Abstract
Coral reefs show high organic and inorganic carbon production and create unique landforms on tropical coastlines. The balance between organic and inorganic carbon production is determined by benthic organisms such as corals, macroalgae, and seagrasses, and also by reef hydrodynamics, which in turn determine CO2 sinks and sources within the ecosystem. Many studies have shown that net organic carbon production in coral reef ecosystems is almost zero (balanced), with net positive calcification resulting in reefs acting as CO2 sources. However, the relationships among productivity, benthic organisms, and hydrodynamics have not been well documented; more detailed information is required from both field observations and coupled physical–biological models. Reef sediments have low organic carbon content (median, 0.35% dry weight), approximately 50% those of tropical and subtropical seagrass beds (median, 0.67%) and 5% those of mangrove forests (median, 6.3%). Sedimentation rates do not vary significantly between these three ecosystems, so organic carbon input and decomposition in the surface sediments are key factors controlling organic carbon burial rates. Coral reefs provide calm conditions that enhance sedimentation of fine sediments, which facilitates the development of seagrass beds and mangrove forests. Seagrass meadows and mangrove forests in turn trap fine sediments from terrestrial sources and prevent high-turbidity water from reaching coral reefs. Coral reefs, seagrass meadows, and mangrove forests are thus interdependent ecosystems; to effectively store and export blue carbon in tropical coastal areas, it is necessary to maintain the health of these ecosystems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Introduction
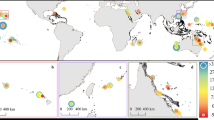
Coral reefs are not explicitly included as part of the blue-carbon ecosystem, so you might wonder why coral reefs are included as a chapter in this book. In the tropics and subtropics, coral reefs often overlap seagrass beds and mangrove forests (Fig. 10.1) and are therefore strongly linked to blue-carbon ecosystems. Often there is also hydrodynamic connectivity or interaction among these tropical coastal habitats (Guannel et al. 2016).
World distribution of coral reefs, mangrove forests, and seagrass beds. The areas with high diversity for each ecosystem overlap, especially around Indonesia, Malaysia, Papua New Guinea, and the Philippines. (Source: https://www.grida.no/resources/7766 (credit: Hugo Ahlenius))
This chapter briefly explains the general characteristics of coral reefs in terms of carbon-cycle components such as primary production and calcification. The resultant CO2 sinks and sources, and carbon storage and export in coral reefs are then addressed. Finally the relationships between coral reefs and other ecosystems, especially with seagrass beds and mangrove forests, are discussed to highlight the necessity of regarding these connected ecosystems together as a blue-carbon ecosystem.
10.2 Carbon Cycling, Storage, and Export in Coral Reefs
10.2.1 Basic Carbonate-Chemistry Changes Due to Calcification and Primary Production
We first briefly explain the basics of carbonate chemistry alterations due to calcification and photosynthesis to provide the background necessary to understand this chapter. For further reading, please refer to Gattuso et al. (1999) or Zeebe and Wolf-Gladrow (2001).
The calcification reaction, which releases CO2, is commonly expressed by the following equation:
The reverse of Eq. (10.1) is the reaction for the dissolution of CaCO3.
Photosynthesis fixes CO2 and is expressed by the following equation:
The reverse of Eq. (10.2) is the reaction for aerobic respiration and decomposition.
There is a so-called “0.6 rule” for seawater, where about 0.6 moles of CO2 is liberated (not the expected 1 mole) when 1 mole of CaCO3 is produced by Eq. (10.1) (Ware et al. 1991; Frankignoulle et al. 1994). In contrast, 1 mole of CO2 is fixed when 1 mole of organic C (CH2O) is produced by Eq. (10.2). Therefore, when the rate of photosynthesis is greater (less) than 60% of the calcification rate, CO2 is fixed (liberated) and the system acts as a sink (source) of CO2. The value 0.6 in the 0.6 rule is due to the buffering capacity of seawater, and it changes with ocean acidification (the trend of increasing CO2 in seawater). This value, termed Ψ (Frankignoulle et al. 1994), is 0.6 when pCO2 (partial pressure of CO2) in seawater is 350 μatm, salinity is 35, and water temperature is 25 °C, but it rapidly increases to 0.78 when pCO2 reaches 1000 μatm under the same conditions. Ψ also changes with temperature (see Fig. 10.2). At constant pCO2, Ψ decreases as water temperature increases. For example, seawater at higher latitudes generally has higher Ψ values and lower buffering capacity.
10.2.2 Calcification and Primary Production in Coral Reefs
One conspicuous characteristic of coral reefs is the huge production of CaCO3 by which tiny corals build the world’s largest living structures, such as the Great Barrier Reef in Australia, which is visible from space. Coral reef environments can be roughly divided into three parts: reef flat with reef crest, inner reef, and outer reef (upper panel in Fig. 10.3). The reef flat with reef crest is the shallowest part of a coral reef and is covered with either corals or macroalgae. Incoming wave energy is effectively dissipated at the reef crest or reef flat. The inner reef is therefore calmer compared to the outer reef, and often has a lagoon (or “moat” when it is shallow [typically shallower than 5 m]), which can harbor corals, seagrasses, and mangroves. The outer reef is a high-energy environment and often covered with corals or coralline algae that are resistant to the high wave energy.
There are three major types of coral reefs: fringing reefs, barrier reefs, and atolls (lower panel in Fig. 10.3). A fringing reef develops right beside an island, whereas a barrier reef has a relatively deep lagoon between an island and the reef flat. Atolls are rings of reefs without a central island; sometimes the reef itself becomes exposed to air and forms a low-lying island.
