Abstract
Vibrio cholerae, the causative agent of the disease cholera still threatens a large proportion of world’s population and is considered as a top priority enteric pathogen. Role of biofilm in V. cholerae pathogenesis is well established as it provides the bacterium with enhanced transmission ability during epidemics and also enhanced tolerance to antimicrobial agents. The clinical efficacy of many existing antibiotics is being threatened by the emergence of multi-drug resistant V. cholerae. The rapidly increasing number of cholera outbreaks in several developing countries and emergence of multidrug resistant V. cholerae necessitates the development of an alternative strategy rather than the existing antibiotic therapy to control the pathogen. In the present chapter, we discuss the different quorum sensing pathways in V. cholerae, the common quorum quenching molecules that targets these pathways and a novel strategy of biofilm inhibition in V. cholerae using antibiofilm compounds in combination with antibiotics to control the disease. Co-dosing strategy reduce the dosage of antibiotics and such a combination therapy can in turn be used to control the spread of antibiotic resistance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Antibiofilm compounds
- Antibiotic resistance
- Combinatorial therapy
- Quorum sensing
- Quorum quenching
- Vibrio cholerae
1 Introduction
Vibrio cholerae is the causative agent of the acute diarrheal illness cholera, once the most feared of all pandemics. Today, cholera is managed by oral rehydration therapy and broad spectrum antibiotics, yet the disease is insuperable during epidemics. In 2015, case fatality ratio (CFR) of the disease reported from 42 countries was 0.8%, which includes a total of 172,454 cases with 1304 deaths (World Health Organization 2016). Cholera outbreaks were reported from 16 countries in Africa, 13 in Asia, 1 in Oceania, 6 each in Europe and America. Globally it has been estimated that a total of 21,000–143,000 deaths occur every year from 1.3–4.0 million cholera cases (Ali et al. 2015). However, exact number of cases remains unknown because of the lack in a strong surveillance programs to monitor the disease in developing countries.
V. cholerae is commonly found in saline water bodies, but act as a facultative pathogen when sufficient infective dose reach the human host. There are about 206 serogroups of V. cholerae out of which O1 and O139 are known to cause outbreaks. V. cholerae O1 are further divided as classical and El Tor biotypes based on phenotypic and genotypic differences. The other serogroups of the bacteria are collectively known as non O1/O139 V. cholerae as they do not agglutinate either O1 or O139 polysera. These non O1/O139 V. cholerae were considered to be non-toxigenic until it caused a colossal outbreak in1992 (Dutta et al. 2013). However, though improper sanitation has been found to be an important factor for sudden cholera outbreaks, detailed understanding about the role of other factors that play role in sudden outbreaks are still unclear. There have been two hypotheses on the occurrence of sudden cholera outbreaks, the first being that the onset of infection in the population is by transmission of the pathogen from asymptomatic carriers to healthy individuals. This hypothesis explicates that the asymptomatic individuals infect local water bodies leading to explosive sporadic outbreaks of cholera (Frerichs et al. 2012; Eppinger et al. 2014). The second hypothesis assumes that the sporadic sudden cholera cases are initiated when a healthy individual acquires the pathogen from environmental autochthonous toxigenic vibrios (Huq et al. 1983; Alam et al. 2006). Temperature, salinity, nutrients and precipitation are important factors which influences the persistence of vibrios in environmental waters. Also, the pathogen comprises a major component of the commensal flora of phyto-zooplankton persisting as biofilms on them (Jutla et al. 2013).
