Abstract
The prognostication of post-cardiac-arrest patients remains a challenge. Electroencephalography (EEG) is a promising modality; however, conventional EEG is difficult for non-neurologists to interpret. Amplitude-integrated EEG (aEEG) is quantitative EEG that is easy to interpret. aEEG is derived from continuous EEG with reduced electrode montage, with an easy setup. aEEG patterns have been useful in the prognostication of comatose post-cardiac-arrest patients. EEG is important in monitoring seizure activity in post-cardiac-arrest care. At the 44th Annual Meeting of the Japanese Society of Critical Care Medicine, we reported the incidence and characteristics of status epilepticus among patients treated with target temperature management and monitored with aEEG using a single bipolar frontal hairline lead. Seven of 61 patients (11%) revealed status epileptics, and 1 patient with continuous normal voltage before status epilepticus showed a good neurological outcome. aEEG monitoring with reduced leads has limited, but substantial, utility for detecting generalized seizure. Status epilepticus during post-cardiac-arrest care is not uniform: patients with status epilepticus are categorized into two groups based on the background pattern of aEEG before status epilepticus. Status epilepticus from continuous normal voltage is not always associated with poor outcomes. Knowledge of the background pattern can be gained, via aEEG monitoring, from the early phase after return of spontaneous circulation (ROSC). This could help identify targets of aggressive anticonvulsant therapy. Furthermore, aEEG monitoring is a useful tool for intensive and emergency physicians who treat post-cardiac-arrest patients at the bedside.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Amplitude-integrated electroencephalography
- Post-cardiac-arrest care
- Hypoxic encephalopathy
- Prognosis
- Status epilepticus
1.1 Background
1.1.1 Why Do We Need Amplitude–Integrated Electroencephalography?
The prognostication of neurological outcomes of comatose post-cardiac-arrest patients remains a challenge. Current guidelines recommend treatment with at least 24 h of target temperature management (TTM), and patients need to be sedated during TTM. Prognosis based on neurological examination needs to be delayed in patients treated with TTM. Early prognostication is ideal to customize post-cardiac-arrest care for each patient. Several modalities are useful in early prognostication, including conventional or continuous electroencephalography (EEG), which is an extensively studied promising test. EEG recordings in post-cardiac-arrest patients are crudely classified as benign or malignant. The malignant pattern includes complete or near-complete suppression, burst suppression, generalized periodic discharge, status epilepticus, and the absence of reactivity to stimuli. These patterns predict a poor neurological outcome. The normal continuous pattern, diffuse slow pattern with reactivity to stimuli, and lack of malignant pattern are relatively benign and associated with good neurological outcomes [1,2,3,4]. However, conventional EEG or continuous EEG (cEEG) is difficult for non-neurologists to interpret. Furthermore, standardization of the interpretation is not sufficient, and there are substantial interobserver differences. In some sophisticated centers, cEEG recordings are interpreted by neurologists online immediately. To the best of our knowledge, there is no center that uses this system in Japan; most emergency and intensive care physicians engaged in post-cardiac-arrest care are not familiar with interpreting EEG. We need some modalities to use cEEG recordings in real time at the bedside.
1.1.2 What Is Amplitude–Integrated Electroencephalography?
There is a trend to quantify EEG recordings and make interpretation easier for non-neurologists. Amplitude-integrated EEG (aEEG) is a type of quantitative EEG that may provide a solution.
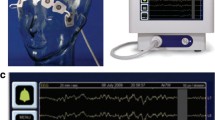
aEEG features the amplitude of conventional EEG and is usually derived from one or two EEG channels with reduced electrode montage. aEEG has been used widely in neonatal care. In this field, electrodes are placed at the C3, C4, P3, and P4 positions of the international 10–20 system. In adults, the use of the single frontal hairline lead is reported, in addition to C3, C4, P3, and P4. The minimum and maximum amplitudes in a short period are displayed as longitudinal bandwidths. aEEG uses a band-pass filter that removes extremely low and high frequencies to avoid artifacts. The upper and lower limits of the band show the maximum and minimum amplitudes, respectively. Amplitudes of <10 μV and >10 μV are displayed on linear and logarithmic scales, respectively. This semilogarithmic scale helped detect small changes at lower amplitudes and avoid overloading the display at higher amplitudes. These bands are arranged using a compressed timescale, which is typically 6 cm/h (Fig. 1.1). Therefore, reviewing longer periods of hours or days is possible. The reduced simplified montage is set up easily and acceptable in the busy environment of intensive care. In addition, the simplified information from the cEEG and compressed timescale enables physicians to interpret and monitor brain function in patients continuously at the bedside.
