Abstract
Latent heat-based energy storage systems provide a convenient way of storing energy when it is adequately available for waste energy recovery, and supply the same during the requirement. The stored energy may be used for domestic and agro-industrial applications such as space heating, air-conditioning systems, and drying applications. Phase change materials (PCMs) are commonly used for latent heat storage due to their ability to absorb thermal energy during phase change that can be extracted at a constant uniform temperature. PCMs melt at their melting point by absorbing the excess heat during charging. The stored heat is supplied back while discharging when the temperature falls below the melting point of the PCM. However, due to the limitations in technology and storage materials’ property, alternate methods have to be adopted to improve energy storage capacity and supply thereafter. This chapter presents the advances in PCM-based latent heat energy storage systems for waste heat recovery and harnessing excess solar energy. Energy economy achieved by using latent heat-based energy storage systems using PCMs and recent developments to achieve high storage density with higher efficiency are also highlighted.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
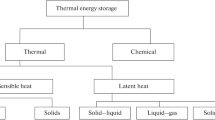
With increase in demand for energy, there is also an increased need for storing it. There has been a new outlook toward renewable sources of energy to meet the increasing demand. It is also important because these sources are environment friendly and available at free of cost. However, the intermittent nature of these sources makes it necessary to have proper storage mechanism. Several methods have been developed to store different forms of energy. Thermal energy is an attractive form of renewable energy [1]. Thermal energy may be stored in the form of sensible and latent heat and as thermochemical reactions. Major drawback to the development of solar thermal applications arises from the space and weight constraints that would be encountered during the storage of the collected energy [2]. The latent heat storage is advantageous over sensible heat storage due to their high energy storage density [3]. The thermochemical storage systems which are still in research phase have even superior energy storage density than the latent heat storage. Both sensible and latent heat storage systems are applicable in wide variety of thermal systems.
Latent heat storage system (LHS) is gaining much attention due to its ability to store and release heat isothermally. In a typical solid–liquid PCM, heat storage system heat is absorbed by the PCM during charging where it gains its latent heat of melting. During the discharging process, the stored heat is released back to the surrounding (or working fluid) that is at temperature below the freezing point of the PCM material. The energy released during its discharge can be used for applications such as building heating, heat pumps, drying applications in agriculture and industries, and several other applications.
This review provides an overview on the methods and developments in the latent heat storage systems using phase change materials for storing thermal energy from low temperature sources, viz., solar energy and waste heat.
2 Selection Criteria of PCM for Use in Latent Heat Storage Systems
PCMs may be classified into organic, inorganic, and eutectics. Organic PCMs can undergo repeated cycles of melting and freezing without causing any segregation in its phases which could cause degradation of its latent heat of fusion [4, 5]. They also have self-nucleating property which enables them to crystalize with almost no supercooling. Their non-corrosive nature offers the flexibility of choosing from different storage materials [6]. Common phase change materials are salt hydrates, metallics, paraffins and non-paraffin organic materials, and eutectics. The desirable properties of any PCM includes: (1) high latent heat of fusion, (2) high thermal conductivity, (3) moderate melting point, (4) stability at different temperatures, (5) high specific heat, Cp (6) ability to undergo repeated cycles of melting and solidification without significant change in its properties, and (7) non-toxicity and non-flammability [6, 7]. Latent heat of melting dictates on the amount of heat a given mass of PCM can store. Therefore, it is desirable to have a high latent heat of melting to provide high energy storage density. Large latent heat values of PCMs make them an attractive means for thermal energy storage [8]. The rate of storage and release of heat by a PCM is influenced by its thermal conductivity. A higher thermal conductivity gives faster rate of absorption and release of heat. Enhancement of heat transfer in PCM is essential to increase domain of its application in thermal systems [9]. A higher specific heat implies a higher heat storage density for sensible part of the heat stored. An ideal PCM should also possess thermal stability when it is subjected to several cycles of melting and solidification. This enables the PCM to be used for longer period of time without replacement.
