become net sinks for atmospheric CO2. (Modified from Kuwae et al. 2016)
11.3.1 Wastewater Treatment
Urban and agricultural nutrient loading and wastewater treatment have a major influence on a SCE’s biogeochemical cycling (Grimm et al. 2008; Kaushal and Belt 2012). The following two points are particularly relevant to air–seawater CO2 gas exchanges and wastewater treatment. First, in the most common wastewater treatment method (i.e., the activated sludge method), carbon is removed more efficiently than nitrogen and phosphorus from wastewater (e.g., Sedlak 1991). Hence, the treated water has relatively less carbon than nitrogen and phosphorous. When such treated water flows into a SCE, primary production is promoted due to the abundant nutrients, while decomposition and mineralization are suppressed by less abundant organic carbon. This means that wastewater treatment suppresses the rise in CO2 concentration in the water column of a SCE.
The second important point is that organic matter in the treated water is refractory (Kubo et al. 2015), because labile organic matter has already been decomposed and removed during wastewater treatment. Therefore, further decomposition and mineralization of the organic matter contained in the treated water is slow, resulting in suppression of the rise in CO2 concentration.
Through these two mechanisms, CO2 concentration in seawater is lowered and uptake of CO2 from the atmosphere is promoted. That is, both nutrient loads derived from urban/agricultural land uses and wastewater treatment that reduces the labile organic carbon load are important factors in a human-impacted SCE becoming a net sink for atmospheric CO2.
However, the inflow of treated water may accelerate decomposition of refractory organic matter in a SCE and may somewhat slow the decrease of CO2 concentration in seawater. This is due to the “priming effect” of decomposing refractory organic matter, which is enhanced at high nutrient concentrations (Taylor and Townsend 2010; Jiao et al. 2014). This phenomenon occurs due to the presence of bacteria that decompose and mineralize refractory organic matter using nutrients in the natural environment. Therefore, how the interaction between refractory organic matter and nutrients affects carbon storage is complex.
11.3.2 Freshwater Inflow, Stratification, and Hypoxic Water Mass
The large inflow of fresh water, stratification (the state where water bodies with different properties, such as temperature or salinity, are layered without mixing), and oxygen-depleted (hypoxic) conditions of human-impacted SCEs are also deeply involved in CO2 gas exchange. Urban centers often divert rivers to meet their demand for fresh water. An increase in the inflow of fresh water and heated effluents from urban areas strengthen the stratification structure and promote seawater exchange in SCEs. Changes in the physical oceanographic structure caused by such human impacts have an indirect but major influence on the SCE’s biogeochemical cycling. When a SCE becomes stratified, upwelling of the high concentration of dissolved inorganic carbon (DIC) in the bottom layer and the subsequent rise in the surface CO2 concentration, which is exchanged with the atmosphere, are suppressed (Fig. 11.5). In turn, POC as a source for increasing the surface CO2 concentration gradually precipitates due to its own weight. Thus, even if there is stratification, the SCE’s sedimentation is not disturbed (Kone et al. 2009).
Because SCEs are shallow, POC sinks and reaches the sea bottom in a short time, leading to less mineralization during sinking in the water column. This also suppresses a rise in the CO2 concentration in surface water. Furthermore, sediments are often resuspended due to the effects of wind-driven waves in SCEs, but the resuspension is suppressed when stratification develops. This suppression decreases the turbidity of the surface water and increases the light intensity available for photosynthesis, and the increased photosynthesis by phytoplankton lowers the CO2 concentration in the surface water (Chen et al. 2008).
Stratification occurs seasonally: it develops in the summer when the surface water is heated with strong sunlight. This seasonality also plays an important role in CO2 gas exchange. During the summer, upwelling of the bottom layer water containing a high DIC concentration is blocked due to stratification. Seawater is well mixed vertically in other seasons when stratification does not develop. As a result of this mixing, the surface CO2 concentration rises. However, because the water temperature is lower and solubility of CO2 is higher in seasons other than summer, less CO2 is emitted into the atmosphere.
