Abstract
Methane oxidation in landfill biocover is a complex process involving water, gas and heat transport as well as microbial oxidation. Such technology is useful for dealing with landfill gases. With the aim of finding the most suitable design configuration for actively-aerated methane biocover, an air injection system is proposed in this work to ensure that oxygen availability is no longer a limitation for methane degradation. A numerical model is developed that incorporates water-gas-heat coupled transport in layered landfill biocover with the consideration of methane oxidation and air actively-aerated injection. The model is verified and calibrated using published data from a laboratory soil column test. Moreover, parametric studies are carried out to investigate the influences of air injection amount and location. It is found that injecting air into the biocover through several inlets along the biocover bed is a promising approach to enhance methane oxidation in the cover.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Landfill is a site stacking the municipal solid waste (MSW) and has wide application worldwide due to its low cost and environmental-friendly characteristics [1]. The cover system, as the essential structure of landfill, is designed to impede the penetration of rainfall and emission of landfill gas (LFG). One main component of LGF is methane (CH4) which is one of the greenhouse gases and has much higher global warming potential than carbon dioxide (CO2) [2]. Therefore, it is necessary to mitigate CH4 emission from landfill effectively and economically.
Landfill biocover is one type of landfill cover, which is effective in impeding the emission of methane from landfill into atmosphere using biotic methane oxidation. It is quite useful in landfills, especially those lacking landfill gas extraction system [3]. The oxidation that occurs in biocover can consume CH4, generate CO2 and water, and release heat, which can be described by the following equation:
where -CH2O- is organics and x is the reaction coefficient.
The oxidation reaction is affected by numerous factors, such as CH4 concentration, O2 concentration, temperature, moisture and other properties. The heat released by reaction can reach 632 kJ/mol [4]. Passive aeration is usually used in traditional biocover. O2 naturally intrudes into the cover by diffusion and provides the required O2 for oxidation reaction. The diffusion of O2 depends on the O2 concentration gradient in cover soils [5]. Recent researches reveal that the maximum reaction depth of CH4 is approximately 30 cm, which is limited by the O2 distribution in cover. Increasing O2 amount in cover artificially is a possible way to enhance the methane oxidation [6].
In this study, a new cover structure is proposed. The new cover structure can adjust gas distribution in cover using actively-aerated injection system, which is beneficial for deepening the O2 penetration depth and reducing LFG emission. A numerical model is built to simulate water-gas-heat transport in the new landfill cover structure.
2 Conceptual Model of Actively-Aerated Landfill Cover System
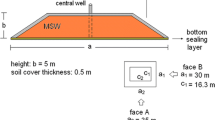
In order to better reduce methane emission, a new landfill cover structure is proposed here. As shown in Fig. 1, the landfill cover consists of three layers with different functions. At the bottom, a gravel layer is used to make gas uniformly distributed. The gravel layer is overlain by a clay layer whose function is impeding methane emission. The silt layer is the reaction layer, which is located at the top. As the major place where the methanobacteria grows, the silt layer also provides the environment for the growth of vegetation. Additionally, the silt layer also helps to avoid cracking of the clay layer under dry condition.
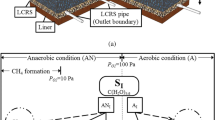
In this study, soil is assumed to be a three-phase porous medium containing liquid, gas and solid particles. The liquid phase is water, while the gas phase is a four-component gas mixture (CO2, O2, CH4 and N2). Gas migration involves advection and diffusion. After the methane migrates from the MSW to the silt layer, methane oxidation reaction occurs, generating water, carbon dioxide and organic matter. The heat released by the reaction in turn affects the biochemical reaction rate. Thus it is a water-gas-heat coupled process.
In the silt layer, O2 is required for the oxidation reaction. Therefore, O2 concentration has significant impact on the oxidation reaction efficiency. In traditional landfill cover, the available O2 for oxidation reaction in the landfill cover relies on the diffusion of O2 from atmosphere to the landfill cover, which makes the O2 concentration becomes the main restriction for the oxidation reaction. To overcome the limitation, horizontal aeration wells are laid in silt layer injecting air under low pressure. In this way, O2 concentration in landfill cover can be increased artificially to improve oxidation reaction efficiency.
