Abstract
Static chamber method is widely used to measure emission of landfill gas by deploying the chamber at the surface of landfill cover soil. However, there will be errors between the measured fluxes and the real fluxes due to the increase of gas pressure in the chamber. A numerical model based on dust gas model was developed to investigate the factors affecting the errors. It is found that, height of static chamber is the most sensitive factor. When the height of chamber increases from 0.12 to 0.5 m, the error decreases from 32% to 10%. It will be easier for the chambers of smaller sizes to accumulate higher concentration and lead to greater errors. The proposed numerical model was successfully used in the analysis of the field static chamber tests carried out at the Xi’an landfill site. The evaluated errors for the field tests can be 30%. The model would be very useful for the error assessment of the static chamber tests.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Methane (CH4) and carbon dioxide (CO2) are the major components of landfill gas (LFG) generated from degradation of municipal solid waste (MSW). Landfill gas will transport from the cover layer to atmosphere. Before taking further measurements to control LFG or analyzing the influence of it on the environment, efforts should be made to determine actual gas emission flux. The static chamber method is widely used to measure landfill gas emission flux from the MSWs [1]. It is easy to operate, economic, and is available for point measurement. The static chamber is placed on the surface of landfill covers and gas concentration in it is measured at certain time intervals to obtain the gas emission flux. Many factors such as improper operation, varying gas pressure and temperature will result in errors of measured flux. Many works is have been focused on optimal chamber design, including using a fan to mix the gas, using a vent tube to minimize pressure change in the chamber, selecting a proper size of chamber and decreasing time interval [2,3,4]. However, it is assumed that gas concentration at the soil surface keeps constant after deployment static chamber. In fact, gas concentration will increase when gas migrates into the chamber. This will lead to a decrease of vertical concentration gradient and gas transport rate. This is also called “chamber effect”. Therefore, the error between measured flux and actual flux should be investigated.
Senevirathna demonstrated that the error was related to the height of the chamber, real flux and deployment time [5]. Livingston developed the non-linear relationship between gas concentration and flux based on Fick’s law and Laplace transform [6]. However, the disadvantage of the relationship is that advection was not considered. Senevirathna developed a two-dimensional model to simulate carbon dioxide transport from a two-layer soil cover to static chamber [7], which also considered methane oxidation Sahoo developed a two-dimensional model considering gas transport in chamber space [8]. However, the effect of advection was also not considered. In addition, most of the proposed models did not consider interaction of different component gas diffusion, i.e., the multi-component gas diffusion. Dust Gas Model (DGM) can describe gas transport and gas concentration in the chamber when the gas consists of many components (e.g., LFG) [9]. The aim of the paper is to develop a numerical model for simulating multi-component gas diffusion and advection in the soil and the static chamber. The proposed model based on DGM can also be used to get the relationship between real flux and measured flux at field.
2 Model Description
2.1 Governing Equations
After static chamber was installed at the soil surface, gas concentration in the chamber was measured at specific time. The flux of component i, N i,measure can be obtained from the gradient of C i [5]:
where V chamber and A chamber are chamber volume (m3) and based area (m2); and C i,chamber (mol/m3) represents mole concentration of component i in the chamber.
Because of “chamber effect”, gas will accumulate in the chamber and result in the decrease of the vertical concentration gradient. The error induced by this effect is [5]:
where \( Error \) is the error rate, N (mol/m2/s) is actual flux without static chamber; and N measure (mol/m2/s) is flux calculated by concentration-time curve.
It is assumed that gas is mixed well in the chamber and gas concentration in chamber is equal to that of the soil surface. Because the model developed is one-dimensional, lateral gas transport is neglected. This will lead to an error when test time is long. The other basic assumptions are as follows: (1) the soil was homogeneous; (2) gas flux distributed uniformly at the bottom of cover soil and kept constant; and (3) effects of atmospheric pressure and temperature fluctuation were neglected. Based on these assumptions, adopting mass balance equation and equation of continuity, the transient model was developed to describe the relationship between gas concentration and gas flux [10]:
where θ g is air volume ratio; z is the depth. C i (mol/m3) is mole concentration of component i; R (m3/Pa · K/mol) is ideal gas constant; T (K) is the absolute temperature; and N i (mol/m2/s) is flux of component i consisting of advection flux \( N_{i}^{V} \) and diffusion flux \( N_{i}^{D} \) [11]:
Advection flux can be described by [12]
where k rg and k i (m2) are relative and intrinsic permeability, respectively; μ (Pa s) is the gas viscosity; and x i is the mole fraction of component i:
The relative gas permeability depends on the effective degree of saturation [13]:
where α can be obtained from soil water characteristic curve test and S e is effective degree of saturation:
where S is degree of saturation, S r is residual degree of saturation.
