Abstract
Electrospinning and electrospraying technologies provide an accessible and universal synthesis method for the continuous preparation of nanostructured materials. This chapter introduces recent uses of electrospun and electrosprayed scaffolds for tissue regeneration applications. More recent in vitro and in vivo of electrospun fibers are also discussed in relation to soft and hard tissue engineering applications. The focus is made on the bone, vascular, skin, neural and soft tissue regeneration. An introduction is presented regarding the production of biomaterials made by synthetic and natural polymers and inorganic and metallic materials for use in the production of scaffolds for regenerative medicine. For this proposal, the following techniques are discussed: electrospraying, co-axial and emulsion electrospinning and bio-electrospraying. Tissue engineering is an exciting and rapidly developing field for the understanding of how to regenerate the human body.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Electrohydrodynamic techniques, namely electrospraying and electrospinning, are very powerful tools for developing and producing materials with the structural features necessary for tissue engineering (TE) applications. By definition, TE is a multidisciplinary field, integrating engineering principles, materials science, with chemistry, biology, and medicine, with the aim of either restoring or enhancing tissue or organ functions [56]. A tissue engineered construct is commonly made of materials and cells. The materials are often presented as porous biodegradable scaffolds which provide structural support for the cells. The materials used to produce the scaffold are a major area of study in TE. Natural materials offer the advantage of presenting structures and sequences that stimulate cell proliferation and adhesion but are highly variable from batch to batch or difficult to obtain on a large scale. Synthetic polymers allow for the control of various parameters, such as molecular weight, hydrophobicity and degradation time, but on the other hand, do not allow for good cell adhesion, proliferation or maintenance of the differentiated state [108]. The materials can be processed by various techniques in order to obtain the scaffolds, including electrospinning and electrospraying.
Electrospraying and electrospinning are technologies which use high electric fields for the production of particles and fibers, respectively. In the technique, a polymer solution jet is accelerated and drawn through an electric field. Depending on the apparatus, electric field strength, preparation conditions and physical properties of the solution, the stretched jet can break, causing droplets that produce micro/nanoparticles or remain as a filament which, after drying, produce nanometric/micro diameter fibers [90, 99, 134].
The first study that described the application of high electrical potentials to generate aerosols from drops of fluids was published in 1745 [14]. An application of the electrospraying technique was patented in 1902. Regarding electrospinning, which follows the same physical principles of electrospraying, the first patent which described the technique was reported in 1934 [100].
The conventional setup of an electrospinning and electrospraying process is illustrated in Fig. 5.1. The technology consists of three major components: a source of the electric field (high voltage power supply), a spinneret (nozzle coupled with a syringe pulsed with a pump) and a counter electrode (normally a metal collector plate). The solution is put through a pump and a difference in an electrical voltage is applied between the nozzle and the counter electrode. The flow rate of the polymer solution and the applied voltage need to be optimized, depending on the type of the solution used. Because of the high voltage that is applied (in the range of 1 to 30 kV), the drop of the polymer solution becomes highly electrified and it causes a cone-shaped deformation because of the surface tension, known as Taylor cone [14, 100]. Under the electrostatic forces the electric field becomes greater, the cone-shaped deformation breaks into highly charged droplets and by selecting the suitable conditions, the droplets reach to micro or nano-size level [101, 106]. Along the path that traverses the electrified jet ejector nozzle to the collector, the stretching process takes place and, depending on the physical characteristics of the polymer solution, the jet can break up into drops or remain as a filament [90, 100]. On this route to the counter electrode, the evaporation of the solvent and solidification of the polymer also occurs, leading to the formation of particles or solid continuous filaments with a reduced diameter [14, 90, 100].
Schematic diagram of the electrospraying (a) and electrospinning (b) processes for the production of particles and fibers, respectively. The polymer solution is pumped into the syringe and passes through a spinneret. This nozzle is connected to one terminal of the power supply and a metal collector to the opposite terminal. The jets of polymer solution ejected from the capillary tube may form polymeric particles or filaments, depending on the physical properties of the polymer solution. (c) co-axial and (d) emulsion electrospinning. Photographs kindly provided by the Stem Cell Laboratory archives, Universidade Federal do Rio Grande do Sul
The fiber formation in an electrospinning process depends on several parameters, which includes solution parameters (molecular weight, viscosity, surface tension, electric conductivity and dielectric effect of the solvent), ambient condition (humidity, temperature, pressure and type of atmosphere), processing conditions (voltage, flow rate, feed rate, diameter of spinneret and distance between the spinneret and collector) [90].
A video of electrospinning fundamentals regarding optimizing solutions and apparatus parameters in TE can be seen in Leach and collaborators [59] as well as the demonstration of electrospinning/electrospraying polymer solutions for biomedical applications [51].
Several polymers have been used industrially, such as nylon, polyester, polyacrylonitrile, polyvinyl alcohol, polyurethane, polylactic acid etc. Conventionally, the electrospinning technique mainly uses a polymer solution in organic solvents, such as chloroform, formic acid, tetrahydrofuran (THF), dimethylformamide (DMF), acetone and alcoholic solvents.
Currently, various modified electrospinning techniques have been developed, such as coaxial electrospinning, emulsion electrospinning, core-shell electrospinning, melt-spinning, blow assisted electrospinning and post-modification electrospinning [134]. The schematic diagrams show the co-axial electrospinning (Fig. 5.1c) and emulsion electrospinning techniques (Fig. 5.1d).
Electrospraying is a versatile way to make nanoparticles. Electrospraying, also called electrohydrodynamic atomization, represents a modified form of electrospinning and is a technique for the preparation of micro- and nanoparticles instead of fibers. Electrospraying has been widely applied to develop different types of commercial technologies, such as mass spectrometry, focused ion beam instruments and electrostatic precipitation of nanoparticles [11].
The particles and filaments produced by the electrospraying and electrospinning techniques can be used in various research and industry areas, as in TE in the production of biomaterials which are useful for treatment and also for diagnosis in the areas of pharmaceuticals, food, cosmetics, etc. [53, 134].
2 Electrospun and Electrosprayed Scaffolds for Tissue Engineering
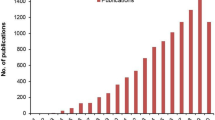
The total number of papers published in the PubMed database (http://www.pubmed.gov) with the keywords “tissue engineering” and “electrospinning” or “electrospun” in the field search “Title/Abstract” is 2215 since their first use in 2001 until 2017 (see Fig. 5.2). The number of papers containing these keywords has increased each year, especially in 2009, from 108 to 127, 163, 171, 214, 270, 266 and 296 in each subsequent year, up to 373 in 2017. A search in the PubMed database with the keywords “tissue engineering” and “electrosprayed” or “electrospray” in the field “Title/Abstract” resulted in 41 original papers from 2001 to 2017.
2.1 Types of Electrospun and Electrosprayed Materials for Tissue Engineering
Natural and synthetic polymers, together with their respective blends and composites, can be processed by both procedures of electrospinning and electrospraying, resulting in either fibers or particles with specific engineered properties. There are three individual groups of biomaterials used in the production of scaffolds for TE: natural polymers, synthetic polymers, and ceramics. Each biomaterial groups has advantages and disadvantages, so the use of composite scaffolds comprised of different types of biomaterials is becoming increasingly habitual [108].
This subsection illustrates the types of materials used for electrospinning and electrospraying, using some examples; however, it does not intended to present a list of all the materials belonging to this category.
2.1.1 Natural Polymers
In order to serve as a temporary extracellular matrix (ECM) for cells involved in the regenerative processes, the scaffold has to present some of the advantageous features of the natural ECM. There are two types of natural polymers derived from natural sources typically used as scaffolds in TE: (1) proteins such as collagen, gelatin, fibrinogen and silk fibroin and (2) polysaccharides as for example hyaluronic acid, chitosan, and alginate. These naturally occurring proteins and polysaccharides have been extensively used in the production of electrospun fibrous scaffolds [76]. Proteins such as collagen, fibrinogen and silk fibroin, which are able to form fibers in nature, are highly recommended for electrospinning and during the process, they easily assemble in fibers. Moreover, these proteins are also biocompatible and biodegradable, and some of them can also present antibacterial and anti-inflammatory properties [73].
The principal component of the ECM of various tissue types is generally collagen and the ratios of different collagen types and their organization define the mechanical properties and structure of the developing and growing tissue. An ideal scaffold should imitate the structure of the natural collagen found in the target organ [57, 73]. Typically, collagen can be electrospun from solutions based on either fluoroalcohols or water-ethanol mixtures [15]. The first study of electrospinning scaffolds using collagen was carried out by Huang and collaborators in 2001 with the electrospinning of collagen–polyethylene oxide (PEO) nanofibers [42]. Fluoroalcohols are still the solvents of choice, although some concerns have arisen concerning the possible effects of these solvents on collagen denaturation. However, cross-linking procedures are needed due to the fact that collagen nanofibers have poor mechanical resistance and a high degradation rate. In order to enhance the resistance different methods have been tested, as, for example, stabilization with either glutaraldehyde, epoxy compounds, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and exposure to ultraviolet light [31]. These methods can be employed during and after the process of electrospinning. On the other hand, the conversion of collagen into nanoparticles is challenging, but it is possible using one-step electrospray deposition. Gelatin is a mixture of products resulting from the degradation of collagen frequently used for biomedical applications. Fibers of gelatin were produced by electrospinning from solutions using HFIP, TFE, acetic and formic acid, and subsequently crosslinked for better mechanical resistance [44, 76, 86].