The coral/algal reef flats display a wide range of calcification rates (5–126 mol CaCO3 m−2 year−1 or 0.5–12.6 kg CaCO3 m−2 year−1; Gattuso et al. 1998). The value gets progressively higher as the water depths decrease over the reef flat zone (shallowest part of coral reefs) independent of domination by corals or coralline algae (Sorokin 1993). On average, the shallow seaward reef flat zone produces 40 moles (or 4 kg) of CaCO3 m−2 year−1. Considering the “0.6 rule” explained in Sect. 10.2.1, net primary production on the “average reef” would have to exceed 24 moles (or 288 g) C m−2 year−1 to act as a CO2 sink. Gross primary production (GPP) on reef flats is quite high, varying from 79 to 584 moles (or 948–7008 g) C m−2 year−1 (Gattuso et al. 1998), but community respiration is also high and net primary production is thought to be close to zero. In this case, calcification overwhelms the net primary production on the reef flat, and the reef flat is believed to act as a source of CO2.
10.2.3 CO2 Sinks and Sources in Coral Reefs
The debate on whether coral reefs act as a sink or source of atmospheric CO2 began in the 1990s. Gattuso’s group (Gattuso et al. 1993, 1996b; Frankignoulle et al. 1996) reported that most coral reef flats are sources of CO2 to the atmosphere. Their study sites were the Tiahura barrier reef (Moorea, French Polynesia) and Yonge Reef (northern Great Barrier Reef). Both sites are classified as barrier reef flats. Gattuso et al. (1996b) reported that the Tiahura reef supported abundant corals on the seaward side and abundant macroalgae on the back reef area, whereas the Yonge Reef flat was mainly dominated by a single community characterized by the coral species Acropora palifera. In contrast, Kayanne’s group (Kayanne et al. 1995) reported that coral reef flats act as sinks for atmospheric CO2. Their study site was the Shiraho reef (Ishigaki Island, Japan), which is a fringing reef flat. The dominant coral species around the monitoring site were Montipora digitate, Porites cylindrica, and Heliopora coerulea (Kayanne et al. 2002), and algal turf and brown algae were on the offshore side of the reef crest (Kayanne et al. 1995). Since Kayanne et al. (1995) indicated that coral reef flats are a net sink for atmospheric CO2, there have been many comments and papers published regarding the CO2 sink–source issue (Buddemeier 1996; Gattuso et al. 1996a, 1997; Kayanne 1996; Kraines et al. 1997; Ohde and van Woesik 1999; Bates et al. 2001; Bates 2002; Kayanne et al. 2005), and the debate has been reviewed extensively (Gattuso et al. 1999; Suzuki and Kawahata 2004). Here we summarize the points of the debate and list some of the more recent literature published after those reviews.
Table 10.1 summarizes the CO2 sinks and sources in coral reefs, separately listing reef flats and lagoons. Positive CO2 flux values indicate sources and negative values indicate sinks. Although the values vary widely, the majority of reef flats studied acted as sources of CO2, with a median CO2 flux value of 2.2 mmol m−2 day−1. The majority of relatively deep lagoons also act as sources of CO2 with a median value of 0.7 mmol m−2 day−1. These values are relatively small and comparable to the CO2 flux for open ocean in most of the subtropical and tropical areas where coral reefs exist, which typically exhibit fluxes of −1 to 1 mol m−2 year−1 (or −2.7 to 2.7 mmol m−2 day−1, Takahashi et al. 2009). Some reefs absorb CO2 from the atmosphere, but many researchers insist that the reef flats absorbing CO2 are mainly fringing reefs (see Fig. 10.3 and Sect. 10.2.2). The hypothesis is that fringing reefs receive substantial amounts of nutrients from adjacent lands, conditions more favorable for the growth of seagrasses or macroalgae than corals. With the reef area covered by these autotrophs, net primary production exceeds calcification and the system overall absorbs CO2. Gattuso et al. (1997) tested this hypothesis by measuring community metabolism and air–sea CO2 fluxes on a fringing reef at Moorea. The results showed that this reef flat was a sink for CO2 up to 10 mmol m−2 day−1, whereas the neighboring barrier reef flat was a CO2 source (Gattuso et al. 1993, 1996b; Frankignoulle et al. 1996). These contrasting results from the fringing reef and the barrier reef of Moorea led to the concept that “algal” reefs absorb CO2 whereas “coral” reefs emit CO2 to the atmosphere.
More recently, different views have been offered regarding the air–sea CO2 flux in coral reefs resulting from long-term or continuous monitoring at the same sites. For example, Kayanne et al. (2005) showed that the Shiraho fringing reef became a source of atmospheric CO2 following coral bleachingFootnote 1 in 1998, although the reef was a CO2 sink during other non-bleached periods. Massaro et al. (2012) presented continuous CO2 data covering 2.5 years in southern Kaneohe Bay, Hawaii, a semi-enclosed tropical coral reef ecosystem. They showed that local climatic forcing strongly affected the biogeochemistry, water-column properties, and air–sea CO2 gas exchange. Large drawdowns of CO2 following storms occasionally caused the bay waters to switch from a CO2 source to a sink. These results indicate that even the same reef can dynamically shift from sink to source depending on reef conditions (e.g. coral and macroalgal coverage) as well as on external forcing (e.g. storms and subsequent supply of nutrients). These kinds of dynamic features can be more important than the static sink–source views of coral reefs under rapidly changing reef conditions due to global climate change and local environmental changes.
More studies are needed for the mechanistic understanding of CO2 dynamics in coral reefs. It is recommended that future works incorporate following points.
-
1.
Measurements should include reef metabolisms (ecosystem primary production, respiration, calcification, and carbonate dissolution) and organic-matter flux along with air–sea CO2 fluxes.
-
2.
The benthic condition of reef areas of interest should be quantitatively monitored. The biomass balance between corals, macroalgae, and seagrasses can be especially important.