However, these combinations of properties are not available in any single material. This has led to several research and modification in design of PCM-based LHS (Latent Heat Storage) systems. Choice of PCM also depends on its application: Temperature being the most important factor because different materials undergo phase change at different temperature. This necessitates to make a choice of the PCM material based on the temperature that a particular application requires.
3 Developments in Latent Heat Storage System for Storing Solar Energy
Storage of solar thermal energy and methods to utilize it in most efficient ways have been sought out in several studies. This section discusses some of the recent developments in the utilization of solar thermal energy for various purposes using PCM as the latent heat storage material.
Youssef et al. [10] designed an indirect expansion solar-assisted heat pump system using a PCM heat exchanger for domestic use. This energy was used in the heat pump cycle during the night. The schematic is shown in Fig. 1. The system worked on two different loops. First loop was made to bypass the PCM heat exchanger tank and used for heating the water in the water storage tank without the application of the heat pump. In the second loop, the solar heat from the solar collector was made to pass through the evaporator of the heat pump whose condenser was used for heating the water in the storage tank.
Schematic diagram of the test rig [10]
An automated control was used for efficient system operation that kept the temperature at the outlet of the water storage tank fixed at 55 °C. Incorporation of the PCM heat storage system increased the mean COP of the heat pump by 6.1% on sunny days and 14% on cloudy days. Qi et al. [11] proposed a new model for cooling car cabin (shown in Fig. 2a) where solar energy was converted into electricity in solar panels for powering air and water pumps. A PCM cabinet, as shown in Fig. 2b, was designed such that it could use ambient air for charging PCM during the daytime and cool water to discharge it during the night. The hot ambient air while passing through the PCM chamber would release its heat and charge the PCM, thus itself getting cooled. This cooled air was then passed into the car cabin to maintain a lower temperature. During night hours, the water from the water storage area was pumped into the PCM chamber which would discharge the PCM and prepare it for next day. This model had an advantage of wireless power transmission from the solar panels to the super-capacitor which avoided any modifications in the car body structures. A temperature drop of up to 30 °C was obtained between the inlet and the outlet air through this system.
a Schematic of the solar-powered cooling system for car cabin. b Schematic of internal PCM chambers [11]
Rabha and Muthukumar [12] used two double-pass solar air heaters to heat the PCM used in drying of red chilli using solar heat only (Fig. 3). The air from the solar air heaters was passed through a shell and tube heat exchanger where the PCM was contained in the shell. The hot air charged the PCM and was forced through a parallel flow drying chamber for drying chilli using a blower. The presence of PCM module allowed a constant temperature of air at drying chamber even when the sky was cloudy during intermediate hours.
Schematic diagram of the forced convective solar dryer; 1. Parallel flow tunnel dryer, 2. Shell and tube heat exchanger (energy storage), 3. Solar air heater, 4. Air blower, 5. Ball valve, 6. Pyranometer, 7. Thermocouples, 8. Flow meter, 9. Energy meter, 10. Data acquisition system [12]
Eswaramoorthy [13] studied the thermal performance of a V-trough solar air heater for drying applications using paraffin wax as the latent heat storage material. Experiments were conducted with and without the PCM module. It was seen that the thermal performance of the system was 10% higher with the incorporation of the thermal storage than without it. The schematic of the V-trough solar air heater is shown in Fig. 4. Okello et al. [14] experimentally investigated the use of PCM and rock particles for a comparative study on the thermal storage with rock only and rock with PCM. A vertical downward flow of hot air was made through the energy storage unit. It was observed that use of copper cylinders containing PCM increased the total heat content of the heat storage unit. Also, the high thermal conductivity of copper tubes led to a thermal equalization in the storage tank which caused a faster rise in temperature at the bottom of the tank.