In addition to collagen and gelatin, fibrinogen is a candidate for the production of scaffolds by electrospinning for TE. Similar to collagen, fibrinogen is a protein that is present in the blood and plays an important role in wound healing, where during hemostasis, it is converted into fibrin fibers that are insoluble. Fibrinogen was first electrospun by Wnek and collaborators using a mixture of hexaflouroisopropanol and minimal essential medium as electrospinning solvent; the fibrinogen nanofibers presented a strong variation of the fiber diameter in the range of 80–700 nm (Gary E. [31, 32]). A study that used fibrinogen showed that fibers that were electrospun were characterized by elasticity and extensibility higher than collagen fibers [6]. In order to better control the mechanical resistance or the rate of degradation of fibrinogen scaffolds the fibers were either cross-linked with chemical compounds, such as glutaraldehyde or the cell culture medium was supplemented with aprotin.
Silk from the silkworm has been used as a medical suture for many centuries. The silk fibers present particularly remarkable mechanical properties and represent an option for many clinical applications [2]. The protein produced by the silkworm Bombyx mori is fibrous. In nature, silk is protected by a coat of sericin which is a glue-like protein. The raw silk fibers need to undergo degumming procedures in order to make the protein available and avoid possible biocompatibility problems due to contamination from residual sericin [2]. It is well known that fibroin possesses excellent biocompatibility and shows minimal immunogenicity and anti-inflammatory activity. This protein also plays important roles in re-epithelialization and elimination of scarring by enhancing the biosynthesis of collagen. For this reason, studies have been directed towards producing fibroin fibers through electrospinning in combination with other active principles, such as vitamin C or green grape seed extracts [28, 65].
Polysaccharides, such as alginate, cellulose, and chitosan can also be electrospun, but the process of electrospinning and electrospraying present some challenges and in most cases require additives.
Chitosan is a polysaccharide obtained through the deacetylation of chitin, which is the second most abundant polysaccharide in nature (after cellulose). Besides this, chitin is the major structural component of the exoskeleton of crustacean, as shrimps and crabs, and of the cell walls of fungi [76, 93]. As mentioned above, polymeric additives and also different acidic solutions, such as acetic acid, trifluoroacetic acid, formic acid and hydrochloric acid, are necessary for electrospinning chitosan [76]. Due to the presence of many amino groups in its backbone that gives to the molecule a polycationic character chitosan is difficult to electrospin. This polycationic nature increases the surface tension of the solution and a high electrical charge becomes necessary to produce electrospun chitosan nanofibers. During the electrospinning, process particles are often formed, probably due to the repulsive forces between ionic groups in the chitosan backbone in an acidic solution [60]. The resulting scaffolds are characterized by haemostatic and antibacterial properties, low immunogenicity and biocompatibility. Methacrylate glycol chitosan, carboxymethyl chitosan, and carboxyethyl chitosan are examples of water-soluble derivatives of chitosan that have been synthesized and electrospun, for TE applications [94]. For example, cinnamon oil, which exhibits antibacterial activity has been incorporated into chitosan/poly(ethylene oxide) (PEO) fibers. The electrospun fibers of cinnamon oil/chitosan were able to release the essential oil in vitro [92]. Electrosprayed chitosan microspheres were also produced and represent a potential carrier for the controlled release of drugs [111]. As chitosan is a mucoadhesive polymer the chitosan microspheres can attach to the mucosal surfaces and therefore may prolong the residence time and improve specific localization of absorption of the target drug.
Hyaluronic acid (HA) is a polyanionic polymer component of the ECM of connective tissue, such as umbilical cord, synovial fluid, vitreous, etc. The polyanionic surfaces of HA are highly hydrophilic. When the material is used as cell support, HA does not favour cellular interaction and attachment due to its thermodynamical features. Consequently, this material does not promote tissue formation. Therefore, in order to improve cell attachment onto HA-based biomaterials, ECM proteins such as type I collagen and fibronectin are coated onto HA surfaces, and HA microporous scaffolds are produced, which serve to direct the growth of cells within the scaffold [49]. As mentioned already in the case of protein-based biomaterials, HA is also often mixed with other polymers or needs to be dissolved in the suitable solvent mixture be to be able to form fibers when submitted to electrospinning procedures [49].
Alginate is an anionic polysaccharide derived from brown seaweed and produced by bacteria [60]. The sodium salt of alginate is soluble in water and forms highly viscous solutions even at very low polymer concentrations (2–3 wt%). Alginate is a very feasible biomaterial, because it is ableto form beads, sponges, and microfibers, which have been used for many TE approaches, such as cartilage, skin, liver, bone and cardiac tissue regeneration [60]. Alginate has many advantages in its use as a biomaterial for scaffolds, such as excellent biocompatibility, and the fact that it does not stimulate the immune response. It is also low cost, and has low toxicity, and can be transformed into a gel with the help of divalent cations, especially Ca2+ and Mg2+. Being of a polyanionic nature, the same challenge, namely the repulsive force among the polyanionic alginate chains, has to be overcome by performing electrospinning only in the presence of synthetic polymers, such as PEO and PVA [9, 68]. Generally, electrospinnability of alginate increases with the increase of synthetic polymers concentration, and nanofibers have been electrospun with compositions that were rich in synthetic polymers, as for example an alginate/synthetic polymer ratio of 50/50. However, the resulting electrospun fibrous alginate scaffolds present a high water solubility which limits their stability in aqueous environments. This instability in water-containing environments is overcome by cross-linking the fibers with glutaraldehyde, divalent ions, and TFA [60, 76].
Polyhydroxyalkanoates (PHA) are biodegradable polymers synthesized by microorganisms such as the bacterium Burkholderia xenovorans [1, 63]. The typical production techniques of PHA scaffolds include electrospinning, salt-leaching, solution casting and 3D printing [63]. One study compared the fabrication of a type of PHA, the poly-hydroxybutyrate (PHB), using electrospinning and salt-leaching techniques [74]. It was found that the nanofibrous scaffolds had better mechanical properties and Vero cells proliferated more on the electrospun PHB scaffold when compared to the PHB salt-leached scaffold. It was concluded that nanofibrous scaffolds were a better choice overall [74]. Another study showed that PHB electrospun membrane obtained by bacterial synthesis from a mutant strain of Azotobacter vinelandii promoted an increase in the cell density when compared to the cast film, suggesting that the fibrous morphology allows for better nutrient transference [95]. The study comparing electrospun fibers aligned to poly-(3-hydroxybutyrate-co-3-hydroxyhexanoate) meshes increased elasticity, tensile stress and MSC proliferation compared with randomly-oriented studies [116].
Decellularized ECM is gaining attention as a biomaterial for TE applications. The goal of decellularization is the removal of all the cells from a tissue or organ once cellular antigens are recognized as foreign by the host and, thereafter, the induction an inflammatory response [87]. Recent studies demonstrated that it is possible to produce hybrid scaffolds based on decellularized ECM and fibrous polymer meshes by electrospinning [113]. For example, Kim and collaborators developed nanofibrous electrospun from heart decellularized ECM-based hybrid scaffolds with poly(l-lactide-co-caprolactone) (PLCL) as a wound dressing for reducing scarring in wound healing [54]. Aslan and collaborators evaluated the use of a combined construct for corneal regeneration consisting of a collagen foam, a poly(l-lactic acid) (PLA) nanofiber mesh and decellularized matrices [5]. Goyal and collaborators developed hybrid scaffolds with decellularization derived from cultivated cells deposited within synthetic polymeric fibers [36].
However, a natural ECM component or its derivatives may not represent the ideal scaffold for biomedical applications because the scaffold should be able to accelerate the regeneration process that is normally encountered during the natural processes.
2.1.2 Synthetic Polymers
A great variety of synthetic polymers have been used to produce fibrous scaffolds by electrospinning, such as: poly(caprolactone) (PCL), poly(glycolic acid) (PGA), poly(lactic acid) (PLA), poly(D,L-lactide-co-glycolide) (PLGA), PLCL, poly(L-lactic acid) (PLLA), poly(carbonate), poly(urethane) (PU), poly(ethylene oxide) and poly(ethylene glycol) (PEG), among others [19]. The most important advantages of synthetic polymers over natural polymers are the possibility of customizing their mechanical properties, the controlled degradation process and the fact that can be synthesized into the desired form. Moreover, they are generally low-cost and have highly reliable characteristics [46].