-
3.
Monitoring should include terrestrial loads such as submarine ground water or river water inputs to the reefs, their chemistry (e.g. nutrients and carbonate chemistry), and their advection in the reef, together with hydrodynamic features of the reefs (e.g. residence time of seawater).
-
4.
The reef as a CO2 sink or source should be addressed by comparison with the CO2 in the open ocean impinging on reefs, and not necessarily directly with atmospheric CO2. The reason is simple: the open ocean is not necessarily in equilibrium with atmospheric CO2, and the capacity of a coral reef as a CO2 sink or source should be discussed relative to CO2 levels in the source water (mainly open ocean seawater in many cases). This would require adequate monitoring of conditions offshore of the reefs as well.
Achieving these points will require a combination of hydrodynamic–biogeochemical modeling as well as the appropriate fieldwork to properly constrain the models. These topics are discussed in more detail in Sect. 10.4.
10.2.4 Carbon Storage in Coral Reefs
Coral reef sediments are known to contain small amounts of organic carbon (OC). Table 10.2 compiles data for the OC content of reef sediment. For example, Sorokin (1993) reviewed OC contents in reef bottom sediments and reported values ranging from 0.09% to 0.6% (% dry weight). The OC in reef sediments rarely exceeds 1%, and the mean and median values from previous studies are 0.46% and 0.35%, respectively. The low OC content of reef sands is thought to reflect background OC contained in carbonates mainly originating from corals, foraminifera, and calcareous algae, which typically have values less than 0.2% to 0.4% (Miyajima et al. 1998).
Table 10.2 also summarizes the OC content of seagrass bed and mangrove forest sediments. Please also refer to Chaps. 2 and 3 for discussions of OC in seagrass beds (Miyajima and Hamaguchi 2018) and mangrove sediments (Inoue 2018), respectively. OC in seagrass sediments shows large variability, ranging from 0.1% to 11% (Kennedy et al. 2010). Most tropical and subtropical seagrass beds have a relatively low OC content of 0.6% or 0.7%; the median of the values surveyed here is 0.67%, which is about twice that of coral reef sediments (0.35%). Coral reef sands vegetated by seagrasses have OC contents 2–5 times those of unvegetated reef sands, possibly from the accumulation of detrital OC in seagrass beds (Miyajima et al. 2015). OC in mangrove sediments also shows large variability, ranging from 0.6% to 36% (Bouillon et al. 2003; Breithaupt et al. 2012). The median of values surveyed here is 6.3%, which is close to the median mangrove sediment OC content of 7.0% reported by Breithaupt et al. (2012) and about 18 times the OC content of reef sediments.
The differences between OC concentrations in coral reef, seagrass meadow, and mangrove sediments can be explained in terms of the supply and preservation of organic carbon in each ecosystem. The sedimentation rates compiled from previous studies are not very different across these ecosystems (Table 10.2): 0.26 g cm−2 year−1 for coral reefs, 0.12 g cm−2 year−1 for seagrass beds (although only limited data are reported), and 0.28 g cm−2 year−1 for mangrove forests. The OC burial rates, however, are much higher in mangroves and in seagrass beds than in coral reefs; the median values are 12.8 gC m−2 year−1 for coral reefs, 83.0 gC m−2 year−1 for seagrass beds, and 139 gC m−2 year−1 for mangrove forests. This difference results not only from the supply of organic matter, but also from the differences in decomposition and preservation of organic matter in the sediments. In coral reef sediments, the top several centimeters of sediments are usually supplied with oxygen (Werner et al. 2006; Yamamoto et al. 2015) mainly through pore-water advection due to highly permeable sediments with relatively large grain size (Werner et al. 2006). This oxygen supply can facilitate carbon mineralization in the surface sediments (Miyajima and Hamaguchi 2018). This mechanism can help maintain low OC content in reef sediments when the supply of organic matter is not too high.
10.2.5 Carbon Export from Coral Reefs
Coral reefs can export organic matter from primary production, either to their internal sediments or to the open ocean. As explained in Sect. 10.2.2, coral reefs have high GPP as well as respiration, but many reef flats are slightly autotrophic and show positive net primary production (NPP) (for example Gattuso et al. 1993, 1996b; Kayanne et al. 1995, 2005; Ohde and van Woesik 1999; Hata et al. 2002). Positive NPP means that OC can be either stored within the ecosystem or exported to adjacent systems such as the open ocean. Note that the pCO2 decrease due to NPP can often be offset by net calcification in coral reefs, which increases pCO2. Corals release the excess primary production as mucus, either as dissolved organic carbon (DOC) or particulate organic carbon (POC) (Wild et al. 2004; Tanaka et al. 2008). According to Wild et al. (2004), much of the mucus released into seawater efficiently traps organic matter from the water column, which is rapidly carried to the lagoon sediment and filtered through the lagoon sands. Mucus transports energy to the lagoon sediments and then the sediments rapidly recycle the organic matter to nutrients, thus serving as a mechanism for retaining energy and nutrients within the reef ecosystem.
Some portion of the excess production can be transported offshore of the reef. Delesalle et al. (1998) studied the organic carbon and carbonate export for Tiahura reef, French Polynesia, and reported an offshore transport of about 47% and 21% of the excess organic and inorganic carbon production, respectively. Hata et al. (1998) studied the organic carbon flux around a barrier reef in Palau in the western Pacific. They estimated that 7% of carbon from GPP on the reef flat is deposited in the lagoon, 4% is exported to the open ocean, and 0.6% is transferred below the thermocline (150-m depth) of the inshore open ocean (Fig. 10.4). Hata et al. (2002) simultaneously studied organic carbon fluxes and community production rates on the Shiraho coral reef for a week. They found that 6–7% of GPP and a majority of NPP (almost 100%) were exported offshore as POC and DOC, and about 14–20% of the POC and 0.2% of GPP exported from the reef flat reached 1 km offshore and 40-m depth.