Biofilms are surface attached, matrix enclosed, multicellular communities of bacteria found in association with both biotic and abiotic surfaces. V. cholerae exist in both planktonic and biofilm state during intestinal and aquatic phase of its life cycle. Role of biofilm in V. cholerae pathogenesis is well established as it provides the bacterium with enhanced transmission ability during epidemics and also enhanced tolerance to antimicrobial agents. V. cholerae biofilms, in its environmental phase provides protection from environmental stresses such as grazing protozoa, bacteriophages and nutrient limitation (Matz et al. 2005). Also, V. cholerae biofilms on chitin surfaces induces its natural competence whereby the cells acquire new genetic materials such as resistance genes (Meibom et al. 2005). During inter epidemic periods, metabolically quiescent V. cholerae persist in the environment in biofilm state known as Viable but Non Culturable vibrios (VBNC) which cannot be cultured by normal traditional culturing methods. These cells become active only after a passage through the host or from signals produced by active cells present in the environment (Colwell et al. 1996; Bari et al. 2013). The pathogen biofilms in environment have important biological relevance as it provide protection to harsh environmental stresses and also increase the infective dose of cells entering a host capable of initiating the infection. Though development of V. cholerae biofilms within host is poorly understood, recent studies using rabbit ileal loop infection models have identified clusters of V. cholerae in microcolonies, supporting the fact that V. cholerae forms biofilms in vivo which will be subsequently excreted in stool thereby increasing the transmission of the disease cholera (Faruque et al. 2006). In the present chapter, we discuss the role of biofim in V. cholerae antibiotic resistance, the different quorum sensing systems in the bacteria and different quorum quenching mechanisms. The chapter highlights on the quorum quenching/antibiofilm therapy as an alternative imminent strategy to control the increasing antibiotic use and its probable advantages in decreasing the burden of antibiotic resistance in the pathogen.
2 Association of Antibiotic Resistance and Biofilm
Lately, V. cholerae strains resistant to these commonly used antibiotics have appeared with increasing frequency in India, as well in as other countries (Bilecen et al. 2015; Gupta et al. 2016). The pathogen develops resistance to antibiotics through several mechanisms. Biofilm formation in V. cholerae has been associated with its resistance gene acquisition. Horizontal gene transfer promotes evolution and genetic diversity of the pathogen. Gene transfer among the environmental and clinical strains in the natural environments has led to the emergence of multidrug-resistant bacteria (Martínez 2012). Also, previous studies have demonstrated that EPS matrix prevents diffusion of antimicrobial agents thus providing a protective niche to the bacterial pathogen which is best known to increase transmission via biofilm microcolonies (Teschler et al. 2015). The common antibiotics taken against cholera such as the quinolone antibiotics, tetracyclines and erythromycin does not reach the bacteria that resides within the biofilm. However these cells get exposed to sub-inhibitory concentrations of the antibiotics leading to adaptive evolution of the bacteria to these antibiotics (Bengtsson-Palme and Larsson 2016). Draft genome sequence analysis of an evolved Haitian variant V. cholerae strain isolated from a recent outbreak in south India revealed the presence of aminoglycoside gene, strB and strA, sulphonamide resistance gene, sul2 and phenicol resistance genes, floR and catB9 suggesting the real time evolution of V. cholerae to the commonly used antibiotics (Narendrakumar et al. 2017).
3 Genetic Determinants of V. cholerae Biofilm Formation
V. cholerae biofilm formation is initiated by the attachment of cells to biotic or abiotic surfaces by Type IV pili (TFP). The bacteria have three types of TFP namely, toxin co-regulated pili (TCP), mannose –sensitive hemagglutinin (MSHA) pilin and chitin- regulated pili (chiRP) of which MSHA pili play a major role in biofilm development. After surface attachment, the pathogen produces an extrapolymeric matrix composed of proteins, nucleic acids and Vibrio exopolysaccharide (VPS). Matrix proteins such as RbmA, RbmC, Bap1 along with VPS maintain the integrity of these biofilms. The VPS genes are clustered as vpsI and vpsII and located at two different positions in the large chromosome of V. cholerae separated by the rbm gene cluster. vpsI cluster comprises of vpsA-K genes (VC0917-27) and vpsII cluster comprises of vpsL-Q (VC0934-9) (Fong et al. 2010). Several studies have reported that the VPS genes are important for the colonization and biofilm development both in-vitro and in-vivo. Expression of genes such as vpsA (VC0917), vpsB (VC0918), vpsC (VC0919) and vpsN (VC0936) are found to be up-regulated during the initial colonization stages and final stages of infection in animal model experiments. VpsH (VC0924) was identified to be at detectable levels in cholera patients on In vivo-induced antigen technology (IVIAT) (Hang et al. 2003). In frame deletion mutation of vps gene clusters significantly reduced the ability of V. cholerae to produce biofilm. However, not all genes in the vpsI and vpsII gene clusters were important for its biofilm formation. V. cholerae vps clusters are positively and negatively regulated by VpsR, VpsT and HapR, CytR respectively (Teschler et al. 2015). VpsR and VpsT were identified to be important for the maximal expression of vps genes and mutation studies in either of the genes significantly reduced the biofilm formation in V. cholerae (Yildiz et al. 2001). The positive regulators VpsR and VpsT have been identified to be homologous to the two-component regulatory systems that are involved in sensing and responding to environmental stimuli with a sensor histidine kinase that regulates vps genes expression.