Although there is debate on the topic, the decomposition and mineralization rates of organic matter are generally considered to be faster when the oxygen concentration is higher (Canfield 1994; Hartnett et al. 1998; Miyajima and Hamaguchi 2018). In addition, the rate of decomposition of organic matter increases where conditions fluctuate between aerobic and anaerobic, thus promoting symbiosis between aerobic and anaerobic heterotrophic bacteria (Zonneveld et al. 2010). This suggests that the presence of diverse chemical and biological environments may promote the decomposition of more diverse organic matter. For example, if labile organic matter is first decomposed and mineralized under aerobic conditions in the bottom water and sedimentary surface layer and then undecomposed organic matter is transported to the deeper anaerobic environment and further mineralized, the mineralization rate per unit area may increase as a whole.
Nevertheless, when hypoxic conditions occur in the bottom water during stratification, the aerobic sediment surface layer becomes anaerobic throughout the sediment layers, and the decomposition and mineralization rates of organic matter decrease. Consequently, organic matter accumulates at the seabed at a faster rate. Also, because decomposition of organic matter by benthic animals is suppressed under hypoxic conditions, the presence of hypoxia facilitates the accumulation of organic matter in sediments (Koho et al. 2013).
Hypoxic water masses are usually seen as purely detrimental, as hypoxia causes mortality of benthic macrofauna such as fish and shellfish. From the viewpoint of carbon storage or climate change mitigation, however, hypoxic water masses have some positive effects, as we have explained. However, hypoxic water masses may promote the production of other greenhouse gases, such as N2O and CH4, and further research on the topic is warranted.
Increased turbidity may function to either increase or decrease the CO2 concentration of surface water, depending on the cause. If the source of turbidity is phytoplankton, primary production is promoted and the surface CO2 concentration is reduced. If the source of turbidity is inorganic mineral particles (sand and mud), photosynthesis is suppressed due to decreased light intensity in water and the concentration of CO2 increases (Chen et al. 2012).
Furthermore, the residence time (exchange) of seawater is also an important factor determining CO2 and organic matter concentrations (Gazeau et al. 2005). These depend on the concentrations in and amount of incoming river water and offshore seawater. Thus, changes in the residence time of seawater may function both in increasing and decreasing CO2 and organic matter concentrations.
11.3.3 Evidence from Field Studies
The air–seawater CO2 exchange in the world’s SCEs was summarized by Borges and Abril (2011), who noted only one case serving as a net sink for atmospheric CO2, and by Laruelle et al. (2013) and Regnier et al. (2013), who concluding that SCEs serve as a net emitter worldwide. In light of the growing literature after those summaries were published, however, we used the Google Scholar and Scopus databases to identify new reported cases of SCEs serving as net sinks for CO2 (Table 11.2) to clarify the characteristics of these exceptional SCE cases.
First, a SCE serving as a net sink of atmospheric CO2 is often located next to an urbanized area or agricultural lands. These findings support our hypothesis that human-impacted SCEs can act as a sink for atmospheric CO2. Second, such SCEs are often affected by wastewater treatment, stratification, and hypoxia. These three characteristics are consistent with the Japanese cases of Tokyo Bay (Fig. 11.6) (Kubo et al. 2017) and Osaka Bay (Fig. 11.7) (Fujii et al. 2013). The effluent flowing into human-impacted SCEs has high nutrient and phytoplankton (chlorophyll a) concentrations and high primary production due to loads derived from urban and agricultural activities. In addition, previous studies revealed that net uptake of atmospheric CO2 occurs when net ecosystem production increases (Maher and Eyre 2012; Tokoro et al. 2014, 2018).
