Abstract
Cancer has become one of the leading causes of death worldwide. Presently, available chemotherapeutic agents have several limitations including severe side effects. Curcumin (diferuloylmethane) is a polyphenol derived from the plant Curcuma longa. Curcumin has been used extensively as spice in many Asian countries and in Ayurvedic medicines. It is nontoxic and has shown to possess various medicinal properties including antioxidant, anti-inflammatory, and antibacterial. Recent investigations have shown that curcumin exerts anticancer properties in various cancer cell models and targets variety of biological pathways involved in cell cycle regulation, apoptosis, mutagenesis, angiogenesis, and metastasis. NF-κB, p53, Nrf2, NFAT, MMPs, STATs, and uPA are important molecular targets of curcumin in multiple cancer models. Enzymes involved in redox balance inside the cells including superoxide dismutases, catalase, and glutathione peroxidase are modulated by curcumin. However, bioavailability, water insolubility, short life span, and rapid systemic clearance of curcumin have posed limitations in developing curcumin as an effective chemotherapeutic agent. To address these challenges, curcumin has been used in combinations with many other chemotherapeutic drugs which have shown encouraging results. This chapter deals with the current information available for the cancer chemopreventive activities of curcumin in various in vitro and in vivo cancer models including epidemiological studies and human trials. Also, molecular pathways involved in the manifestation of biological activities of curcumin against various processes of cancer development have been discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
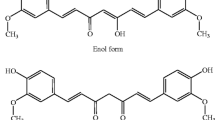
Curcumin is a yellow-colored compound derived from Curcuma longa commonly known as turmeric. Turmeric has been used for many years in several herbal remedies. The compound is one of the most studied component of turmeric with more than 9000 citations in the literature [1]. About two centuries back, curcumin was isolated from the ground rhizome of Curcuma longa L. (family Zingiberaceae) by Vogel and Pelletier [2]. Structurally this polyphenolic compound is diferuloylmethane[(1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] and exists in two tautomeric forms (Fig. 16.1).
Curcumin is a multi-targeting compound and has been reported to possess a wide range of preventive or putative therapeutic properties of antioxidant, anti-inflammatory, anticancer, antiarthritic, antiaging, antidiabetic, antimicrobial, antiviral, anti-atherosclerotic, antidepressant, wound healing, and memory-enhancing activities against variety of diseases [3,4,5]. These nutraceutical properties of curcumin are possibly attributed to methoxy, hydroxyl, α, β-unsaturated carbonyl moiety or diketone groups [6].
Among various pharmacological activities, curcumin had proved its credential as a vital source of chemopreventive agent against varieties of cancer. Curcumin has been studied in multiple human carcinomas including melanoma, head and neck, pancreatic, prostate, breast, colon, and ovarian cancers [7,8,9,10,11,12,13,14,15]. Many in vitro studies have been conducted on several cancer cell lines showing strong efficacy of curcumin against several human cancers. Curcumin served as a strong inhibitor of cell proliferation in Burkitt’s lymphoma cell lines expressing Bax protein [16]. In colon cancer, curcumin enhanced the sensitivity of overexpressed glucose-regulated protein 78 (the basic regulator of endoplasmic reticulum) in HT-29 cells lines and induced apoptosis in control cells compared to GRP 78 kD DLD-1 cells during cell cycle examination and TUNEL assay [17].
Curcumin prevented progression of colon cancer through inhibiting cell proliferation and cell survival, improving cellular uptake, activating the cascade of caspases, exhibiting controlled release at physiological pH, and promoting intrinsic apoptotic signaling [18]. Another study evaluated the anticancer effect of curcumin in cervical cancer where curcumin controlled the function and expression of P-glycoprotein (Pgp) in multidrug-resistant (MDR) human cervical carcinoma (KB-V1) cells [19]. It also enhanced the cisplatin-induced apoptosis by sensitizing the SiHa cells which are cisplatin-resistant. Thus, curcumin has the potential to reverse the MDR in cervical cancer cells. Goel et al. [3] suggested that 0.5–12 g orally administrated curcumin in a daily dose for 3 months leads to histologic improvement in uterine and cervical intraepithelial carcinoma’s precancerous lesions. This improvement in lesions were observed in one out of four patients that have uterine and cervical carcinomas [20]. In other reports, curcuminoids were found to potentiate cytotoxic function of natural killer cells and induce apoptosis [21, 22]. In addition to curcumin, difluorinated curcumin (CDF), an analog of curcumin found in turmeric, has been reported to decrease cell survival, invasive cell migration, formation of pancreatospheres, clonogenicity, and cancer stem cell function in human pancreatic cancer cells.
Bioavailability and tissue distribution of curcumin and CDF can be increased with cyclodextrin [23]. Effect of curcumin on cancer cells was linked with the activation of caspase 3 enzyme in the apoptotic cascade which induces apoptosis in prostate cancer [12]. According to epidemiological studies, consumption of curcumin rich food products greatly reduces the risk of developing cancer of different origins. In one report of breast cancer, effect of curcumin was studied for apoptosis on two MCF-7/TH and MCF-10A cell lines. MCF-7/TH cells displayed a remarkable apoptosis level when treated with certain curcumin concentrations, but the same concentrations of drug showed insignificant level of apoptosis on mammary epithelial cells [24]. Curcumin depicts downregulation of mutant p53 mRNAs, PCNA, and Ki67 as its chemopreventive action in breast cancer cells [25].
In another study the effects of curcumin were investigated in both in vivo and in vitro tests against matrix metalloproteinases, i.e., MMP2 and MMP9, which revealed the range of molecular mechanisms involving the inhibition of NF-κB and nitric oxide production, effects on transcription factors, and intervention in signaling pathways exhibited by curcumin’s action to induce apoptosis [26]. Both in vitro and in vivo studies have proven curcumin as an apoptosis inducer against the growth of head and neck cancer through suppression of IKK-mediated NF-κB activation and through NF-κB-regulated gene expressions [8].
2 Curcumin Chemoprevention by Inhibition of NF-κB
The ability to sense and adjust to external environment is crucial for the survival of all living beings. Regulation through inducible gene expression makes the organisms to acclimatize to the fluctuating outside environment. Different transcription factors play various roles in accomplishing these essential effects. NF-κB was discovered for its role in immunity, which was later found to be involved in various mechanisms that impact cell survival, differentiation, and proliferation. Thus, dysregulation of NF-κB can cause several consequences and diseases including autoimmune diseases, neurodegenerative diseases, cardiovascular disease, diabetes, and cancer [27]. Even though NF-κB target genes that have been extensively studied for their role in inflammation and immune responses, these transcription factors are also involved in the regulation of genes responsible for cell proliferation, survival, migration, and apoptosis (Fig. 16.2). In numerous cancer types, these are found to be constitutively expressed making them important targets for anticancer therapy [28].
2.1 Regulation of NF-κB
Nuclear factor κB is a family of protein dimers which bind to the common DNA motif known as κB site. In resting stage or in the absence of stimuli, NF-κB is located inside the cytoplasm associated with IκB proteins. Once the stimulus is triggered, IκB proteins undergo phosphorylation and ubiquitination leading to their proteasomal degradation, thus leaving NF-κB free to translocate into the nucleus resulting in transcription of target genes. IKK complex which comprises of NF-κB dimers and IκB is the crucial part of NF-κB signaling pathway (Fig. 16.3).
Overview of inhibition of NF-κB pathway mediated through curcumin treatment. Numerous ligands can activate NF-κB by inhibiting its interaction with IκB and increasing its translocation inside the nucleus thus promoting transcription of many genes including cancer promoting factors and cancer. Curcumin blocks IκB phosphorylation and its ubiquitin-mediated proteasomal degradation
Mammalian NF-κB family has five members RelA/p65, RelB, c-Rel, p50 (NF-κB1), and p52 (NF-κB2) [29]. NF-κB binds to the target gene in the form of either homodimers or heterodimers and may cause both positive and negative effects on the level of transcription. Crystal structures of NF-κB dimers have shown a common RHD domain (Rel Homology Domain) which is present in all the NF-κB family members. This RHD domain promotes the association of NF to the κB sites and also assists in the subunit dimerization process. Only three of the members (RelA/p65, RelB, c-Rel) possess C-terminal transactivation domain (TADs) that have the ability to initiate the process of transcription. The other two proteins, viz., p50 and p52, do not have TADs; however, they still can initiate transcription through heterodimerization with TADs containing proteins or with non-Rel proteins that have transactivation efficiency. The other key regulatory proteins of NF-κB pathway are IκB proteins, which are marked by multiple ankyrin repeat domains. IκB family consists of IκB proteins IκBa, IκBb, IκBe, IκBz, Bcl-3 (B-cell lymphoma 3), and IκBns and the precursor proteins p100 (NF-κB2) and p105 (NF-κB1). The most important role of IκB proteins is to sequester the NF complexes inside the cytoplasm, thus preventing their translocation into the nucleus and binding to κB sites [30].
Phytochemicals have shown effective anticancer potential against different cancer types. Many of them are also found to inhibit NF-κB pathway, thus limiting the transcription of genes involved in cancer progression. Melanoma is one of the deadly cancers which have shown to constitutively express NF-κB, likely playing a central role in disease progression. In melanoma, IκB is also found to be activated through phosphorylation resulting in its degradation, causing increased localization of NF-κB into the nucleus [31]. Curcumin has shown to possess promising inhibitory activity against NF-κB. Curcumin (10–50 μmol/l) inhibited the transactivation of NF-κB in 4046T melanoma cells compared to normal melanocytes. Curcumin has also decreased the phospho-IκBα in these tumor cells, which correlated with the decrease in NF-κB activity. Additionally decrease in the expression of two target genes of NF-κB, i.e., COX-2 and cyclin D1, was also observed. Curcumin caused apoptosis selectively in 4046T cancer cells via cleavage of caspase 3 [32]. In case of colon cancer HCT 116 cells, curcumin triggered apoptosis in time- and dose-dependent fashion and this was linked to NF-κB inhibition. HCT 116 cells were transiently transfected with 3EnhConALuc reporter vector, this vector carried the luciferase reporter gene, and treatment with curcumin caused significant decrease in NF-κB-dependent luciferase activity.
2.2 MAPK-NF-κB
Activation of JNK, p38, and ERK through phosphorylation has been investigated in the presence and absence of curcumin, and it was found that curcumin has caused sustained phosphorylation and activation of both JNK and p38 proteins. JNK activation and inhibition of NF-κB promote apoptosis in HCT 116 colorectal cancer cells. However, curcumin did not affect the state of ERK, which is constitutively activated in these cells [33]. One of the most predominant forms found in many cells is the active p65/p50 NF-κB heterodimer [34]. p65/RelA subunit of NF-κB can have effect on cell survival due to activation of NF-κB attributed to upregulation of anti-apoptotic genes including X-linked inhibitor of apoptosis (XIAP), Bcl-xL and A20 and inhibition of sustained activation of c-Jun N-terminal kinase (JNK). However, forced overexpression of p65/Rel A did not affect curcumin-induced JNK activation through phosphorylation but has caused increased apoptosis and cleavage of PARP. This additive effect of p65 upon curcumin-mediated apoptosis was blocked by IκB super-repressor (Delta NIκB) transfection. Additionally, curcumin treatment caused downregulation of anti-apoptotic genes targeted through NF-κB in mock-transfected and p65-overexpressed cells, although expression level was found to be higher in p65-overexpressed cells. Conclusively, the study showed that curcumin-induced apoptosis and JNK activation do not require p65 inhibition but p65 can potentiate the apoptotic effect of curcumin in HCT 116 cells [35]. Many stimuli act as an activator of NF-κB pathway which include inflammatory cytokines (e.g., tumor necrosis factor (TNF-α), lymphotoxin, and interleukin-1), oxidative stress (H2O2), mitogens, bacterial products, UV light, and phorbol ester (PMA) [36, 37].
In an in vitro study, TNF-α was employed to activate NF-κB pathway in human myeloid ML-1a cells, and curcumin was employed to block the activation. Blockade of NF-κB activation could be beneficial in many complications including cancer. The results of this study indicated that curcumin (2–60 μM) has significantly blocked the activation of NF-κB. H2O2 and PMA are potent activators of NF-κB; thus, curcumin was employed at 50 μM concentration which interestingly completely blocked NF-κB activation. Curcumin also inhibited TNF-dependent phosphorylation, and proteolysis of IκBα protein, thus, inhibited the translocation of NF-κB inside the nucleus [38]. It has been previously shown that phosphorylation of inducible IκBα is not enough for its dissociation from NF-κB. However, the degradation of IκBα is a necessary event in the activation of NF-κB, and protease inhibitors employed in the study prevented its activation. Protease inhibitors (tosyl phenylalanyl chloromethyl ketone (TPCK), tosyl-L-lysyl-chloromethane hydrochloride (TLCK), N-benzoyl-L-tyrosine ethyl ester (BTEE), or 3,4-dichloroisocoumarin (DCIC)) employed in the study also inhibited inducible phosphorylation of IκBα, indicating a more complex role of proteases in the activation of NF-κB [39]. It was also shown that agents like TPCK can cause alteration in the sulfhydryl group preventing activation of NF-κB; however, this inhibition was prevented in the presence of reducing agents like DTT. DTT can also reverse the inhibitory effect of phenylarsine oxide (a potent protein tyrosine phosphatase inhibitor) on NF-κB activation [39].
To investigate, if presence of reducing agents can reverse the effect of curcumin on the activation of NF-κB by TNF, human myeloid ML-1a cells were exposed to curcumin alone or in combination with DTT. Interestingly, DTT did not reverse the inhibition caused by curcumin, however, significantly reversed the inhibition caused by phenylarsine oxide, indicating that curcumin acts differently than protein tyrosine phosphatase inhibitor in these cells. All compounds (DTT, DMP, phenylarsine oxide) used in this study caused an increase in reactive oxygen intermediates; thus, it is possible that the effect of curcumin is through scavenging these reactive intermediates. Furthermore, inhibitors of respiratory chain complex have also shown to block TNF-mediated NF-κB activation, further suggesting the role of reactive oxygen intermediates [38].