4 Quorum Sensing and Biofilm Formation
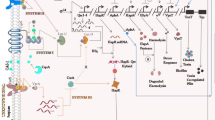
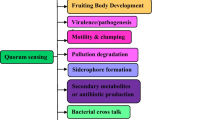
Cell to cell communication in bacteria for the regulation and expression of specific genes is known as bacterial quorum sensing (QS). Quorum sensing is dependent on the bacterial cell density and concentration of chemical signal molecules known as autoinducers (AIs) produced by the bacteria. These AIs are released into the environment which accumulate and signals other bacteria of the same kind for collective expression of specific genes for definite functions such as virulence, biofilm formation, bioluminescence etc (Williams and Camara 2009). Many of the molecular mechanisms for intracellular signaling in bacteria are now well studied. Different quorum sensing small molecules produced by microbes include Acyl Homoserine Lactones (AHL), Peptide auto-inducers and Auto-inducer 2.
4.1 Acyl Homoserine Lactones (AHL)
AHLs are produced within the bacterial cells and released into the environment. AHLs produced by different bacteria differ in the R-group side chain length which can vary from 4 to 18 carbon atoms and the carbonyl group at the third carbon. AHL signals contains a homoserine lactone linked by a amide bond to the acyl side chain. The first identified AHL molecule in QS was in the marine bacterium Vibrio fischeri which were responsible for the bioluminescence in the light organs of Hawaiian bobtail squid, Euprymna scolopes (Ruby 1996). After this discovery, AHL signal molecules were identified from different Gram- negative bacteria including V. cholerae. AHL signal molecules are chiefly catalyzed by LuxI enzyme family and perceived by LuxR cytoplasmic DNA binding proteins. Based on the length of the side chain, AHL molecules can be distinguished as short side-chain AHL moleculaes and long side- chain AHL molecules. Short chain AHLs diffuse freely across the cell membrane whereas the long chain AHLs requires active efflux pumps to export them from within the cell. Different bacteria are known to produce different AHL molecules and few of them produce diverse AHL signals (Huma et al. 2011). There are also reports of bacteria like Pseudomonas simiae and Pseudomonas brenneri isolated from Ny-Alesund, Arctic at 79°N producing varied AHL molecules at different temperatures (Kalia et al. 2011; Dharmaprakash et al. 2016).
4.2 Peptide Auto-inducers
Many Gram-positive bacteria utilize peptides as signal molecules for QS. They are usually secreted oligopeptides which results from post-translational modifications. Peptide signals can differ in size from 5 to 87 amino acids and also can contain modifications like lactone or thiolactone linkages. These peptide auto-inducers require special export mechanisms like ATP binding cassette transporters. After the accumulation of sufficient peptide auto-inducer in the environment, the signal molecules are perceived by histidine sensor kinase protein of a two-component regulatory system in the bacteria leading to expression of specific genes. Competence signal peptides (CSP) produced by streptococcal species is an example for peptide auto-inducer (De Spiegeleer et al. 2015). Bacteriocins are also categorized to be a peptide AI (Zhao and Kuipers 2016). ComX bacterial peptide AI induces sporulation in Bacillus subtilis by activating the ComP/ComA two-component phosphorylation cascade. Activation of the ComP/ComA component in turn leads to increased gene expression of the transcriptional activator ComK which in turn activates the expression of genes required for sporulation (van Sinderen et al. 1995). In Staphylococcus aureus, peptide AI system agr play an important role in virulence and pathogenicity of the organism (Baldry et al. 2016). The S. aureus peptide AI is detected by AgrC/AgrA sensor kinase/response regulator which gets phosphorylated. The phosphorylated ArgA upregulates RNAIII which positively regulates the expression of virulence genes of S. aureus.