The CO2 concentration in seawater in Tokyo Bay. The dotted line shows the concentration of CO2 in the atmosphere. When the concentration is below the dotted line, CO2 in the atmosphere is taken up by the seawater. Except for the site near the incoming river, atmospheric CO2 is taken up in the seawater throughout the year in Tokyo Bay. The bay becomes a sink even when considering the annual average of CO2 gas exchange over its entire area (ca. 140 g CO2/m2/year). (Modified from Kubo et al. 2017)
Atmospheric CO2 uptake rate in Osaka Bay. Atmospheric CO2 is taken up throughout the year (ca. 133 g CO2/m2/year). The numbered dots indicate the sampling locations. (Modified from Fujii et al. 2013)
As cases of vegetated ecosystems acting as net sinks of atmospheric CO2, seagrass meadows and one kelp bed were extracted. The uptake rate in the seagrass meadows was 24.6 ± 44.1 mmol C/m2/day and that in the kelp bed was 59.4 mmol C/m2/day (Ikawa and Oechel 2015), all of which were faster than the uptake rate of SCEs without seagrass meadows (9.6 ± 6.7 mmol C/m2/day). There were also cases of coral reefs in which the CO2 concentration in water was undersaturated and the system acted as a sink (Kayanne et al. 1995, 2005; Delille et al. 2009), although the uptake rate was not described.
The global average of the net CO2 emission rate from SCEs is about 40–50 mmol C/m2/day (Laruelle et al. 2013). However, most of the data for these statistics were acquired intermittently at fragmented spatial scales; there are very few cases for which fluctuations in CO2 concentration were measured continuously at various time scales, such as 24 h or throughout the year. Therefore, these statistics include large uncertainty and bias. In particular, in low-salinity waters, total alkalinity is generally low and the buffer effect of carbonate chemistry is weak, causing high temporal variability in CO2 in water.
11.4 Future Studies
11.4.1 Enhancement of CO2 Gas Exchange Data
Compared to terrestrial and open oceans, the data for CO2 gas exchange in SCEs are limited (Laruelle et al. 2013), and there has been no description of SCE gas exchange even in the latest assessment report of the Intergovernmental Panel on Climate Change (IPCC 2013). In order to evaluate whether each SCE is a net sink or source, key data for carbon cycling such as CO2 gas exchange, carbon chemistry in water, and the dynamics of organic carbon are indispensable (Maher and Eyre 2012; Obrador and Pretus 2012; Tokoro et al. 2014; Watanabe and Kuwae 2015). Furthermore, because the range and uncertainty of the gas exchange rate differ depending on the measurement period, long-term data are important for predicting future gas exchange rates and the extent of human impacts (Crosswell et al. 2017). Indeed, a numerical simulation predicted that the uptake rate of atmospheric CO2 in areas from estuaries to shelves will be accelerated in the future due to an increase in atmospheric CO2 concentration and increased nutrient loads (Andersson and Mackenzie 2004).
11.4.2 Revaluation of Stored Carbon
Many studies on the importance of blue carbon ecosystems and their conservation are based on the premise that if the ecosystem degrades or disappears, all of the carbon stored will be mineralized and released into the atmosphere as CO2 (e.g., McLeod et al. 2011). This assumption, however, is a worst case scenario and clearly overestimates emissions (Pendleton et al. 2012; Macreadie et al. 2014; Lovelock et al. 2017). Thus, further research is needed to examine the relationship between the degree of degradation or extinction of the ecosystem and indices such as the ecosystem area, vegetation biomass, net ecosystem production rate, and amount of carbon storage (Kuwae and Hori 2018).
As green carbon is stored in SCEs together with blue carbon, there is room for discussion as to whether the land-derived green carbon should also be included in the blue carbon storage function. It may be reasonable to include green carbon if the decision is based on the site where carbon is stored. Similarly, it may be reasonable to exclude green carbon if it is based on the site where CO2 is first captured from the atmosphere. Indeed, some studies have estimated the contribution of blue carbon and green carbon separately (Middelburg et al. 1997; Kennedy et al. 2010; Dubois et al. 2012; Miyajima et al. 2015; Watanabe and Kuwae 2015; Kubo and Kanda 2017). Likewise, there needs to be discussion as to whether particulate blue carbon (POC) and macrophyte drifts that flow out of the SCE and are stored at the seabed of the shelf or the open ocean is also included as SCE blue carbon storage (Krause-Jensen and Duarte 2016; Duarte and Krause-Jensen 2017; Abo et al. 2018).