Diffusive flux can be described by the DGM model [11]:
where D ij (m2/s) is binary diffusion coefficient between component i and j [14]; \( D_{iM}^{e} \) (m2/s) is Knudsen diffusion coefficient of component i and \( \tau \) is tortuosity coefficient [15]:
where n is the porosity of soil.
Knudsen diffusion coefficient can be calculated as a function of Klinkenberg parameter b i , permeability, viscosity and molecular mass M i [14]:
where b i is Klinkenberg parameter of gas component i:
and M air and M i (g/mol) are molecular mass of air and component i, respectively.
For idea gas, the partial pressure is related to mole concentration [16]:
where P i is partial pressure of component i.
2.2 Boundary and Initial Conditions
The boundary condition at soil cover bottom is assumed to be a constant flux boundary:
The bottom flux of LFG N bottom is equal to gas production from waste layer, while flux of O2 and N2 is 0.
The top boundary for the case without static chamber can be constant concentration:
where P atm is atmospheric pressure.
The top boundary for the case with static chamber needs to be considered when gas enters the chamber and gas concentration increases with time:
where the first item in Eq. (16) represents atmospheric concentration; and the second item which expressed in integral form represents mole concentration of species i entering the chamber during time t.
This model was separated by two steps. Firstly, the steady condition for the case without static chamber is calculated. The calculated gas concentration was then used as an initial condition of second step for the case with static chamber. It is assumed that the soil cover was initially filled by air. The initial condition for first step calculation is the same as the top boundary in the case without chamber. The initial conditions of the second step are based on the results of the first step calculation.
3 Model Verification
The proposed numerical model was verified by the experimental data from a laboratory static chamber [17]. Details on the experiment were given in this research [17]. CO2 was used for gas transport in the compacted soil specimen and the static chamber. The porosity, degree of saturation, gas permeability of the soil are n = 0.38, S = 25%, kg = 1×10−10 m2, respectively. The test was firstly carried out without the static chamber. The gas concentration at the top boundary was atmospheric concentration, i.e., \( {\text{P}} = 101\,{\text{Kpa}}, \) \( x_{{CO_{2} }} \):\( x_{{N_{2} }} \):\( x_{{O_{2} }} \) = 0.03:78.5:21.5. The bottom flux of CO2 is \( 5.2 \times 10^{ - 5}\,{\text{mol}}/{\text{m}}^{2} /{\text{s}} \). When the gas concentration in the soil column remained constant, steady state was achieved. The CO2 concentration profile was obtained at the steady state. The calculated CO2 concentration profile agrees relatively well with the experimental data (see Fig. 1).
Secondly, different sizes of static chambers were deployed at the soil surface. Gas concentration in the chamber was recorded at a certain time interval. For different sizes of chambers, the test data and the calculated results by the proposed model were shown in Fig. 2a, b and c. It can be found that when the chamber is the smallest (h: 0.05 m; id: 0.1 m), the results of the proposed model consists well with the experimental data in the first 500 s. At 800 s, the difference of those two is about 3%. When the chamber is the biggest one (h: 0.16 m; id: 0.25 m), with a height of 0.165 m, result of model calculation and experiment consists well until 20 min. For medium size chamber, with a height of 0.12 m, results of model calculation and experiment consist well in first 600 s. The difference of them is 0.5% at 800 s.
4 Application of Proposed Model in Field
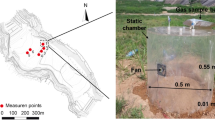
The field test was conducted at a landfill located at Xi’an (Northwest China). The static chamber was installed into the final cover of the landfill. A layer of 0.9 m compacted loess was the main material of final cover of the landfill. Gas permeability of loess at field is 2.86 × 10−13 m2 [18]. Two points in the field were selected randomly to conduct static chamber test.