Organic carbon flux around a coral reef (based on Hata et al. 1998). The organic carbon flux from the reef flat to the open ocean is estimated at 74 mol C m−1 day−1, which is about 7% of the gross primary production (GPP) of the reef. The net export of organic carbon from the reef flat is 37 mol C m−1 day−1, which is about 4% of the GPP, and about 6 mol C m−1 day−1 is carried to a depth of 150 m (below the thermocline) in the inshore open ocean, which is about 0.6% of the GPP
Carbon export studies from coral reefs are obviously limited and need a more integrated approach in the future. First, the carbon fluxes should be studied together with in-reef productivity measurements to understand their interrelationships. Seasonal variations as well as tidal effects on the carbon export should also be examined; a higher carbon flux can be anticipated during spring tide compared to neap tide. Characterization of the organic matter will also be required. The C/N ratios of the organic particles captured in sediment traps have been reported (Delesalle et al. 1998; Hata et al. 1998, 2002), but it will be necessary to know the fatty acid composition or isotopic signatures of the particles to determine the origins of the organic matter (Hata et al. 2002).
10.3 Relationships Between Coral Reefs and Other Tropical and Subtropical Coastal Ecosystems
As noted in the Introduction, the distribution of coral reefs overlaps with those of mangroves and tropical/subtropical seagrasses (Fig. 10.1), and we can expect close linkages among these ecosystems. Mangroves and seagrass beds interrupt freshwater discharge, are sinks for organic and inorganic materials as well as pollutants, and can generate an environment with clear, nutrient poor water that promotes the growth of coral reefs offshore (Fig. 10.5 and references such as Moberg and Folke 1999; Hemminga and Duarte 2000; Duke and Wolanski 2001; Unsworth and Cullen 2010). Coral reefs in turn dissipate wave energy and create favorable conditions for the growth of seagrasses and mangrove ecosystems (Birkeland 1985; Ogden 1988). Here we briefly summarize the physical and biogeochemical relationships between coral reefs and associated landscapes such as seagrass meadows and mangrove forests. We also explain the shift of reef flats from coral communities to macroalgal communities (“phase shift”) and the implications of this shift to changes in biogeochemical cycles.
Conceptual illustration showing the turbid waters and sediment plume from (a) an undisturbed terrestrial catchment, (b) a disturbed terrestrial catchment for a rural area only, (c) a terrestrial catchment including rural, port, and city areas disturbed by clearing mangrove vegetation, and (d) a catchment in a port and city area rehabilitated from the conditions in (c) with mangroves and riparian vegetation. Arrows indicate prevailing wave direction and relative strength. Figures are after Duke and Wolanski (2001) with slight modifications
10.3.1 Relationship Between Coral Reefs and Seagrass Beds
Lamb et al. (2017) showed recently that when seagrass meadows are present in a reef ecosystem there is a 50% reduction in the relative abundance of pathogens potentially capable of causing diseases in humans and marine organisms. Their field surveys showed that disease levels in more than 8000 reef-building corals located adjacent to seagrass meadows were lower by a factor of two compared to corals at sites without adjacent seagrass meadows.
Biogeochemical interactions between seagrasses and corals have recently been proposed. Unsworth et al. (2012) found that 83% of seagrass meadows in the Indo-Pacific have a positive NPP and can increase seawater pH, which could buffer coral reef calcification against future ocean acidification. Future laboratory and field work should quantify the buffering capacity of seagrass relative to ocean acidification.
Coral reefs in turn serve as physical buffers against oceanic currents and waves, creating a suitable environment for seagrass beds over geologic time (Moberg and Folke 1999). However, as Saunders et al. (2014) suggested, this shelter effect can be threatened by increases of water depth in the lagoon (or moat) from sea-level rise. They indicate that the rates of carbonate accretion typical of modern reef flats (up to 3 mm year−1) will not be sufficient to maintain suitable conditions for reef seagrasses in the future. These climate change (i.e. ocean acidification and sea-level rise) impacts on connected coral reef–seagrass landscapes should be considered when planning conservation efforts.
10.3.2 Relationships Between Coral Reefs and Mangrove Forests
Mangrove forests act as natural filters to trap fine sediments and improve water clarity (Fig. 10.5; Duke and Wolanski 2001). Mangrove forests typically occur in turbid waters where the turbidity mainly comes from a terrestrial catchment. When the catchment still has natural forests, grasslands, or freshwater wetlands, mangroves can filter the turbid water, and any remaining slightly turbid water does not reach that far into the coastal areas, permitting the co-existence of offshore seagrass meadows and coral reefs (Fig. 10.5a). If a river catchment includes disturbances in rural areas from clearing vegetation for grazing and agriculture, the turbid waters and sediment plume can extend far offshore, resulting in seagrass dieback (Fig. 10.5b). Mangroves can be largely unaffected by these disturbances, or even become established on new depositional banks, achieving a net gain in areal extent. In cases where even the mangrove forests are cleared, slightly turbid water can extend farther toward reef areas (Fig. 10.5c). Seagrass dieback occurs in turbid waters and coral damage in slightly turbid water. In the Great Barrier Reef, there has been a shift from pristine conditions (Fig. 10.5a) to disturbed conditions (Fig. 10.5b, c) within the last 200 years, since European settlement. Rehabilitation of upstream ecosystems is considered the only way of restoring downstream marine ecosystems (Fig. 10.5d). The maintenance of healthy mangrove forests can therefore be seen as a prerequisite for keeping coral reefs (and seagrass meadows) productive, and thus they should be rehabilitated or conserved together as a connected seascape.