3 Numerical Model of Actively-Aerated Landfill Cover System
In this section, a multi-field coupled model is developed to describe the water-gas-heat transport processes in the landfill cover.
3.1 Governing Equation
In this model, gas is regarded as the ideal gas. Heat balance is assumed to exist between two phases, so each phase has the same temperature at the same location. Thus only one heat transfer governing equation is required [7].
Based on the conservation of energy and mass, water transfer governing equation can be expressed as
where ρ w is the water density; ρ DB is the dry density of soil; v w is the water flow velocity; θ w is the volumetric water content; MH2O is the molar mass of water; r w is the reaction rate of water.
The gas transfer governing equation can be expressed as
where ϕ is the soil porosity; N g is the diffusive flux of gas; S w is the saturation degree; v g is the gas mixture advective velocity; c g is the gas molar concentration; H w is the gas molar concentration dissolved in water; r g is the gas reaction rate in methane oxidation; “+” indicates gas generation such as CO2; and “−” denotes gas consumption such as O2 and CH4.
The heat transfer controlled biochemical equation can be expressed as
where W is the soil heat capacity (J m−3°C −1); T r is the temperature (°C) and its value takes 22 °C in this study; H conv is the heat per unit area (J m−2 s−1); H oxi is the heat generation rate of the methane oxidation (J m−3 s−1); and λ T is the thermal conductivity (J m−1 s−1°C−1) and the function of water content.
Microbial aerobic methane oxidation (MAMO) is controlled by the concentrations of CH4 and O2, temperature and water content in soil. MAMO can be described by Eq. (1) mentioned. That x = 0.5 is adopted in Eq. (1) gives:
It is necessary to determine the methane oxidation rate r CH4 g (kg m−3 s−1) which can help to investigate the effect of methane oxidation on water, gas and heat transfer:
where Vmax is the maximum reaction rate; \( y_{{{\text{CH}}_{ 4} }} \) is the CH4 concentration; and K m is the half-saturated constant for CH4.
Methane oxidation efficiency is used to evaluate the performance of the biocover (dimensionless), which can be calculated as follows:
where C in and C out are CH4 inlet and outlet concentrations, respectively; Q in and Q out are the CH4 flow discharges at the inlet and outlet, respectively.
3.2 Numerical Implementation and Model Assumptions
The model is solved based on ANSYS Fluent platform and selected “mixture” model to solve multiphase conservation equation. The coupled pressure-velocity computation used the “phase-coupled SIMPLE” algorithm, and biochemical and kinetic equations are solved with the first-order implicit algorithm.
Since the main mode of gas transfer is convection and diffusion, the gas in the model is considered as laminar flow instead of turbulent flow, which is also determined by the rate of migration. Soil is considered as an ideal porous medium with uniform pore distribution.
4 Numerical Examples
Since researches about actively-aerated landfill cover system are quite limited. In this part, a soil column test [1] (Fig. 2) is analyzed, which can be viewed as a simple actively-aerated landfill cover.
As shown in Fig. 2, a 2D finite element model with a height of 90 cm and a width of 7.5 cm is used to simulate the soil column test. The holes are used to simulate the aeration probes. CH4 gas is introduced at the bottom of the model. The boundaries can transfer heat, but not gas and liquid. The top of the column is the exit of gas, which can simulate the real atmosphere environment. The initial soil moisture content is 0.236 and the initial methane and water vapor contents are zero, which are the same as the values in the test. The other needed parameters are summarized in Table 1.
First, the experimental test results [1] are adopted to verify the numerical model. Parametric study is then conducted to investigate the influences of air injection amount and location on CH4 oxidation efficiency.
4.1 Model Calibration and Verification
In the test, air aeration is applied through A and B equally (Fig. 2). The daily injection flux of CH4 was 1235.41 g while the total amount of air aeration was 10 times that of CH4. The test lasted for 200 days. The comparison of gas concentration profiles on the 28th day is shown in Fig. 3a. It can be found that the calculated results agree with the experimental results reasonably well. O2 concentration increases firstly and then decreases with depth, indicating that the horizontal aeration wells can effectively increase O2 in the cover. CH4 concentration keeps decreasing with depth, which reflects that CH4 is consumed in the whole cover domain. CO2, as the product of oxidation reaction, increases from the bottom to the top. N2 concentration distribution is stable and only affected by the concentration change in the other gases.