PGA is the simplest polyester, it is biodegradable, and presents high crystallinity. PGA has slow degradation rate and is water insoluble. Its solubility depends on the molecular weight and typically, high molecular weight PGA is insoluble in most organic solvents except fluorinated solvents (e.g., hexafluoroisopropanol). Another synthetic polymer, PLLA, represents one of the most promising materials in TE because of its excellent mechanical properties, processability, and very good biocompatibility and biodegradability. PLLA is commonly used in various suture and implantation applications and due to its good mechanical properties, it can last for the sufficient time period in mechanical stresses in vivo [38]. In addition, PLLA electrospun fibrous scaffolds have been used in different biomedical applications. PLA itself presents high crystallinity (ca. 40%) and rigidity and a slow degradation, which limits its use as a suture material. Therefore, lactic acid is very often copolymerized with other biodegradable monomers as, for example, glycolic acid in order to achieve the required properties [38].
PLGA is a synthetic copolymer that is biodegradable and biocompatible and is very often used in electrospinning and electrospraying. Various characteristics can be obtained using the polylactides/glycolide by manipulating four key variables: the co-monomer ratio, the monomer stereochemistry, the linearity of the polymer chain, and the molecular weight [17]. The mechanism of degradation of the copolymer is the simple hydrolysis of the ester links, with crystallinity and water uptake being two key factors in determining the rates of in vivo degradation [17]. The crystallinity of the PLGA is related to its swelling behavior, mechanical strength, biodegradation rate, hydrolysis capability, which further depends on the molecular weight, and the molar ratio of the lactic and glycolic in the polymer chain. The lactide/glycolide polymer chains are cleaved by hydrolysis to the monomeric acids and are eliminated from the body through the Krebs cycle, primarily as carbon dioxide and in urine. The rate of hydrolysis of the PLGA is dependent only on changes in temperature and pH or the presence of a catalyst, therefore very little difference is observed in the degradation rate at different body sites [17]. PLGA is approved by both the European Medicine Agency and the US Food and Drugs Administration as a material that can be used in implantable biomedical applications and also for designing drug delivery systems. Moreover, PLGA is frequently used worldwide for the preparation of intravenous drug delivery systems and biomimetic materials and it has extensive application possibilities in TE.
PCL is a biodegradable and biocompatible polymer, which is chemically stable, mechanically strong, semi-crystalline and with a glass transition temperature of −60 °C. Due to these properties, PCL is approved by the Food and Drug Administration (FDA) for its application in drug delivery systems and TE [46].
The synthetic polymers mentioned here are being widely used in drug delivery systems, biomedical devices, and TE applications in both electrospun and electrosprayed preparations.
2.1.3 Polymeric Composites
Most of the polymers used to produce electrospun TE scaffolds are in their pure, single component form. However, very often one polymer does not meet all the requirements for various TE applications [57, 73].
As mentioned earlier, natural polymers, such as collagen, gelatin, silk fibroin, fibrinogen, chitosan, HA and their combinations, have been electrospun into nanofibers. However, these electrospun scaffolds show poor mechanical properties and lose their 3D structure in an aqueous environment. This problem can be overcome by blending the synthetic and natural polymers, which results in composite materials that present a combination of both good biocompatibility and mechanical strength, combining the advantages of two types of materials [22]. The combination of naturally occurring polymers on the surface of the composite nanofibers provides continued cell recognition signals, which is important for cell functioning during regeneration [22]. Natural bone matrix is an example of composite material, consisting of collagen (organic) and mineral components (inorganic), which provides an excellent balance between strength and toughness, superior to either of its individual components. The composite scaffolds with polymer and inorganic part are very advantageous scaffolds for bone TE.
In one study, Cui and collaborators produced PDLLA fibers by electrospinning and investigated their physical properties. They found that the fibers were hydrophobic, and could not support the initial adhesion and further growth of the cells, probably due to the surface erosion and dimensional shrinkage [21]. They, therefore, produced PDLLA and PEG composite electrospun fibers by blending different amounts of PEG into PDLLA [21]. PCL/HA composite nanofibrous scaffolds produced by electrospraying HA on PCL or PCL/collagen nanofibers enhanced the differentiation of mesenchymal stem cells into osteogenic lineage, indicating the use for bone TE [115]. Zheng and collaborators electrospun membranes with different gelatin/PCL ratios. The results show that three kinds of membranes with various gelatin/PCL ratios exhibited biocompatibility with chondrocytes and that electrospun gelatin/PCL is a good candidate for cartilage and other tissue regeneration [130].
Composite polymer/carbon nanotubes are another example of a combination of the excellent mechanical and electronic properties of the carbon nanotubes with the biocompatibility and degradability of synthetic polymers. For instance, the single carbon nanotube has a modulus as high as several thousands of gigapascal (GPa) and a tensile strength of several tens of GPa. However, carbon nanotubes are very difficult to align when they are used as mechanical reinforcement in composite fabrication and, therefore, the resulting composite does not exhibit the mechanical properties as one would expect. The alignment is a crucial step and electrospinning is presented as one method to align the carbon nanotubes in fibers [43, 79]. A number of research groups have tried to yield such nanofibers in recent years, by making PCL/gold or ZnO, polyacrylnitrile (PAN)/TiO2, PVA/Silica, and Nylon6/montmorillonite (Mt) ultrafine fibers, respectively.
2.1.4 Inorganic and Metallic Materials
Electrospinning applications are mostly limited to the fabrication of nanofibers from natural and synthetic polymers because of the accessibility in preparing a polymer solution with appropriate physical properties required for electrospinning. Inorganic materials, as for example ceramics, are usually considered not to be suitable for electrospinning. However, it is possible, to electrospin ceramic nanofibers from their melts by using extremely high temperatures [61]. In order to prepare ceramic fibers by electrospinning, some extra steps are required, steps that are normally not necessary when electrospinning natural or synthetic polymers; they are the following: preparation of an inorganic solution containing a matrix polymer together with an alkoxide, polymer precursor or a salt, followed by electrospinning of the solution to produce composite fibers and, finally, calcination, sintering, or chemical conversion of the precursor into the desired ceramic at high temperature, with removal of all organic components from the precursor fibers [61].
Several groups have shown that inorganic sols prepared by hydrolysis and condensation could be directly used for electrospinning [58, 61]. Fibers constructed of Al2O3, PbZrxTi1 − xO3, SiO2, and TiO2/SiO2 have been successfully produced in this manner.
Not only fibers but also inorganic nanoparticles have been developed for various biomedical applications due to their nanosize and biological properties. Several studies have reported encapsulation of inorganic nanoparticles such as titanium, silica, alumina, calcium carbonate and magnetic iron oxides onto a polymeric matrix, but their biomedical applications are sparse [40].
3 Tissue Engineering Applications
The facility of fabricating micro/nanofibers or particles and the wide variety of biocompatible polymers that can be used in electrospinning and electrospraying have revealed their potential applications in TE. One advantage of scaffolds produced by electrospinning is that their surface can be adjusted by controlling the parameters, thereby allowing for the topography that best fits the application. Another advantage is that nanofiber sheets can be shaped into almost any form-in accordance with the desired application. Electrospun scaffolds have assisted in the regeneration of a variety of tissue, such as skin, vasculature, neural, bone, ligament, and tendon ([105]a; [62]).
Regarding papers using electrospinning for TE published until 2017 in the PubMed database, it was possible to verify that approximately 30% have been used for the regeneration or reconstruction of bone; 16% for soft tissue; 13% for vascular; 13% for wound healing/skin/wound dressing; 11% for neural; 6% for cardiac; 6% for cartilage and a lower percentage for suture, bladder, corneal, liver, urinary incontinence and conjunctival regeneration (Fig. 5.3).
Tissue engineering areas of major application of electrospinning, according to research realized in the PubMed database until December 2017. The keywords used in the field Title/Abstract were: “tissue engineering” and “electrospinning” or “electrospun” and the organs and tissue applications (“cartilage” or “trachea”, “heart” or “cardiac”, “nervous” or “nerve” or “neural”, “skin” or “wound healing” or “wound dressing”, “vascular” or “vessels”, “soft tissue” or “tendon” or “valve” or “muscle”, “bone”, “suture”, “bladder”, “corneal”, “liver” or “hepatic”, “urinary incontinence”, “conjunctival”)
For TE, the research carried out on electrospun nanofibers quantified in terms of journal publications is much more in comparison with that of electrosprayed nanoparticles.
3.1 Bone Tissue Engineering
For bone repair, autograft is considerate the gold standard, however, there are supply limitations in its use [26]. In this context, the use of electrospun scaffolds for bone TE has become a rapidly expanding research field. Some of the most current materials and approaches used in electrospinning scaffolds for bone TE are summarized in Table 5.1.
3.2 Soft Tissue Engineering
In the field of soft TE, there is an immediate need to develop biomaterials with a high capacity for mechanical and biological performance [3]. A variety of scaffolds are investigated to promote soft TE by electrospinning; some current materials and approaches used are summarized in Table 5.2.