2.3 Cancer Stem Cells and NF-κB
Cancer stem cells (CSCs) have important implications in many different cancers including hepatocellular carcinomas (HCC). NF-κB is one of the key pathways which is generally found to be constitutively activated in CSCs of HCC. The characteristic features of HCC are high recurrence rate and multidrug resistance which are found to be associated with CSCs. Thus, drugs which can target CSCs may play pivotal role in managing HCC. The effect of curcumin has been investigated on different HCC-sensitive and HCC-resistant cells [40]. Furthermore, it was also reported that NF-κB is central to inflammation-fibrosis-cancer axis in hepatocellular carcinogenesis [41]. It was found that the differential response to curcumin was directly related to NF-κB inhibition and its downstream signaling including STAT3, cyclin D1, and JNK. In sensitive cells, NF-κB inhibition caused significant decrease in potency of sphere formation and tumorigenicity. In case of resistant cells, curcumin treatment along with histone deacetylase inhibitor, trichostatin A (TSA), made cells susceptible to curcumin treatment [42]. Several studies have shown that HDAC and NF-κB signaling are interrelated [43, 44]. Conclusively, the disruption of NF-κB signaling by curcumin can play an important role in managing prognostically adverse HCCs.
3 Chemoprevention by Regulation of p53
Despite numerous genes involved in tumorigenesis, p53 protein (encoded by the human gene TP53) is a master regulator and an established tumor suppressor protein. p53 is a transcription factor that has been reported to play crucial role in cell cycle regulation and apoptosis; thus, dysregulation of p53 can lead to cancer [45, 46]. Additionally, mutations in p53 not only render its tumor-suppressive ability but can also evoke oncogenic functions including invasion, migration, metastasis, cell proliferation, and cell survival [47]. Mdm2 is a p53-specific E3 ubiquitin ligase and cellular antagonist of p53 which does not allow p53 to perform its growth-suppressive function in normal conditions. Under normal conditions, Mdm2 causes ubiquitination and directs proteasomal degradation of p53, thus maintaining low level of p53 protein inside the cells. However, in event of DNA damage, hypoxia, telomerase shortening, and other cellular stresses, p53 is phosphorylated by different proteins including ATM. Phosphorylation of p53 prevents Mdm2 and p53 association, thus, preventing ubiquitination of p53 that results in increased levels of p53 inside the cell. This increased level of p53 causes increased transcription of many proteins that play role in cell cycle arrest, one important target of p53 is p21, a cyclin-dependent kinase inhibitor, which may cause cell cycle arrest [48] (Fig. 16.4).
In case, where DNA damage is so severe and repair machinery is unable to repair the damage, p53 can activate apoptosis pathways, which mainly comprises of its ability to regulate transcription of pro-apoptotic members of the Bcl-2 family. Bcl-2 family members harbor consensus p53 response elements in promoters of their genes [49]. p53 is an important target of curcumin, and various studies have shown that curcumin regulates the expression of p53 in vivo and in vitro models. Curcumin caused apoptosis in human breast cancer cells in a p53-dependent fashion. In MCF-7 cells, in which expression of wild-type p53 can be induced, curcumin (10 μM) treatment caused increase in p53 protein level as early as 4 h of treatment. This increased p53 level started causing increase in the level of Bax protein after 8 h, and the highest level was found after 24 h. To establish the role of p53 in inducing apoptosis, p53-null (MDAH041) and tetracycline-regulated wild-type p53-expressing cell lines (TR9-7) were employed and treated with curcumin. In p53-null (MDAH041) cells, curcumin failed to induce apoptosis. In TR9-7 cells, curcumin moderately induced apoptosis in low p53-expressing condition, i.e., in the presence of tetracycline, which downregulates wild-type p53 expression. However, when TR9-7 cells were released from tetracycline-inhibited p53 expression, curcumin induced a high level of apoptosis. This study indicates clearly that curcumin-induced apoptosis was p53-dependent [50]. Similarly, curcumin induced apoptosis at G2/M phase with deregulated cyclin D1 in mammary epithelial carcinoma cells, but not in their normal counterparts. Curcumin selectively caused increase in p53 levels at G2 phase of the cell cycle and release of cytochrome c from mitochondria into the cytosol in cancer cells.
Furthermore, experiments conducted with p53-null and dominant-negative and wild-type p53-transfected cells have established that curcumin-induced apoptosis was through p53-dependent pathway. However, curcumin also caused reversible arrest in normal mammary epithelial cells by downregulating cyclin D1 expression and disruption of their association with CDK4/6; curcumin also inhibited phosphorylation and, thus, caused inactivation of retinoblastoma protein. In normal cells, curcumin caused upregulation of p21/Waf1, which lead to arrest of cells in G0 phase of cell cycle and thus escaped from apoptosis in G2 phase. Conversely, in cancer cells cyclin D1 expression remained high, and thus they reached to G2 phase and had undergone apoptosis [51]. In patients with colorectal cancer (CRC), curcumin administration has shown significant effect on weight loss, decreased level of tumor necrosis factor (TNFα) in serum, and induction of apoptosis. Additionally, curcumin administration also increased p53 and Bax levels and decreased Bcl-2 significantly, explaining that p53 is an important target of curcumin in causing apoptosis in CRC [52]. Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death worldwide. miRNAs play vital roles in numerous steps of carcinogenesis and serve as feasible therapeutic cancer targets. In H460 and A427 cells, curcumin stimulated global miRNA levels. Importantly, miR-192-5p and miR-215 (miR-192-5p/215) are putative anti-oncogenic miRNAs in NSCLC, and curcumin caused significant change in the levels of these two miRNAs. Interestingly, curcumin-induced upregulation of these miRNAs was p53-dependent. Conditional knockdown of wild-type p53 disrupts the miR-192-5p/215 response in H460, A427, and A549 cells. Conversely, conditional expression of wild-type but not R273H mutant p53 caused upregulation of miR-192-5p/215 when treated with curcumin in p53-null H1299 cells. Additionally, miR-192-5p/215 targets XIAP for induction of apoptosis in these cells [53].
So far, we have discussed the studies which reported that curcumin-induced anticancer effects in various cancer models were dependent on p53. However, some groups have reported p53-independent pathways in curcumin-induced cell cycle arrest and apoptosis. Curcumin-induced cytotoxicity was investigated in p53+/+ and p53-/- HCT116 colon cancer cells, as well as mutant p53 HT-29 colon cancer cells. Curcumin caused significant decrease in cell proliferation and increase in number of dead cells in both the cell lines in a dose (2.5–160 μM)- and time (24–72 h)-dependent fashion. Curcumin also caused chromatin condensation in both p53+/+ and p53-/- HCT116 colon cancer cells. Curcumin treatment caused phosphorylation and induction of p53 in wild-type HCT116 cells; however, apoptosis was also observed in p53-/- HCT116 colon cancer cells indicating that the effect was independent of p53 status. A dose-dependent increase in the generation of superoxide anion in both the cell lines was reported, indicating the involvement of oxidative stress for eliciting apoptosis [54]. Similarly, curcumin caused dose-dependent apoptosis in eight melanoma cell lines, four with wild-type p53, while other four with mutant p53. Curcumin did not induce p53, indicating that curcumin-induced apoptosis was independent of p53 and was mediated through Fas receptor/caspase 8 pathway [55]. Curcumin selectively inhibited the proliferation of BKS-2, an immature B-cell lymphoma, and also induced apoptosis in dose- and time-dependent fashion. Curcumin decreased the level of p53, along with egr-1, c-myc, and Bcl-xL, indicating these effects were not dependent on p53 [56, 57].
4 Nrf2 and Curcumin
A major defense mechanism against oxidative stress is the activation of Nrf2 antioxidant response element signaling pathway, which regulates the expression of genes involved in removal of reactive oxygen species. Though, the regulatory mechanisms involved are not fully understood, but cytosolic proteins like Keap1 are some of the key players involved in the regulation of Nrf2. In normal conditions, Keap1 binds to Nrf2 which causes ubiquitin-mediated degradation of Nrf2. However, in case of oxidative stress, Nrf2 is phosphorylated that causes dissociation from Keap1 and translocation of Nrf2 into the nucleus. After entering the nucleus, Nrf2 may bind to small adaptor proteins such as musculoaponeurotic fibrosarcoma (Maf) and finally targets antioxidant response element (ARE). ARE is a cis-acting response element found in the promoter region of the genes involved in the detoxification of reactive oxygen species and maintaining homeostasis (Fig. 16.5).
Targets of Nrf2 involve GSTA2 (glutathione S-transferase A2), NQO1 (NADPH:quinine oxidoreductase 1), glutathione S-transferase Ya subunit, heme oxygenase1 (HO-1), and ϒ-glutamyl cysteine synthase [58]. Fen1 endonuclease, a DNA repair enzyme, is also closely associated with cell proliferation and is generally found in the development of breast cancer and is found upregulated in many cancers including breast cancer. The link between Fen1 overexpression and Nrf2 is not well established. MCF-7 breast cancer cells were treated with curcumin and a decrease in cell proliferation was observed. Additionally, curcumin treatment caused upregulation of Nrf2 with concomitant downregulation of Fen1 expression. Curcumin treatment also caused nuclear localization of Nrf2 that decreased promoter activity of Fen1 [59].
In case of renal epithelial NRK-52E cells, Nrf2 was upregulated when cells were exposed to low concentrations of curcumin. Curcumin also stimulated Nrf2 activity, and this effect was completely inhibited by the natural repressor Keap1. Curcumin caused translocation of Nrf2 from cytoplasm to nucleus and stimulation of Nrf2 association with ARE sequence and a significant increase in HO-1 expression and heme oxygenase activity levels [60]. PhIP (Amino-1-methyl-6-phenylimidazo [4,5-b]pyridine) is a heterocyclic amine (HCA) and has shown genotoxic and carcinogenic effects. PhIP is formed when meat products are cooked at high temperatures. PhIP generates reactive oxygen species, which in turn may cause DNA damage through formation of DNA adducts and DNA strand breaks. Curcumin blocks the PhIP-induced DNA damage by inhibiting ROS generation. MCF-10A breast epithelial cells were used to screen different dietary phytochemicals that may have potential to inhibit cytotoxicity caused by PhIP. PhIP increased ROS generation in a dose-dependent fashion; however, curcumin co-treatment blocked the ROS generation caused by PhIP. Curcumin also decreased the formation of DNA adducts and inhibited DNA strand breaks caused by PhIP exposure. Oxidative stress marker Nrf2 and its target genes such as NQO-1, GPX-1 (glutathione peroxidase), and GSR (glutathione reductase) and FOXO (forkhead box protein) and their targets such as catalase, GADD-45 (growth arrest and DNA damage-inducible 45), and PRDX-3 (thioredoxin-dependent peroxide reductase) have been studied. DNA damage markers γ-H2A.X and BRCA-1 were induced by PhIP, and curcumin treatment blocked the expression of these genes. p16 (cyclin-dependent kinase inhibitor 2A) expression was inhibited by PhIP treatment; however, curcumin treatment restored its expression. Curcumin targets genes that are involved in the management of oxidative stress. Nrf2 was also found to be an important target of curcumin in MCF-10A breast epithelial cells [60, 61].
5 NFAT as a Target of Curcumin
There are many regulatory mechanisms in cells, among them transcription factors are responsible to control the mRNA levels during the transcription process. They have DNA binding sites where they bind at a particular sequence of the genes to control their expression pattern. This regulation could be either ways by positively through the help of activators or negatively through repressors. NFAT (nuclear factor of activated T cells) a transcription factor initially reported in T lymphocytes with its five members (NFAT1–NFAT5); it acts as the transcription activator of IL-2 promoter and regulator of T cells [62, 63]. Recently, it was found that this protein was expressed in both immune and non-immune cells such as adipocytes, cartilage, pancreatic, cardiac and breast cells other than T lymphocytes [64,65,66,67,68,69,70]. From the discovery of this molecule, its role in multiple biological processes such as cell cycle, apoptosis, metastasis and angiogenesis was observed in invertebrates and vertebrates [69, 71,72,73]. NFAT is well known for controlling gene expression at the time of cell activation and differentiation in T lymphocytes [71, 74]. Calcineurin signaling is responsible for the regulation of NFAT in T cells [75].
5.1 Structure and Function of NFAT in Normal Physiological Condition
NFAT is composed of two conserved regions, i.e., NFAT homology region (NHR) which shares 22–36% and DNA binding domain (DBD) up to 64–72%, identical sequences among all the five families. NFAT in its inactive form in resting cells is localized in cytosol, mediated by highly NHR phosphorylation. This is activated by dephosphorylation of NHR region by calcium-/calmodulin-dependent serine/threonine phosphatase enzyme calcineurin, whenever there is an elevation in the level of intracellular calcium. Subsequently, the nuclear localization sequence (NLS) gets free after dephosphorylation, NFAT1-4 translocate into the nucleus, binds to the promoter site, and regulates gene expression. NFAT-mediated transcriptional activity is regulated by the reversible activation/inactivation shuttles operated between nucleus and cytosol by the dephosphorylation and phosphorylation enzymatic system [76]. All the NFAT are regulated by calcium-/calcineurin-dependent pathway except NFAT-5, which is regulated during osmotic stress conditions. NFAT-3 is the exception which is not expressed in the immune system, whereas others do [74, 77].
5.2 Curcumin Regulates Transcription Factor NFAT
T cells have a major role in organ transplant biology. The immune-suppressive action has immense importance during organ transplant where immune rejection or acceptance plays a convincing role for the successful transplant. From a longer duration, cyclosporin A (CsA) and FK-506 are widely used as key immune suppressor during transplantation, but the rising of CsA-resistant T cells along with toxic effects decreased their efficacy and the implanted organs become more prone to rejection. There are reports of immune-suppressive function of curcumin where its pretreatment enhanced survival of skin-grafted animal and also inhibited CsA-resistant T cells [78, 79]. The mechanism behind the suppressive action of curcumin on T cells is via NFAT where calcium (Ca) mobilization leads to NFAT inactivation and blocked NFAT-dependent cytokine expression [80].