Mangrove forests can enhance the biomass of coral reef fishes. Mumby et al. (2004) showed that mangroves in the Caribbean strongly influence the community structure of fish on neighboring coral reefs, and the biomass of some commercially important fish is more than doubled when the adult fish habitat is connected to mangroves. More recently, Serafy et al. (2015) pointed out that at a regional scale in the Caribbean, a greater expanse of mangrove forest generally functions to increase the densities on neighboring reefs of those fishes that use these shallow, vegetated habitats as nurseries.
10.3.3 Relationships Between Corals and Macroalgal Communities: Phase Shift
Several reefs around the world have been degraded and shifted from a coral-dominated phase to a macroalgae-dominated phase. This phase shift has been reported in Caribbean reefs and was attributed to increased nutrient loading as a result of changed land-use and intensive fishing, which reduced the numbers of herbivorous fish species (Scheffer et al. 2001).
The phase shift toward macroalgae could influence the carbon cycle in the reefs. For example, Haas et al. (2013) found that macroalgae released more DOC than hermatypic corals, but the exudates from macroalgae and corals had different impacts on neighboring ecosystems. Coral exudates increased the net planktonic microbial community production and enhanced autotrophic benthic microbial community production, thus shifting toward a net autotrophic system. In contrast, macroalgal exudates stimulated heterotrophic organic carbon consumption rates by the planktonic and benthic microbial community, thus there was an overall shift toward a microbial community metabolism that was substantially more heterotrophic.
10.4 Directions for Future Study of Blue–Carbon Dynamics in Coral Reefs and Connected Ecosystems
Modeling can be a strong approach to understanding blue-carbon dynamics in coral reefs under both current and future conditions. Considering the large spatiotemporal variability of carbon dynamics caused by the heterogeneous distribution of benthic organisms and the resultant biogeochemical cycles in coral reefs, it is difficult to accurately represent blue-carbon dynamics solely from field data. Recently biogeochemical models have been coupled with hydrodynamic models for coral reefs (Zhang et al. 2011; Falter et al. 2013; Watanabe et al. 2013; Nakamura et al. 2017). For example, Watanabe et al. (2013) developed a carbonate-system dynamics model driven by coral and seagrass photosynthesis and calcification, and described the air–sea CO2 fluxes under various hydrodynamic and benthic conditions. They clarified that the status of the fringing reef studied as a CO2 sink or source was greatly influenced by neap and spring tides (Fig. 10.6). During neap tide, the tidal exchange becomes sluggish and the seawater residence time inside the reef increases, which allows the effects of reef metabolism to remain more within the reef.
Spatial distribution of CO2 sinks and sources around a coral reef at Ishigaki Island, Japan during (a) neap tide and (b) spring tide, simulated using a carbonate-system dynamics model coupled with a three-dimensional hydrodynamics model (Watanabe et al. 2013). (Source: Watanabe et al. 2013 with slight modifications)
The model by Watanabe et al. (2013) did not consider the feedback from water quality to coral metabolism, so Nakamura et al. (2017) further refined the model by incorporating these feedback interactions so that, for example, the modeled coral could respond to ocean acidification (OA). They also incorporated the effects of seawater flow over the reef on mass transfer in the model. Higher bottom velocity and hence higher bottom shear stress induces higher mass transfer velocity, which in turn enhances diffusive material exchange between corals and ambient seawater. Using their model, they examined coral calcification of inner reef corals under present conditions and under various future OA and sea-level-rise (SLR) scenarios in the year 2100 (Fig. 10.7). In general, calcification rates decreased as a result of OA, but increased in some nearshore reef flat areas because of enhanced mass exchange due to SLR. The more efficient water exchange due to SLR supplies more dissolved oxygen to corals and enhances respiration, which increases ATP synthesis and therefore increases calcification rates in the model.
Spatial distribution of coral polyp calcification rates (G, %) of inner-reef corals relative to the present rate under various future Intergovernmental Panel on Climate Change (IPCC) climate-change scenarios for the year 2100 around a coral reef at Ishigaki Island, Japan (Nakamura et al. 2017). (a) CO2 421 ppm and sea level rise (SLR) 0.4 m (IPCC representative concentration pathway [RCP] 2.6); (b) CO2 538 ppm, SLR 0.47 m (RCP 4.5); (c) CO2 670 ppm, SLR 0.48 m (RCP 6.0); (d) CO2 936 ppm, SLR 0.63 m (RCP 8.5). (Source: Nakamura et al. 2017)
Many things need to be improved or added for such ecosystem models to be applied to the analysis of blue-carbon dynamics (Fig. 10.8). First, it will be necessary to properly model organic matter production and decomposition. This is critically important to understanding whether the carbon produced within the blue-carbon ecosystem can be exported outside the system (i.e. to the deep layers of the open ocean or within reef sediments) (Abo et al. 2018; Kuwae et al. 2018). An initial simulation of DOC exports using the carbon dynamics model (Fig. 10.8) from a fringing coral reef is shown in Fig. 10.9. Second, interactions between sediment and water-column should be modeled. Carbon burial and sequestration in the sediments can be the key factor determining blue-carbon dynamics (Endo and Otani 2018; Inoue 2018; Miyajima and Hamaguchi 2018). Third, the model should properly address seagrass, macroalgae, and mangrove biogeochemical carbon cycles. Fourth, modeling should incorporate terrestrial carbon (so called “green carbon”) dynamics in the coastal area, including the dynamics of suspended solids. The transport and accumulation of green carbon in coastal areas should be evaluated together with blue-carbon dynamics to quantify the relative importance of these carbons and to highlight the importance of the blue carbon. Fifth, regional three-dimensional models should incorporate horizontal and vertical carbon exports in the open ocean. Export of dissolved inorganic carbon, DOC, or POC from coastal ecosystems to the interior of the open ocean can be considered long-term storage of blue carbon, but is difficult to quantify solely from observations. We should therefore model the blue-carbon exports to the ocean interior, which can be validated from sediment-trap observations. All of these considerations can be challenging given the different time scales and models used, but they could be achieved through transdisciplinary approaches involving specialists in hydrology, geochemistry, oceanography, marine ecology, and ecological modeling.