11.4.3 Mitigation of Climate Change Through Wastewater Treatment
In this chapter, we noted that CO2 emission from human-impacted SCEs is suppressed because carbon flowing into the SCE has been largely removed by wastewater treatment. This means that CO2 that would be emitted from the sea surface is instead emitted from the wastewater treatment plant. In other words, if we view the land and SCE as an integrated system, the amount of CO2 taken up by the SCE may be canceled out by the emission from decomposition of organic matter in the open aeration tank of the wastewater treatment plant. However, by appropriate management of wastewater treatment, we are able to suppress CO2 emission from the treatment plant or capture carbon (Fig. 11.8). For example, the generated sludge can be used as fuel. In addition, by collecting CO2 generated by wastewater treatment and introducing it into a culture tank of algae, CO2 can be absorbed by algae. The oils extracted from the algal bodies can also be used as an alternative fuel and industrial material. Moreover, by using an anaerobic treatment method (e.g., methane fermentation), the generated gas can also be converted into fuel (Parkin and Owen 1986). Furthermore, it is also possible to adjust the quality of the treated water, such as the carbon and nutrient concentrations, by regulating the extent of the treatment as well as selecting the treatment method, including removal of phosphorus by the coagulating sedimentation method and removal of nitrogen by the anaerobic-anoxic-oxic (A2O) method.
The complexity of the relationship between wastewater treatment and CO2 gas exchange in SCEs reflects the complex relationship between the social system and adjacent ecosystem. Therefore, biogeochemical models and numerical simulations are necessary to enact appropriate ecosystem-based mitigation measures.
11.5 Conclusions
In this chapter we discussed how human-impacted SCEs can be managed to help mitigate climate change. Through a detailed review of past findings and in situ case studies, we provided a mechanistic explanation of how SCEs can serve as net sinks for atmospheric CO2. Furthermore, we showed that the environmental conditions necessary for a net sink match with those of SCEs affected by human impacts. That is, by coordinating the interrelationships between social systems and ecosystems, we can create new means of utilizing human-impacted SCEs to mitigate climate change (as a carbon reservoir and as a sink of atmospheric CO2). In particular, vegetated SCEs or blue carbon ecosystems (e.g., mangroves, salt marshes, and seagrass meadows) are important because of their strong capability for carbon accumulation and long-term storage.
In addition, technology to mitigate climate change through conservation and restoration of SCEs, that is, technology utilizing blue carbon ecosystems, is both feasible and more sustainable than other mitigation measures (e.g., marine iron fertilization and carbon capture and storage) in terms of technical difficulty, cost, ecological risk, social acceptance, operation, and ethics (Nellemann et al. 2009; Cusack et al. 2014). Furthermore, the conservation and restoration of SCEs can result in not only the mitigation of climate change but also other ecosystem services (co-benefits), including an improved food supply, water purification, tourism, recreation, and disaster prevention (Kuwae and Hori 2018). However, because ecosystem-based mitigation technologies use natural systems, there are large diurnal, seasonal, and annual fluctuations and high uncertainty. Therefore, as we develop systems for the utilization of SCEs to help mitigate climate change, it is necessary to gather field data enabling the evaluation of uncertainty as well as to improve coupled geophysical–biogeochemical modeling for future projections.