In another report, curcumin’s cardioprotective activity was observed to be NFAT-dependent. Trypanosoma cruzi infection causes Chagas cardiomyopathy where cascade of endothelin-1 (ET-1) and Ca2+/NFAT activation occurs in cardiomyocytes and induces cyclooxygenase type 2 (COX-2) with release of prostanoids and prohypertrophic peptides. Curcumin hampered Chagas pathogenesis through interfering transcriptional activity of Ca2+-dependent NFAT2/NFATc1 and COX-2 and thereby declining prostaglandin levels in cardiomyocytes. BNP (brain natriuretic peptide) overexpression is a strong indicator of Chagas-mediated cardiac pathogenesis and is reduced by curcumin in both infected and uninfected cells [81]. In human colonic myofibroblasts, canonical transient receptor potential (TRPC) proteins responsible for sensation are inhibited by curcumin via TNF-alpha attenuation in NFAT-dependent manner [82]. Overall, the anti-inflammatory properties of curcumin in the form of TNF-alpha, COX-2, and calcium mobilization regulate NFAT-mediated response in cells. Thus, curcumin is an essential polyphenol phytochemical that regulates NFAT transcription factor during normal as well as pathological conditions.
6 Regulation of Oxidant and Antioxidant Enzymes
Oxidative stress which results in an imbalance between oxidants and antioxidants is a major player in the regulation of pathogenesis of various diseases such as neuronal cell injury, hemorrhage and shock, myocardial ischemia, cerebral brain ischemia-reperfusion injury, hypoxia, and cancer. Several natural products have been used for the management of oxidative stress and related disease. Curcumin has caught scientific attention as a potential therapeutic agent due to its bifunctional antioxidant properties. It has been reported that curcumin directly reacts with reactive species and induces an upregulation of various cytoprotective and antioxidant proteins. It is able to scavenge hydroxyl radicals (·OH), superoxide anions (O2·), hydrogen peroxide (H2O2), nitric oxide, singlet oxygen, peroxynitrite, and peroxyl radicals (ROO·) [83]. The presence of phenolic groups in the structure of curcumin might explain its ability to react with reactive oxygen species (ROS) and reactive nitrogen species (RNS).
6.1 Curcumin Regulates Oxidant Enzymes
The imbalance of oxidation and reduction in tissues during different steps of chronic inflammation contributes to a variety of human cancers [84]. Oxidants may lead to tumor initiation or progression due to overproduction of ROS by the member of NADPH oxidase (NOX) family of protein [85]. However, other potential sources of ROS such as the mitochondrial electron transport chain, the cytochrome P450 system, xanthine oxidase, myeloperoxidase, and uncoupled nitric oxidase synthase can also make substantial contributions to the oxidative atmosphere of the tumor microenvironment [86, 87]. Although curcumin is a powerful antioxidant, it has been shown that it can produce prooxidant effect at higher doses. At higher doses the phenolic hydroxyl group of curcumin and transition metal such as Cu (II) form a complex through chelation. After intermolecular electron transfer reaction, the complex leads to the formation of semiquinone, superoxide and hydroxyl radicals, respectively [88]. Moreover, the activity of thioredoxin reductase can be modified dose dependently by curcumin, leading to oxidation of NADPH by oxygen and subsequently formation of ROS [89].
6.2 Curcumin Regulates Antioxidant Enzymes
There are several enzymes expressed in normal and cancer cells that contribute to the production and degradation of ROS. The interaction and regulation of some of these enzymes by curcumin are as follows.
6.3 Superoxide Dismutase
The superoxide dismutases (SOD) are the major enzymes that catalyze the dismutation of the superoxide radicals into molecular oxygen or hydrogen peroxide. There are three different isoforms of SOD that have been identified, namely, cytosolic copper-/zinc-containing SOD (SOD-1 or CuZnSOD), mitochondrial manganese-containing SOD (SOD-2 or MnSOD), and the extracellular SOD (SOD-3 or ecSOD). SOD-3 is also a copper-/zinc-containing enzyme, mainly produced and secreted by vascular smooth muscle cells and also reported to bind with glycosaminoglycans present in vascular extracellular matrix on the cell surface of endothelial cells [90]. Single-charged anions such as fluoride and azide are fixed by all types of SOD, but they are distinct in their susceptibilities of Fe (I), Mn (I), or CuZnSODs [91, 92].
The decreased activity of antioxidant enzymes during oxidative stress can result in human cancers. The first line of defense against superoxide radicals is provided by SODs which have been studied in human cancers. The activity of CuZnSOD is found significantly lower in gastric adenocarcinoma samples than normal mucosa [93]. Similarly, the activity of MnSOD is often dysregulated during cancer development. In human esophageal cancers, the decreased MnSOD is associated with increased incidence of esophageal adenocarcinoma [94]. In human oral cancers, a high-level expression of MnSOD was associated with better disease-specific survival, especially in early-stage buccal mucosal squamous cell carcinoma and for patients with moderate or poor cell differentiation [95]. Current studies suggest that ecSOD may play important role in human lung carcinoma. In lung carcinomas the expression level of ecSOD was found downregulated which is the result of ecSOD promoter methylation and loss of heterozygosity [96]. In hepatocellular carcinoma, a virus-mediated tumor suppressor MnSOD gene was delivered which resulted in overexpression of MnSOD and induced cytotoxicity in hepatocellular carcinoma cells [97]. The overexpression of MnSOD has shown radioprotection as well as chemoprotection against antioxidant-based therapeutics [98], while in colorectal cancer, the increased mitochondrial DNA instability and elevated MnSOD expression were also observed [99]. Indeed, the overexpression of MnSOD in human cancer cell lines can cause increased H2O2 production and reduce tumor cell proliferation in the absence of anticancer agent [100].
6.4 Effect of Curcumin on SOD
A recent study has shown that curcumin oil preserves the expression of MnSOD in esophageal cancer cell and delayed the bile-induced esophageal injury and potential cancer progression in vitro [101]. A herbal medicine Curcuma aromatica has shown anticarcinogenic properties in a variety of cancer cell lines and animal models. In further studies, the esophagoduodenal anastomosis (EDA) rats were treated with Curcuma aromatica oil and the enzymatic activity and protein level of MnSOD found similar to the control. Hence, curcuma aromatica oil induces MnSOD expression and activity, which is associated with potential protection from incidence of esophageal adenocarcinoma [102]. In human breast epithelial cells which is transformed by the effect of radiation in the presence of estrogen, curcumin enhances the MnSOD protein and catalase expression that protect the cells from oxidative stress damages [103]. Das et al. reported that the expression of SOD in liver of Dalton’s lymphoma (DL) and DL+DMSO mice was significantly downregulated compared to normal. In DL and DL+DMSO mice, the expression and activity of both MnSOD and CuZn-SOD were found significantly downregulated. The activity of MnSOD in DL and DL+DMSO mice was 65% and 63% than normal mice, respectively. After treatment of curcumin with different doses such as 50–150 mg/kg body weight, MnSOD activity increased in a dose-dependent manner as up to 1.3–1.6-fold of DL+DMSO mice, respectively. Similarly, the activity of CuZn-SOD was found 61% decreased of normal mice. After treatment with curcumin, the activity of CuZn-SOD increases in a similar dose-dependent manner, up to 1.2–1.5-fold of DL+DMSO mice with 50–150 mg/kg body weight, respectively [104]. Hence, curcumin has potential to elevate the expression and activity of SOD in DL mice.
6.5 Glutathione Peroxidase and Catalase
Glutathione peroxidase (GPx) is a family of antioxidant enzymes that catalyze the reduction of H2O2 or organic peroxides such as lipid peroxide to water or corresponding alcohols typically using glutathione as a reductant [105]. Glutathione peroxidase has eight variants, out of which four GPx1-4 that have selenoproteins in their catalytic center are found in all mammalian cells. GPx6 is a selenoprotein found in human but not in other mammals [106, 107]. The presence of selenocysteine residue in each of the four identical subunits was suggested to ensure a quick reaction with the hydroperoxide and fast glutathione reduction [108]. GPx have an antioxidant function at different cellular compartments; GPx1 is most abundant in cytosol and mitochondria, GPx2 in gastrointestinal epithelium, and GPx3 abundant in plasma. GPx4 has several isoforms in vertebrates and have varied location and specificity. GPx5 containing cysteine, instead of selenocysteine in its active center, is a protein found in the epididymis [109]. GPx6 is expressed in olfactory epithelium [109]. GPx7 and GPx8 are cysteine GPx with low GPx activity.
Catalase is an antioxidant enzyme which is mainly located in the peroxisome of mammalian cells and, to some extent, in the cytosol. It has two enzymatic activities that depend on the concentration of H2O2. It removes H2O2 by forming water and molecular oxygen when concentration of H2O2 is high; however, at lower concentration of H2O2 and in the presence of suitable hydrogen donor (such as phenol, methanol, ethanol, and others), it acts paradoxically by removing H2O2 but oxidizing its substrate [110]. By removing H2O2, it indirectly detoxifies superoxide radicals which were turned into H2O2 by SOD.
It is reported that H2O2 induces apoptosis in vitro via activation of caspase cascade in a wide range of cancer cells [111]. Other reports have described a lower level of catalase expression in a large variety of tumor and cancer cell lines compared to normal cells [112, 113]. In healthy cells, i.e., transformed with T-antigen of SV-40 or Ras, catalase was found downregulated, but the underlying mechanism of this downregulation is still unknown [114, 115]. Cancer cell lines resistant to chemotherapeutic agents or hydrogen peroxide, the expression level of catalase was found to be altered [116, 117].
6.6 Effect of Curcumin on Glutathione Peroxidase and Catalase
During initial phase of metastasis, expression of various molecules is activated by NF-κB in both liver cells and tumor cells through H2O2-mediated pathways. The removal of H2O2 is considered to be an effective approach to inhibit metastasis [118]. The antioxidant enzyme GPx and catalase play important role in detoxification of H2O2. GPx also detoxify the lipid hydroperoxides using glutathione [119]. Catalase expression is generally suppressed in elevated ROS condition mainly by H2O2 at the site of cancer microenvironment and oxygen free radicals. Das et al. reported the low activity of catalase and GPx (GPx1, GPx2, GPx4, and Gpx5) in the liver of cancerous mouse compared to normal mouse, which confirms the depleted expression of antioxidant defense system. They also reported that GPx3 was detected as a faint and thin activity band only in DL- and DMSO-treated DL mice liver. However, in curcumin-treated DL mice and normal mice, these isoforms could not be detected. They also reported that curcumin shifted the activities of all isoforms of GPx and catalase toward normal. Curcumin upregulates the expression of Gpx1 in the liver of lymphoma-bearing mice. Further, the expression of catalase was also found to be upregulated in curcumin-treated DL mice. Thus, curcumin recovers the defected antioxidant defense system by upregulating the activity and expression of antioxidant enzymes. Moreover, curcumin treatment downregulates the p65/p50 and p50/p50 complexes in lymphoma-bearing mice. Also, curcumin treatment decreased the binding of both subunits to DNA in DL mice indicating that curcumin downregulates the NF-κB activation. The overexpression of GPx1 in curcumin-treated DL cancerous mouse was associated with inhibition of NF-κB activation in vivo. Hence, curcumin inhibits H2O2 signaling-mediated NF-κB activation in the liver of DL-bearing mice by upregulating the expression and activity of endogenous antioxidant enzymes GPx and catalase as well as by decreasing the oxidative status [120].
In a similar study, it was shown that curcumin reduced the DNA damage which was induced by inorganic arsenic (As III) in Swiss albino mice liver cells. Moreover, the As III exposure caused depletion of antioxidant enzyme such as catalase, SOD, GPx, GR and GST, and antioxidants like GSH were significantly increased by the treatment of curcumin in Swiss albino mice liver cells. In conclusion, this study shows the efficacy of curcumin in preventing DNA damage and quenching of ROS induced by As III in Swiss albino mice [121].
7 Anti-angiogenic Properties of Curcumin
Angiogenesis is a process of new blood capillary formation from the preexisting blood vessels. Angiogenesis is crucial for the tumor growth and expansion. Tumor cells import nutrients and oxygen for their uncontrolled growth through the tumor angiogenesis process. Tumor cells have capability to form new blood vessel through constitutive expression of proangiogenic factors such as the vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Angiogenesis is strictly controlled by anti-angiogenic molecules in mammals. There are several reports suggesting curcumin has the ability to modulate the tumor angiogenesis process by inducing anti-angiogenic stimulators [122].
Curcumin downregulates the expression of VEGF isomers such as VEGF-A, VEGF-C, and VEGFR2 and decreases microvessel density in breast cancer cell-implanted mice, which leads to inhibition of breast tumor growth. Rodrigues et al. reported that overexpression of osteopontin (OPN), a chemokine-like ECM-associated small integrin-binding ligand N-linked glycoprotein, is one of the major markers of cancer progression in several cancers including breast cancer [123, 124]. Moreover, the targeting of OPN and its downstream signaling pathways has shown promising therapeutic efficacy in various cancers including breast cancer [125]. It is also demonstrated that OPN enhances VEGF expression and hence promotes VEGF-dependent angiogenesis in breast cancer [126]. Philip et al. reported that curcumin suppressed OPN-induced tumor growth in nude mice, by suppressing the pro-MMP2 expression. They demonstrated that curcumin potentially suppressed the OPN-induced cell migration, tumor growth, and NF-κB-mediated pro-MMP2 activation by blocking the IKK/IκBα signaling pathways [127] Chakraborty et al. reported that curcumin inhibits the VEGF expression by abrogating the activity of NF-κB/ATF4 in OPN-induced MDA-MB231 implanted in nude mice. This result clearly suggests that curcumin acts as a potent anti-angiogenic agent in OPN-induced tumor angiogenesis in human breast cancer [128].
Another potent angiogenic molecule is bFGF which plays role in both new blood vessel formation and migration of cancer cells. It is reported that curcumin inhibits the neovascularization even in induced bFGF condition of mouse cornea. Moreover, in TPA-induced transformed human keratinocyte HaCaT cells, curcumin showed no effect on VEGF expression. These results suggest that curcumin acts as an angiogenesis inhibitor [129]. In contrary, Gururaj et al. reported that curcumin downregulates the VEGF mRNA and protein expression level in EAT cells [130]. They also reported the downregulation of Ang1 and Ang2 (newly identified angiogenic ligand) by curcumin in EAT cells.
Perry et al. reported curcumin can cross blood-brain barrier and inhibit angiogenesis in in vivo study. Thus curcumin decreased the gelatinolytic activities of MMP9. Furthermore, curcumin inhibited glioma-induced angiogenesis by decreasing endothelial cell marker such as CD31 and CD105 from newly formed vessels and also by decreasing hemoglobin content in glioma tumor [131]. These results indicate that curcumin may act as an inhibitor of angiogenesis in in vivo.