Schematic diagram of the V-trough solar air heater [13]
Reyes et al. [15] constructed a hybrid solar dryer for mushroom dehydration (Fig. 5). The setup consisted of a wavy solar collector is made from zinc with fins to increase the surface area. The dryer operated in a closed-circuit mode with an electric heater in the main circuit and 70 and 80% of the air being recirculated while the difference was supplied through the solar collector and solar accumulator. The solar energy accumulator came into operation in the evening when it was able to raise the air to 20 °C above the ambient temperature for 2 h. For the daytime, the required heat was supplied by the solar collector and the electric heaters. This helped in constantly supplying a heated air at a higher temperature to the dryer.
Hybrid-solar dryer, (A) solar panel, (B) solar energy accumulator, (C) electrical heater, (D) drying chamber, (E) centrifugal fan: Ti air temperature, Vi valves, ΔP pressure drop [15]
4 Advances in Latent Heat Storage Systems for Waste Heat Recovery
In this section, we present some of the recent advancements in recovering waste energy through the use PCMs. Exhausts of several energy conversion devices carry a significant amount of energy which goes into the ambient air. Recapture of this heat can save huge amount of fuels that would otherwise be utilized to produce the same heat. Pandiyarajan et al. [16] used a finned shell and tube heat exchanger to experimentally study the recovery of heat from diesel engine exhaust gases using cylindrical PCM capsules. This system used castor oil as heat transfer fluid between the exhaust gas and the storage tank which contained paraffin stored in 48 capsules. This offered dual advantage of sensible and latent heat storage. Copper fins were installed on the exterior of the tubes in the shell and tube heat exchanger to increase the heat exchange area available on the gas side. Through this system it was possible to save 10–15% of the heat contained in the exhaust gases. High thermal conductivity of stainless steel used as storage tank for PCM enabled a uniform distribution of heat throughout the TES (Thermal Energy Storage) tank. The charging rate and efficiency were found to be higher at higher loads. Gopal et al. [17] conducted a similar study on a two-cylinder diesel engine and carried out energy and exergy analyses of the complete system. The schematic of the experimental work and the exhaust heat exchanger is shown in Fig. 6a, b, respectively. They incorporated water as a heat transfer fluid to carry heat from the exhaust gases to the TES unit using an exhaust heat exchanger. The heat exchanger consisted of a mild steel tube around which copper tubes that carry water were wound. Lathe scrap was placed in the empty spaces around the mild steel tube to promote heat transfer to the copper tubes. The charging of PCM occurred by the natural circulation of water due to temperature difference between the exhaust heat exchanger and TES unit. The use of PCM storage unit enabled 6.13% of energy savings from the fuel input and 0.14% of the chemical exergy of the fuel.
a Schematic diagram of the experimental setup. b Schematic diagram of the exhaust heat exchanger [17]
Shon et al. [18] studied on the methods for heat storage rate improvement and heat exchanger efficiency when the heat exchanger was filled with PCM for an automobile coolant waste heat recovery system. The time required and the heat transfer rate were theoretically evaluated in a PCM-filled tube heat exchanger. It was observed that the natural convective heat transfer on the PCM side decreased drastically with the formation of the liquid layer. Hence, the liquid layer should be thin for better heat transfer through natural convection. A high viscosity resulted in a lower heat transfer by natural convection in the liquid phase of the PCM.
Jia and Lee [19] conducted an experiment under laboratory conditions to recover heat from an air-conditioning system by use of PCM. The test facility consisted of two chambers to simulate the indoors and outdoors conditions (Fig. 7a). The indoor conditions were kept at 22 °C and 55% relative humidity while the outdoor temperature was varied between 25 and 35 °C with 5 °C intervals. A cylindrical heat recovery water tank was placed between the compressor and the condenser of the air-conditioning unit. The heat exchanger consisted of helical coils of copper containing a cylindrical PCM container with longitudinal fins inside the helical coils. The cross section of the PCM cylinder is shown in Fig. 7b. The performance of the air-conditioning system was tested in with PCM and without PCM scenarios. An overall gain of 6.9–9.8% was observed for the with PCM scenario while the heat retention time of the water tank increased by 21.1%. Sun et al. [20] conducted an analysis of a TES unit for recovery of heat from industrial residual water. The schematic of the facility is shown in Fig. 8. A TES unit using PCM was designed for supplying the required heating load to the workshop for 3.6 h when the production unit was not operated.