Integrated model for describing blue-carbon dynamics at local and regional scales around a coral reef at Ishigaki Island, Japan. A coral polyp model, sediment model, seagrass model, and macroalgae model (and eventually mangrove model) are incorporated into a local-scale hydrodynamic- biogeochemical model coupled with a watershed model which calculates green carbon flux. The model system calculates the local, reef scale primary production, calcification, and mucus release rate and then calculates dissolved and particulate organic carbon exports to the open ocean. A regional scale hydrodynamic model, which is downscaled from analysis/reanalysis products of global HYCOM (Hybrid Coordinate Ocean Model) using multi-nesting approach, is coupled with biogeochemical and sediment models to track the fate of organic C exported from the local scale model
Finally, field and experimental data are indispensable for verifying a blue-carbon dynamics model. The fate of organic matter should be assessed experimentally through decomposition experiments and empirically using sediment traps. Sediment organic carbon contents and sedimentation rates should be measured across different reef environments with and without mangrove forests and seagrass meadows.
Notes
- 1.
Coral bleaching: Corals have symbiotic algae called zooxanthellae inside their tissue. When corals are stressed from high water temperature or other causes, they release or digest their zooxanthellae and lose their color, making the white coral skeleton visible. This phenomenon is called coral bleaching.
References
Abo K, Sugimatsu K, Hori M, Yoshida G, Shimabukuro H, Yagi H, Nakayama A, Tarutani K (2018) Quantifying the fate of captured carbon: from seagrass meadow to deep sea. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 251–271
Bates NR (2002) Seasonal variability of the effect of coral reefs on seawater CO2 and air–sea CO2 exchange. Limnol Oceanogr 47:43–52
Bates NR, Samuels L, Merlivat L (2001) Biogeochemical and physical factors influencing seawater fCO2 and air–sea CO2 exchange on the Bermuda coral reef. Limnol Oceanogr 46:833–846
Birkeland C (1985) Ecological interactions between tropical coastal ecosystems. UNEP Reg Seas Rep Stud 73:1–26
Bouillon S, Dahdouh-Guebas F, Rao AVVS, Koedam N, Dehairs F (2003) Sources of organic carbon in mangrove sediments: variability and possible ecological implications. Hydrobiologia 495:33–39
Breithaupt JL, Smoak JM, Smith TJ III, Sanders CJ, Hoare A (2012) Organic carbon burial rates in mangrove sediments: strengthening the global budget. Glob Biogeochem Cycles 26:GB3011. https://doi.org/10.1029/2012GB004375
Buddemeier RW (1996) Coral reefs and carbon dioxide. Science 271:1298–1299
Cyronak T, Santos IR, Erler DV, Maher DT, Eyre BD (2014) Drivers of pCO2 variability in two contrasting coral reef lagoons: the influence of submarine groundwater discharge. Glob Biogeochem Cycles 28:398–414
Dai M, Lu Z, Zhai W, Baoshan C, Zhimian C, Kuanbo Z, Wei-Jun C, Chen-Tung AC (2009) Diurnal variations of surface seawater pCO2 in contrasting coastal environments. Limnol Oceanogr 54:735–745
Delesalle B, Buscail R, Carbonne J, Courp T, Dufour V, Heussner S, Monaco A, Schrimm M (1998) Direct measurements of carbon and carbonate export from a coral reef ecosystem (Moorea Island, French Polynesia). Coral Reefs 17:121–132
Drupp PS, De Carlo EH, Mackenzie FT, Sabine CL, Feely RA, Shamberger KE (2013) Comparison of CO2 dynamics and air-sea gas exchange in differing tropical reef environments. Aquat Geochem 19:371–397
Duke NC, Wolanski E (2001) Muddy coastal waters and depleted mangrove coastlines – depleted seagrass and coral reefs. In: Wolanski E (ed) Oceanographic processes of coral reefs. Physical and biology links in the Great Barrier Reef. CRC Press, Washington, DC, pp 77–91
Endo T, Otani S (2018) Chapter 8. Carbon storage in tidal flats. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 129–151
Fagan KE, Mackenzie FT (2007) Air-sea CO2 exchange in a subtropical estuarine-coral reef system, Kaneohe Bay, Oahu, Hawaii. Mar Chem 106:174–191
Falter JL, Lowe RJ, Zhang Z, McCulloch M (2013) Physical and biological controls on the carbonate chemistry of coral reef waters: effects of metabolism, wave forcing, sea level, and geomorphology. PLoS One 8:e53303
Frankignoulle M, Canon C, Gattuso J-P (1994) Marine calcification as a source of carbon dioxide: positive feedback of increasing atmospheric CO2. Limnol Oceanogr 39(2):458–462
Frankignoulle M, Gattuso J-P, Biondo R, Bourge I, Copin-Montégut G, Pichon M (1996) Carbon fluxes in coral reefs. II. Eulerian study of inorganic carbon dynamics and measurement of air–sea CO2 exchanges. Mar Ecol Prog Ser 145:123–132
Gattuso J-P, Pichon M, Delesalle B, Frankignoulle M (1993) Community metabolism and air–sea CO2 fluxes in a coral reef ecosystem (Moorea, French Polynesia). Mar Ecol Prog Ser 96:259–267