Scheme of an amplitude-integrated electroencephalography. The minimum and maximum amplitudes in a short period are displayed as longitudinal bandwidths. The upper and lower limit of the bands shows the maximum and minimum amplitudes, respectively. Amplitudes <10 and > 10 μV are displayed on linear and logarithmic scales, respectively. These bands are arranged with a compressed timescale, which is usually 6 cm/h
Raw EEG traces are available in modern devices, and it is important to refer to the raw trace to avoid any misinterpretation caused by noise. Furthermore, raw EEG traces can assist with the interpretation of seizure activity.
1.1.2.1 aEEG in Neonatal Care
aEEG traces are classified into patterns according to background activity. Physicians can monitor brain function by recognizing these simple patterns. Several classification systems have been suggested for use with neonates. In neonatal care, the gestational age significantly affects EEG. Sleep-wake cycling plays an important role in interpreting brain function together with background activity. Hellstrom et al. have developed a classification that consists of five background patterns, that is, continuous, discontinuous, burst suppression, low voltage, and flat. In their classification, the sleep-wake cycle and seizure are also considered [5]. aEEG is useful in predicting the neurological outcome of hypoxic encephalopathy in neonates. A randomized trial that evaluated the efficacy of hypothermia in neonates with hypoxic encephalopathy used aEEG pattern as part of the inclusion criteria of the study [6]. In addition, aEEG is useful in detecting status epilepticus. Seizure activity displays as abrupt rises in the lower and upper margin of the band in aEEG traces. The raw EEG is referenced to confirm seizure activity.
1.1.2.2 aEEG in Adult Post–Cardiac–Arrest Care
Recently, aEEG patterns have been reported as useful in adult post-cardiac-arrest care. Rundgren et al. monitored comatose post-cardiac-arrest patients with aEEG from admission to the intensive care unit during the completion of therapeutic hypothermia. They classified aEEG patterns into four simple background patterns: continuous, suppression burst, flat, and status epilepticus (Fig. 1.2). The aEEG was created from two bipolar channels. They measured aEEG in 100 patients: 29 of 31 (90%) with continuous aEEG patterns at the start of monitoring, and 54 of 62 patients (87%) at normothermia (36 h after cardiac arrest) gained consciousness. No patients who exhibited suppression-burst patterns at registration and normothermia regained consciousness. Flat aEEG patterns at registration were not associated with poor neurological outcomes; however, flat patterns at normothermia were more associated with poor outcomes [7].
Oh et al. monitored consecutive aEEG in 55 comatose post-cardiac-arrest patients with different methods from those used by Rundgren et al. They monitored aEEG using the bipolar frontal hairline lead. In addition, they applied the neonatal care classification system of aEEG with more detailed voltage criteria. They classified aEEG patterns into continuous normal voltage (CNV), discontinuous normal voltage (DNV), low voltage (LV), burst suppression, and status epilepticus (Fig. 1.3). The continuous pattern classification used by Rundgren et al. included the CNV, DNV, and LV patterns used by Oh et al. In their study, 16 of 17 patients (94%) with CNV at the start of monitoring had a cerebral performance category (CPC) score of 1 or 2 at hospital discharge. Conversely, all 21 patients who did not show CNV patterns during monitoring period had poor neurological outcomes [8]. These studies showed that aEEG monitoring can be performed and play an important role in the prognostication for adult post-cardiac-arrest care. aEEG patterns could predict good neurological outcomes at the very early phase after ROSC and predict poor neurological outcomes by monitoring changes in aEEG patterns. Therefore, intensivists and emergency physicians who are unfamiliar with conventional EEG can use aEEG bedside prognostication.
Considering these results, recovery of the normal aEEG trace is essential to obtaining a good neurological outcome. However, some patients who recover a normal trace after an extended period do not have a good neurological outcome. Therefore, the particular cutoff time for predicting a good neurological outcome from ROSC to recovery of a normal aEEG trace remains unknown. In infants with asphyxia, Thoresen et al. have reported that time to a normal aEEG trace is a good predictor of neurological outcomes [9].
Oh et al. prospectively studied 130 adult comatose post-cardiac-arrest patients. They found that the recovery of CNV within 24 h after ROSC was strongly associated with good neurological outcomes [10]. In a previous study, we evaluated 30 adult survivors of cardiac arrest with initial shockable rhythm and found that the optical cutoff of CNV recovery time after ROSC to predict a good neurological outcome is 23 h. These two studies report similar results and evaluated patients sedated with midazolam. Furthermore Oh et al. reported in their study that a time to normal trace from ROSC >36 h predicted a poor neurological outcome with 100% specificity.