As reported earlier, the HGF/c-Met pathway promotes tumor angiogenesis and hence cancer progression. When HGF binds to its receptor, c-Met (expressed in endothelial cells) stimulates endothelial cell growth, invasion, and motility [132]. Inhibition of c-Met activity can impair the survival, invasion, and tubulogenesis of HUVECs in vitro and reduces neovascularization and formation of microvessel in tumor model [133, 134]. Jiao et al. reported curcumin suppressed HGF-induced phosphorylation of c-Met/AKT/mTOR in human lung cancer cells. Moreover, curcumin suppressed the EMT in c-Met-overexpressed A549 and PC-9 cells and also reduced the expression of vimentin and increased the expression of E-cadherin [135]. It is reported that HGF promotes angiogenesis by upregulating VEGF expression [136]. Their results show, curcumin significantly decreased the expression of VEGF and microvessel density.
In pancreatic cancer, curcumin has shown to downregulate the NF-κB-regulated gene products such as VEGF, MMP9, cyclin D, and IKKα/β, hence induce apoptosis, and reduce angiogenesis in in vitro and in vivo models [137].
Chen et al. reported that curcumin suppressed the secretion of VEGF in U937 cells and Raji cells that was upregulated by TNFα treatment. The mRNA expression of VEGF165 and VEGF121 were increased by TNFα and reduced by curcumin. They also reported that VEGF and TNFα can induce angiogenesis and curcumin can inhibit angiogenesis in ECV304 cells [138]. Overexpression of COX-2 and VEGF are reported in HepG2 cell line [139]. COX-2 can induce angiogenic factor and can directly take part in angiogenesis. Curcumin decreased the tumor-induced overexpression of VEGF and COX-2 via inhibiting NF-κB signaling pathway [140, 141]. In an in vivo study, Sabrina et al. reported that curcumin inhibited the angiogenesis and tumor growth in a heterotrophic mouse model of breast cancer by downregulating NF-κB-regulated genes such as cyclin D1, p65, and PECAM-1 [142].
Further, hypoxia which is normally detected in the central part of solid tumor can be an important cause of angiogenesis via activating the expression of angiogenic factors [143, 144]. Hypoxia-inducible factor (HIF) is a main factor of this process. HIF consisting HIF-1α and HIF-1β homodimers can regulate the transcription of hypoxia-activated genes. In normal oxygenic condition, α subunit of HIF-1 is rapidly degraded but stable under hypoxia while HIF-1β constitutively expressed [145]. It is reported that curcumin strongly reduces the expression and activity of HIF-1α but did not affect the mRNA of HIF-1α. This suggests that the curcumin reduced the stability of HIF-1α at protein level. To confirming this notion, proteasome inhibitor LLnL was added to the HepG2 cells and checked the protein level of HIF-1α. The treatment of LLnL restored the HIF-1α protein level which was decreased by curcumin. This result indicates curcumin-mediated degradation of HIF-1α is probably proteasome-dependent. Moreover, curcumin reduced the hypoxia-induced mRNA and protein expression of VEGF. These results suggest that curcumin reduces the transactivation function of HIF-1α. These results suggest that hypoxia-induced angiogenesis is inhibited by curcumin via downregulating the activity of HIF-1α [146].
It is reported that COX-2 and COX-derived prostaglandins, induced by VEGF, play important role in HIMEC angiogenesis [147]. Binion et al. reported that the pretreatment of curcumin inhibits the angiogenic effects of VEGF-induced COX-2 mRNA and protein expression as well as PGE2 production in HIMECs. Pretreatment of HIMECs with NS398, a COX-2 inhibitor, reduced the angiogenesis in vitro. These results support the idea that COX-2 and COX-derived prostaglandins play essential role in angiogenesis. They concluded that the inhibitory effect of curcumin could be due to the suppression of PGI2 through the inhibition of COX-2 expression [148]. Curcumin has also shown to inhibit the endothelial nitric oxide synthase (eNOS) expression in endothelial cells which contribute to impaired endothelial tube formation [149]. Taken together, these results suggest that curcumin inhibits angiogenesis by modulating multiple pathways in endothelial cells.
Similarly, Yoysungnoen et al. reported the tumor neocapillary density (NCD) which was significantly increased in HepG2 group of BALB/c nude mice and this increment of neocapillary density was characterized as a time-dependent manner. Moreover, the tissue perfusion on day basis also supported the increased NCD value. The increase NCD and tissue perfusion of HepG2-group was remarkably reduced by curcumin treatment. However, the upregulated COX-2 expression in HepG2 group was suppressed by curcumin treatment in a dose-dependent manner. Interestingly, the increased serum VEGF in HepG2 group was downregulated by the treatment of curcumin in a dose-dependent manner as well [140]. These results also suggest that curcumin could inhibit tumor angiogenesis through reduction of angiogenic biomarkers such as COX-2 and VEGF.
The effect of curcumin can influence the expression profile of micro-RNAs (miRs) in angiogenesis. Recently, miRs came in focus as a powerful mechanism in regulation of gene expression after transcription. In a study, the expression profile of miRs in HUVECs was extensively investigated, and most significantly dysexpressed miRs were selected for their supposed role in the anti-angiogenic effect of curcumin on HUVECs. miR-1246 has been reported to promote angiogenesis by activating Smad signaling in endothelial cells [150], and miR-1275 has been reported to contribute in tissue heterogeneity establishment via epigenetic mechanisms [151]. By bioinformatics means the targets of miR-1275 and miR-1246 were detected VEGF and NKAP, respectively. The NF-κB p65 activation was detected by inhibiting the miR-1246 inhibitor. It also upregulated NF-κB p65 level leading to increased expression of cyclin D1, MMP9, and VEGFB, which finally induced angiogenesis [152]. The anti-angiogenic effect of curcumin with regard to the function of miR-1246 was contrary to the previous report by Yamada et al. [150], in which miR-1246 promotes angiogenesis via activation of Smad pathways.
8 Anti-metastatic Properties of Curcumin
8.1 Anti-metastatic Effects of Curcumin on Activation of Nuclear Factor-Kappa B (NF-κB)
NF-κB is a transcription factor which regulates the group of genes that control cell proliferation and survival. Tumor invasion-related proteins such as MMPs, urokinase-type plasminogen activator (uPA), interleukin-8 (IL-8), and many other chemokines are regulated by NF-κB [153, 154]. NF-κB activation can be induced by many cancer promoter molecules such as tumor necrosis factor and hydrogen peroxide, and the most common is phorbol ester [155, 156]. Curcumin has the potential to inhibit this activation proved by several works. It is reported that curcumin inhibits the activation of NF-κB through phosphorylation and degradation of IκBα [157, 158]. In estrogen receptor (ER)-negative breast cancer cells and prostate cancer cells, curcumin has shown to inhibit the activation of p65, a member of NF-κB transcription factor family [159, 160]. In breast cancer cells, curcumin downregulates the NF-κB. This downregulation of NF-κB leads to downregulation of many cancer proliferative genes and induces apoptosis, hence inhibiting cancer cell invasion and metastasis [161].
8.2 Effect of Curcumin on Signal Transducer and Activator of Transcription 3 (STAT3)
STAT3 is a transcription factor that is constitutively expressed/activated in cancer cells and regulates the cell survival, cell cycle, angiogenesis, and metastasis [162]. In particular, the relation of activated STAT3 with metastasis has been reported in many cancer tumors. Curcumin inhibited the SCLC cell invasion through downregulation of the expression of STAT3-regulated gene products involved in the invasion (e.g., MMP2 and MMP7). Curcumin also downregulates VEGF which explains its anti-metastatic activities. The role of JAK has been concerned in activation of STAT3; curcumin suppressed the phosphorylation of JAK-1, JAK-2, and JAK-3 (upstream proteins of STAT3) in SCLC cells [163]. Curcumin blocked the activation loop of JAK and suppresses the interleukin-6 (IL-6) cytokine signaling pathway and, hence, blocks the signaling required for phosphorylation and activation of STAT3 [163]. As curcumin significantly suppressed the STAT3- and STAT-regulated genes, hence, curcumin treatment reduces the rate of metastasis in SCLC cells. Curcumin also inhibits IL-6-induced phosphorylation of STAT3 in myeloma cells [156, 164] as shown in Fig. 16.6.
Curcumin inhibits invasion, migration, and angiogenesis. Curcumin blocks the activation loop of JAK and suppresses the interleukin-6 (IL-6)-mediated cytokine signaling pathway and blocks phosphorylation and activation of STAT3. STAT3 modulates transcription of many genes involved in various processes leading to invasion, migration, and angiogenesis
8.3 Effect of Curcumin on Inflammatory Cytokines
In many cases, cancer development and progression are influenced by chronic inflammation. Inflammatory cytokines have the ability to enhance cancer progression and development by providing growth factors and attracting the mononuclear cells to the cancer site. Angiogenesis is an important process in the cancer progression; the inflammatory cytokines such as CXCL1 and CXCL2 were found to be involved in this process [165]. CXCL1 has been shown to promote migration of breast cancer cells in vitro [166]. It has been identified that CXCL1 and CXCL2 are the major targets of curcumin as anticancer agents in breast cancer cells. In this study, curcumin has shown to downregulate the mRNA and protein expression of CXCL1 and CXCL2 in a metastatic breast cancer cell line MDA-MB-231. In another study, Helbig et al. has shown that the expression of chemokine receptor CXCR4 is directly induced by NF-κB to promote migration and metastasis in breast cancer cells [167]. Hence, curcumin reduces breast cancer metastasis through decreasing NF-κB-mediated expression of two inflammatory cytokines, namely, CXCL1 and CXCL2, and also reducing the chemotactic receptor CXCR4. It has been reported that the promoter regions of both CXCL1 and CXCL2 genes are identical and in proximity and contain a perfect binding site for NF-κB; hence, curcumin downregulates these inflammatory cytokines by targeting NF-κB in metastatic prostate cancer cells [158].
8.4 Effect of Curcumin on Urokinase Plasminogen Activator (uPA) Expression
Cancer cells release a serine-specific protease uPA, which after binding to its receptor uPAR, activates plasmin; this plasmin then mediates the degradation of extracellular matrix (ECM) [168]. The excessive degradation of ECM from primary tumor is a crucial step for cancer invasion and metastasis. uPA secretion is regulated by NF-κB. In breast cancer cells, curcumin significantly reduced the uPA expression and NF-κB DNA binding activity. In colon cancer cell line, curcumin decreased the expression of uPA and MMP9 through NF-κB inhibition. And also the NF-κB inhibition is mediated by activation of AMPK (AMP-activated protein kinase). Moreover, authors have concluded that curcumin suppressed the binding of p65 NF-κB to the uPA and MMP9 promoter region [169]. Based on above results, it can be concluded that curcumin inhibits the invasion and adhesion of breast and colon cancer cells through downregulating the expression of uPA and MMP9 via inhibiting NF-κB activation.
8.5 Effect of Curcumin on the Expression of Matrix Metalloproteinase (MMPs)
MMPs are calcium-dependent zinc-containing endopeptidases, capable of degrading all kind of extracellular matrix (ECM) proteins [168]. They play a major role in cell proliferation, migration, angiogenesis, and apoptosis. The expression of MMPs is finely controlled and regulated by tissue inhibitors of metalloproteinase (TIMPs) during normal physiological conditions. However, the overexpression of MMPs is the result of disturbance in balance between MMPs and TIMPs, which further results in various pathogenic processes including tumor invasion and metastasis [170, 171]. Most of the human cancer cells overexpress MMPs, which is related to metastasis. The two main MMPs which degrade the major components of basement membrane are MMP2 and 9 [172]. Hence, these two MMPs are the main therapeutic target for anti-metastatic drugs.
In human lung cancer cells (A549), curcumin inhibited the MMP2 and 9 and VEGF in a dose-dependent manner and hence inhibited the invasion and migration [173]. The anti-invasive properties of curcumin are reported in human lung cancer 801D cells in vitro and in vivo in a mouse model. In this study, curcumin inhibited the Rac-dependent signaling pathways and downregulated the expression of MMP2 and 9, hence inhibiting tumor invasion and migration in both in vivo and in vitro [174]. Overexpression of Ras homolog gene family member A (RhoA) induces the expression of MMPs, which finally promotes the invasion of cancer cells [175]. Breast cancer cells have poor prognosis due to its invasion and migration. Lysophosphatidic acid (LPA) is an inducer of cancer cell invasion and metastasis by activation of Rho/ROCK/MMP signaling pathway in breast cancer. Curcumin inhibits LPA-induced invasion by attenuating Rho/ROCK/MMPs pathway in breast cancer cells [175].
12-O-Tetradecanoylphorbol-13 acetate (TPA) is a potent tumor promoter. TPA promotes tumor invasion of breast cancer cells by inducing MMP9 via protein kinase C pathway [176, 177]. Curcumin has shown inhibition of TPA-induced MMP9 expression and, hence, cell invasion through downregulation of NF-κB and AP1 activation. TPA-induced phosphorylation of p38 and JNK and translocation of PKCα from cytosol to membrane are also strongly inhibited by curcumin [176].
The anti-invasive property of curcumin is also demonstrated in ER-negative breast cancer cell line (MDA-MB231) in a dose-dependent manner. Curcumin has shown to downregulate MMP2 and upregulate TIMP1 and 2 [178]. Additionally, curcumin was shown to inhibit the expression of VEGF and basic fibroblast growth factor in ER-negative breast cancer MDA-MB231 cells [178]. In another study, curcumin was shown to downregulate the expression of MMP2 and 9 and upregulate the expression of TIMP1 and 4 in ER-negative cell lines in a dose- and time-dependent manner. Based on the above findings, it is concluded that curcumin significantly inhibits MMP9 activity by downregulating its expression and upregulating TIMP1 and 4 gene expression in breast cancer cells.