4.3 Auto-inducer 2
Auto-inducer 2 is a furanosyl borate diester signal molecule. AI-2 is produced by many Gram positive and Gram negative bacteria and is believed to be an evolutionary link between the two QS systems. AI-2 facilitates cross species communication in bacteria. The AI-2 system was also first reported from the marine bacterium V. fischeri. For bacteria like E. coli and Salmonella typhimurium, a group of genes called lsr gene cassette are induced that encode AI-2 components of the machinery (Pereira et al. 2013). Periplasmic protein LsrB act as the receptor for AI-2 signal molecules which will be delivered inside to the cell cytoplasm by an ABC transporter. This transportation of the signal molecule into the cytoplasm is coupled to its phosphorylation by LsrK kinase (Xavier and Bassler 2005). The prime function of the phosphorylated AI-2 is to deactivate the transcriptional repressor LsrR which in turn activates the positive feedback of AI-2. The synthesis of AI-2 is facilitated by the enzyme LuxS which converts S- ribosyl homocysteine to homocystein and 4,5-dihydroxy-2,3-pentanedione (DPD), the precursor compound of AI-2 (Marques et al. 2011).
4.4 Quorum Sensing Signals in Vibrionaceae Family and Biofilm Formation
Vibrionaceae comes under the Gammaproteobacteriae comprising facultative anaerobic Gram negative bacteria such as V. cholerae, V. parahaemolitycus, V. harveyii, V. anguillarum, V. vulnificus, V. fischeri etc. The family includes Vibrio and Photobacterium genera. Of these genera are many top priority human diarrheal pathogens such as V. cholerae and V. parahaemolyticus and fish pathogens such as V. anguillarum and V. vulnificus. There are also many bacterial symbionts such as V. pomeroyi, V. aestuarianus, and V. fischeri in this family (Yang et al. 2011). QS systems have been identified in many bacteria of the Vibrionaceae family such as V. harveyi (Bassler et al. 1997), V. cholerae (Zhu et al. 2002), V. anguillarum (Milton et al. 1997; Buchholtz et al. 2006), A. salmonicida (Bruhn et al. 2005), V. vulnificus (Valiente et al. 2009) and Photobacterium phosphoreum (Flodgaard et al. 2005). In a study to determine global phylogenetic distribution of AHL molecules in bacteria belonging to Vibrionaceae family using biological monitors and LC-MS identification, it was identified that acyl homoserine lactones, including AHLs with odd numbers of carbon, was the most abundant signaling molecule. The study also revealed an AHL fingerprint correlated to specific phylogenetic subclades in Vibrionaceae family (Rasmussen et al. 2014). However, quorum sensing systems rapidly diverge in nature and signal orthogonality and mutual inhibition frequently occur among closely related diverging systems of Vibrionaceae. Different homologues of LuxI/LuxR proteins have been identified that respond to each of the different AHL molecules. Also, the degree of sensitivity of LuxI/LuxR to different AHL molecules differ (Tashiro et al. 2016).
Most of the members in the Vibrionaceae family are also good biofilm formers. QS controls biofilm formation in Vibrionaceae by different mechanisms such as regulating coordinated behavior and synchronized environmental response (Mireille Aye et al. 2015; Okutsu et al. 2015), regulating the synthesis of biofilm matrix (Tseng et al. 2016), indirectly upregulating biofilm formation by increasing bacterial motility (Yang et al. 2014) and also regulate dispersal of matured biofilm to initiate a new developmental cycle of biofilm formation (Emerenini et al. 2015). A recent study has proved the ability of Vibrio parahaemolyticus to regulate biofilm formation and enhancement of colonization by QS (Vinoj et al. 2014). Also studies on genetic basis of QS mediated regulation of virulence related gene Hfq, motility/extracellular protein Pep and colony phenotype intermediated protein valR was studied in biofilm forming V. alginolyticus (Chang et al. 2010; Cao et al. 2011; Liu et al. 2011). VanT, a homologue of Vibrio harveyi LuxR, is known to regulate biofilm formation in V. anguillarum (Croxatto et al. 2002). AphA, DNA binding regulators in vibrios belonging to padR family proteins which is important for the QS in vibrios at low cell density is known to regulate biofilm formation, motility and virulence in pandemic V. parahaemolyticus (Wang et al. 2013). It is also interesting to note that there is about 85% homology between the AphA protein of V. parahaemolyticus and V. cholerae suggesting a possibility of similar regulation in V. cholerae also.