1.1.2.3 aEEG Monitoring Methods in Adult Post–Cardiac–Arrest Care
No classification system of aEEG in adult post-cardiac arrest care has been established. Rundgren et al. have suggested a very simple classification system that is easy to understand (Fig. 1.2). Compared with this classification, Oh et al., who used the neonatal care classification system, have developed a more precise set of voltage criteria (Fig. 1.3). Moreover, this classification is still easy for non-neurologists to interpret. In the studies mentioned above, Oh et al. reported higher specificity for both good and poor neurological outcomes. This difference is related, in part, to the aEEG classification system used in both studies. Higher specificity is desirable for prognostication; therefore, we have used the classification reported by Oh et al. in our practice.
Simplicity and ease of setup are important factors in monitoring aEEG during post-cardiac-arrest care because many procedures have to be performed in this situation. The complexity of preparation could become a major barrier to monitoring cEEG. Typically, a montage with reduced electrodes is used to aid with this. Oh et al. used a bipolar channel of frontal hairline lead. They argue that this method is sufficient to monitor global brain function and is useful in prognostication. The frontal hairline lead can be positioned easily. Expert EEG technicians are not available full time in most centers in Japan; however, physicians or nurses in intensive care units (ICU) can attach the frontal hairline electrode in a very short time and set up aEEG monitoring. We use the Fp1-Fp2 bipolar channel to monitor aEEG, with disposable hydrogel electrodes (Fig. 1.4). Using this method, it is possible to begin aEEG monitoring in 5 min.
Monitoring seizure activity is another important role of cEEG. The monitoring and treatment of status epilepticus have been increasingly emphasized in post-cardiac-arrest care. Current guidelines recommend the use of frequent conventional EEG or continuous EEG to detect seizure in these patients [11, 12]. The role of aEEG in monitoring seizure in post-cardiac-arrest patients has not been established. We recently reported 65 cases that were monitored with aEEG using frontal hairline montage during post-cardiac-arrest care after ROSC at our center at the 44th annual meeting of Japanese Society of Critical Care Medicine. We evaluated the incidence and characteristics of post-cardiac-arrest patients with electrographic status epilepticus.
1.2 Methods
Comatose survivors after cardiac arrest who were treated with TTM and monitored with aEEG at our center from April 2013 to December 2016 were evaluated. Patients were excluded from the study if they were under 18 years of age, aEEG monitoring was not started within 24 h after ROSC, or aEEG recordings could not be interpreted because of noise.
During TTM, patients were maintained at 34 °C for 24 h and gradually rewarmed to 36 °C during the next 12 h. An external cooling system was applied as a cooling device, but the heat exchanger in circuit was used in patients treated with extracorporeal membrane oxygenation. Midazolam and fentanyl were used for sedation and analgesia, and most patients were paralyzed with vecuronium during hypothermia to control shivering. Administration of midazolam and vecuronium was stopped when the patient’s temperature reached 36 °C.
We used the classification system of aEEG patterns previously described by Oh et al. (Fig. 1.3). This classification includes CNV, DNV, LV, flat, BS, and status epilepticus (SE). The aEEG monitoring was performed with bipolar channel Fp1-Fp2 (frontal hairline lead). We used the diagnostic criteria for status epilepticus previously described in the study by Rundgren et al. Status epilepticus was suspected from aEEG traces and confirmed by referencing the raw bipolar channel Fp1-Fp2 trace. There are two different diagnostic criteria for status epilepticus, from continuous background and burst suppression. In patients with a continuous pattern before status epilepticus, electrographic status epilepticus was diagnosed when the repetitive epileptiform discharge with a frequency and amplitude >1 Hz and >50 μV, respectively, continued for >30 min. And in patients with a suppression-burst pattern, electrographic status epilepticus was diagnosed when repetitive epileptiform discharge with a frequency and amplitude >1 Hz and >50 μV, respectively, occupied >50% of a 30-min period [7].
1.3 Results
Sixty-five patients were eligible for the study. The median age was 63, 86% were male, 66% had an initial shockable rhythm, and 88% underwent coronary angiography after admission. Median arrest time was 24 min, median time from ROSC to the start of aEEG monitoring was 5.7 h, and median monitoring period was 53 h. Among these patients, 22 patients (44%) had a good neurological outcome (Table 1.1).
Status epilepticus was recognized in seven patients (11%). Backgrounds and clinical characteristics of patients with status epilepticus were not significantly different from those without status epilepticus (Table 1.1). CNV, flat, and burst suppression patterns led to status epilepticus in one, one, and three patients, respectively. Two patients showed status epilepticus at the start of monitoring. Among these patients, one patient had a good neurological outcome at hospital discharge.