8.6 Effect of Curcumin on Epithelial to Mesenchymal Transition (EMT)
Epithelial to mesenchymal transition (EMT) is an important event in the metastatic cascade where the cells acquire migratory and invasive capabilities [179]. The effect of curcumin on EMT and related gene in breast cancer cells has been evaluated. For this study Marcela Gallardo and Gloriam M calaf [180] used triple-positive and triple-negative breast cancer cell lines for ER, PgR, and Erb-3 in relation to EMT process. Curcumin decreased the expression of EMT markers, namely, E-cadherin, Slug, AXL, and Twist1, in MCF-10F (triple-negative) while N-cadherin, β-catenin, Slug, AXL, Twist1, vimentin, fibronectin, ZEB2, EZH2, and STAT3 in tumor 2 (triple-positive) and E-cadherin, N-cadherin, Twist1, AXL, and fibronectin in MDA-MB231 (triple-negative) cells compared to its counterpart. Since MDA-MB231 cells after treatment with curcumin decreased the expression of EMT gene and induced morphological changes, hence, curcumin triggered EMT in MDA-MB231 cells. In this study curcumin inhibited Slug expression which affects E-cadherin and vimentin resulting in decreased cancer cell invasion and metastasis [180].
It is reported earlier that LPS (lipopolysaccharide) mediated the EMT in breast cancer cells by modifying NF-κB signaling [181]. Hunag et al. reported that curcumin inhibits the LPS-induced EMT in breast cancer cells. They have also suggested the ability of curcumin to inhibit tumor invasion is associated with inactivation of NF-κB-Snail signaling and regulate the expression of the important downstream EMT marker, E-cadherin (upregulation) and vimentin (downregulation) [182]. Similarly Chen et al. reported that curcumin suppresses doxorubicin-induced EMT via inhibiting the TGF-β and PI3K/AKT signaling pathways in a triple-negative (ER, PR, and HER2-negative) breast cancer cells [183].
9 Synergistic Effect of Curcumin with Other Chemopreventive Agents, Chemotherapeutic Agents, and Radiation Therapy
The rise of cancer burden is huge worldwide, where 14.1 million new cases with 8.2 million deaths occurred in the year 2012 [184]. Despite depending on available cancer treatments including radiotherapy, surgery, cytotoxic therapy, and hormonal and targeted immunotherapies, still there is need of effective treatment modalities to achieve a better cure [185]. Cancer is a complex process operated via variable multiple signaling networks in the cells, which is very difficult to target by any single drug used presently. Thus the success rates of these therapies are not so encouraging and bring secondary complications in patients during and after treatments [186]. Cancer cells developed various escaping methods such as increasing drug efflux pumps, quicker drug metabolism, increasing self-repair pathways, and expressing newer or modified drug targets against the available conventional treatments [187]. Therefore, the use of multidrug phenomena in different combinations could be able to suppress most of the survival mechanisms raised by cancers and may enhance the efficacy. In clinics, combinatorial treatment approach has been adopted to maximize the overall effect on cancer therapeutics [187, 188]. Employing combinatorial therapy will target multiple pathways which will make difficult to survival of cancer cells. Thus the combinatorial therapy may solve the shortcomings of cancer therapeutics. Still this approach is not very easy to apply because of maintenance of proper doses and their unknown interaction and solubility. However, overall, the combinatorial treatment strategy will be a better application than the other conventional treatments if proper combination and safety measures will be taken during the application [189].
Despite curcumin’s superior antitumor properties, still its use is restricted because of poor bioavailability, water insolubility, short-life span, rapid systemic clearance, and quick metabolism in the gastrointestinal tract [190]. The reason behind the insolubility in water is due to its molecular structure [(1E,6E)-(1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione)] which is a keto form in acidic, neutral, and solid conditions. The pKa value which lies at 8.54 makes it difficult to dissolve in aqueous solution and therefore reduces bioavailability [191]. Therefore, to overcome these problems, curcumin was used in combination with chemopreventive agents and radiotherapy. In this section, we have summarized the use and effect of various combinatorial agents along with curcumin for cancer treatments.
9.1 Curcumin Used in Combination with Other Chemopreventive Agents
Chemoprevention concept in recent years is found to occupy one of the major places in cancer treatment due to its potential anticancer activity with lesser side effects [192]. Many of them (COX-2 inhibitors, green and black tea derivatives, β-carotene, vitamins, isothiocyanates, and farnesyl transferase inhibitors) have been used as common food stuff or in ancient medicine among different populations worldwide [193].
Presently, we are aware about the chemopreventive potential of curcumin and its limitations against various cancers through several mechanisms. So, in order to improve its anticancer efficacy to a much significant level, combination of other known chemopreventive agents with curcumin is required. Here, we are going to address such combinations used against cancers with their effective anticancer effects.
9.1.1 Curcumin-Phospho-sulindac
There were various reports showing the cancer prevention and metastasis by the use of NSAIDs (Nonsteroidal anti-inflammatory drugs), but it was associated with renal and gastrointestinal toxicities. Considering this problem, one group of scientists developed a novel phospho form of NSAIDs, for example, phospho-sulindac (PS, OXT-328), with inhibitory action against breast and colon cancer. PS alone was unable to prevent human lung cancer xenografts but strongly inhibited when curcumin in micellar nanoparticulate formulation was orally supplied to mice prior to PS. This combination showed a promising drug combination which synergistically suppressed tumor growth and improved the pharmacokinetics of PS. Thus, it was observed that curcumin may play a role in enhancing cellular uptake and bioavailability of PS in lung cancer [194].
9.1.2 Curcumin-Aspirin-Sulforaphane
In this study, the combination of curcumin, aspirin, and sulforaphane was encapsulated with the help of solid lipid nanoparticle, and the effect was observed on pancreatic cancer cells Panc-1s and MIA PaCa-2. This encapsulation was used to improve the sustained release of drug for longer duration. Synergistic effect was observed both in MTS cell viability and apoptosis studies where the significance rate was comparatively higher than the treatments alone. Thus, curcumin successfully played a major role in reducing pancreatic cancer progression synergistically in the presence of aspirin and sulforaphane [195].
9.1.3 Curcumin-Leflunomide-Perindopril
Here, curcumin was combined with two anti-angiogenic drugs leflunomide and perindopril where the combinations were used to target angiogenic pathways in mice, developed with diethylnitrosamine (DEN)-induced hepatocellular carcinoma (HCC). The effect was observed on the expression of CD31-positive endothelial cells and hepatic microvessel density in mice with HCC as compared to the normal liver. The elevated level of hepatic VEGF and HIF-1α in the DEN-treated mice was synergistically abrogated in the presence of the combinations, as compared to the singular treatments of leflunomide and perindopril. Curcumin potentiated the anti-angiogenic activity of leflunomide and perindopril in HCC mice model and improved chemoprevention [196].
9.1.4 Curcumin-Catechins
In this study, curcumin and green tea catechin that have anticancer activities against wide varieties of cancers were used together as combinatorial treatment approach against colon cancer. This combination significantly reduced the number of aberrant crypt foci per rat; however, there was no change in foci numbers with curcumin and catechins when treated separately. The colon cancer proliferation index was synergistically inhibited to a significantly higher level in combinatorial treatment as compared to other treatment groups. This showed the improvised efficiency in the chemopreventive value of catechins in the presence of curcumin in colon carcinogenesis [197].
9.1.5 Curcumin-(–)-Epigallocatechin-3-gallate (EGCG)
In another combinatorial treatment, EGCG- combined with curcumin wastested in human oral epithelial cells (normal, premalignant, and malignant). Here, the benefits obtained after combining these two agents were the reduction of 4.4–8.5-fold dose of EGCG and 2.2–2.8-fold in case of curcumin. Apart from this, they showed synergistic inhibitory effect on cell proliferation and cell cycle arrest and proved curcumin as a significant partner as a chemopreventive agent [198].
9.1.6 Curcumin-Silymarin
Two popular anticancer phytochemicals, silymarin and curcumin, when used in combination to treat colon cancer, synergistic effects were found on cell proliferation inhibition and increased apoptosis with membrane blebbing. The caspase 3/7 activity was almost doubled from threefold to fivefold when combinations were employed compared to curcumin or silymarin treatment alone in colon cancer. Thus, curcumin was found to sensitize colon cancer cells to silymarin treatment and to improve chemopreventive status in colon cancer [199].
9.1.7 Curcumin Used in Combination with Other Chemotherapeutic Agents
Chemotherapy used presently has mild to severe toxicity; however, these agents are still in use as major treatments for cancer. To improve the quality of cancer treatment with lesser side effects, it is now used in combination with other treatments such as surgery, radiotherapy, etc. In combination, chemopreventive agents are emerging candidates in cancer therapy that are well suited to support chemotherapeutic agents in an efficient manner at present [200]. Recent studies such as DEN-induced HCC inhibition along with various reports with dose- and time-dependent inhibition of stomach, duodenal, esophageal, colon, and oral cancer support curcumin to be a very good chemopreventive and chemotherapeutic agent against cancers [201]. To further improve the efficacy and to reduce the side effects of chemotherapeutic agents used during cancer treatment, curcumin was used in combinations with doxorubicin, fenretinide, temozolomide, carboplatin, paclitaxel, resveratrol, etc. We have discussed some of the combinatorial studies which showed to be promising aspect in future anticancer treatment regimens.
9.1.8 Curcumin-Doxorubicin
Chemosensitizers are small molecules having significant role in chemotherapy where MDR developed by cancer cells gets reversed and enhances the chemotherapeutic potentials of drugs in vitro. But at the same time, chemosensitizers are not much successful candidates with in vivo cancer studies because MDR response was not always effectively reduced. To minimize the limitations and improve the chemosensitizer response of two chemotherapeutic agents, curcumin and doxorubicin were used together with the help of nanotechnology. This strategy was found to be effectively causing sensitization of tumors with proper loading, co-delivery, and co-release of chemotherapeutic agents which solve the problems of conventional chemosensitizers. Subsequently, curcumin augmented the cytotoxicity of doxorubicin against MCF-7/ADR cells. Thus, the combinatorial approach enhanced the antitumor efficacy of these chemotherapeutic agents against MDR cancers [202]. In another study, these drug combinations successfully achieved the synergistic anticancer activity through chemosensitization of MDR tumors and DEN-induced HCC tumor model [203].
9.1.9 Curcumin-Fenretinide
Curcumin and fenretinide in combinatorial are extensively used as chemotherapeutic agents against non-small cell lung cancer and have showed significant elevated cytotoxicity in vitro as well as in vivo. This is associated with synergistic overexpression on pro-apoptotic molecule cleaved PARP and also regulates glucose-regulated protein 78 (GRP78), chaperon protein induced by curcumin of endoplasmic reticulum. Therefore, curcumin and fenretinide combination was found to be kpromising in the treatment of lung cancer [204].
9.1.10 Curcumin-Temozolomide
Temozolomide is one of the most important DNA alkylating agents that has been used as a chemotherapeutic drug in glioblastoma treatment. However, its use in this cancer has been limited due to the development of resistance. The role of curcumin as a chemosensitizer in various cancers encouraged the combination of these drugs against glioblastomas. It was found that curcumin potentiates the apoptosis effect of temozolomide to a synergistically higher level than the apoptosis with any particular treatments alone. This is based on the pronounced combinatorial effect by ROS generation and p-Akt/mTOR pathway inhibition which leads to an optimized therapy in glioblastoma treatment [205].
9.1.11 Curcumin-Carboplatin
Carboplatin is a commonly used chemotherapeutic agent against non-small cell lung cancer (NSCLC), but its efficacy is retarded by the survival signaling pathways along with drug resistance cell development in tumors. Here, the use of curcumin acts as a chemosensitizer when used with carboplatin and produces improved efficacy against lung cancer cells. The synergism of these compounds was found to be efficient against various events of cancer such as on cell proliferation, apoptosis, migration, and invasion in NSCLC cell line, A549. This study again provides support that curcumin can be a strong candidate in the field of cancer treatment with carboplatin [206].
9.2 Curcumin Used in Combination with Radiotherapy
Ionizing radiation either from external or internal sources is one of the three major pillars of cancer therapy including surgery and chemotherapy [207]. The cytotoxicity obtained through radiotherapy is an effective approach to treat cancers but the limitations starts with severe side effects. This is very challenging to deliver a maximum dose to the malignant cells without affecting the normal healthy cells surrounding them, which consequently reduces the amount of radiation. Another major limitation with radiotherapy is the development of resistance, i.e., radioresistance, similarly like chemoresistance during chemotherapy [208]. The above major limitations of radiotherapy can be broadly avoidable by considering two objectives such as radiosensitizers and radioprotectors [209]. Curcumin has both these properties established with its significant contributions when combined with radiation therapy against various tumor cells [210]. Here, we have discussed some of the studies where radiotherapy is used in combination with curcumin with maximizing tumor killing efficiency along with minimizing side effects.
9.2.1 Curcumin as a Radio-sensitizer
The role of radio-sensitizers is to reduce the irradiation doses which will lower the chance of affecting nearby healthy cells and at the same time will enhance cytotoxicity specifically on the tumors [211]. Curcumin in combination with radiation was found to target receptor of growth factor signaling, cell cycle, and angiogenic and epigenetic modulation pathways in glioblastoma cells, thereby synergistically inducing cell death through apoptosis as well as autophagy. This treatment also hinders the chemo- and radio-resistance phenomena adapted by glioblastoma cells and makes them sensitize toward death [209]. Radio-sensitization of curcumin was also found in nasopharyngeal carcinoma where it was reducing the expression of multidrug resistance gene 1 (MDR1) by increasing miR 593. This radio-sensitization was obtained both in vitro and in vivo studies where 100 mg/kg of curcumin was combined with 4 Gy irradiation [212]. In in vitro study on hepatocellular carcinoma; when treated with curcumin and radiation, cell proliferation, NF-B activity, and metastasis-related pathway were inhibited significantly [213]. In another study, the dual nature of curcumin and trans-resveratrol was found in the form of radio-protector on non-cycling lymphocytes, whereas radio-sensitizer on cycling lymphocytes [214]. These are some of the evidences which support the role of curcumin as a radio-sensitizer in the form of an adjunct small molecule to improve the main stream radiation therapy in cancer treatment.