3.3 Vascular Tissue Engineering
Patients with cardiovascular disease have greatly benefited from the development of devices such as tissue-engineered vascular graft (TEVG) [30]. Vascular TE usually involves the fabrication of electrospun tubular scaffolds. Some current materials and approaches used in electrospinning scaffolds for vascular TE are summarized in Table 5.3.
3.4 Skin Tissue Engineering
Skin grafts are usually autografts, allografts, allogeneic, or xenogeneic. With the use of TE, it is possible to develop an improved approach to wound healing [81]. Some current materials used in electrospinning scaffolds for skin TE wound healing and wound dressing are summarized in Table 5.4.
3.5 Neural Tissue Engineering
Peripheral nerve tissue can self-regenerate if the external injuries are small. However, a number of challenges lie in restoring nerve tissue. Various kinds of scaffolds have been applied for neural TE, such as electrospun nanofibers [134] (Table 5.5).
4 Electrospray as Drug Delivery System
Electrospraying is recognized as an important and one of the most efficient techniques for the preparation of nanoparticles with respect to pharmaceutical applications. The entrapment of drugs into a biocompatible and biodegradable polymer matrix has been the focus of interest for the production of sustained drug release applications. The polymer takes the drug to the target, reduces the metabolic drug degradation, provides a continuous release, increases the active pharmaceutical ingredient activity and reduces the side effects of the drug [106]. Moreover, the nanoparticles have a wider range of applications because of their “zero” dimensional nature, whereas nanofibers are more restricted due to their two-dimensional nature [106].
Many different types of biodegradable polymers have been developed with differences in biodegradability. Drug delivery systems based on polymeric nanoparticles have the advantages of scalability, biodegradability, biocompatibility, cheaper cost, targeted delivery, sustainability in the release of encapsulated drugs that will result in improved efficacy. Among these polymers, polyesters such as PLGA, PCL, PLA and their derivatives, polyorthoesters and polyanhydrides, are being extensively used in a wide range of clinical applications as they are approved by the Food and Drug Administration for their biocompatibility and low toxicity [25, 82]. Bohr and collaborators successfully electrosprayed PLGA fabricated microspheres containing celecoxib, a non-steroidal anti-inflammatory drug; the compound proved to be amorphous and physically stable for more than 8 months [12]. Yu and collaborators prepared nanoparticles by a modified electrospraying process using polyvinylpyrrolidone (PVP) as a hydrophilic polymer matrix and ketoprofen as a model drug [127]. Ketoprofen is widely used for the treatment of inflammation, pain, or rheumatism and its short biological half-life leads to an increase of the frequency of medicine intake. In another example, a coating of nanoparticles composed of carbonated calcium deficient hydroxyapatite and PLA were deposited on a PLA substrate surface via electrospraying [132]. The deposited coating was also applied as a carrier to assist alendronate sodium, an approved bisphosphonate drug used for the treatment of osteoporosis, through local release. Paclitaxel, an antineoplastic chemotherapy drug which is widely used for the treatment of ovarian, breast, lung and pancreatic cancer was also successfully encapsulated into different biodegradable polymers, such as PLA, PCL or PLGA with high encapsulation efficiency through electrospraying [82].
Moreover, natural polymers of either protein or carbohydrate were found to produce stable micro/nanoparticles without any loss of their bioactivity of either the drug or encapsulating biomolecules [25, 37].
Electrospraying has been shown to meet the requirements for production of aerosols because monodisperse particles with controllable size and shape can be produced [82]. Ijsebaert and collaborators developed an aerosol generator of beclomethasone dipropionate by using electrospraying [45]. Electrospray nebulizers were used for producing microparticles of a size range of 2–5 μm and the particles serve the purpose of inhaling drugs through the lungs [106].
Another application of electrospraying as a drug delivery system is its potential in delivering more than one drug. Multiple drugs in a fixed dose combination could be delivered and released at the target sites [82]. The main benefit of the fixed-dose combination is that co-delivery of various therapeutic agents in the same delivery vehicles can bring the advantage of synergy, suppress drug resistance and be more convenient for patients (simplified dose regimen for daily treatment). An example is the work of Sakuma and collaborators who used electrospraying to enhance oral absorption of lopinavir through co-encapsulation with ritonavir [98]. Lopinavir, a human immunodeficiency virus (HIV) protease inhibitor, is used for the treatment of HIV infection. Low bioavailability and fast elimination are observed when lopinavir alone is orally administered; however, co-administration with ritonavir dramatically improves the poor pharmacokinetic properties of lopinavir [98].
5 Co-Axial Electrospinning and Electrospraying
The co-axial electrospinning method (Fig. 5.1c) is a modification of the traditional single spinneret electrospinning set up. This innovative method was first reported by Loscertales and collaborators in 2002 [67]. Loscertales and collaborators produced the capsules with diameters ranging from 150 nm to 10 μm by use of electrospraying. Sun and collaborators first used this set up to prepare nanofibers with core-sheath structures and called this technique ‘co-electrospinning’ [110]. The main purpose of co-axial electrospinning is to obtain fibers with a core-shell structure. The single spinneret is replaced by two co-axial capillaries in which two channels are connected to two reservoirs [69]. This technique is mainly used to obtain fibers with a core-shell structure having the specific desired drug encapsulated in the core of the fibers, which, due to the controlled degradation of the shell polymer, leads to a continued and controlled drug release over a long period. For drug delivery purposes various molecules have been successfully loaded into the co-axial fibers, as for example, antibiotics, proteins, enzymes and growth factors, The main advantage of this technique is that the core-shell fiber structure offers protection for the molecule loaded into the core and the bioactivity of the growth factor or drug, remains preserved [19, 105]. The fact of the drug or biological molecule being in the inner jet while the electrospinning process is in progress gives protection and enhances its the enhancing functionality or maintains the bioactivity of unstable compounds. Another advantage of the core-shell system is that it improves the sustained release of drugs [19]. The co-axial fibers present several advantages regarding the materials for the preparation of scaffolds, namely due to the possibility of combining a core with the desired mechanical properties with a shell, prepared from biocompatible materials, which will establish appropriate interactions with the host.
Various parameters can influence the encapsulation of drugs and biomolecules in the core of the co-axial fibers, such as concentrations of the core polymer and shell polymer, the relative flow rate of the core and shell solutions and the molecular weight and drug concentration [101]. Depending on the degradation rate of the shell polymer, when necessary an accelerated transport of core molecules into the environment is achieved by incorporating low molecular weight PEG as a porogen into the shell.
Co-axial electrospraying allows for the production of bilayered nano and microparticles by using a high electric field between the coaxial capillary needle and the ground. In this technique, the resultant electrical shear stress elongates the core and the shell liquid menisci at the needle outlet to form the Taylor cone; after this phenomenon, the jet of the liquid elongates enough until it is broken into multilayer droplets owing to the electrohydrodynamic forces [129]. Table 5.6 shows some recent examples of possible TE applications of co-axial fibers.
6 Emulsion Electrospinning
Emulsion electrospinning (Fig. 5.1d) is a simple variation of electrospinning to produce core-shell nanofibers by using a stable polymer emulsion, which has raised increasing interest, as the process is considered more stable. The advantage of emulsion electrospinning over the other blending techniques is that the drug of interest and the polymer are each solubilized in appropriate solvents, thus eliminating the need for a solvent that is suitable for the drug and polymer at the same time [101].
The emulsion electrospinning technique is a good alternative as it allows for the encapsulation of lipophilic molecules using hydrophilic polymers and avoids the use of organic solvents [83]. Emulsion electrospinning relies on chemical means of separation through the creation of an emulsion within a single solution and the subsequent organization of the emulsified droplets into two distinct phases as the solvent evaporates from the electrospun fibers. However, the method lacks well-defined control over the placement of the therapeutic agent within either the core or shell of the structure.
Yang and collaborators assessed the potential use of emulsion electrospinning to prepare core-shell fibers as carriers for therapeutic proteins [123]. Bovine serum albumin (BSA) was selected as a model protein and PDLLA as a polymer. The ultrafine fibers prepared by emulsion showed higher structural integrity of the core-shell fibers [123]. Table 5.7 shows some of the most recent studies investigating TE applications of scaffolds produced by emulsion electrospinning.
7 Bio-Electrospraying
Bio-electrospraying is a development of electrospraying that allows for producing matrices of cells inserted in scaffolds that could form engineered tissues and organs. Bio-electrospraying was first developed in 2005, and since then has been employed in some studies which further refined its use and made it evolve as a novel, direct in vivo TE and regenerative medicine strategy [39, 47, 48, 77, 84]. As the name suggests, the bio-electrospraying involves the spraying of living cells under the application of an electrical potential difference. Jayasinghe and collaborators have electrosprayed two types of cells, namely human blood and Jurkat cells, assessed for their viability after the procedure using trypan blue staining, they demonstrated that the cells could be maintained viable [47, 77]. Bartolovic and collaborators established a protocol for bio-electrospraying hematopoietic stem cells, showing that the cells retained both their viability and stem cell characteristics [7].