Patients with good and poor neurological outcomes had different clinical characteristics. Patients with a good neurological outcome had shorter arrest times compared with those with a poor outcome and had an initial shockable rhythm. He recovered CNV before status epilepticus. Conversely, six patients with poor prognosis exhibited noncontinuous patterns, such as burst suppression and flat patterns, before status epilepticus, or exhibited status epilepticus at the start of monitoring. Among them, status epilepticus began during hypothermia, with a median time of 18 h after ROSC (Table 1.2).
Here, we present a more detailed clinical course of patients with good neurological outcomes. A 59-year-old man suffered from sudden cardiac arrest and received bystander cardiopulmonary resuscitation. He revealed a ventricular fibrillation when paramedics arrived at the scene. ROSC was achieved 22 min after collapse. Coronary angiography was performed after the admission to our center, and a diagnosis of vasospastic angina was given. He underwent TTM, and aEEG monitoring began 3 h after ROSC. He revealed LV at the start of aEEG monitoring; however, the amplitude developed gradually and CNV was recovered 23 h after ROSC. He was maintained at 34 °C for 24 h and then rewarmed. Midazolam was discontinued after his temperature reached 36 °C. Following this, he exhibited status epilepticus 44 h after ROSC; however, he received no anticonvulsant treatment. He regained consciousness approximately 96 h after ROSC, with a CPC score of 2 at hospital discharge.
1.4 Discussion
The frequency of status epilepticus in comatose post-cardiac-arrest care patients is approximately 10–30% [13,14,15]. In addition, epileptiform activity, such as electrographic seizures or interictal epileptiform discharges, is recognized in approximately 40% of patients [15]. The status epilepticus in the post-cardiac-arrest period is associated with poor neurological outcomes [13,14,15,16]. However, a small number of patients who reveal status epilepticus after ROSC have good neurological outcomes. Legriel et al. reported that 2 of 31 patients with status epilepticus (6%) during post-cardiac-arrest care had a CPC score of 1 or 2 at 1 year after cardiac arrest. These two patients demonstrated background reactivity to stimuli. Legriel et al. discussed that the prognosis of hypoxic encephalopathy with status epilepticus might be improved compared to that in the era before hypothermia treatment, and therapeutic hypothermia and sedatives with anticonvulsant effects might be beneficial in treating status epilepticus in these post-cardiac-arrest patients [14]. Rundgren et al. categorized patients with status epilepticus into two groups in their study to evaluate the efficacy of aEEG in comatose post-cardiac-arrest patients. The electrographic status epilepticus was recognized in 26 of 95 patients (27%). Of these patients, 16 developed electrographic status epilepticus from a suppression burst, and 10 patients developed electrographic status epilepticus after they revealed a continuous pattern in aEEG. A group of patients with a suppression-burst pattern developed status epilepticus at a relatively early phase during hypothermia. None of these 16 patients regained consciousness. Conversely, two patients who exhibited a continuous pattern regained consciousness. Electrographic status epilepticus occurred after rewarming in these two patients. One of these patients received no anticonvulsant treatment other than propofol for sedation, and he recovered to CPC 2. Another patient was administered midazolam, fosphenytoin, diazepam, and topiramate. She recovered to CPC 3 after 6 months. This study shows that the continuous pattern of background activity before status epilepticus is a key factor to obtaining a good neurological outcome. This was consistent with the results of our study. It is important to understand the process of status epilepticus development (Figs. 1.5 and 1.6). For this reason, cEEG monitoring from an early phase after ROSC is necessary. Furthermore, aEEG with reduced montage could play an important role in providing information on background activity changes. Intensivists and emergency physicians could understand this process at the bedside. This could lead to prompt anticonvulsant therapy for patients with the potential for good neurological recovery.
Frieberg et al. mentioned in their clinical review that “A major question, yet to be answered, is whether post-anoxic ESE is a condition that causes further brain injury, or is simply a sign of the hypoxic-ischemic encephalopathy” [17].
Current guidelines recommend anticonvulsant therapy for seizures after cardiac arrest, which is based on evidence that anticonvulsant therapy is recommended to prevent damage of the brain caused by prolonged status epilepticus. Seizures may increase the cerebral metabolic rate and exacerbate brain injury caused by cardiac arrest. However there has been no definite evidence to recommend anticonvulsant therapy vs no treatment in status epilepticus among post-cardiac-arrest patients. As mentioned above, one patient in the study by Rundgren et al. had a good neurological outcome without aggressive anticonvulsant therapy for status epilepticus. In addition, one patient who developed electrographic status epilepticus after rewarming and cessation of midazolam had a CPC score of 2 at hospital discharge without anticonvulsant therapy in our study. It remains uncertain whether he might have developed better cerebral function if he were treated aggressively with anticonvulsant therapy.