9.2.2 Curcumin as a Radio-protector
Cancer radiation therapy will boost up not only by radiosensitizers but also needs support from radioprotectors. As we have seen in the previous section, curcumin was showing its radio-sensitizing effects on various cancers; it also possess radio-protecting action on non-tumorigenic organs [208]. This section will comprise some studies showing the radio-protective role of curcumin. An initial in vivo study has been performed on mice with head and neck cancer where the mice were categorized into four groups on the basis of treatments before irradiation of 20 Gy. The groups containing curcumin- and lycopene-treated mice had reduced damage significantly on parotid glands as compared to the DMSO or control mice with irradiation [215]. Curcumin analog diarylpentanoid curcumin was found to be a very good radioprotective agent from gamma radiation to the intestinal mucosa of mice [216]. Curcumin and trans-resveratrol are observed to be radio-protective antioxidant polyphenols with protection for non-cycling lymphocytes [212]. There are very few reports available on radio-protective role of curcumin. For the beginning, the above mentioned evidences are sufficient to proceed further for the evaluation of radio-protective role of curcumin and associated mechanisms in various cancer models.
10 Curcumin in Clinical Trials
Turmeric which is a readily available source of vital curcumin is a popular spice, food coloring, and flavoring agent in several Asian cuisines. Dose of curcumin estimated in people consuming high levels of turmeric in their diet is nearly about 150 mg/day [217, 218] which is approximately 20 times lower than the recommended. In a short-term human trial of 3–4 months, suggested concentrations of 3600 mg/day induced low level of toxicity and is safe for future cancer chemoprevention clinical trials [219,220,221]. Inhibitory effect of curcumin on carcinogenesis has been evident in hundreds of scientific research on several animal models of various tumor types of different origin including mammary carcinoma, oral cancer, intestinal tumors, leukemia, prostate, breast, pancreatic, colorectal, cervical and colon cancers. Here in this section, we are going to discuss some information on these clinical trial studies. Numerous preclinical trials, in vitro studies and animal model testing have verified curcumin as a chemopreventive agent which intervene the array of cell signal transduction pathways and molecular mechanisms involved in cancer progression. Safety, tolerability, bioavailability, and mechanism of action of curcumin against multiple human chronic diseases have been claimed through comprehensive clinical trials over the past quarter century. These pre-clinical and clinical trials suggest low bioavailability of curcumin in patients following oral application and readily elimination from body. The key factors which are primarily considered to design cancer chemoprevention clinical trials are (i) the doses at which curcumin should be supplemented, (ii) the selection of the participants for the trials, and (iii) the duration of the trials and respective follow-ups.
10.1 Prostate Cancer
A pilot-scaled phase II was designed after successfully completing phase I to assess the efficacy of docetaxel/curcumin in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (CRPC). The study was conducted within a group of 30 patients with progressing CRPC and a rising prostate-specific antigen (PSA) who received docetaxel/prednisone in standard conditions for 6 cycles in combination with orally supplied curcumin, 6000 mg/day (day –4 to day +2 of docetaxel). The result revealed that 26 patients received the scheduled treatment, 2 progressed and 2 died before the end of treatment. Fifty-nine percent of patients responded toward PSA within first three cycles (88% of responders), 40% patient partially responded, and 20 patients were 100% curcumin compliant. The study produced a high response rate, good tolerability, and patient acceptability for randomized trial [222].
10.2 Cervical Cancer
A phase II randomized four intervention arm controlled study was performed to evaluate the efficacy of Basant polyherbal vaginal cream that contained extracts of curcumin, reetha, amla, and aloe vera and of curcumin vaginal capsules to eliminate HPV infection from the cervix. Screening of women was done by Pap smear and HPV DNA test. HPV-positive women without high-grade cervical neoplasias (N=287) were treated with vaginal Basant cream, vaginal placebo cream, curcumin vaginal capsules, and placebo vaginal capsules, respectively. The assigned formulation was applied once per day for 1 month except during menstruation and recalled within a week of the last application for repeat HPV test, cytology and colposcopy. HPV clearance rate which was 87.7% in Basant arm was significantly higher than 73.3% of combined placebo arms. Rate of clearance in curcumin application was 81.3% than placebo. Mild to moderate vaginal itching and irritation was slightly higher after Basant application [223].
10.3 Breast Cancer
Research on open-label phase I dose trial in 14 patients with advanced and metastatic breast cancer was conducted in order to find maximal tolerated dose of the combination of dose-escalating curcumin and docetaxel. Six cycles of 100 mg/m2 docetaxel were administered, and curcumin was orally given from 500 mg/d for seven consecutive days by cycle. Three dose-limiting toxicities were observed at the last dose level of curcumin. Two out of three patients at this dose level refused to continue treatment, leading maximal tolerated dose of curcumin at 8000 mg/d. Eight patients out of 14 had measurable lesions according to RECIST criteria, with 5 PR and 3 SD. The recommended dose of curcumin was 6000 mg/d for seven consecutive days every 3 weeks in combination [224].
10.4 Colon Cancer
A pilot-scale study was performed to quantify levels of curcuminoids in colorectal mucosa on 26 patients undergoing colorectal endoscopy or surgical resection and to obtain information on the acceptability and compliance. Patients were treated with curcumin C3 complex (2.35 g) once daily for 14 days prior to endoscopic biopsy or colonic resection. Curcuminoid levels were analyzed in plasma, urine, and colonic mucosa by UPLC-UV (ultra high performance liquid chromatography with UV detection) with characterization by tandem LC-MS/MS. Twenty-four patients commencing curcumin completed the course, out of which six patients reported mild gastrointestinal adverse events. Curcuminoids were detectable in 9/24 plasma samples, in 24/24 urine samples, and in the colonic mucosa of all 23 biopsied participants. Mean tissue levels were found to be 48.4 μg/g (127.8 nmol/g) of parent curcuminoids. Curcumin glucuronide a major conjugate was detectable in 29/35 biopsies. High levels of topical curcumin persisted in the mucosa for up to 40 h postadministration. Sixteen participants agreed to take curcumin in long-term trials should it be of proven benefit. Pharmacologically active levels of curcumin were recovered from colonic mucosa [225].
10.5 Colorectal Cancer
In a study of herbal preparation, MB-6 that is composed of curcumin, fermented soybean extract, green tea extract, grape seed extract, Antrodia camphorata mycelia and Spirulina was evaluated for its effectiveness of leucovorin/5-fluorouracil chemotherapy in patients with colorectal cancer. MB-6, in combination with leucovorin/5-fluorouracil chemotherapy supplied to colon cancer tumor-bearing BALB/c mice, showed increased survival rate and life span compared to chemotherapy alone. In a proof of concept, 16-week randomized clinical study was executed on 72 patients with metastatic colorectal cancer that were treated with leucovorin, 5-fluorouracil and oxaliplatin in combination with either MB-6 or placebo. The follow-up taken after 77 weeks of treatment showed no significant difference in the best overall response rate (primary outcome) and overall survival (secondary outcome) was observed between the two groups. Patients in the MB-6 group had a significantly lower disease progression rate (secondary outcome) than patients in the placebo group. The placebo group had a significantly higher incidence of adverse events (secondary outcome) at least grade 4 compared with the MB-6 group and a significantly higher occurrence of increased serum creatinine compared with the MB-6 group. The result revealed herbal formulation MB-6 as a potential adjunct to chemotherapy in patients with metastatic colorectal cancer [226].
In another study of dose-escalation pilot clinical trial of advanced colorectal human cancer, a proprietary capsule of curcumin with dosage between 440 and 2200 mg/day, containing 36–180 mg of curcumin, was tested on 15 patients for 4 months. Glutathione S-transferase activity showed 59% decrease in blood samples after 440 mg curcuma extract ingestion for 29 days. The effect was not observed for higher-dose concentration. Leukocytic DNA adduct malondialdehyde levels were constant within each patient and unaffected by treatment. For 2–4 months of treatment, disease was found radiologically stable in five patients. The result suggested safe administration of Curcuma extract up to 2.2 g daily equivalent to 180 mg of curcumin. Investigation on intestinal metabolism revealed low oral bioavailability of curcumin [227]. Another similar study on the above designed trial 0.45 and 3.6 g of curcumin was orally administered by patients. Three biomarkers of curcumin activity suggested in preclinical trials that are glutathione S-transferase activity, levels of DNA adduct malondialdehyde and inhibition of prostaglandin E2 (PGE2) production induced ex vivo. Chromatography analysis detected curcumin and its glucuronide and sulfate metabolites in plasma and urine. A dose of 3.6 g/day curcumin caused 62% and 57% decreases in inducible PGE2 production in blood samples taken 1 h after dose on days 1 and 29, respectively. A daily oral dose of 3.6 g of curcumin is recommended for phase II trials in the prevention or treatment of cancers ex vivo [227].
10.6 Pancreatic Cancer
A clinical trial was evaluated for biological effects of curcumin in advanced pancreatic cancer. In a study of 21 patients, 8 g curcumin was administered orally until disease progression with restaging every 2 months. Serum cytokine levels for interleukin (IL)-6, IL-8, IL-10 and IL-1 receptor antagonists and peripheral blood mononuclear cell expression of curcumin nuclear factor NF-κB and cyclooxygenase-2 were monitored. Curcumin was detectable in glucuronide and sulfate forms at low steady-state levels that suggest poor oral bioavailability. Two patients showed clinical biological activity. One patient had ongoing stable disease for more than 18 months and one patient depicted 73% tumor regression accompanied by serum cytokine levels. Curcumin downregulated expression of NF-κB, COX-2 and phosphorylated STAT3 in blood from patients. There were interpatient variation in plasma curcumin levels and drug levels (22–41 ng/mL) remained constant over the first 4 weeks. Oral curcumin was well tolerated and had limited absorption with biological activity in some patients with pancreatic cancer [228].
In a clinical trial on gemcitabine resistant pancreatic cancer patients, curcumin was studied in combination with gemcitabine-based chemotherapy. No dose-limiting toxicities were observed in the phase I study and oral curcumin 8 g per day was selected as the recommended dose for the phase II study. No dose-limiting toxicities, no intolerability, and 100% compliance rate were observed. Patient’s survival time after initiation of curcumin was 161 days and annual survival rate was 19%. Evaluation of phase II resulted in safe and feasible uptake of suggested curcumin dose [229].
In a similar kind of another clinical trial on advanced pancreatic cancer, 17 patients received 8 g curcumin per day along with gemcitabine 1000 mg/m(2) IV weekly × 3 of 4 weeks. Five patients discontinued the trial because of intractable abdominal fullness or pain and in two patients dose was reduced to 4 g/day due to abdominal complaints. Nine percent of patient showed partial response, 36% of patient had stable disease condition, and 55% of patient had tumor progression. The study suggested to prevent the use of high doses of oral curcumin needed to achieve systemic effect [230].
10.7 Leukemia
A study was designed to evaluate nitric oxide clearance activity of imatinib along with curcumin in 50 patients of chronic myeloid leukemia. The first group received imatinib alone and the second group received turmeric powder with imatinib for 6 weeks. Significant decrease in nitric oxide level was noted in curcumin-aided imatinib group as compared to imatinib alone and thus demonstrated curcumin as an adjuvant to imatinib which could help in the treatment of chronic myeloid leukemia patients [231] (Table 16.1).
11 Conclusions
Currently, the alternative and less toxic chemotherapeutic and chemopreventive agents are essentially desired due to lack of effective cancer therapy. Multiple molecular pathways such as NF-κB activation, Nrf2, NFAT, p53, MMP-mediated metastasis and VEGF-mediated angiogenesis are deregulated in various cancer types and represent potential therapeutic targets. Curcumin has shown to target many mechanisms deregulated in various malignancies. Many encouraging results have been obtained employing curcumin against various cancer models in vitro and in vivo. However, certain limiting biophysical properties such as bioavailability, water insolubility, short life span, rapid systemic clearance and quick metabolism in the gastrointestinal tract have limited the use of curcumin as an effective chemotherapeutics. In order to enhance its anticancer efficacy to a significant level, combination of other known chemotherapeutics and chemopreventive agents with curcumin have shown positive results. Thus, curcumin has cancer-preventive potential and can be developed as an effective chemotherapeutic agent in coming years which would provide less toxic and cheap medical alternatives for the management of cancer.