To analyze error of measured flux at field, the proposed model was used on the basis of the parameters obtained in the field site. The parameter values were obtained from the field site test at a landfill in west China (see Table 1). Because LFG mainly consists of CH4 and CO2, the 4 components, i.e., CH4, CO2, O2 and N2 were considered. The Gas properties were shown in Table 2.
Figure 3 shows the errors of methane and carbon dioxide flux for chambers of different heights. The errors decreased with the increase of the height of the static chamber. When the height of chamber is 0.12 m, the error for the flux of CH4 and CO2 are both higher than 20% at 1200 s. When the height of the chamber is 0.5 m, the error is 9% at 1200 s. These results demonstrated that chamber effect cannot be neglected. Even the chamber is relatively high (e.g., 0.5 m). The measured flux still needs to be corrected. The reason for the higher errors for the cases with relatively small heights is that the concentration in the static chamber increases more quickly due to the lower volumes. For the same chamber, the error for CO2 was smaller than that of CH4, especially when the chamber is small. When the height of chamber is 0.12 m, difference between the errors of those two gases is 3% at 1200 s. This is due to the fact that the binary diffusion coefficients for CO2 are smaller than that of CH4.
The static chamber, with a height of 55 cm and diameter of 50 cm, consisted of a glass cylinder and equipped with a small fan for internal air recirculation. An external and flexible pedestal was used to seal the static chamber to the ground, thus preventing gases exchange between the chamber and the atmosphere when the groove in the steel pedestal was filled with water (see Fig. 4). Shut off all the valves, waited for accumulation of the gas in the chamber, sampled the gas into Tedlar bags every 10 min for three times (0 min, 10 min, 20 min) and then methane fluxes were determined (the slope of the gas concentration increase versus time). Gas concentration in those gas sampling bags was analyzed by gas chromatograph (GC9800) in laboratory.
By inputting gas concentrations data of different time into Eq. (1), N measure , the measured emission flux can be obtained. The flux can be corrected by assuming a flux error. The gas concentrations corresponding to the corrected flux can then be obtained by inputting the corrected flux to the model. The calculated gas concentrations were compared to the measured data to evaluate the ranges of the error. In Fig. 5a, when assuming that the errors are 20% and 30%, the corrected concentration predicted by the proposed model is within the range of measured concentrations of methane and carbon dioxide. For the test point 2, the errors of the measured CO2 and CH4 data was 0-20% (see Fig. 5b). These results indicate that the errors with respect to field static chamber tests can be quite high and it should be modified by the numerical model.
5 Conclusions
A one-dimensional model for gas transport from the soils to the static chamber was developed. The proposed model is verified by the experimental data. It can be used to analyze effect of chamber height, real flux and degree of saturation on the errors induced by the chamber effect. The main conclusions are as follows:
-
1.
Chamber size is the main factor affecting the measurement error. When the height of chamber ranges from 0.12 to 0.5 m, the error ranges from 32% to 10%. It will be easier for the chambers of smaller sizes to accumulate higher concentration and lead to greater errors. For chamber height of 0.12 m, the error can be 30% at 1200 s. However, the deployment time for smaller chamber will be shorter. The optimal deployment time for small chambers needs further research.
-
2.
The proposed numerical model was successfully used in the analysis of the field static chamber tests carried out at the Xi’an landfill site. The evaluated errors for the field tests can be 30%. The model would be very useful for the error assessment of the static chamber tests.