Status epilepticus in post-cardiac-arrest patients is often refractory; therefore, aggressive anticonvulsant therapies, such as large amounts of anesthetic agents like midazolam, propofol, or barbiturates, are required to control electrographic seizure activity. It is difficult to decide whether these kinds of anesthetic agents should be used in each situation. The sedative effects of these drugs lead to difficulties in early prognostication and more complicated post-cardiac arrest care. Patients sedated with barbiturates usually need a long period before regaining consciousness. Furthermore, deep sedation caused by these agents may lead to various complications, such as circulatory collapse; severe nosocomial infections, like pneumonia; ICU-acquired weakness; and deep vein thrombosis. These complications may be detrimental for the prognosis of post-cardiac-arrest patients.
A multicenter randomized controlled trial, “TELSTAR,” is recruiting participants. This study recruits comatose post-cardiac-arrest patients who are admitted to ICUs, monitored with cEEG, and report electrographic status epilepticus after ROSC. Patients are randomly assigned to aggressive anticonvulsant therapy to suppress all seizure activity or no treatment. The aggressive therapy includes both antiepileptic and anesthetic drugs, like thiopental. The primary outcome is neurological outcome at 3 months after cardiac arrest [18].
This study is recruiting all patients with status epilepticus and is not considering the background activity before status epilepticus. The frequency of patients with the potential for recovery, i.e., patients with status epilepticus from a continuous background, is very small. We argue that over half of the patients with status epilepticus exhibit burst suppression or noncontinuous patterns as background activity, and these patients usually have very severe brain damage with a poor prognosis. If these patients are included in the study, anticonvulsant treatment might not show any benefit.
We suggest that the efficacy of anticonvulsant treatment should be evaluated only in patients who exhibit continuous background aEEG patterns before status epilepticus. However, these patients would be a relatively small population within the population of patients with hypoxic encephalopathy.
In addition, the role of aEEG in this field is challenging. cEEG with standard montage is the gold standard to detect nonconvulsive status epilepticus. In some specialized centers, cEEG recordings are sent to neurophysiologists online and interpreted continuously. However, most centers do not have these sophisticated systems, and recordings are interpreted by experts on a daily basis. This causes delays in diagnosis and therapeutic intervention; therefore, the detection of status epilepticus with monitoring of trends in quantitative EEG by non-neurologists is desired.
qEEG is a visual representation of statistically transformed raw EEG signals. Although aEEG is a representative method, other qEEGs have been used to detect seizure activity. The compressed spectral array is another common method, which consists of a color display representing power in different frequency bands. Other qEEG methods include techniques that display EEG data based on rhythmicity or spectral symmetry.
Seizure activity causes an abrupt elevation of lower and upper margin of the band when monitoring aEEG. There are several studies that examined the efficacy of aEEG monitoring to detect electrographic seizure activity; however, the results of these studies are not optimistic.
In neonates, aEEG is used to detect seizure activity and monitoring cerebral activity in various encephalopathies. aEEG monitoring can help detect clinical and nonclinical seizures; however, its sensitivity and specificity have been as insufficient when compared with conventional cEEG. Rakshabhuvankar et al. conducted a systematic review that included 10 studies and 433 patients. These studies compared the simultaneous recording of aEEG and cEEG. The sensitivity and specificity for individual seizure detection were 76% and 85%, respectively, for aEEG with a raw trace, and 39% and 95%, respectively, for aEEG without a raw trace. They concluded that aEEG has a relatively low and variable sensitivity for seizure detection and cannot be recommended as the sole method for the management of neonatal seizures [19].
A few small single-center studies have evaluated the efficacy of aEEG for seizure detection in the management of seizures in adult patients. Nitzschke et al. examined whether aEEG recordings derived from a frontal single-channel EEG could detect seizures compared with raw EEG recordings. The sensitivity and specificity of aEEG were 40% and 89%, respectively. In this study, aEEG was evaluated by intensive care physicians who received training in aEEG interpretation [19]. Another study by Dericioglu et al. examined the ability of nonexpert ICU physicians and nurses to detect seizures. In this study, raw EEG recordings were converted to aEEG and density spectral array (DSA), and raters interpreted the recordings of aEEG and DSA without the raw trace. They found that the sensitivity and specificity for seizure detection were high and inter-rater reliability was acceptable. In addition, there was no difference between physician and nurses, and they concluded that ICU fellows and residents can achieve acceptable levels of accuracy for seizure identification using the digital EEG trend analysis methods following brief training [20].