References
Abo K, Sugimatsu K, Hori M et al (2018) Quantifying the fate of captured carbon: from seagrass meadow to deep sea. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 251–271
Akhand A, Chanda A, Manna S et al (2016) A comparison of CO2 dynamics and air-water fluxes in a river-dominated estuary and a mangrove-dominated marine estuary. Geophys Res Lett 43:11726–11735
Akhand A, Chanda A, Das S, Hazra S, Kuwae T (2018) CO2 fluxes in mangrove ecosystems. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 185–221
Andersson AJ, Mackenzie FT (2004) Shallow-water oceans: a source or sink of atmospheric CO2? Front Ecol Environ 2:348–353
Arrieta JM, Mayol E, Hansman RL, Herndl GJ, Dittmar T, Duarte CM (2015) Dilution limits dissolved organic carbon utilization in the deep ocean. Science 348:331–333
Bakker DC, de Baar HJ, de Wilde HP (1996) Dissolved carbon dioxide in Dutch coastal waters. Mar Chem 55:247–263
Bauer JE, Cai WJ, Raymond PA, Bianchi TS, Hopkinson CS, Regnier PA (2013) The changing carbon cycle of the coastal ocean. Nature 504:61–70
Borges A, Abril G (2011) Carbon dioxide and methane dynamics in estuaries. In: Wolanski E, McLusky D (eds) Treatise on estuarine and coastal science, volume 5: biogeochemistry. Academic, Waltham, pp 119–161
Borges AV, Delille B, Frankignoulle M (2005) Budgeting sinks and sources of CO2 in the coastal ocean: diversity of ecosystem counts. Geophys Res Lett 32:L14601
Cai WJ (2011) Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annu Rev Mar Sci 3:123–145
Canfield DE (1994) Factors influencing organic carbon preservation in marine sediments. Chem Geol 114:315–329
Chambers JQ, Higuchi N, Tribuzy ES, Trumbore SR (2001) Carbon sinks for a century. Nature 410:429
Chen CTA, Zhai W, Dai M (2008) Riverine input and air–sea CO2 exchanges near the Changjiang (Yangtze River) estuary: status quo and implication on possible future changes in metabolic status. Cont Shelf Res 28:1476–1482
Chen CTA, Huang TH, Fu YH, Bai Y, He X (2012) Strong sources of CO2 in upper estuaries become sinks of CO2 in large river plumes. Curr Opin Environ Sustain 4:179–185
Chen CTA, Huang TH, Chen YC, Bai Y, He X, Kang Y (2013) Air–sea exchange of CO2 in world’s coastal seas. Biogeosciences 10:6509–6544
Cole JJ, Prairie YT, Caraco NF et al (2007) Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10:172–185
Cotovicz LC Jr, Knoppers BA, Brandini N, Santos SC, Abril G (2015) A strong CO2 sink enhanced by eutrophication in a tropical coastal embayment (Guanabara Bay, Rio de Janeiro, Brazil). Biogeosciences 12:6125–6146
Crosswell JR, Wetz MS, Hales B, Paerl HW (2012) Air–water CO2 fluxes in the microtidal Neuse River estuary, North Carolina. J Geophys Res 117:C8
Crosswell JR, Anderson IC, Stanhope JW et al (2017) Carbon budget of a shallow, lagoonal estuary: transformations and source-sink dynamics along the river-estuary-ocean continuum. Limnol Oceanogr 62:S29–S45
Cusack DF, Axsen J, Shwom R, Hartzell-Nichols L, White S, Mackey KRM (2014) An interdisciplinary assessment of climate engineering strategies. Front Ecol Environ 12:280–287
Delille B, Borges AV, Delille D (2009) Influence of giant kelp beds (Macrocystis pyrifera) on diel cycles of pCO2 and DIC in the Sub-Antarctic coastal area. Estuar Coast Shelf Sci 81:114–122
Duarte CM, Krause-Jensen D (2017) Export from seagrass meadows contributes to marine carbon sequestration. Front Mar Sci 4:13
Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N (2013) The role of coastal plant communities for climate change mitigation and adaptation. Nat Clim Chang 3:961–968
Dubois S, Savoye N, Grémare A et al (2012) Origin and composition of sediment organic matter in a coastal semi-enclosed ecosystem: an elemental and isotopic study at the ecosystem space scale. J Mar Syst 94:64–73