Gattuso J-P, Frankignoulle M, Smith SV, Ware JR, Wollast R (1996a) Coral reefs and carbon dioxide. Science 271:1298
Gattuso J-P, Pichon M, Delesalle B, Canon C, Frankignoulle M (1996b) Carbon fluxes in coral reefs. I. Lagrangian measurement of community metabolism and resulting air–sea CO2 disequilibrium. Mar Ecol Prog Ser 145:109–121
Gattuso J-P, Payri CE, Pichon M, Delesalle B, Frankignoulle M (1997) Primary production, calcification, and air–sea CO2 fluxes of a macroalgal-dominated coral reef community (Moorea, French Polynesia). J Phycol 33:729–738
Gattuso J-P, Frankignoulle M, Wollast R (1998) Carbon and carbonate metabolism in coastal aquatic ecosystems. Annu Rev Ecol Syst 29:405–434
Gattuso J-P, Allemand D, Frankignoulle M (1999) Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Am Zool 39:160–183
Guannel G, Arkema K, Ruggiero P et al (2016) The power of three: coral reefs, seagrasses and mangroves protect coastal regions and increase their resilience. PLoS One 11(7):e0158094. https://doi.org/10.1371/journal.pone.0158094
Haas AF, Nelson CE, Rohwer F, Wegley-Kelly L, Quistad SD, Carlson CA, Leichter JJ, Hatay M, Smith JE (2013) Influence of coral and algal exudates on microbially mediated reef metabolism. PeerJ 1:e108. https://doi.org/10.7717/peerj.108
Hata H, Suzuki A, Maruyama T, Kurano N, Miyachi S, Ikeda Y, Kayanne H (1998) Carbon flux by suspended and sinking particles around the barrier reef of Palau, western Pacific. Limnol Oceanogr 43(8):1883–1893
Hata H, Kudo S, Yamano H, Kurano N, Kayanne H (2002) Organic carbon flux in Shiraho coral reef (Ishigaki Island, Japan). Mar Ecol Prog Ser 232:129–140
Hemminga MA, Duarte CM (2000) Seagrass ecology. Cambridge University Press, Cambridge
Inoue T (2018) Carbon sequestration in mangroves. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 73–99
Jiang ZP, Huang JC, Dai M, Kao SJ, Hydes DJ, Chou WC, Jan S (2011) Short-term dynamics of oxygen and carbon in productive nearshore shallow seawater systems off Taiwan: observations and modeling. Limnol Oceanogr 56:1832–1849
Kayanne H (1996) Coral reefs and carbon dioxide- reply. Science 271:1299–1300
Kayanne H, Suzuki A, Saito H (1995) Diurnal changes in the partial pressure of carbon dioxide in coral reef water. Science 269:214–216
Kayanne H, Harii S, Ide Y, Akimoto F (2002) Recovery of coral populations after the 1998 bleaching on Shiraho Reef, in the southern Ryukyus, NW pacific. Mar Ecol Prog Ser 239:93–103
Kayanne H, Hata H, Kudo S, Yamano H, Watanabe A, Ikeda Y, Nozaki K, Kato K, Negishi A, Saito H (2005) Seasonal and bleaching-induced changes in coral reef metabolism and CO2 flux. Glob Biogeochem Cycles 19:GB3015. https://doi.org/10.1029/2004GB002400
Kennedy H, Beggins J, Duarte CM, Fourqurean JW, Holmer M, Marbà N, Middelburg JJ (2010) Seagrass sediments as a global carbon sink: isotopic constraints. Global Biogeochem Cycles 24:GB4026. https://doi.org/10.1029/2010GB003848
Kraines S, Suzuki Y, Omori T, Shitashima K, Kanahara S, Komiyama H (1997) Carbonate dynamics of the coral reef systems at Bora Bay, Miyako Island. Mar Ecol Prog Ser 156:1–16
Kuwae T, Kanda J, Kubo A, Nakajima F, Ogawa H, Sohma A, Suzumura M (2018) CO2 uptake in the shallow coastal ecosystems affected by anthropogenic impacts. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 295–319
Lamb JB, van de Water JAJM, Bourne DG, Altier C, Hein MY, Fiorenza EA, Abu N, Jompa J, Harvell CD (2017) Seagrass ecosystems reduce exposure to bacterial pathogens of humans, fishes, and invertebrates. Science 355:731–733
Longhini CM, Souza MFL, Silva AM (2015) Net ecosystem production, calcification and CO2 fluxes on a reef flat in Northeastern Brazil. Estuar Coast Shelf Sci 166:13–23
Massaro RFS, De Carlo EH, Drupp PS, Mackenzie FT, Jones SM, Shamberger KE, Sabine CL, Feely RA (2012) Multiple factors driving variability of CO2 exchange between the ocean and atmosphere in a tropical coral reef environment. Aquat Geochem 18:357–386
McGowan HA, MacKellar MC, Gray MA (2016) Direct measurements of air-sea CO2 exchange over a coral reef. Geophys Res Lett 43:4602–4608
Miyajima T, Hamaguchi M (2018) Carbon sequestration in sediment as an ecosystem function of seagrass meadows. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems: carbon dynamics, policy, and implementation. Springer, Singapore, pp 33–71
Miyajima T, Koike I, Yamano H, Iizumi H (1998) Accumulation and transport of seagrass-derived organic matter in reef flat sediment of Green Island, Great Barrier Reef. Mar Ecol Prog Ser 175:251–259
Miyajima T, Hori M, Hamaguchi M, Shimabukuro H, Adachi H, Yamano H, Nakaoka M (2015) Geographic variability in organic carbon stock and accumulation rate in sediments of East and Southeast Asian seagrass meadows. Global Biogeochem Cycles 29:397–415. https://doi.org/10.1002/2014GB004979
Moberg F, Folke C (1999) Ecological goods and services of coral reef ecosystems. Ecol Econ 29:215–233