References
Pihlatie, M.K., Christiansen, J.R., Aaltonen, H., Korhonen, J.F.J., Nordbo, A., Rasilo, T., Benanti, G., Giebels, M., Helmy, M., Sheehy, J., Jones, S., Juszczak, R., Klefoth, R., Lobo-do-Vale, R., Rosa, A.P., Schreiber, P., Serça, D., Vicca, S., Wolf, B., Pumpanen, J.: Comparison of static chambers to measure CH4 emissions from soils. Agric. For. Meteorol. 171–172, 124–136 (2013)
Christiansen, J.R., Korhonen, J.F., Juszczak, R., Giebels, M., Pihlatie, M.: Assessing the effects of chamber placement, manual sampling and headspace mixing on CH4 flux in a laboratory experiment. Plant Soil 343(1–2), 171–185 (2011)
Hutchinson, G.L., Livingston, G.P.: Soil-atmosphere gas exchange. In: Dane, J.H., Topp, G.C. (eds.) Methods of soil analysis. Part 4. SSSA Book Series 5, pp. 1159–1182. SSSA, Madison (2002)
Rochette, P., Eriksenhamel, N.S.: Chamber measurements of soil nitrous oxide flux: are absolute values reliable? Soil Sci. Soc. Am. J. 72(2), 331–342 (2008)
Senevirathna, D.G.M., Achari, G., Hettiaratchi, J.P.A.: A laboratory evaluation of errors associated with the determination of landfill gas emissions. Can. J. Civ. Eng. 33(3), 240–244 (2006)
Livingston, G.P., Hutchinson, G.L., Spartalian, K.: Trace gas emission in chambers: a non-steady-state diffusion model. Soil Sci. Soc. Am. J. 70(5), 1459–1469 (2006)
Senevirathna, D.G.M., Achari, G., Hettiaratchi, J.P.A.: A mathematical model to estimate errors associated with closed flux chambers. Environ. Model. Assess. 12(1), 1–11 (2007)
Sahoo, B.K., Mayya, Y.S.: Two dimensional diffusion theory of trace gas emission into soil chambers for flux measurements. Agric. For. Meteorol. 150(9), 1211–1224 (2010)
Molins, S., Mayer, K.U.: Coupling between geochemical reactions and multicomponent gas and solute transport in unsaturated media: a reactive transport modeling study. Water Resour. Res. 43(5), 687–696 (2007)
Binning, P.J., Postma, D., Russell, T.F., Wesselingh, J.A., Boulin, P.F.: Advective and diffusive contributions to reactive gas transport during pyrite oxidation in the unsaturated zone. Water Resour. Res. 43(2), 329–335 (2007)
Mason, E.A., Malinauskas, A.P.: Gas Transport in Porous Media: The Dusty-Gas Model. Elsevier, Amsterdam (1983)
Clifford, K.H., Webb, S.W.: Gas transport in porous media. Encycl. Ecol. 14(8–9), 3576–3582 (2006)
Parker, J.C.: Multiphase flow and transport in porous media. Rev. Geophys. 27(3), 311–328 (1989)
Thorstenson, D.C., Pollock, D.W.: Gas transport in unsaturated zones: Multicomponent systems and the adequacy of Ficks Law. Water Resour. Res. 25(3), 477–507 (1989)
Moldrup, P., Olesen, T., Gamst, J., Schjønning, P., Yamaguchi, T., Rolston, D.E.: Predicting the gas diffusion coefficient in repacked soil: water-induced linear reduction model. Soil Sci. Soc. Am. J. 64(1), 1588–1594 (2000)
Reid, R.C., Prausnitz, J.M., Sherwood, T.K.: The Properties of Gases and Liquids. McGrawHill, New York (1977)
Perera, M.D.N., Hettiaratchi, J.P.A., Achari, G.: A mathematical modeling approach to improve the point estimation of landfill gas surface emissions using the flux chamber technique. J. Environ. Eng. Sci. 1(1), 451–463 (2002)
Zhan, L.T., Qiu, Q.W., Xu, W.J., et al.: Field measurement of gas permeability of compacted loess used as an earthen final cover for a municipal solid waste landfill. J. Zhejiang Univ.-Sci. A 17(7), 541–552 (2016)
Acknowledgements
The financial supports from the National Natural Science Foundation of China (Grants Nos. 41672288, 51478427, 51625805, 51278452, and 51008274), and the Fundamental Research Funds for the Central Universities (Grant No. 2017QNA4028) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Zuo, X., Shen, S., Xie, H., Chen, Y. (2018). A Numerical Model for Error Analyses of Static Chamber Method Used at Landfill Site. In: Farid, A., Chen, H. (eds) Proceedings of GeoShanghai 2018 International Conference: Geoenvironment and Geohazard. GSIC 2018. Springer, Singapore. https://doi.org/10.1007/978-981-13-0128-5_61
Download citation
DOI: https://doi.org/10.1007/978-981-13-0128-5_61
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0127-8
Online ISBN: 978-981-13-0128-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)