Endo T, Otani S (2018) Carbon storage in tidal flats. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 129–151
Evans W, Hales B, Strutton PG (2013) pCO2 distributions and air–water CO2 fluxes in the Columbia River estuary. Estuar Coast Shelf Sci 117:260–272
Fourqurean JW, Duarte CM, Kennedy H et al (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 5:505–509
Fujii T, Fujiwara T, Nakayama K (2013) Fluxes of carbon dioxide in the eastern regions of Osaka Bay. JSCE Ann J Coast Eng 69:1111–1115 (in Japanese, English summary)
Gattuso JP, Frankignoulle M, Wollast R (1998) Carbon and carbonate metabolism in coastal aquatic ecosystems. Annu Rev Ecol Syst 29:405–434
Gazeau F, Borges AV, Barron C et al (2005) Net ecosystem metabolism in a micro-tidal estuary (Randers Fjord, Denmark): evaluation of methods. Mar Ecol Prog Ser 301:23–41
Grimm NB, Faeth SH, Golubiewski NE et al (2008) Global change and the ecology of cities. Science 319:756–760
Hartnett HE, Keil RG, Hedges JI, Devol AH (1998) Influence of oxygen exposure time on organic carbon preservation in continental margin sediments. Nature 391:572–574
Hendriks IE, Sintes T, Bouma T, Duarte CM (2007) Experimental assessment and modeling evaluation of the effects of seagrass (P. oceanica) on flow and particle trapping. Mar Ecol Prog Ser 356:163–173
Hunt CW, Salisbury JE, Vandemark D, McGillis W (2011) Contrasting carbon dioxide inputs and exchange in three adjacent New England estuaries. Estuar Coasts 34:68–77
Ikawa H, Oechel WC (2015) Temporal variations in air-sea CO2 exchange near large kelp beds near San Diego, California. J Geophys Res Ocean 120:50–63
Inoue T (2018) Carbon sequestration in mangroves. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 73–99
IPCC (2013) Fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva
Jiao N, Robinson C, Azam F et al (2014) Mechanisms of microbial carbon sequestration in the ocean: future research directions. Biogeosciences 11:5285–5306
Kaushal SS, Belt KT (2012) The urban watershed continuum: evolving spatial and temporal dimensions. Urban Ecosyst 15:409–435
Kayanne H, Suzuki A, Saito H (1995) Diurnal changes in the partial pressure of carbon dioxide in coral reef water. Science 269:214–216
Kayanne H, Hata H, Kudo S et al (2005) Seasonal and bleaching-induced changes in coral reef metabolism and CO2 flux. Glob Biogeochem Cycles 19:GB3015
Kennedy H, Beggins J, Duarte CM et al (2010) Seagrass sediments as a global carbon sink: isotopic constraints. Glob Biogeochem Cycles 24:GB4026
Koho KA, Nierop KGJ, Moodley L et al (2013) Microbial bioavailability regulates organic matter preservation in marine sediments. Biogeosciences 10:1131–1141
Kone YJM, Abril G, Kouadio KN, Delille B, Borges AV (2009) Seasonal variability of carbon dioxide in the rivers and lagoons of Ivory Coast (West Africa). Estuar Coasts 32:246–260
Kouame KV, Yapo OB, Mambo V, Seka A, Tidou AS, Houenou P (2009) Physicochemical characterization of the waters of the coastal rivers and the lagoonal system of Cote d’Ivoire. J Appl Sci 9:1517–1523
Kragh T, Søndergaard M (2009) Production and decomposition of new DOC by marine plankton communities: carbohydrates, refractory components and nutrient limitation. Biogeochemistry 96:177–187
Krause-Jensen D, Duarte CM (2016) Substantial role of macroalgae in marine carbon sequestration. Nat Geosci 9:737–742
Kubo A, Kanda J (2017) Seasonal variations and sources of sedimentary organic carbon in Tokyo Bay. Mar Pollut Bull 114:637–643
Kubo A, Kawai MY, Kanda J (2015) Seasonal variations in concentration and composition of dissolved organic carbon in Tokyo Bay. Biogeosciences 12:269–279