Mumby PJ, Edwards AJ, Ernesto Arias-González JE, Lindeman KC, Blackwell PG, Gall A, Gorczynska MI, Harborne AR, Pescod CL, Renken H, Wabnitz CCC, Llewellyn G (2004) Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 427:533–536
Nakamura T, Nadaoka K, Watanabe A, Yamamoto T, Miyajima T, Blanco AC (2017) Reef-scale modeling of coral calcification responses to ocean acidification and sea-level rise. Coral Reefs. https://doi.org/10.1007/s00338-017-1632-3
Ogden JC (1988) The influence of adjacent systems on the structure and function of coral reefs. Proceedings of the sixth international coral reef symposium. 1
Ohde S, van Woesik R (1999) Carbon dioxide flux and metabolic processes of a coral reef. Okinawa Bull Mar Sci 65:559–576
Saunders MI, Leon JX, Callaghan DP, Roelfsema CM, Hamylton S, Brown CJ, Baldock T, Golshani A, Phinn SR, Lovelock CE, Hoegh-Guldberg O, Woodroffe CD, Mumby PJ (2014) Interdependency of tropical marine ecosystems in response to climate change. Nat Clim Chang 4(8):724–729
Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413:591–596
Serafy JE, Shideler GS, Araújo RJ, Nagelkerken I (2015) Mangroves enhance reef fish abundance at the Caribbean regional scale. PLoS One 10(11):e0142022. https://doi.org/10.1371/journal.pone.0142022
Shaw EC, McNeil BI (2014) Seasonal variability in carbonate chemistry and air-sea CO2 fluxes in the southern Great Barrier Reef. Mar Chem 158:49–58
Smith SV, Pesret F (1974) Processes of carbon dioxide flux in the Fanning Island lagoon. Pac Sci 28:225–245
Smith SV, Chandra S, Kwitko L, Schneider RC, Schoonmaker J, Seeto J, Tebano T, Tribble GW (1984) Chemical stoichiometry of lagoon metabolism: preliminary report on an environmental chemistry survey of Christmas Island, Kiribati. University of Hawaii/University of South Pacific International Sea Grant Programme Cooperative Report. UNIHISEAGRANT-CR-84-02
Sorokin YI (1993) Coral reef ecology. Springer-Verlag (Ecological studies 102)
Suzuki A, Kawahata H (2004) Reef water CO2 system and carbon production of coral reefs: topographic control of system-level performance. In: Global environmental change in the ocean and on land. pp 229–248
Takahashi T, Sutherland SC, Wanninkhof R, others (2009) Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans. Deep-Sea Res II 56:554–577
Tanaka Y, Miyajima T, Koike I, Hayashibara T, Ogawa H (2008) Production of dissolved and particulate organic matter by the reef-building corals Porites cylindrica and Acropora pulchra. Bull Mar Sci 82:237–245
Unsworth RKF, Cullen LC (2010) Recognising the necessity for Indo-Pacific seagrass conservation. Conserv Lett 3:63–73
Unsworth RKF, Collier CJ, Henderson GM, McKenzie LJ (2012) Tropical seagrass meadows modify seawater carbon chemistry: implications for coral reefs impacted by ocean acidification. Environ Res Lett 7:024026
Ware JR, Smith SV, Reaka-Kudla ML (1991) Coral reefs: sources or sinks of atmospheric CO2? Coral Reefs 11:127–130
Watanabe A, Yamamoto T, Nadaoka K, Maeda Y, Miyajima T, Tanaka Y, Blanco AC (2013) Spatiotemporal variations in CO2 flux in a fringing reef simulated using a novel carbonate system dynamics model. Coral Reefs 32:239–254
Werner U, Bird P, Wild C, Ferdelman T, Polerecky L, Eickert G, Jonstone R, Hoegh-Guldberg O, de Beer D (2006) Spatial patterns of aerobic and anaerobic mineralization rates and oxygen penetration dynamics in coral reef sediments. Mar Ecol Prog Ser 309:93–105
Wild C, Huettel M, Klueter A, Kremb SG, Rasheed MYM, Jorgensen BB (2004) Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428:66–70
Yamamoto S, Kayanne H, Tokoro T, Kuwae T, Watanabe A (2015) Total alkalinity flux in coral reefs estimated from eddy covariance and sediment pore-water profiles. Limnol Oceanogr 60:229–241
Yan H, Yu K, Shi Q, Tan YH, Zhang HL, Zhao MX, Li S, Chen TR, Huang LY, Wang PX (2011) Coral reef ecosystems in the South China Sea as a source of atmospheric CO2 in summer. Chin Sci Bull 56:676–684
Yan H, Yu K, Shi Q, Tan Y, Liu G, Zhao M, Li S, Chen T, Wang Y (2016) Seasonal variations of seawater pCO2 and sea-air CO2 fluxes in a fringing coral reef, northern South China Sea. J Geophys Res Oceans 121:998–1008
Zeebe RE, Wolf-Gladrow D (2001) CO2 in seawater: equilibrium, kinetics, isotopes. Elsevier, Paperback ISBN: 9780444509468
Zhang Z, Lowe R, Falter J, Ivey G (2011) A numerical model of wave- and current-driven nutrient uptake by coral reef communities. Ecol Model 222:1456–1470
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Watanabe, A., Nakamura, T. (2019). Carbon Dynamics in Coral Reefs. In: Kuwae, T., Hori, M. (eds) Blue Carbon in Shallow Coastal Ecosystems. Springer, Singapore. https://doi.org/10.1007/978-981-13-1295-3_10
Download citation
DOI: https://doi.org/10.1007/978-981-13-1295-3_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1294-6
Online ISBN: 978-981-13-1295-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)