References
Kunnumakkara AB et al (2016) Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol 174:1325–1348
Prasad S, Tyagi AK, Aggarwal BB (2014) Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 46(1):2–18
Goel A, Kunnumakkara AB, Aggarwal BB (2008) Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol 75(4):787–809
Gupta SC et al (2012) Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 39(3):283–299
Rainey N et al (2015) Curcumin hormesis mediates a cross-talk between autophagy and cell death. Cell Death Dis 6:e2003
Aggarwal BB, Gupta SC, Sung B (2013) Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol 169(8):1672–1692
Siwak DR et al (2005) Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer 104(4):879–890
Aggarwal S et al (2004) Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer 111(5):679–692
LoTempio MM et al (2005) Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res 11(19 Pt 1):6994–7002
Wang Z et al (2008) Synergistic effects of multiple natural products in pancreatic cancer cells. Life Sci 83(7-8):293–300
Elattar TM, Virji AS (2000) The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Anticancer Res 20(3a):1733–1738
Mukhopadhyay A et al (2001) Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene 20(52):7597–7609
Mehta K et al (1997) Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anti-Cancer Drugs 8(5):470–481
Hanif R et al (1997) Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med 130(6):576–584
Lin YG et al (2007) Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res 13(11):3423–3430
Hussain AR et al (2008) Curcumin suppresses constitutive activation of nuclear factor-kappa B and requires functional Bax to induce apoptosis in Burkitt's lymphoma cell lines. Mol Cancer Ther 7(10):3318–3329
Shakibaei M et al (2014) Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS One 9(1):e85397
Waghela BN et al (2015) Curcumin conjugated with PLGA potentiates sustainability, anti-proliferative activity and apoptosis in human colon carcinoma cells. PLoS One 10(2):e0117526
Chearwae W et al (2004) Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem Pharmacol 68(10):2043–2052
Singh M, Singh N (2009) Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol Cell Biochem 325(1-2):107–119
Halder RC et al (2015) Curcuminoids and omega-3 fatty acids with anti-oxidants potentiate cytotoxicity of natural killer cells against pancreatic ductal adenocarcinoma cells and inhibit interferon gamma production. Front Physiol 6:129
Fiala M (2015) Curcumin and omega-3 fatty acids enhance NK cell-induced apoptosis of pancreatic cancer cells but curcumin inhibits interferon-gamma production: benefits of omega-3 with curcumin against cancer. Molecules 20(2):3020–3026
Imran M et al (2016) Cucurmin; anticancer and antitumor perspectives – a comprehensive review. Crit Rev Food Sci Nutr 58:1271–1293
Labbozzetta M et al (2009) Curcumin as a possible lead compound against hormone-independent, multidrug-resistant breast cancer. Ann N Y Acad Sci 1155:278–283
Ramachandran C, You W (1999) Differential sensitivity of human mammary epithelial and breast carcinoma cell lines to curcumin. Breast Cancer Res Treat 54(3):269–278
Onoda M, Inano H (2000) Effect of curcumin on the production of nitric oxide by cultured rat mammary gland. Nitric Oxide 4(5):505–515
Hayden MS, Ghosh S (2012) NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 26(3):203–234
Karin M (2009) NF-κB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 1(5):a000141
Karin M et al (2002) NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2(4):301–310
Karin M (1999) How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene 18(49):6867–6874
Dhawan P, Richmond A (2002) A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J Biol Chem 277(10):7920–7928
Marin YE et al (2007) Curcumin downregulates the constitutive activity of NF-kappaB and induces apoptosis in novel mouse melanoma cells. Melanoma Res 17(5):274–283
Collett GP, Campbell FC (2004) Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis 25(11):2183–2189
Sun Z, Andersson R (2002) NF-kappaB activation and inhibition: a review. Shock 18(2):99–106
Collett GP, Campbell FC (2006) Overexpression of p65/RelA potentiates curcumin-induced apoptosis in HCT116 human colon cancer cells. Carcinogenesis 27(6):1285–1291
Baeuerle PA (1991) The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta 1072(1):63–80
Naumann M et al (1997) Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor κB and activator protein 1 and the induction of inflammatory cytokines. J Exp Med 186(2):247–258
Singh S, Aggarwal BB (1995) Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem 270(42):24995–25000
Finco TS, Beg AA, Baldwin AS (1994) Inducible phosphorylation of I kappa B alpha is not sufficient for its dissociation from NF-kappa B and is inhibited by protease inhibitors. Proc Natl Acad Sci U S A 91(25):11884–11888
Shostak K, Chariot A (2011) NF-κB, stem cells and breast cancer: the links get stronger. Breast Cancer Res: BCR 13(4):214–214
Luedde T, Schwabe RF (2011) NF-kappaB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 8(2):108–118
Marquardt JU et al (2015) Curcumin effectively inhibits oncogenic NF-kappaB signaling and restrains stemness features in liver cancer. J Hepatol 63(3):661–669
Anest V et al (2003) A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature 423(6940):659–663
Kuo HP et al (2013) Epigenetic roles of MLL oncoproteins are dependent on NF-kappaB. Cancer Cell 24(4):423–437
Shaw PH (1996) The role of p53 in cell cycle regulation. Pathol Res Pract 192(7):669–675
Amaral JD et al (2010) The role of p53 in apoptosis. Discov Med 9(45):145–152
Rivlin N et al (2011) Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes Cancer 2(4):466–474
Meek DW (1999) Mechanisms of switching on p53: a role for covalent modification? Oncogene 18(53):7666–7675
Fridman JS, Lowe SW (2003) Control of apoptosis by p53. Oncogene 22(56):9030–9040
Choudhuri T et al (2002) Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett 512(1-3):334–340
Choudhuri T et al (2005) Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem 280(20):20059–20068
He ZY et al (2011) Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Investig 29(3):208–213
Ye M et al (2015) Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett 357(1):196–205
Watson JL et al (2010) Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett 297(1):1–8
Bush JA, Cheung KJ Jr, Li G (2001) Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res 271(2):305–314
Han SS et al (1999) Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53. Clin Immunol 93(2):152–161
Ramakrishnan SK et al (2014) Expression of targeted ribozyme against telomerase RNA causes altered expression of several other genes in tumor cells. Tumour Biol 35(6):5539–5550
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116
Chen B et al (2014) Curcumin inhibits proliferation of breast cancer cells through Nrf2-mediated down-regulation of Fen1 expression. J Steroid Biochem Mol Biol 143:11–18
Balogun E et al (2003) Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J 371(Pt 3):887–895
Jain A et al (2015) Curcumin inhibits PhIP induced cytotoxicity in breast epithelial cells through multiple molecular targets. Cancer Lett 365(1):122–131
Shaw JP et al (1988) Identification of a putative regulator of early T cell activation genes. Science 241:202–205. J Immunol, 2010. 185(9): p. 4972–4975
Durand DB et al (1988) Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol 8(4):1715–1724
Shukla U et al (2009) Tyrosine phosphorylation of 3BP2 regulates B cell receptor-mediated activation of NFAT. J Biol Chem 284(49):33719–33728
Zanoni I et al (2009) CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 460(7252):264–268
Ranger AM et al (2000) The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J Exp Med 191(1):9–22
Ho IC et al (1998) A potential role for the nuclear factor of activated T cells family of transcriptional regulatory proteins in adipogenesis. Proc Natl Acad Sci U S A 95(26):15537–15541
Buchholz M et al (2006) Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J 25(15):3714–3724
Jauliac S et al (2002) The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol 4(7):540–544
de la Pompa JL et al (1998) Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 392(6672):182–186
Viola JP et al (2005) NFAT transcription factors: from cell cycle to tumor development. Braz J Med Biol Res 38(3):335–344
Baksh S, DeCaprio JA, Burakoff SJ (2000) Calcineurin regulation of the mammalian G0/G1 checkpoint element, cyclin dependent kinase 4. Oncogene 19(24):2820–2827
Hernandez GL et al (2001) Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med 193(5):607–620
Macian F (2005) NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol 5(6):472–484
Schulz RA, Yutzey KE (2004) Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol 266(1):1–16
Mognol GP et al (2016) Cell cycle and apoptosis regulation by NFAT transcription factors: new roles for an old player. Cell Death Dis 7:e2199
Neuhofer W (2010) Role of NFAT5 in inflammatory disorders associated with osmotic stress. Curr Genomics 11(8):584–590
Chueh SC et al (2003) Curcumin enhances the immunosuppressive activity of cyclosporine in rat cardiac allografts and in mixed lymphocyte reactions. Transplant Proc 35(4):1603–1605
Ranjan D et al (2004) Curcumin inhibits mitogen stimulated lymphocyte proliferation, NFkappaB activation, and IL-2 signaling. J Surg Res 121(2):171–177
Kliem C et al (2012) Curcumin suppresses T cell activation by blocking Ca2+ mobilization and nuclear factor of activated T cells (NFAT) activation. J Biol Chem 287(13):10200–10209
Hernandez M, Wicz S, Corral RS (2016) Cardioprotective actions of curcumin on the pathogenic NFAT/COX-2/prostaglandin E2 pathway induced during Trypanosoma cruzi infection. Phytomedicine 23(12):1392–1400
Hai L et al (2011) Counteracting effect of TRPC1-associated Ca2+ influx on TNF-alpha-induced COX-2-dependent prostaglandin E2 production in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol 301(2):G356–G367
Sai Krishna Borra PG, Mahendra J, Jayamathi KM, Cherian C, chand NaR (2013) Antioxidant and free radical scavenging activity of curcumin determined by using different in vitro and ex vivo models. J Med Plants Res 7(36):2680–2690
Wu Y et al (2014) Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett 345(2):164–173
Hussain SP, Hofseth LJ, Harris CC (2003) Radical causes of cancer. Nat Rev Cancer 3(4):276–285
Hamanaka RB, Chandel NS (2011) Cell biology. Warburg effect and redox balance. Science 334(6060):1219–1220
Giannoni E, Parri M, Chiarugi P (2012) EMT and oxidative stress: a bidirectional interplay affecting tumor malignancy. Antioxid Redox Signal 16(11):1248–1263
Chattopadhyay D et al (2014) Dichotomous effect of caffeine, curcumin, and naringenin on genomic DNA of normal and diabetic subjects. Scientifica (Cairo) 2014:649261
Cai W et al (2012) Curcumin targeting the thioredoxin system elevates oxidative stress in HeLa cells. Toxicol Appl Pharmacol 262(3):341–348
Wassmann S, Wassmann K, Nickenig G (2004) Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 44(4):381–386
Leone M et al (1998) Fourier transform infrared analysis of the interaction of azide with the active site of oxidized and reduced bovine Cu, Zn superoxide dismutase. Biochemistry 37(13):4459–4464
Vance CK, Miller AF (1998) Spectroscopic comparisons of the pH dependencies of Fe-substituted (Mn)superoxide dismutase and Fe-superoxide dismutase. Biochemistry 37(16):5518–5527
Monari M et al (2006) Superoxide dismutase in gastric adenocarcinoma: is it a clinical biomarker in the development of cancer? Biomarkers 11(6):574–584
Martin RC et al (2007) Chemoprevention of carcinogenic progression to esophageal adenocarcinoma by the manganese superoxide dismutase supplementation. Clin Cancer Res 13(17):5176–5182
Fu TY et al (2011) Manganese superoxide dismutase and glutathione peroxidase as prognostic markers in patients with buccal mucosal squamous cell carcinomas. Head Neck 33(11):1606–1615
Teoh-Fitzgerald ML et al (2012) Genetic and epigenetic inactivation of extracellular superoxide dismutase promotes an invasive phenotype in human lung cancer by disrupting ECM homeostasis. Mol Cancer Res 10(1):40–51
Huang F et al (2014) Targeting gene-virus-mediated manganese superoxide dismutase effectively suppresses tumor growth in hepatocellular carcinoma in vitro and in vivo. Cancer Biother Radiopharm 29(10):403–411
Tarhini AA et al (2011) A phase I study of concurrent chemotherapy (paclitaxel and carboplatin) and thoracic radiotherapy with swallowed manganese superoxide dismutase plasmid liposome protection in patients with locally advanced stage III non-small-cell lung cancer. Hum Gene Ther 22(3):336–342
Govatati S et al (2016) Manganese-superoxide dismutase (Mn-SOD) overexpression is a common event in colorectal cancers with mitochondrial microsatellite instability. Tumour Biol 37(8):10357–10364
Zhong W et al (1996) Inhibition of cell growth and sensitization to oxidative damage by overexpression of manganese superoxide dismutase in rat glioma cells. Cell Growth Differ 7(9):1175–1186
Schiffman SC, Li Y, Martin RC (2012) The association of manganese superoxide dismutase expression in Barrett’s esophageal progression with MnTBAP and curcumin oil therapy. J Surg Res 176(2):535–541
Li Y et al (2009) Chemoprotective effects of Curcuma aromatica on esophageal carcinogenesis. Ann Surg Oncol 16(2):515–523
Calaf GM et al (2011) Protective role of curcumin in oxidative stress of breast cells. Oncol Rep 26(4):1029–1035
Das L, Vinayak M (2014) Long term effect of curcumin in regulation of glycolytic pathway and angiogenesis via modulation of stress activated genes in prevention of cancer. PLoS One 9(6):e99583
Margis R et al (2008) Glutathione peroxidase family – an evolutionary overview. FEBS J 275(15):3959–3970
Kryukov GV et al (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443
Brigelius-Flohe R, Maiorino M (2013) Glutathione peroxidases. Biochim Biophys Acta 1830(5):3289–3303
Tappel AL (1978) Glutathione peroxidase and hydroperoxides. Methods Enzymol 52:506–513
Ghyselinck NB, Dufaure JP (1990) A mouse cDNA sequence for epididymal androgen-regulated proteins related to glutathione peroxidase. Nucleic Acids Res 18(23):7144
Scibior D, Czeczot H (2006) Catalase: structure, properties, functions. Postepy Hig Med Dosw (Online) 60:170–180
Fang J, Nakamura H, Iyer AK (2007) Tumor-targeted induction of oxystress for cancer therapy. J Drug Target 15(7-8):475–486
Sato K et al (1992) Negative regulation of catalase gene expression in hepatoma cells. Mol Cell Biol 12(6):2525–2533
Kwei KA et al (2004) Transcriptional repression of catalase in mouse skin tumor progression. Neoplasia 6(5):440–448
Sun Y, Colburn NH, Oberley LW (1993) Depression of catalase gene expression after immortalization and transformation of mouse liver cells. Carcinogenesis 14(8):1505–1510
Hoffschir F et al (1993) Decrease in catalase activity and loss of the 11p chromosome arm in the course of SV40 transformation of human fibroblasts. Carcinogenesis 14(8):1569–1572
Xu H et al (2005) Concentration-dependent collateral sensitivity of cisplatin-resistant gastric cancer cell sublines. Biochem Biophys Res Commun 328(2):618–622
Lee HC et al (2004) Increased expression of antioxidant enzymes in radioresistant variant from U251 human glioblastoma cell line. Int J Mol Med 13(6):883–887
Kobayashi Y et al (2008) Hydrogen peroxide-mediated nuclear factor kappaB activation in both liver and tumor cells during initial stages of hepatic metastasis. Cancer Sci 99(8):1546–1552
Vina J et al (1996) Exercise causes blood glutathione oxidation in chronic obstructive pulmonary disease: prevention by O2 therapy. J Appl Physiol (1985) 81(5):2198–2202
Das L, Vinayak M (2012) Anti-carcinogenic action of curcumin by activation of antioxidant defence system and inhibition of NF-kappaB signalling in lymphoma-bearing mice. Biosci Rep 32(2):161–170