Bio-electrospraying has been continuously refined since its development to improve jet stability and continuity, but also to allow for the formation of encapsulations that include cells of various morphologies or multicellular model organisms such as zebrafish. The technique is unique for many reasons, as for example the ability to handle highly concentrated suspensions (>106 cells/mL). Moreover, the technique offers the possibility of forming nano/microstructures, including cells while using large needles, which are necessary in order to limit cell damage arising from shear forces on the cells [4]. One advantage of bio-electrospraying over other techniques such as ink-jet printing and aerosol delivery is that it can process denser cell suspensions and also generate finer droplets [48].
The methodology of bio-electrospraying provides a wide range of applications spanning from bio-analytics to diagnostics, but most importantly, it shows the potential for forming synthetic or artificial tissue, repairing and replacing damaged/aging tissue. One of the possible applications is found in the ability to bio-electrospray whole human blood without affecting the genetic make-up, demonstrating that this technique is a possible diagnostic protocol [77].
Bio-electrospraying allows for the design of constructs and this synthetic construct would require significantly reduced bioreactor time. Moreover, the combination of traditional electrospinning with bio-electrospraying offers the possibility of creating nanofibrous scaffolds with a uniform 3D distribution of cells for use in TE and regenerative medicine.
8 Conclusions and Perspectives
Electrospinning and electrospraying are electrohydrodynamic techniques used for the production of scaffolds or nano/microparticles in several fields of research. Variations of these techniques include electrospraying for drug delivery systems, co-axial electrospinning, and electrospraying, emulsion electrospinning as well as bio-electrospraying. The materials most commonly used for the production of scaffolds for TE or nano/microparticles for drug delivery are natural or synthetic polymers, polymers composites and inorganic and metallic materials.
The ability to produce fibers and particles with well-defined dimensions is an important characteristic of the electrospinning/spraying methods. The morphology of materials can be manipulated by solution parameters, such as molecular weight of the polymer, viscosity, the concentration of the solution, and by manipulating the processing parameters, such as tip to collector distance, conductivity, applied voltage, etc. The variously modified electrospinning and electrospraying techniques allow for the production of a great variety of nanostructured materials with desired properties for the regeneration of tissue and organs.
TE applied for regenerative medicine is an interdisciplinary subject of medicine, biology, engineering and life science. Numerous studies have been devoted to this rapidly developing field, with the aim of developing scaffolds to provide support for cells. It is with the continuous study of TE that we can successfully achieve the necessary groundbreaking techniques for the rebuilding of human tissue and organs.
There are still many challenges to be met regarding the improvement of the materials in terms of guaranteeing their three-dimensional structure along with vascularization and sufficient porosity to allow for the cells to grow and proliferate in the scaffolds. For drug delivery, the systems need further refinement for optimizing the encapsulation of biomolecules, ensuring they attain the appropriate quantity and their delivery in the desired location. Numerous studies are working to provide realistic solutions for these desired improvements to guarantee the successful development of scaffolds or drug delivery systems. The long-term goal for these studies will be a very high standard system for delivering drugs in small but highly efficient doses in the target with a significant decrease of undesired side effects and the elevation of efficiency in tissue regeneration in terms of the repair of lesions in tissue and organs.
References
Acevedo F, Villegas P, Urtuvia V et al (2018) Bacterial polyhydroxybutyrate for electrospun fiber production. Int J Biol Macromol 106:692–697. https://doi.org/10.1016/j.ijbiomac.2017.08.066
Altman GH, Diaz F, Jakuba C et al (2003) Silk-based biomaterials. Biomaterials 24:401–416
Altomare L, Farè S, Tanzi MC (2017) Bio-instructive scaffolds for muscle regeneration. In: Bio-instructive scaffolds for musculoskeletal tissue engineering and regenerative medicine. Elsevier, pp 161–186
Andreu N, Thomas D, Saraiva L et al (2012) In vitro and in vivo interrogation of bio-sprayed cells. Small 8:2495–2500. https://doi.org/10.1002/smll.201200138
Aslan B, Guler S, Tevlek A, Aydin HM (2017) Evaluation of collagen foam, poly( <scp>l</scp> -lactic acid) nanofiber mesh, and decellularized matrices for corneal regeneration. J Biomed Mater Res Part B Appl Biomater. https://doi.org/10.1002/jbm.b.34022
Baker S, Sigley J, Helms CC et al (2012) The mechanical properties of dry, electrospun fibrinogen fibers. Mater Sci Eng C 32:215–221. https://doi.org/10.1016/J.MSEC.2011.10.021
Bartolovic K, Mongkoldhumrongkul N, Waddington SN et al (2010) The differentiation and engraftment potential of mouse hematopoietic stem cells is maintained after bio-electrospray. Analyst 135:157–164. https://doi.org/10.1039/B917813A
Baudequin T, Gaut L, Mueller M et al (2017) The osteogenic and Tenogenic differentiation potential of C3H10T1/2 (mesenchymal stem cell model) cultured on PCL/PLA electrospun scaffolds in the absence of specific differentiation medium. Materials (Basel) 10:1387. https://doi.org/10.3390/ma10121387
Bhattarai N, Li Z, Edmondson D, Zhang M (2006) Alginate-based Nanofibrous scaffolds: structural, mechanical, and biological properties. Adv Mater 18:1463–1467. https://doi.org/10.1002/adma.200502537
Bhowmick S, Rother S, Zimmermann H et al (2017) Biomimetic electrospun scaffolds from main extracellular matrix components for skin tissue engineering application – the role of chondroitin sulfate and sulfated hyaluronan. Mater Sci Eng C 79:15–22. https://doi.org/10.1016/j.msec.2017.05.005
Bock N, Woodruff MA, Hutmacher DW, Dargaville TR (2011) Electrospraying, a reproducible method for production of polymeric microspheres for biomedical applications. Polymers (Basel) 3:131–149. https://doi.org/10.3390/polym3010131
Bohr A, Kristensen J, Dyas M et al (2012) Release profile and characteristics of electrosprayed particles for oral delivery of a practically insoluble drug. J R Soc Interface 9:2437–2449. https://doi.org/10.1098/rsif.2012.0166
Braghirolli DI, Helfer VE, Chagastelles PC et al (2017) Electrospun scaffolds functionalized with heparin and vascular endothelial growth factor increase the proliferation of endothelial progenitor cells. Biomed Mater 12:25003. https://doi.org/10.1088/1748-605X/aa5bbc
Braghirolli DI, Steffens D, Pranke P (2014) Electrospinning for regenerative medicine: a review of the main topics. Drug Discov Today 19:743–753. https://doi.org/10.1016/j.drudis.2014.03.024
Bürck J, Heissler S, Geckle U et al (2013) Resemblance of electrospun collagen nanofibers to their native structure. Langmuir 29:1562–1572. https://doi.org/10.1021/la3033258
Chamundeswari VN, Yuan Siang L, Jin Chuah Y et al (2017) Sustained releasing sponge-like 3D scaffolds for bone tissue engineering applications. Biomed Mater 13:15019. https://doi.org/10.1088/1748-605X/aa8bcd
Chasin M, Langer R (1990) Biodegradable polymers as drug delivery systems. Marcel Dekker, New York
Coimbra P, Santos P, Alves P et al (2017) Coaxial electrospun PCL/gelatin-MA fibers as scaffolds for vascular tissue engineering. Colloids Surfaces B Biointerfaces 159:7–15. https://doi.org/10.1016/j.colsurfb.2017.07.065
Cornejo Bravo JM, Villarreal Gómez LJ, Serrano Medina A (2016) Electrospinning for drug delivery systems: drug incorporation techniques. In: Electrospinning – material, techniques, and biomedical applications. InTech, London