System configuration of a workshop heating system [20]
The heating of the workshop during the non-operation hours of the medicine production unit was achieved by first preheating pool water from 5 to 35 °C using the heat obtained from the drying unit; then this water was passed through the TES where it absorbs heat from PCM. They estimated 10.25% savings through the application of waste heat recovery system that could be achieved by the medicine production plant.
5 New Innovations in Latent Heat Storage Systems for Increasing Storage Density and Capacity of PCM
In this section, the recent innovative approaches and ideas in the PCM-based latent heat storage systems are discussed. Focus is given to the ways to improve the heat storage capacity and heat storage rate.
Akeiber et al. [21] tested three local PCMs extracted from petroleum products in Iraq for building encapsulation to study the energy saving achievable when the rooms are maintained at 24 °C against ambient temperature of 40–44 °C. Three different combinations of oil and wax, (40% oil + 60% wax), (50% oil + 50% wax) and (60% oil + 40% wax), were initially tested to find the best combination. The third combination was selected for building encapsulation due to its higher heat storage capacity as compared to others. Two identical rooms were constructed of equal dimensions (3 m × 2.5 m × 2 m) with identical wall material. One of the rooms was encapsulated with the selected PCM contained in aluminum slabs on the roof and walls. Both rooms were maintained at 24 °C using an air-conditioning system and the difference in cooling load of the air-conditioners was observed for studying the effectiveness of the PCM in saving energy. They reported that the room with PCM (test room) consumed 4.356 kWh as compared to the room without PCM that consumed 7.92 kWh which is 45% more than the test room.
Yamada and Nagano [22] developed and demonstrated the use of heat storage panel for micro- and nano-satellites. The heat storage panel (HSP) consists of carbon-fiber-reinforced polymer (CFRP) in which the PCM was injected. The HSP was used for increasing the heat bearing capacity of the satellite communication equipments where periodic heat generation was encountered. The testing and validation of the HSP were conducted on-orbit testing, model analysis, and thermal vacuum testing. The tests confirmed the temperature stabilization ability of the HSP. Thermal tests showed the temperature uniformity was achieved by the system. The HSP was loaded in Hodoyoshi-4, a Japanese satellite, and was found to function successfully in space. Li and Wu [23] made a numerical study on NaNO3 as PCM inside porous copper matrix. The effects of natural convection, heat conduction, pore density, and porosity were studied numerically. The thermal conductivity was observed to improve with porosity of the copper matrix. However, the natural convection of the liquid PCM was hampered with higher porosity due to the restrictions from the metal frames. The lower pore density on the other hand decreased the melting and solidification rate by reducing the contact area for the PCM with the matrix. In the solid phase, the heat transfer coefficient due to conduction was increased by 28.1 times with the incorporation of the metal matrix, while in the liquid phase it was increased by 3.1 times due to the combined effect of natural convection and conduction. Joybari et al. [24] carried a numerical analysis of simultaneous charging and discharging of PCM with different fin configurations in a triplex tube heat exchanger. They studied the effects of fin thickness, length and number on the melting process of PCM and suggested the most optimal values for different configurations. It was recommended that for simultaneous charging and discharging there should be three internal fins and one external fin while in the absence of simultaneous charging and discharging four fins are required in both internal and external cylinders. The fin thickness did not have significant influence on the heat transfer. However, the fin length and number of fins were crucial for better thermal performance of the configurations.