Figure 3b shows the comparison of CH4 oxidation efficiency. After 18 days, the calculated results remain stable. The calculated results are overall enclosed by the observed values. The difference is caused by the change in bacteria quantity during the aeration process, which is not considered in the numerical model.
4.2 Influences of Air Injection
Figure 4 shows the CH4 oxidation efficiency given different injected air amounts while CH4 flux keeps unchanged. The daily injection flux of CH4 is 1235.41 g and air flux varies from 2 to 10 times that of CH4. Air is injected through A and B equally and lasts for 200 days. The methane oxidation efficiency gradually increases with the aeration process for all the 5 curves and the results remain stable after about 18 days. The methane oxidation efficiency also increases with the air flux level. It is noteworthy that the methane oxidation efficiencies of the 8 times case and 10 times case are very close, indicating that higher air flux level can significantly enhance the oxidation efficiency but it is not economical to further increase the air flux level when it is high enough.
Figure 5 shows the influence of the air injection location on methane oxidation efficiency. Three scenarios are simulated; namely, air injected through A (Scenario 1), A and B equally (Scenario 2), A, B and C equally (Scenario 3). The gas fluxes for the three scenarios are the same, which is 1235.41 g/d for CH4 and 5068 g/d for air. The simulation lasts for 200 days. The methane oxidation efficiencies of Scenarios 1 and 2 are 65.8% and 95.8%, respectively, and that for Scenario 3 is slightly higher than that of Scenario 2.
5 Conclusion
A new landfill biocover with horizontal aeration wells is proposed in this study. A numerical model is developed to simulate the water-gas-heat coupled transport in the cover. The numerical model is verified against existing soil column experimental test results. The main conclusions are as follows:
-
(1)
Laying the aeration well at the top of landfill cover system can effectively increase O2 concentration in the landfill cover, which can enhance the methane oxidation reaction and is beneficial for impeding methane emission to the atmosphere.
-
(2)
Higher air flux level can significantly enhance the oxidation efficiency but it is not economical to further increase the air flux level when it is high enough (e.g., 8 times the methane flux in this study).
-
(3)
Given the same injected air amount, injecting air though more rows of wells gives higher methane oxidation efficiency. In this study, two rows of wells have similar oxidation efficiency as three rows of wells.
References
Ng, C.W.W., Feng, S., Liu, H.W.: A fully coupled model for water-gas-heat reactive transport with methane oxidation in landfill covers. Sci. Total Environ. 508, 307–319 (2014)
Farrokhzadeh, H., Hettiaratchi, J.P.A., Jayasinghe, P., Kumar, S.: Aerated biocovers with multiple-level air injection configurations to enhance biological treatment of methane emissions. Bioresour. Technol. 239, 219–225 (2017)
Clapp, R.B., Hornberger, G.M.: Empirical equations for some soil hydraulic properties. Water Resour. Res. 14(4), 601–604 (1978)
Abu-Hamdeh, N.H., Reeder, R.C.: Soil thermal conductivity effects of density, moisture, salt concentration, and organic matter. Soil Sci. Soc. Am. J. 64(4), 1285–1290 (2000)
Poling, B.E., Prausnitz, J.M., O’connell, J.P.: The Properties of Gases and Liquids. Mcgraw-Hill, New York (2001)
Gebert, J., Groengroeft, A.: Passive landfill gas emission-influence of atmospheric pressure and implications for the operation of methane-oxidising biocovers. Waste Manag. 26(3), 245–251 (2006)
Huber-Humer, M., Gebert, J., Hilger, H.: Biotic systems to mitigate landfill methane emissions. Waste Manag. Res. 26, 33–46 (2008)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Feng, SJ., Wang, TY. (2018). Numerical Simulation of Methane Oxidation in Actively-Aerated Landfill Biocover. In: Farid, A., Chen, H. (eds) Proceedings of GeoShanghai 2018 International Conference: Geoenvironment and Geohazard. GSIC 2018. Springer, Singapore. https://doi.org/10.1007/978-981-13-0128-5_51
Download citation
DOI: https://doi.org/10.1007/978-981-13-0128-5_51
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0127-8
Online ISBN: 978-981-13-0128-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)