CR R, PS S, O M, et al (2017) Nanochitosan enriched poly ε-caprolactone electrospun wound dressing membranes: A fine tuning of physicochemical properties, hemocompatibility and curcumin release profile. Int J Biol Macromol. doi: 10.1016/j.ijbiomac.2017.11.035
Cui W, Li X, Zhou S, Weng J (2008) Degradation patterns and surface wettability of electrospun fibrous mats. Polym Degrad Stab 93:731–738. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2007.12.002
Cui W, Zhou Y, Chang J (2010) Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater 11:14108. https://doi.org/10.1088/1468-6996/11/1/014108
Dadras Chomachayi M, Solouk A, Akbari S et al (2018) Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: thyme essential oil and doxycycline monohydrate release study. J Biomed Mater Res Part A. 106:1092. https://doi.org/10.1002/jbm.a.36303
Damaraju SM, Shen Y, Elele E et al (2017) Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials 149:51–62. https://doi.org/10.1016/j.biomaterials.2017.09.024
De Jong WH, Borm PJA (2008) Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine 3:133–149
Di Martino A, Liverani L, Rainer A et al (2011) Electrospun scaffolds for bone tissue engineering. Musculoskelet Surg 95:69–80. https://doi.org/10.1007/s12306-011-0097-8
Enayati MS, Behzad T, Sajkiewicz P et al (2018) Development of electrospun poly (vinyl alcohol)-based bionanocomposite scaffolds for bone tissue engineering. J Biomed Mater Res Part A. 106:1111. https://doi.org/10.1002/jbm.a.36309
Fan L, Wang H, Zhang K et al (2012) Vitamin C-reinforcing silk fibroin nanofibrous matrices for skin care application. RSC Adv 2:4110. https://doi.org/10.1039/c2ra20302b
Fukunishi T, Best CA, Ong CS et al (2018) Role of bone marrow mononuclear cell seeding for nanofiber vascular grafts. Tissue Eng Part A 24:135–144. https://doi.org/10.1089/ten.tea.2017.0044
Fukunishi T, Shoji T, Shinoka T (2017) Nanofiber composites in vascular tissue engineering. In: Nanofiber composites for biomedical applications. Elsevier, Duxford, pp 455–481
Fullana MJ, Wnek GE (2012) Electrospun collagen and its applications in regenerative medicine. Drug Deliv Transl Res 2:313–322. https://doi.org/10.1007/s13346-012-0087-x
Wnek GE, Carr ME, Simpson DG, Bowlin GL (2002) Electrospinning of nanofiber fibrinogen structures. Nano Lett 3(2):213–216. https://doi.org/10.1021/NL025866C
Ghorbani S, Tiraihi T, Soleimani M (2018) Differentiation of mesenchymal stem cells into neuron-like cells using composite 3D scaffold combined with valproic acid induction. J Biomater Appl 32:702–715. https://doi.org/10.1177/0885328217741903
Gomes S, Rodrigues G, Martins G et al (2017) Evaluation of nanofibrous scaffolds obtained from blends of chitosan, gelatin and polycaprolactone for skin tissue engineering. Int J Biol Macromol 102:1174–1185. https://doi.org/10.1016/j.ijbiomac.2017.05.004
Goonoo N, Bhaw-Luximon A, Jonas U et al (2017) Enhanced differentiation of human Preosteoblasts on electrospun blend Fiber Mats of Polydioxanone and anionic sulfated polysaccharides. ACS Biomater Sci Eng 3:3447–3458. https://doi.org/10.1021/acsbiomaterials.7b00350
Goyal R, Vega ME, Pastino AK et al (2017) Development of hybrid scaffolds with natural extracellular matrix deposited within synthetic polymeric fibers. J Biomed Mater Res Part A 105:2162–2170. https://doi.org/10.1002/jbm.a.36078
Gulfam M, Kim J, Lee JM et al (2012) Anticancer drug-loaded gliadin nanoparticles induce apoptosis in breast cancer cells. Langmuir 28:8216–8223. https://doi.org/10.1021/la300691n
Gupta B, Revagade N, Hilborn J (2007) Poly(lactic acid) fiber: an overview. Prog Polym Sci 32:455–482. https://doi.org/10.1016/J.PROGPOLYMSCI.2007.01.005
Hall RP, Ogilvie CM, Aarons E, Jayasinghe SN (2008) Genetic, genomic and physiological state studies on single-needle bio-electrosprayed human cells. Analyst 133:1347–1351. https://doi.org/10.1039/b806901h
Hanemann T, Szabó DV (2010) Polymer-nanoparticle composites: from synthesis to modern applications. Materials (Basel) 3:3468–3517. https://doi.org/10.3390/ma3063468
Hsu C-N, Lee P-Y, Tuan-Mu H-Y et al (2018) Fabrication of a mechanically anisotropic poly(glycerol sebacate) membrane for tissue engineering. J Biomed Mater Res Part B Appl Biomater 106:760–770. https://doi.org/10.1002/jbm.b.33876
Huang L, Nagapudi K, Apkarian RP, Chaikof EL (2001) Engineered collagen–PEO nanofibers and fabrics. J Biomater Sci Polym Ed 12:979–993. https://doi.org/10.1163/156856201753252516
Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63:2223–2253. https://doi.org/10.1016/S0266-3538(03)00178-7
Huang Z-M, Zhang Y, Ramakrishna S, Lim C (2004) Electrospinning and mechanical characterization of gelatin nanofibers. Polymer (Guildf) 45:5361–5368. https://doi.org/10.1016/J.POLYMER.2004.04.005
Ijsebaert JC, Geerse KB, Marijnissen JCM et al (2001) Electro-hydrodynamic atomization of drug solutions for inhalation purposes. J Appl Physiol 91:2735–2741. https://doi.org/10.1152/jappl.2001.91.6.2735
Jayaraman P, Gandhimathi C, Venugopal JR et al (2015) Controlled release of drugs in electrosprayed nanoparticles for bone tissue engineering. Adv Drug Deliv Rev 94:77–95
Jayasinghe SN, Eagles PAM, Qureshi AN (2006a) Electric field driven jetting: an emerging approach for processing living cells. Biotechnol J 1:86–94. https://doi.org/10.1002/biot.200500025
Jayasinghe SN, Qureshi AN, Eagles PAM (2006b) Electrohydrodynamic jet processing: an advanced electric-field-driven jetting phenomenon for processing living cells. Small 2:216–219. https://doi.org/10.1002/smll.200500291
Ji Y, Ghosh K, Shu XZ et al (2006) Electrospun three-dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials 27:3782–3792. https://doi.org/10.1016/j.biomaterials.2006.02.037
Joy J, Pereira J, Aid-Launais R et al (2018) Gelatin — oxidized carboxymethyl cellulose blend based tubular electrospun scaffold for vascular tissue engineering. Int J Biol Macromol 107:1922–1935. https://doi.org/10.1016/j.ijbiomac.2017.10.071
Kaplan J, Grinstaff M (2015) Fabricating Superhydrophobic polymeric materials for biomedical applications. J Vis Exp. https://doi.org/10.3791/53117
Khalili S, Nouri Khorasani S, Razavi M et al (2018) Nanofibrous scaffolds with biomimetic structure. J Biomed Mater Res Part A 106:370–376. https://doi.org/10.1002/jbm.a.36246
Kijeńska E, Swieszkowski W (2017) General requirements of electrospun materials for tissue engineering: setups and strategy for successful electrospinning in laboratory and industry. In: Electrospun materials for tissue engineering and biomedical applications: research, design and commercialization. Elsevier, Duxford, pp 43–56
Kim TH, Jung Y, Kim SH (2018) Nanofibrous electrospun heart Decellularized extracellular matrix-based hybrid scaffold as wound dressing for reducing scarring in wound healing. Tissue Eng Part A tentea 2017:0318. https://doi.org/10.1089/ten.tea.2017.0318
Kung FH, Sillitti D, Shrirao AB et al (2018) Collagen nanofibre anisotropy induces myotube differentiation and acetylcholine receptor clustering. J Tissue Eng Regen Med 12:e2010. https://doi.org/10.1002/term.2632
Langer R, Vacanti JP (1993) Tissue engineering Science 260:920–926
Lannutti J, Reneker D, Ma T et al (2007) Electrospinning for tissue engineering scaffolds. Mater Sci Eng C 27:504–509. https://doi.org/10.1016/J.MSEC.2006.05.019
Larsen G, Velarde-Ortiz R, Minchow K et al (2003) A method for making inorganic and hybrid (organic/inorganic) fibers and vesicles with diameters in the submicrometer and micrometer range via sol−gel chemistry and electrically forced liquid jets. J Am Chem Soc 125:1154–1155. https://doi.org/10.1021/ja028983i
Leach MK, Feng Z-Q, Tuck SJ, Corey JM (2011) Electrospinning fundamentals: optimizing solution and apparatus parameters. J Vis Exp 2494. https://doi.org/10.3791/2494
Lee KY, Jeong L, Kang YO et al (2009) Electrospinning of polysaccharides for regenerative medicine. Adv Drug Deliv Rev 61:1020–1032. https://doi.org/10.1016/J.ADDR.2009.07.006
Li D, McCann JT, Xia Y, Marquez M (2006) Electrospinning: a simple and versatile technique for producing ceramic nanofibers and nanotubes. J Am Ceram Soc 89:1861–1869. https://doi.org/10.1111/j.1551-2916.2006.00989.x