Biswas J et al (2010) Indian spice curcumin may be an effective strategy to combat the genotoxicity of arsenic in Swiss albino mice. Asian Pac J Cancer Prev 11(1):239–247
Safe S, Kasiappan R (2016) Natural products as mechanism-based anticancer agents: Sp transcription factors as targets. Phytother Res 30(11):1723–1732
Rodrigues LR et al (2007) The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomark Prev 16(6):1087–1097
Kasi PD et al (2016) Molecular targets of curcumin for cancer therapy: an updated review. Tumour Biol 37(10):13017–13028
Jain S et al (2007) Osteopontin: an emerging therapeutic target for anticancer therapy. Expert Opin Ther Targets 11(1):81–90
Chakraborty G, Jain S, Kundu GC (2008) Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res 68(1):152–161
Philip S, Kundu GC (2003) Osteopontin induces nuclear factor kappa B-mediated promatrix metalloproteinase-2 activation through I kappa B alpha/IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J Biol Chem 278(16):14487–14497
Chakraborty G et al (2008) Curcumin suppresses breast tumor angiogenesis by abrogating osteopontin-induced VEGF expression. Mol Med Rep 1(5):641–646
Arbiser JL et al (1998) Curcumin is an in vivo inhibitor of angiogenesis. Mol Med 4(6):376–383
Gururaj AE et al (2002) Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun 297(4):934–942
Perry MC et al (2010) Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol Nutr Food Res 54(8):1192–1201
Ponzo MG et al (2009) Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc Natl Acad Sci U S A 106(31):12903–12908
Heideman DA et al (2004) Suppression of tumor growth, invasion and angiogenesis of human gastric cancer by adenovirus-mediated expression of NK4. J Gene Med 6(3):317–327
Zou HY et al (2007) An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 67(9):4408–4417
Jiao D et al (2016) Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer. Mol Ther Oncolytics 3:16018
Zhang YW et al (2003) Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci U S A 100(22):12718–12723
Bimonte S et al (2013) Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. Biomed Res Int 2013:810423
Chen WH, Chen Y, Cui GH (2005) Effects of TNF-alpha and curcumin on the expression of VEGF in Raji and U937 cells and on angiogenesis in ECV304 cells. Chin Med J 118(24):2052–2057
Millanta F et al (2006) COX-2 expression in canine and feline invasive mammary carcinomas: correlation with clinicopathological features and prognostic molecular markers. Breast Cancer Res Treat 98(1):115–120
Yoysungnoen P et al (2006) Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc 34(1-2):109–115
Li L, Braiteh FS, Kurzrock R (2005) Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 104(6):1322–1331
Bimonte S et al (2015) Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. Biomed Res Int 2015:878134
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3(6):401–410
Vaupel P (2004) The role of hypoxia-induced factors in tumor progression. Oncologist 9(Suppl 5):10–17
Jeong JW et al (2002) Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell 111(5):709–720
Bae MK et al (2006) Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep 15(6):1557–1562
Ogawa H et al (2003) Sodium butyrate inhibits angiogenesis of human intestinal microvascular endothelial cells through COX-2 inhibition. FEBS Lett 554(1-2):88–94
Binion DG, Otterson MF, Rafiee P (2008) Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 57(11):1509–1517
Rossig L et al (2002) Inhibitors of histone deacetylation downregulate the expression of endothelial nitric oxide synthase and compromise endothelial cell function in vasorelaxation and angiogenesis. Circ Res 91(9):837–844
Yamada N et al (2014) Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim Biophys Acta 1839(11):1256–1272
Katsushima K et al (2012) Contribution of microRNA-1275 to Claudin11 protein suppression via a polycomb-mediated silencing mechanism in human glioma stem-like cells. J Biol Chem 287(33):27396–27406
Bai Y et al (2016) Curcumin inhibits angiogenesis by up-regulation of microRNA-1275 and microRNA-1246: a promising therapy for treatment of corneal neovascularization. Cell Prolif 49(6):751–762
Fan Y, Mao R, Yang J (2013) NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 4(3):176–185
Aggarwal BB (2004) Nuclear factor-kappaB: the enemy within. Cancer Cell 6(3):203–208
Shishodia S (2013) Molecular mechanisms of curcumin action: gene expression. Biofactors 39(1):37–55
Kunnumakkara AB, Anand P, Aggarwal BB (2008) Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett 269(2):199–225
Bachmeier BE et al (2008) Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis 29(4):779–789
Killian PH et al (2012) Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis 33(12):2507–2519
Jacobs MD, Harrison SC (1998) Structure of an IkappaBalpha/NF-kappaB complex. Cell 95(6):749–758
Verma IM et al (1995) Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev 9(22):2723–2735
Zong H et al (2012) Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Mol Biol Rep 39(4):4803–4808
Huang S (2007) Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res 13(5):1362–1366
Yang CL et al (2012) Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One 7(5):e37960
Bharti AC, Donato N, Aggarwal BB (2003) Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol 171(7):3863–3871
Strieter RM et al (1995) The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 270(45):27348–27357
Youngs SJ et al (1997) Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer 71(2):257–266
Helbig G et al (2003) NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 278(24):21631–21638
Brooks SA et al (2010) Molecular interactions in cancer cell metastasis. Acta Histochem 112(1):3–25
Tong W et al (2016) Curcumin suppresses colon cancer cell invasion via AMPK-induced inhibition of NF–κB, uPA activator and MMP9. Oncol Lett 12(5):4139–4146
Chirco R et al (2006) Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 25(1):99–113
Mylona E et al (2006) Expression of tissue inhibitor of matrix metalloproteinases (TIMP)-3 protein in invasive breast carcinoma: relation to tumor phenotype and clinical outcome. Breast Cancer Res 8(5):R57
Baldwin AS (2001) Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest 107(3):241–246
Lin SS et al (2009) Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett 285(2):127–133
Chen QY et al (2014) Curcumin inhibits lung cancer cell migration and invasion through Rac1-dependent signaling pathway. J Nutr Biochem 25(2):177–185
Sun K et al (2016) Curcumin inhibits LPA-induced invasion by attenuating RhoA/ROCK/MMPs pathway in MCF7 breast cancer cells. Clin Exp Med 16(1):37–47
Kim JM et al (2012) Curcumin suppresses the TPA-induced invasion through inhibition of PKCalpha-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine 19(12):1085–1092
Lee SO et al (2008) Suppression of PMA-induced tumor cell invasion by capillarisin via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochem Biophys Res Commun 366(4):1019–1024
Shao ZM et al (2002) Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int J Cancer 98(2):234–240
Cowin P, Welch DR (2007) Breast cancer progression: controversies and consensus in the molecular mechanisms of metastasis and EMT. J Mammary Gland Biol Neoplasia 12(2-3):99–102
Gallardo M, Calaf GM (2016) Curcumin inhibits invasive capabilities through epithelial mesenchymal transition in breast cancer cell lines. Int J Oncol 49(3):1019–1027
Chen MC et al (2012) Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun 18(5):685–693
Huang T, Chen Z, Fang L (2013) Curcumin inhibits LPS-induced EMT through downregulation of NF-kappaB-Snail signaling in breast cancer cells. Oncol Rep 29(1):117–124
Chen WC et al (2013) Curcumin suppresses doxorubicin-induced epithelial-mesenchymal transition via the inhibition of TGF-beta and PI3K/AKT signaling pathways in triple-negative breast cancer cells. J Agric Food Chem 61(48):11817–11824
Ferlay J et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Palumbo MO et al (2013) Systemic cancer therapy: achievements and challenges that lie ahead. Front Pharmacol 4:57
Mishra J et al (2013) Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit Rev Oncol Hematol 86(3):232–250
Zhang L et al (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83(5):761–769
Peer D et al (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2(12):751–760
Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46(12 Pt 1):6387–6392
Lee WH et al (2014) Recent advances in curcumin nanoformulation for cancer therapy. Expert Opin Drug Deliv 11(8):1183–1201
Ghosh S, Banerjee S, Sil PC (2015) The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol 83:111–124
Logsdon CD, Abbruzzese JL (2010) Chemoprevention of pancreatic cancer: ready for the clinic? Cancer Prev Res (Phila) 3(11):1375–1378
Cirmi S et al (2016) Chemopreventive agents and inhibitors of cancer hallmarks: may citrus offer new perspectives? Nutrients 8(11):698
Cheng KW et al (2013) Curcumin enhances the lung cancer chemopreventive efficacy of phospho-sulindac by improving its pharmacokinetics. Int J Oncol 43(3):895–902
Sutaria D et al (2012) Chemoprevention of pancreatic cancer using solid-lipid nanoparticulate delivery of a novel aspirin, curcumin and sulforaphane drug combination regimen. Int J Oncol 41(6):2260–2268
Nasr M et al (2014) Targeting different angiogenic pathways with combination of curcumin, leflunomide and perindopril inhibits diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J Pharmacol 723:267–275
Xu G et al (2010) Combination of curcumin and green tea catechins prevents dimethylhydrazine-induced colon carcinogenesis. Food Chem Toxicol 48(1):390–395
Khafif A et al (1998) Quantitation of chemopreventive synergism between (-)-epigallocatechin-3-gallate and curcumin in normal, premalignant and malignant human oral epithelial cells. Carcinogenesis 19(3):419–424
Montgomery A et al (2016) Curcumin sensitizes Silymarin to exert synergistic anticancer activity in colon cancer cells. J Cancer 7(10):1250–1257
Feng S-S, Chien S (2003) Chemotherapeutic engineering: application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem Eng Sci 58(18):4087–4114
Chuang SE et al (2000) Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis 21(2):331–335
Guo S et al (2016) A nanoparticulate pre-chemosensitizer for efficacious chemotherapy of multidrug resistant breast cancer. Sci Rep 6:21459
Zhao X et al (2015) Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J Pharm Biopharm 93:27–36
Chen H et al (2016) Synergistic effect of fenretinide and curcumin for treatment of non-small cell lung cancer. Cancer Biol Ther 17:1–8
Yin H et al (2014) Curcumin sensitizes glioblastoma to temozolomide by simultaneously generating ROS and disrupting AKT/mTOR signaling. Oncol Rep 32(4):1610–1616
Kang JH et al (2015) Curcumin sensitizes human lung cancer cells to apoptosis and metastasis synergistically combined with carboplatin. Exp Biol Med (Maywood) 240(11):1416–1425
Zoller F et al (2009) Endoradiotherapy in cancer treatment—basic concepts and future trends. Eur J Pharmacol 625(1–3):55–62
DeNardo SJ, Denardo GL (2006) Targeted radionuclide therapy for solid tumors: an overview. Int J Radiat Oncol Biol Phys 66(2 Suppl):S89–S95
Zhao J, Zhou M, Li C (2016) Synthetic nanoparticles for delivery of radioisotopes and radiosensitizers in cancer therapy. Cancer Nanotechnol 7(1):9
Verma V (2016) Relationship and interactions of curcumin with radiation therapy. World J Clin Oncol 7(3):275–283
Luthra PM, Lal N (2016) Prospective of curcumin, a pleiotropic signalling molecule from Curcuma longa in the treatment of Glioblastoma. Eur J Med Chem 109:23–35
Fan H et al (2016) MiR-593 mediates curcumin-induced radiosensitization of nasopharyngeal carcinoma cells via MDR1. Oncol Lett 11(6):3729–3734
Hsu F-T et al (2015) Curcumin sensitizes hepatocellular carcinoma cells to radiation via suppression of radiation-induced NF-κB activity. Biomed Res Int 2015:7
Sebastia N et al (2014) Curcumin and trans-resveratrol exert cell cycle-dependent radioprotective or radiosensitizing effects as elucidated by the PCC and G2-assay. Mutat Res 766-767:49–55
Lopez-Jornet P et al (2016) Radioprotective effects of lycopene and curcumin during local irradiation of parotid glands in Sprague Dawley rats. Br J Oral Maxillofac Surg 54(3):275–279
Fukuda K et al (2016) A diarylpentanoid curcumin analog exhibits improved radioprotective potential in the intestinal mucosa. Int J Radiat Biol 92(7):388–394
Sharma RA, Gescher AJ, Steward WP (2005) Curcumin: the story so far. Eur J Cancer 41(13):1955–1968
Eigner D, Scholz D (1999) Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J Ethnopharmacol 67(1):1–6
Johnson JJ, Mukhtar H (2007) Curcumin for chemoprevention of colon cancer. Cancer Lett 255(2):170–181
Hsu CH, Cheng AL (2007) Clinical studies with curcumin. Adv Exp Med Biol 595:471–480
Sharma RA et al (2004) Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10(20):6847–6854
Mahammedi H et al (2016) The new combination Docetaxel, Prednisone and Curcumin in patients with castration-resistant prostate cancer: a pilot phase II study. Oncology 90(2):69–78
Basu P et al (2013) Clearance of cervical human papillomavirus infection by topical application of curcumin and curcumin containing polyherbal cream: a phase II randomized controlled study. Asian Pac J Cancer Prev 14(10):5753–5759
Bayet-Robert M et al (2010) Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther 9(1):8–14
Irving GR et al (2013) Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration–a clinical pilot study including assessment of patient acceptability. Cancer Prev Res (Phila) 6(2):119–128
Chen WT et al (2014) Effectiveness of a novel herbal agent MB-6 as a potential adjunct to 5-fluoracil-based chemotherapy in colorectal cancer. Nutr Res 34(7):585–594
Sharma RA et al (2001) Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res 7(7):1894–1900
Dhillon N et al (2008) Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res 14(14):4491–4499
Kanai M et al (2011) A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol 68(1):157–164
Epelbaum R et al (2010) Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer 62(8):1137–1141
Ghalaut VS et al (2012) Effect of imatinib therapy with and without turmeric powder on nitric oxide levels in chronic myeloid leukemia. J Oncol Pharm Pract 18(2):186–190
Irving GR et al (2015) Combining curcumin (C3-complex, Sabinsa) with standard care FOLFOX chemotherapy in patients with inoperable colorectal cancer (CUFOX): study protocol for a randomised control trial. Trials 16:110
James MI et al (2015) Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett 364(2):135–141
Panahi Y et al (2014) Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytother Res 28(10):1461–1467
Ryan JL et al (2013) Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res 180(1):34–43
Kanai M (2014) Therapeutic applications of curcumin for patients with pancreatic cancer. World J Gastroenterol 20(28):9384–9391
Golombick T et al (2012) Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: a randomized, double-blind placebo-controlled cross-over 4g study and an open-label 8g extension study. Am J Hematol 87(5):455–460
Carroll RE et al (2011) Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 4(3):354–364
Ide H et al (2010) Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 70(10):1127–1133
Golombick T et al (2009) The potential role of curcumin in patients with monoclonal gammopathy of undefined significance–its effect on paraproteinemia and the urinary N-telopeptide of type I collagen bone turnover marker. Clin Cancer Res 15(18):5917–5922
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sabarwal, A., Kumar, K., Shyanti, R., Singh, R.P. (2018). Curcumin in Cancer Prevention. In: Rani, V., Yadav, U. (eds) Functional Food and Human Health. Springer, Singapore. https://doi.org/10.1007/978-981-13-1123-9_16
Download citation
DOI: https://doi.org/10.1007/978-981-13-1123-9_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1122-2
Online ISBN: 978-981-13-1123-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)