Tasnim et al. [25] performed a scale analysis followed by numerical analysis on nano-PCM (cyclohexane + CuO nanoparticles) to study the effects of convection on its melting process. A rectangular enclosure having impermeable walls was considered for numerical study with left wall subjected to a heat source of temperature above the melting point of the PCM and other three walls taken as adiabatic. They reported that the presence of nano-PCM slowed down the process of melting of the PCM. Addition of nanoparticles at fixed Ra (Rayleigh Number) decreased the width of the melt front and reduced the stream function values. Similar study was conducted by Hossain et al. [26] where the top wall of the rectangular enclosure was subjected to a heat source having temperature above the melting point of the PCM (Cyclohexane). The porous medium consisted of aluminum and the nanoparticles used was CuO. They reported that the melting rate was higher at lower porosity of the medium. Higher porosity was associated with higher amount of energy required to melt the PCM. However, a higher volume fraction of the nanoparticles increased the rate of melting for any given porosity of the medium.
6 Conclusions
An overview of the recent developments in latent heat storage systems using PCM as storage medium was presented in this chapter. First, some important criteria for selection of PCM for a particular application have been discussed. Various applications of PCM-based TES including space heating, drying and dehydration, car cabin cooling were discussed along with the system designs and the benefits derived from them. In the second part, some of the recent methods adopted for recovery of waste heat have been discussed and system designs and analyses were presented. Some recent innovative methods incorporated in order to achieve better PCM properties, storage density, and heat storage capacity such as the use of nano-PCM and role of porous medium have also been presented. This chapter is intended to serve as a guideline for designing new thermal energy storage units and help in decision making while selecting the PCM. Future work may be done in the areas of improving the thermal properties of the PCM. Also, methods to improve the heat transfer coefficients in the liquid phase of the PCM have to be developed for achieving faster rate of heat absorption and release.
References
Pelaya, U., Luoa, L., Fan, Y., Stitou, D., Rood, M.: Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 79, 82–100 (2017)
Murray, R., Desgrosseilliers, L., Stewart, J., Osbourne, N., Marin, G., Safatli, A., Groulx, D., White, M.A.: Design of a latent heat energy storage system coupled with a domestic hot water solar thermal system. World renewable energy congress 2011, Sweden. Solar Thermal Applications (2011)
Farid, M.M., Khudhair, A.M., Razack, S.A.K., Al-Hallaj, S.: A review on phase change energy storage: materials and applications. Energy Convers. Manage. 45, 1597–1615 (2004)
Zalba, B., Marın, J.M., Cabeza, L.F., Mehling, H.: Review on thermal energy storage with phase change: materials, heat transfer analysis and applications. Appl. Therm. Eng. 23, 251–283 (2003)
Khudhair, A.M., Farid, M.M.: A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manage. 45, 263–275 (2004)
Sharma, A., Tyagi, V.V., Chen, C.R., Buddhi, D.: Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 13, 318–345 (2009)
Alva, G., Liu, L., Huang, X., Fang, G.: Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 68, 693–706 (2017)
Zhang, Y., Faghri, A.: Analysis of thermal energy storage system with conjugate turbulent forced convection. J. Thermophys. Heat Transf. 9(4) (1995)
Youssef, W.M.K.A.: Experimental and computational study of indirect expansion solar assisted heat pump system with latent heat storage for domestic hot water production. PhD thesis, College of Engineering, Design, and Physical Sciences Brunel University London (2017)