Li Y, Bou-Akl T (2016) Electrospinning in tissue engineering. In: Electrospinning – material, techniques, and biomedical applications. InTech, London
Lim J, You M, Li J, Li Z (2017) Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater Sci Eng C 79:917–929. https://doi.org/10.1016/j.msec.2017.05.132
Lin C, Liu C, Zhang L et al (2017) Interaction of iPSC-derived neural stem cells on poly(L-lactic acid) nanofibrous scaffolds for possible use in neural tissue engineering. Int J Mol Med. https://doi.org/10.3892/ijmm.2017.3299
Lin S, Chen M, Jiang H et al (2016) Green electrospun grape seed extract-loaded silk fibroin nanofibrous mats with excellent cytocompatibility and antioxidant effect. Colloids Surfaces B Biointerfaces 139:156–163. https://doi.org/10.1016/j.colsurfb.2015.12.001
Liu C, Wang C, Zhao Q et al (2018) Incorporation and release of dual growth factors for nerve tissue engineering using nanofibrous bicomponent scaffolds. Biomed Mater 13:044107. https://doi.org/10.1088/1748-605X/aab693
Loscertales IG, Barrero A, Guerrero I et al (2002) Micro/Nano encapsulation via electrified coaxial liquid jets. Science (80- ) 295:1695–1698. https://doi.org/10.1126/science.1067595
Lu J-W, Zhu Y-L, Guo Z-X et al (2006) Electrospinning of sodium alginate with poly(ethylene oxide). Polymer (Guildf) 47:8026–8031. https://doi.org/10.1016/j.polymer.2006.09.027
Lu Y, Huang J, Yu G et al (2016) Coaxial electrospun fibers: applications in drug delivery and tissue engineering. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 8:654–677. https://doi.org/10.1002/wnan.1391
Luo J, Zhang H, Zhu J et al (2017) 3-D mineralized silk fibroin/Polycaprolactone composite scaffold modified with Polyglutamate conjugated with BMP-2 peptide for bone tissue engineering. Colloids Surfaces B Biointerfaces 163:369. https://doi.org/10.1016/j.colsurfb.2017.12.043
Lv F, Wang J, Xu P et al (2017) A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater 60:128–143. https://doi.org/10.1016/j.actbio.2017.07.020
Ma M-X, Liu Q, Ye C et al (2017) Preparation of P3HB4HB/(gelatin + PVA) composite scaffolds by coaxial electrospinning and its biocompatibility evaluation. Biomed Res Int 2017:1–12. https://doi.org/10.1155/2017/9251806
Ma PX (2008) Biomimetic materials for tissue engineering. Adv Drug Deliv Rev 60:184–198
Masaeli E, Morshed M, Rasekhian P et al (2012) Does the tissue engineering architecture of poly(3-hydroxybutyrate) scaffold affects cell-material interactions? J Biomed Mater Res Part A 100A:1907–1918. https://doi.org/10.1002/jbm.a.34131
Maurmann N, Pereira DP, Burguez D et al (2017) Mesenchymal stem cells cultivated on scaffolds formed by 3D printed PCL matrices, coated with PLGA electrospun nanofibers for use in tissue engineering. Biomed Phys Eng Express 3:45005. https://doi.org/10.1088/2057-1976/aa6308
Mele E (2016) Electrospinning of natural polymers for advanced wound care: towards responsive and adaptive dressings. J Mater Chem B 4:4801–4812. https://doi.org/10.1039/C6TB00804F
Mongkoldhumrongkul N, Best S, Aarons E, Jayasinghe SN (2009) Bio-electrospraying whole human blood: analysing cellular viability at a molecular level. J Tissue Eng Regen Med 3:562–566. https://doi.org/10.1002/term.185
Nadim A, Khorasani SN, Kharaziha M, Davoodi SM (2017) Design and characterization of dexamethasone-loaded poly (glycerol sebacate)-poly caprolactone/gelatin scaffold by coaxial electro spinning for soft tissue engineering. Mater Sci Eng C 78:47–58. https://doi.org/10.1016/j.msec.2017.04.047
Naebe M, Lin T, Wang X (2010) Carbon nanotubes reinforced electrospun polymer Nanofibres. In: Nanofibers. InTech, London
Naseri-Nosar M, Salehi M, Hojjati-Emami S (2017) Cellulose acetate/poly lactic acid coaxial wet-electrospun scaffold containing citalopram-loaded gelatin nanocarriers for neural tissue engineering applications. Int J Biol Macromol 103:701–708. https://doi.org/10.1016/j.ijbiomac.2017.05.054
Naves LB, Almeida L, Rajamani L (2017) Nanofiber composites in skin tissue engineering. In: Nanofiber composites for biomedical applications. Elsevier, Duxford, pp 275–300
Nguyen DN, Clasen C, Van den Mooter G (2016) Pharmaceutical applications of Electrospraying. J Pharm Sci 105:2601–2620. https://doi.org/10.1016/j.xphs.2016.04.024
Nikmaram N, Roohinejad S, Hashemi S et al (2017) Emulsion-based systems for fabrication of electrospun nanofibers: food, pharmaceutical and biomedical applications. RSC Adv 7:28951–28964. https://doi.org/10.1039/C7RA00179G
Odenwälder PK, Irvine S, McEwan JR, Jayasinghe SN (2007) Bio-electrosprays: a novel electrified jetting methodology for the safe handling and deployment of primary living organisms. Biotechnol J 2:622–630. https://doi.org/10.1002/biot.200700031
Oftadeh MO, Bakhshandeh B, Dehghan MM, Khojasteh A (2018) Sequential application of mineralized electroconductive scaffold and electrical stimulation for efficient osteogenesis. J Biomed Mater Res Part A. 106:1200. https://doi.org/10.1002/jbm.a.36316
Oraby MA, Waley AI, El-Dewany AI et al (2013) Electrospinning of gelatin functionalized with silver Nanoparticles for nanofiber fabrication. Model Numer Simul Mater Sci 3:95–105. https://doi.org/10.4236/mnsms.2013.34013
Ott HC, Rajab TK (2016) Tissue-derived matrices. In: In Situ Tissue Regeneration. Elsevier, Amsterdam, pp 229–250
Pal P, Dadhich P, Srivas PK et al (2017) Bilayered nanofibrous 3D hierarchy as skin rudiment by emulsion electrospinning for burn wound management. Biomater Sci 5:1786–1799. https://doi.org/10.1039/c7bm00174f
Qu Y, Wang B, Chu B, et al (2018) Injectable and thermo-sensitive hydrogel and PDLLA electrospun nanofiber membrane composites for guided spinal fusion. ACS Appl Mater Interfaces 10(5):4462–4470 acsami.7b17020. doi: https://doi.org/10.1021/acsami.7b17020
Ramakrishna S, Fujihara K, Teo W-E et al (2005) Electrospinning Process. In: An introduction to electrospinning and nanofibers. World Scientific Publishing Company, Singapore, pp 90–154
Ramphul H, Bhaw-Luximon A, Jhurry D (2017) Sugarcane bagasse derived cellulose enhances performance of polylactide and polydioxanone electrospun scaffold for tissue engineering. Carbohydr Polym 178:238–250. https://doi.org/10.1016/j.carbpol.2017.09.046
Rieger KA, Schiffman JD (2014) Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr Polym 113:561–568. https://doi.org/10.1016/j.carbpol.2014.06.075
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632. https://doi.org/10.1016/J.PROGPOLYMSCI.2006.06.001
Romano I, Mele E, Heredia-Guerrero JA et al (2015) Photo-polymerisable electrospun fibres of N-methacrylate glycol chitosan for biomedical applications. RSC Adv 5:24723–24728. https://doi.org/10.1039/C5RA02301G
Romo-Uribe A, Meneses-Acosta A, Domínguez-Díaz M (2017) Viability of HEK 293 cells on poly-β-hydroxybutyrate (PHB) biosynthesized from a mutant Azotobacter vinelandii strain. Cast film and electrospun scaffolds. Mater Sci Eng C 81:236–246. https://doi.org/10.1016/j.msec.2017.07.045
Rosa AR, Steffens D, Santi B et al (2017) Development of VEGF-loaded PLGA matrices in association with mesenchymal stem cells for tissue engineering. Brazilian J Med Biol Res = Rev Bras Pesqui medicas e Biol 50:e5648. https://doi.org/10.1590/1414-431X20175648
Sadeghi-Avalshahr A, Nokhasteh S, Molavi AM et al (2017) Synthesis and characterization of collagen/PLGA biodegradable skin scaffold fibers. Regen Biomater 4:309–314. https://doi.org/10.1093/rb/rbx026
Sakuma S, Matsumoto S, Ishizuka N et al (2015) Enhanced boosting of oral absorption of Lopinavir through electrospray coencapsulation with Ritonavir. J Pharm Sci 104:2977–2985. https://doi.org/10.1002/JPS.24492
Salata O (2005) Tools of nanotechnology: electrospray. Curr Nanosci 1:25–33. https://doi.org/10.2174/1573413052953192
Saul CK, Stori EM, Petzhold CL, et al (2013) Process For producing polymeric structures that have activated surfaces and activated polymeric structures. Amsterdam, Netherlands. Patent number EP2767623A1
Seeram Ramakrishna M, Zamani M, Molamma P Prabhakaran S (2013) Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int J Nanomedicine 8:2997. https://doi.org/10.2147/IJN.S43575