Considering these results, aEEG cannot substitute cEEG in monitoring seizures. Today’s gold standard for monitoring nonclinical or nonconvulsive seizures is cEEG. However, aEEG should play a complementary role to cEEG. In addition, we should not interpret aEEG recordings without the raw trace. For a non-neurologist, aEEG traces suggesting seizure activity should warrant a consultation to the neurologist. Nonetheless, aEEG could help non-neurologists by assisting with continuous monitoring and timely interventions of seizure.
Another problem with seizure monitoring in critical care settings is whether the reduced montage number of channel leads is acceptable for seizure management. The conventional 10–20 system montage with full leads is a standard technique to detect seizure. This montage usually requires specially trained EEG technicians, which are not available 24 h a day and 7 days a week in most centers. Furthermore, the placement of many electrodes is time-consuming, and frequent care for patients in ICU is a major obstacle for the placement of many electrodes. Patients may need to be moved frequently, and typically these patients have many lines or drains. Several studies have evaluated the efficacy of a reduced electrode montage in seizure detection. These studies report relatively low sensitivity (approximately 70%) and high specificity (approximately 90%) of this method for seizure detection [21,22,23,24,25]. Most studies used a reduced montage with 7–8 leads; however, some studies examined the efficacy of subhairline leads. Subhairline leads have advantage of easy placement because hydrogel electrodes can be used in a similar manner to that in electrocardiography. Brenner et al. examined the efficacy of subhairline leads in patients who exhibited altered mental states after seizure in the emergency department. A neurophysiologist could diagnose nonconvulsive status epilepticus in all 12 patients using the subhairline montage compared with the standard 10–20 system [26].
These studies included patients with various backgrounds and clinical conditions. Vanherpe et al. examined the efficacy of the subhairline or eight-lead montage in comatose post-cardiac-arrest patients treated with TTM. They categorized the EEG recordings into three categories: without ictal activity, with interictal activity, and with probable electrographic seizure. The sensitivity for detecting probable electrographic seizure was 92% and 100% for hairline and eight-lead montage, respectively. Vanherpe et al. discussed that global seizures are more common in hypoxic encephalopathy compared with other conditions; therefore, the sensitivity of hairline and eight-lead montage is more acceptable in these populations [27].
In our study, 11% of patients revealed status epilepticus. This fits with the low number reported in previous studies. We used a single bipolar frontal hairline lead in our study. Considering the results of these studies, this method might not have enough sensitivity to detect all patients with status epilepticus. We decided to use this method as the simplest way without obstacles to introduce aEEG in our center. Therefore, ease and simplicity were most highly valued. Our main purpose was to use aEEG as a guide in prognosis. We argue that we will use a montage with more leads to detect seizures with a greater sensitivity. During the very early phase after ROSC, it is difficult to place many leads; however, after a few hours post-ROSC, we could place an additional five or six leads.
The diagnostic criteria of EEG for nonconvulsive status epilepticus are generally defined as definite, if the following conditions are recognized [28]:
-
1.
Focal or generalized spikes, sharp waves, or sharp-and-slow complexes at frequencies of >2.5 Hz
-
2.
Focal or generalized spikes, sharp waves, or sharp-and-slow complexes at frequencies ≤2.5 Hz or rhythmic activity >0.5 Hz and one of the following:
-
Electrographic and clinical improvement after an IV trial of an antiseizure drug
-
Subtle clinical ictal phenomena during the EEG pattern
-
Typical spatiotemporal evolution
-
These criteria should be met during at least 10 consecutive seconds, and abnormal EEG should constitute >50% of the monitoring period.
The diagnostic criteria of aEEG to diagnose electronic status epilepticus have not been developed. Rundgren et al. diagnosed electrographic status epilepticus in their study based on the pattern of aEEG and simultaneous raw EEG recordings, and we used these criteria in our study. They applied different criteria to the electrographic status epilepticus, from continuous pattern and suppression burst. In patients with a continuous pattern before status epilepticus, electrographic status epilepticus was diagnosed when the repetitive epileptiform discharge with a frequency > 1 Hz and amplitude >50 μV continued for more than 30 min and in patients with a suppression-burst pattern, when the repetitive epileptiform discharge with a frequency and amplitude of >1 Hz and >50 μV, respectively, occupies more than 50% of a 30-min period [7]. These criteria are different from the current gold standard because of the time of publication of their study. The incidence of status epilepticus is strongly affected by diagnostic criteria; therefore, while aEEG is a complementary technique to detect seizures, the actual diagnosis of seizure requires the use of the raw cEEG trace, and standard diagnostic criteria should be referenced.