Youssef, W., Ge, Y., Tassou, S.A: Indirect expansion solar assisted heat pump system for hot water production with latent heat storage and applicable control strategy. In: 1st International Conference on Sustainable Energy and Resource Use in Food Chains, ICSEF 2017, Berkshire, UK (2017)
Qi, L., Pan, H., Zhu, X., Zhang, X., Salman, W., Zhang, Z., Li, L., Zhu, M., Yuan, Y., Xiang, B.: A portable solar-powered air-cooling system based on phase-change materials for a vehicle cabin. Energy Convers. Manage. 150, 148–158 (2017)
Rabha, D.K., Muthukumar, P.: Performance studies on a forced convection solar dryer integrated with a paraffin wax-based latent heat storage system. Sol. Energy 149, 214–226 (2017)
Eswaramoorthy, M.: Thermal performance of V-trough solar air heater with the thermal storage for drying applications. Appl. Solar Energy 52(4), 245–250 (2016)
Okello, D., Foong, C.W., Nydal, O.J., Banda, E.J.K.: An experimental investigation on the combined use of phase change material and rock particles for high temperature (350 °C) heat storage. Energy Convers. Manage. 79, 1–8 (2014)
Reyes, A., Mahn, A., Vásquez, F.: Mushrooms dehydration in a hybrid-solar dryer, using a phase change material. Energy Convers. Manage. 83, 241–248 (2014)
Pandiyarajan, V., Pandian, M.C., Malan, E., Velraj, R., Seeniraj, R.V.: Experimental investigation on heat recovery from diesel engine exhaust using finned shell and tube heat exchanger and thermal storage system. Appl. Energy 88, 77–87 (2011)
Gopal, K.N., Subbarao, R., Pandiyarajan, V., Velraj, R.: Thermodynamic analysis of a diesel engine integrated with a PCM based energy storage system. Int. J. Thermodyn. 13(1), 15–21 (2010)
Shon, J., Kim, H., Lee, K.: Improved heat storage rate for an automobile coolant waste heat recovery system using phase-change material in a fin–tube heat exchanger. Appl. Energy 113, 680–689 (2014)
Jia, J., Lee, W.L.: Experimental investigations on using phase change material for performance improvement of storage enhanced heat recovery room air-conditioner. Energy 93, 1394–1403 (2015)
Sun, W., Zhao, Z., Wang, Y.: Thermal analysis of a thermal energy storage unit to enhance a workshop heating system driven by industrial residual water. Energies 10, 219 (2017). https://doi.org/10.3390/en10020219
Akeiber, H.J., Hosseini, S.E., Hussen, H.M., Wahid, M.A., Mohammad, A.T.: Thermal performance and economic evaluation of a newly developed phase change material for effective building encapsulation. Energy Convers. Manage. 150, 48–61 (2017)
Yamada, K., Nagano, H.: Development of a heat storage panel for micro/nano-satellites and demonstration in orbit. Appl. Therm. Eng. 91, 894–900 (2015)
Li, Z., Wu, Z.G.: Numerical study on the thermal behavior of phase change materials (PCMs) embedded in porous metal matrix. Sol. Energy 99, 172–184 (2014)
Joybari, M.M., Haghighata, F., Seddegh, S., Al-Abidi, A.A.: Heat transfer enhancement of phase change materials by fins under simultaneous charging and discharging. Energy Convers. Manage. 152, 136–156 (2017)
Tasnim, S.H., Hossain, R., Mahmud, S., Dutta, A.: Convection effect on the melting process of nano-PCM inside porous enclosure. Int. J. Heat Mass Transf. 85, 206–220 (2015)
Hossain, R., Mahmud, S., Dutta, A., Pop, I.: Energy storage system based on nanoparticle-enhanced phase change material inside porous medium. Int. J. Therm. Sci. 91, 49–58 (2015)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Mishra, L., Sinha, A., Gupta, R. (2019). Recent Developments in Latent Heat Energy Storage Systems Using Phase Change Materials (PCMs)—A Review. In: Drück, H., Pillai, R., Tharian, M., Majeed, A. (eds) Green Buildings and Sustainable Engineering. Springer Transactions in Civil and Environmental Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-13-1202-1_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-1202-1_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1201-4
Online ISBN: 978-981-13-1202-1
eBook Packages: EngineeringEngineering (R0)