Kubo A, Maeda Y, Kanda J (2017) A significant net sink for CO2 in Tokyo Bay. Sci Rep 7:44355
Kuwae T, Hori M (2018) The future of blue carbon: addressing global environmental issues. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 347–373
Kuwae T, Kanda J, Kubo A et al (2016) Blue carbon in human-dominated estuarine and shallow coastal systems. Ambio 45:290–301
Laruelle GG, Durr HH, Lauerwald R et al (2013) Global multi-scale segmentation of continental and coastal waters from the watersheds to the continental margins. Hydrol Earth Syst Sci 17:2029–2051
Lønborg C, Álvarez-Salgado XA, Davidson K, Miller AE (2009) Production of bioavailable and refractory dissolved organic matter by coastal heterotrophic microbial populations. Estuar Coast Shelf Sci 82:682–688
Longhurst AR, Harrison WG (1989) The biological pump: profiles of plankton production and consumption in the upper ocean. Prog Oceanogr 22:47–123
Lovelock CE, Atwood T, Baldock J et al (2017) Assessing the risk of carbon dioxide emissions from blue carbon ecosystems. Front Ecol Environ 15:257–265
Macreadie PI, Baird ME, Trevathan-Tackett SM, Larkum AWD, Ralph PJ (2014) Quantifying and modelling the carbon sequestration capacity of seagrass meadows: a critical assessment. Mar Pollut Bull 83:430–439
Maher DT, Eyre BD (2012) Carbon budgets for three autotrophic Australian estuaries: implications for global estimates of the coastal air-water CO2 flux. Glob Biogeochem Cycles 26:GB1032
McIntyre NE, Knowles-Yánez K, Hope D (2000) Urban ecology as an interdisciplinary field: differences in the use of “urban” between the social and natural sciences. Urban Ecosyst 4:5–24
McLeod E, Chmura GL, Bouillon S et al (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560
Middelburg JJ, Nieuwenhuize J, Lubberts RK, van de Plassche O (1997) Organic carbon isotope systematics of coastal marshes. Estuar Coast Shelf Sci 45:681–687
Miyajima T, Hamaguchi M (2018) Carbon sequestration in sediment as an ecosystem function of seagrass meadows. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 33–71
Miyajima T, Hori M, Hamaguchi M et al (2015) Geographic variability in organic carbon stock and accumulation rate in sediments of East and Southeast Asian seagrass meadows. Glob Biogeochem Cycles 29:397–415
Miyajima T, Hori M, Hamaguchi M, Shimabukuro H, Yoshida G (2017) Geophysical constraints for organic carbon sequestration capacity of Zostera marina seagrass meadows and surrounding habitats. Limnol Oceanogr 62:954–972
Moran MA, Sheldon WM, Zepp RG (2000) Carbon loss and optical property changes during long-term photochemical and biological degradation of estuarine dissolved organic matter. Limnol Oceanogr 45:1254–1264
Nellemann C, Corcoran E, Duarte CM et al (2009) Blue carbon: a rapid response assessment. United Nations Environmental Programme, GRID-Arendal, Birkeland Trykkeri AS, Birkeland
Obrador B, Pretus JL (2012) Budgets of organic and inorganic carbon in a Mediterranean coastal lagoon dominated by submerged vegetation. Hydrobiology 699:35–54
Ogawa H, Amagai Y, Koike I, Kaiser K, Benner R (2001) Production of refractory dissolved organic matter by bacteria. Science 292:917–920
Otani S, Endo T (2018) CO2 flux in tidal flats and salt marshes. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 223–250
Parkin GF, Owen WF (1986) Fundamentals of anaerobic digestion of wastewater sludges. J Environ Eng 112:867–920
Pendleton L, Donato DC, Murray BC et al (2012) Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS One 7:e43542
Le Quéré C, Moriarty R, Andrew RM et al (2018) Global Carbon Budget 2017. Earth Syst Sci Data 10:405–448