Periodic discharges are often recognized during ictal or interictal periods in post-cardiac-arrest patients. aEEG may have a limited ability to detect the periodic discharges. When seizures start, the lower and upper margin of the band of the aEEG trace elevates suddenly. However, periodic low-frequency discharge might not show remarkable changes on an aEEG trace. The upper margin of the band may increase; however, this change may be difficult to notice when periodic discharges continue for long periods (Fig. 1.7). These aEEG traces, with periodic discharges, might be recorded as continuous normal voltage or continuous pattern traces. Periodic discharges have been reported to be associated with poor outcome in some studies [28]; therefore, we should pay attention to periodic discharges when using aEEG in prognostication.
1.5 Conclusion (Perspective from Our Study)
Our study showed that aEEG can help to identify patients with status epilepticus with limited sensitivity and provide important information regarding background activity before status epilepticus. This may lead to a better prognostication of patients with status epilepticus and aid to determine an indication for aggressive anticonvulsant therapy for each patient. With this aim, we have to begin aEEG monitoring from the early phase after ROSC.
The sensitivity of aEEG with a single frontal lead alone is not sufficient. Additional leads should be placed once patients have been stabilized to detect seizure activity. The efficacy of anticonvulsant therapy in post-cardiac-arrest patients with status epilepticus remains unknown. We believe that patients with a continuous background trace before status epilepticus should be treated aggressively.
References
Westhall E, Rossetti AO, van Rootselaar A-F, Wesenberg Kjaer T, Horn J, Ullén S, et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology. 2016;86(16):1482–90. https://doi.org/10.1212/WNL.0000000000002462.
Lamartine Monteiro M, Taccone FS, Depondt C, Lamanna I, Gaspard N, Ligot N, et al. The prognostic value of 48-h continuous EEG during therapeutic hypothermia after cardiac arrest. Neurocrit Care. 2016;24(2):153–62. https://doi.org/10.1007/s12028-015-0215-9.
Spalletti M, Carrai R, Scarpino M, Cossu C, Ammannati A, Ciapetti M, et al. Single electroencephalographic patterns as specific and time-dependent indicators of good and poor outcome after cardiac arrest. Clin Neurophysiol. 2016;127(7):2610–7. https://doi.org/10.1016/j.clinph.2016.04.008.
Tjepkema-Cloostermans MC, Hofmeijer J, Trof RJ, Blans MJ, Beishuizen A, van Putten MJAM. Electroencephalogram predicts outcome in patients with postanoxic coma during mild therapeutic hypothermia. Crit Care Med. 2015;43(1):159–67. https://doi.org/10.1097/CCM.0000000000000626.
Hellström-westas L. Amplitude-integrated EEG classification and interpretation in preterm and term infants. NeoReviews. 2006;7(2):76–97.
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–70. https://doi.org/10.1016/S0140-6736(05)17946-X.
Rundgren M, Westhall E, Cronberg T, Rosén I, Friberg H. Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med. 2010;38(9):1838–44. https://doi.org/10.1097/CCM.0b013e3181eaa1e7.
Oh SH, Park KN, Kim YM, Kim HJ, Youn CS, Kim SH, et al. The prognostic value of continuous amplitude-integrated electroencephalogram applied immediately after return of spontaneous circulation in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation. 2013;84(2):200–5. https://doi.org/10.1016/j.resuscitation.2012.09.031.
Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126(1):e131–9. https://doi.org/10.1542/peds.2009-2938.
Oh SH, Park KN, Shon YM, Kim YM, Kim HJ, Youn CS, et al. Continuous amplitude-integrated electroencephalographic monitoring is a useful prognostic tool for hypothermia-treated cardiac arrest patients. Circulation. 2015;132(12):1094–103. https://doi.org/10.1161/CIRCULATIONAHA.115.015754.
Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for post-resuscitation care 2015: section 5 of the European resuscitation council guidelines for resuscitation. Resuscitation. 2015;95:202–22. https://doi.org/10.1016/j.resuscitation.2015.07.018.
Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: post–cardiac arrest care. Circulation. 2015;132(18 suppl 2):S465–82. https://doi.org/10.1161/CIR.0000000000000262.
Rittenberger JC, Popescu A, Brenner RP, Guyette FX, Callaway CW. Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocrit Care. 2012;16(1):114–22. https://doi.org/10.1007/s12028-011-9565-0.
Legriel S, Hilly-Ginoux J, Resche-Rigon M, Merceron S, Pinoteau J, Henry-Lagarrigue M, et al. Prognostic value of electrographic postanoxic status epilepticus in comatose cardiac-arrest survivors in the therapeutic hypothermia era. Resuscitation. 2013;84(3):343–50. https://doi.org/10.1016/j.resuscitation.2012.11.001.