Sevivas N, Teixeira FG, Portugal R et al (2017) Mesenchymal Stem Cell Secretome Improves Tendon Cell Viability In Vitro and Tendon-Bone Healing In Vivo When a Tissue Engineering Strategy Is Used in a Rat Model of Chronic Massive Rotator Cuff Tear Am J Sports Med 46:36354651773585. https://doi.org/10.1177/0363546517735850
Shamirzaei Jeshvaghani E, Ghasemi-Mobarakeh L, Mansurnezhad R et al (2017) Fabrication, characterization, and biocompatibility assessment of a novel elastomeric nanofibrous scaffold: a potential scaffold for soft tissue engineering. J Biomed Mater Res Part B Appl Biomater. https://doi.org/10.1002/jbm.b.34043
Sperling LE, Reis KP, Pozzobon LG et al (2017) Influence of random and oriented electrospun fibrous poly(lactic-co-glycolic acid) scaffolds on neural differentiation of mouse embryonic stem cells. J Biomed Mater Res - Part A 105:1333. https://doi.org/10.1002/jbm.a.36012
Sperling LE, Reis KP, Pranke P, Wendorff JH (2016) Advantages and challenges offered by biofunctional core-shell fiber systems for tissue engineering and drug delivery. Drug Discov Today 21:1243. https://doi.org/10.1016/j.drudis.2016.04.024
Sridhar R, Ramakrishna S (2013) Electrosprayed nanoparticles for drug delivery and pharmaceutical applications. Biomatter 3:e24281. https://doi.org/10.4161/biom.24281
Steffens D, Mathor MB, Soster PR d L et al (2017) Treatment of a burn animal model with functionalized tridimensional electrospun biomaterials. J Biomater Appl 32:663–676. https://doi.org/10.1177/0885328217735933
Steffens D, Braghirolli DI, Maurmann N, Pranke P (2018) Update on the main use of biomaterials and techniques associated with tissue engineering. Drug Discov Today. https://doi.org/10.1016/j.drudis.2018.03.013
Strobel HA, Calamari EL, Beliveau A et al (2018) Fabrication and characterization of electrospun polycaprolactone and gelatin composite cuffs for tissue engineered blood vessels. J Biomed Mater Res Part B Appl Biomater 106:817–826. https://doi.org/10.1002/jbm.b.33871
Sun Z, Zussman E, Yarin AL et al (2003) Compound core–shell polymer nanofibers by co-electrospinning. Adv Mater 15:1929–1932. https://doi.org/10.1002/adma.200305136
Suvannasara P, Siralertmukul K, Muangsin N (2014) Electrosprayed 4-carboxybenzenesulfonamide-chitosan microspheres for acetazolamide delivery. Int J Biol Macromol 64:240–246. https://doi.org/10.1016/J.IJBIOMAC.2013.12.012
Tan Z, Gao X, Liu T et al (2017) Electrospun vein grafts with high cell infiltration for vascular tissue engineering. Mater Sci Eng C 81:407–415. https://doi.org/10.1016/j.msec.2017.08.034
Thakkar S, Fernandes H, Moroni L (2015) Decellularized extracellular matrix scaffolds for cartilage regeneration. Methods Mol Biol 1340:133–151
Vatankhah E, Prabhakaran MP, Ramakrishna S (2017) Biomimetic microenvironment complexity to redress the balance between biodegradation and de novo matrix synthesis during early phase of vascular tissue engineering. Mater Sci Eng C 81:39–47. https://doi.org/10.1016/j.msec.2017.06.021
Venugopal J, Rajeswari R, Shayanti M et al (2012) Electrosprayed hydroxyapatite on polymer nanofibers to differentiate mesenchymal stem cells to osteogenesis. J Biomater Sci Polym Ed ahead-of-print:1–15. https://doi.org/10.1163/156856212X629845
Wang Y, Gao R, Wang P-P et al (2012) The differential effects of aligned electrospun PHBHHx fibers on adipogenic and osteogenic potential of MSCs through the regulation of PPARγ signaling. Biomaterials 33:485–493. https://doi.org/10.1016/j.biomaterials.2011.09.089
Wang Z-H, Chang Y-Y, Wu J-G et al (2017) Novel 3D neuron regeneration scaffolds based on synthetic polypeptide containing neuron cue. Macromol Biosci 1700251. https://doi.org/10.1002/mabi.201700251
Wright MEE, Wong AT, Levitt D et al (2018) Influence of ciprofloxacin-based additives on the hydrolysis of nanofiber polyurethane membranes. J Biomed Mater Res Part A 106:1211. https://doi.org/10.1002/jbm.a.36318
Wu S, Peng H, Li X et al (2017) Effect of scaffold morphology and cell co-culture on tenogenic differentiation of HADMSC on centrifugal melt electrospun poly (L-lactic acid) fibrous meshes. Biofabrication 9:44106. https://doi.org/10.1088/1758-5090/aa8fb8
Wu T, Zhang J, Wang Y et al (2018) Fabrication and preliminary study of a biomimetic tri-layer tubular graft based on fibers and fiber yarns for vascular tissue engineering. Mater Sci Eng C 82:121–129. https://doi.org/10.1016/j.msec.2017.08.072
Xu W, Shen R, Yan Y, Gao J (2017) Preparation and characterization of electrospun alginate/PLA nanofibers as tissue engineering material by emulsion eletrospinning. J Mech Behav Biomed Mater 65:428–438. https://doi.org/10.1016/j.jmbbm.2016.09.012
Yan Y, Sencadas V, Jin T et al (2017) Tailoring the wettability and mechanical properties of electrospun poly(l -lactic acid)-poly(glycerol sebacate) core-shell membranes for biomedical applications. J Colloid Interface Sci 508:87–94. https://doi.org/10.1016/j.jcis.2017.08.033
Yang Y, Li X, Cui W et al (2008) Structural stability and release profiles of proteins from core-shell poly (DL-lactide) ultrafine fibers prepared by emulsion electrospinning. J Biomed Mater Res Part A 86A:374–385. https://doi.org/10.1002/jbm.a.31595
Yang Y, Yang Q, Zhou F et al (2016) Electrospun PELCL membranes loaded with QK peptide for enhancement of vascular endothelial cell growth. J Mater Sci Mater Med 27:106. https://doi.org/10.1007/s10856-016-5705-6
Yin A, Luo R, Li J et al (2017a) Coaxial electrospinning multicomponent functional controlled-release vascular graft: optimization of graft properties. Colloids Surfaces B Biointerfaces 152:432–439. https://doi.org/10.1016/J.COLSURFB.2017.01.045
Yin H, Wang J, Gu Z et al (2017b) Evaluation of the potential of kartogenin encapsulated poly(L-lactic acid-co-caprolactone)/collagen nanofibers for tracheal cartilage regeneration. J Biomater Appl 32:331–341. https://doi.org/10.1177/0885328217717077
Yu D-G, Williams GR, Wang X et al (2011) Polymer-based nanoparticulate solid dispersions prepared by a modified electrospraying process. J Biomed Sci Eng 4:741–749. https://doi.org/10.4236/jbise.2011.412091
Zhang C, Wang X, Zhang E et al (2018) An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomater 66:141–156. https://doi.org/10.1016/j.actbio.2017.09.036
Zhang L, Huang J, Si T, Xu RX (2012) Coaxial electrospray of microparticles and nanoparticles for biomedical applications. Expert Rev Med Devices 9:595–612. https://doi.org/10.1586/erd.12.58
Zheng R, Duan H, Xue J et al (2014) The influence of gelatin/PCL ratio and 3-D construct shape of electrospun membranes on cartilage regeneration. Biomaterials 35:152–164. https://doi.org/10.1016/j.biomaterials.2013.09.082
Zhou F, Jia X, Yang Y et al (2016) Nanofiber-mediated microRNA-126 delivery to vascular endothelial cells for blood vessel regeneration. Acta Biomater 43:303–313. https://doi.org/10.1016/j.actbio.2016.07.048
Zhou H, Bhaduri SB (2013) Deposition of PLA/CDHA composite coating via electrospraying. J Biomater Sci Polym Ed 24:784–796. https://doi.org/10.1080/09205063.2012.714726
Zhou T, Li G, Lin S et al (2017) Electrospun poly(3-hydroxybutyrate- co −4-hydroxybutyrate)/graphene oxide scaffold: enhanced properties and promoted in vivo bone repair in rats. ACS Appl Mater Interfaces 9:42589–42600. https://doi.org/10.1021/acsami.7b14267
Zong H, Xia X, Liang Y et al (2017) Designing function-oriented artificial nanomaterials and membranes via electrospinning and electrospraying techniques. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2017.11.007
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Maurmann, N., Sperling, LE., Pranke, P. (2018). Electrospun and Electrosprayed Scaffolds for Tissue Engineering. In: Chun, H., Park, C., Kwon, I., Khang, G. (eds) Cutting-Edge Enabling Technologies for Regenerative Medicine. Advances in Experimental Medicine and Biology, vol 1078. Springer, Singapore. https://doi.org/10.1007/978-981-13-0950-2_5
Download citation
DOI: https://doi.org/10.1007/978-981-13-0950-2_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0949-6
Online ISBN: 978-981-13-0950-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)