Raymond PA, Bauer JE, Cole JJ (2000) Atmospheric CO2 evasion, dissolved inorganic carbon production, and net heterotrophy in the York River estuary. Limnol Oceanogr 45:1707–1717
Raymond PA, Hartmann J, Lauerwald R et al (2013) Global carbon dioxide emissions from inland waters. Nature 503:355–359
Regnier PAG, Friedlingstein P, Ciais P et al (2013) Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat Geosci 6:597–607
Rysgaard S, Mortensen J, Juul-Pedersen T et al (2012) High air–sea CO2 uptake rates in nearshore and shelf areas of southern Greenland: temporal and spatial variability. Mar Chem 128:26–33
Sedlak RI (1991) Phosphorus and nitrogen removal from municipal wastewater: principles and practice. CRC Press, Boca Raton
Sholkovitz ER (1976) Flocculation of dissolved organic and inorganic matter during the mixing of river water and seawater. Geochim Cosmochim Acta 40:831–845
Smith SV (1981) Marine macrophytes as a global carbon sink. Science 211:838–840
Sugimatsu K, Yagi H, Abo K et al (2015) A coupled particle tracking– carbon cycle modeling system for sedimentary organic carbon derived from drafting seagrass in Seto Inland Sea. JSCE Annu J Coast Eng 71:1387–1392 (in Japanese, English summary)
Tanaka Y, Ogawa H, Miyajima T (2011a) Bacterial decomposition of coral mucus as evaluated by long-term and quantitative observation. Coral Reefs 30:443–449
Tanaka Y, Ogawa H, Miyajima T (2011b) Production and bacterial decomposition of dissolved organic matter in a fringing coral reef. J Oceanogr 67:427–437
Taylor PG, Townsend AR (2010) Stoichiometric control of organic carbon–nitrate relationships from soils to the sea. Nature 464:1178–1181
Tokoro T, Hosokawa S, Miyoshi E et al (2014) Net uptake of atmospheric CO2 by coastal submerged aquatic vegetation. Glob Chang Biol 20:1873–1884
Tokoro T, Watanabe K, Tada K, Kuwae T (2018) Air–water CO2 flux in shallow coastal waters: theoretical background, measurement methods, and mechanisms. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 153–184
Tsunogai S, Watanabe S, Sato T (1999) Is there a continental shelf pump for the absorption of atmospheric CO2? Tellus 51:701–712
Wada S, Aoki MN, Mikami A et al (2008) Bioavailability of macroalgal dissolved organic matter in seawater. Mar Ecol Prog Ser 370:33–44
Wanninkhof R (1992) Relationship between wind-speed and gas-exchange over the ocean. J Geophys Res 97:7373–7382
Watanabe K, Kuwae T (2015) How organic carbon derived from multiple sources contributes to carbon sequestration processes in a shallow coastal system. Glob Chang Biol 21:2612–2623
Watanabe A, Nakamura T (2018) Carbon dynamics in coral reefs. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 273–293
Watanabe A, Yamamoto T, Nadaoka K et al (2013) Spatiotemporal variations in CO2 flux in a fringing reef simulated using a novel carbonate system dynamics model. Coral Reefs 32:239–254
Zhai W, Dai M (2009) On the seasonal variation of air–sea CO2 fluxes in the outer Changjiang (Yangtze River) Estuary, East China Sea. Mar Chem 117:2–10
Zonneveld KAF, Versteegh GJM, Kasten S et al (2010) Selective preservation of organic matter in marine environments: processes and impact on the sedimentary record. Biogeosciences 7:483–511
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kuwae, T. et al. (2019). CO2 Uptake in the Shallow Coastal Ecosystems Affected by Anthropogenic Impacts. In: Kuwae, T., Hori, M. (eds) Blue Carbon in Shallow Coastal Ecosystems. Springer, Singapore. https://doi.org/10.1007/978-981-13-1295-3_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-1295-3_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1294-6
Online ISBN: 978-981-13-1295-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)