Mani R, Schmitt SE, Mazer M, Putt ME, Gaieski DF. The frequency and timing of epileptiform activity on continuous electroencephalogram in comatose post-cardiac arrest syndrome patients treated with therapeutic hypothermia. Resuscitation. 2012;83(7):840–7. https://doi.org/10.1016/j.resuscitation.2012.02.015.
Rossetti AO, Logroscino G, Liaudet L, Ruffieux C, Ribordy V, Schaller MD, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology. 2007;69(3):255–60. https://doi.org/10.1212/01.wnl.0000265819.36639.e0.
Friberg H, Westhall E, Rosén I, Rundgren M, Nielsen N, Cronberg T. Clinical review: continuous and simplified electroencephalography to monitor brain recovery after cardiac arrest. Crit Care. 2013;17(4):233. https://doi.org/10.1186/cc12699.
Ruijter BJ, Van Putten MJ, Horn J, Blans MJ, Beishuizen A, Van Rootselaar A-F, et al. Treatment of electroencephalographic status epilepticus after cardiopulmonary resuscitation (TELSTAR): study protocol for a randomized controlled trial. Trials. 2014;6(15):433. https://doi.org/10.1186/1745-6215-15-433.
Nitzschke R, Müller J, Engelhardt R, Schmidt GN. Single-channel amplitude integrated EEG recording for the identification of epileptic seizures by nonexpert physicians in the adult acute care setting. J Clin Monit Comput. 2011;25(5):329–37. https://doi.org/10.1007/s10877-011-9312-2.
Dericioglu N, Yetim E, Bas DF, Bilgen N, Caglar G, Arsava EM, et al. Non-expert use of quantitative EEG displays for seizure identification in the adult neuro-intensive care unit. Epilepsy Res. 2015;109:48–56. https://doi.org/10.1016/j.eplepsyres.2014.10.013.
Rubin MN, Jeffery OJ, Fugate JE, Britton JW, Cascino GD, Worrell GA, et al. Efficacy of a reduced electroencephalography electrode array for detection of seizures. Neurohospitalist. 2014;4(1):6–8. https://doi.org/10.1177/1941874413507930.
Ma BB, Johnson EL, Ritzl EK. Sensitivity of a reduced EEG montage for seizure detection in the neurocritical care setting. J Clin Neurophysiol. 2018;1:256. https://doi.org/10.1097/WNP.0000000000000463.
Karakis I, Montouris GD, Otis JAD, Douglass LM, Jonas R, Velez-Ruiz N, et al. A quick and reliable EEG montage for the detection of seizures in the critical care setting. J Clin Neurophysiol. 2010;27(2):100–5. https://doi.org/10.1097/WNP.0b013e3181d649e4.
Kolls BJ, Husain AM. Assessment of hairline EEG as a screening tool for nonconvulsive status epilepticus. Epilepsia. 2007;48(5):959–65. https://doi.org/10.1111/j.1528-1167.2007.01078.x.
Young GB, Sharpe MD, Savard M, Al Thenayan E, Norton L, Davies-Schinkel C. Seizure detection with a commercially available bedside EEG monitor and the subhairline montage. Neurocrit Care. 2009;11(3):411–6. https://doi.org/10.1007/s12028-009-9248-2.
Brenner JM, Kent P, Wojcik SM, Grant W. Rapid diagnosis of nonconvulsive status epilepticus using reduced-lead electroencephalography. West J Emerg Med. 2015;16(3):442–6. https://doi.org/10.5811/westjem.2015.3.24137.
Vanherpe P, Schrooten M. Minimal EEG montage with high yield for the detection of status epilepticus in the setting of postanoxic brain damage. Acta Neurol Belg. 2017;117(1):145–52. https://doi.org/10.1007/s13760-016-0663-9.
Leitinger M, Beniczky S, Rohracher A, Gardella E, Kalss G, Qerama E, et al. Salzburg consensus criteria for non-convulsive status epilepticus—approach to clinical application. Epilepsy Behav. 2015;49:158–63. https://doi.org/10.1016/j.yebeh.2015.05.007.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sugiyama, K., Hamabe, Y. (2018). A Single-Center Study on Nonconvulsive Status Epilepticus After Cardiac Arrest. In: Aibiki, M., Yamashita, S. (eds) A Perspective on Post-Cardiac Arrest Syndrome. Springer, Singapore. https://doi.org/10.1007/978-981-13-1099-7_1
Download citation
DOI: https://doi.org/10.1007/978-981-13-1099-7_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1098-0
Online ISBN: 978-981-13-1099-7
eBook Packages: MedicineMedicine (R0)