4.5 V. cholerae Quorum Sensing Systems
V. cholerae posseses two well characterized and one predicted quorum sensing mechanism as compared to V. fischeri. The well-established systems of V. cholerae includes the CqsA/CAI-1/CqsS system and LuxS/AI2/LuxPQ system (Ke et al. 2014). In V. cholerae, there are two AIs such as the Cholera Auto-inducer (CAI), S-3-hydroxy-tridecan-4-one and Auto-inducer-2 (2S, 4S- 2- methyl-2,3,3,4-tetrahydroxy tetrahydrofuran-borate) and two cognate receptors. The CAI-1 signals are synthesized by CqsA and sensed by CqsS system sensor (Ng and Bassler 2009). AI-2 is synthesized by LuxS and the cognate sensor is LuxP/Q. The components of the third predicted QS system in V. cholerae is unknown.
At low bacterial cell density when the AIs are at minimum concentration, the QS system sensors CqsS and LuxP/Q transfers a phosphate group to the cytoplasmic integral protein LuxU which in turn phosphorylates LuxO (Milton 2006). The phosphorylated LuxO in association with the σ54 initiate the repression of hapR via the activation of a putative repressor. HapR is a transcriptional repressor of the genes which have specific functions in V. cholerae biofilm formation and virulence (Amy et al. 2009). Previous studies have reported that a deletion mutant of hapR produces strong biofilm compared to its wild type counterpart. At a higher cell density, the AIs exported out reaches the maximum threshold level converting the LuxQ and CqsS kinases to phosphatases. This backflow of phosphate group from the LuxO destabilizes the repression of putative protein on hapR activating its expression. HapR activates Haemagglutinin Protease A (HapA) which disperses the V. cholerae in biofilm to planktonic cells.
Another major component that determines the biofilm formation of V. cholerae is the concentration of a secondary messenger, c-di-GMP (Cotter and Stibitz 2007). c-di- GMP messengers are synthesized by proteins containing specific GGDEF motifs and its degradation is carried out by proteins containing domains with EAL or HD-GYP motifs. Previous studies reports the presence of 62 genes that encode proteins governing c-di-GMP levels in V. cholerae (Galperin 2004). Increase in the concentration of the secondary messenger within the bacterial cell induces the activation of the transcriptional activator vpsT. VpsT in turn activates the genes in the vps clusters leading to the increase in extracellular matrix production and increased biofilm. However, the master transcriptional repressor gene HapR is identified to regulate the c-di-GMP mediated biofilm formation at two levels. HapR has been identified to repress 14 genes that encodes for proteins that synthesis c-di-GMP. Also, HapR directly represses the vpsT gene which prevents the downstream activation of vps genes.
5 Quorum Quenching
Quorum quenching (QQ) or QS interference is a strategy wherein the cell to cell communication of bacteria is interrupted by specific compouds. QQ strategy came up with the understanding that bacterial cells have the ability to communicate with each other to collectively regulate various important traits such as virulence, biofilm formation, antimicrobial resistance (Sharma and Jangid 2015; Shiva Krishna et al. 2015). This communication occurs via various signaling molecules which are recognized by specific receptors. The signal molecule binding to the receptor further activates a cascade of molecular signaling which ultimately results in the specific gene regulation. Theoretically, mechanisms that can effectually interfere this signaling/communication between the bacteria can be used as a QQ molecule. A QQ molecule can act on either the signaling molecule, receptor to the signal, regulatory proteins in the signaling cascade.
5.1 Quorum Quenching by Signal Molecule Degradation
There are QQ enzymes like AHL-lactonase, AHL-acylase and paraoxonases (PONs), which degrade AHL signals.
5.1.1 Quorum Quenching by AHL-Lactonase
AHL- lactonase act on the QS signal, AHL by hydrolyzing the homoserine lactone ring (Dong and Zhang 2005). The first AHL-lactonase, encoded by the aiiA gene was identified from a Bacillus sp. isolate 240B1. The enzyme has two zinc ions at their active site which initiates a nucleophilic attack at the substrate’s carbonyl carbon group. The lactone ring interact with the enzymes Zn1 and the substrate’s carbonyl oxygen interact with Zn2 thereby weakening the carbonyl bond breaking the lactone ring to yield an open-ring product. Due to its specificity in action, AHL- lactonase is the most precise AHL-degradation enzyme which can hydrolyse both short chain and long chain AHLs efficiently. The ability of AiiA to disrupt QS in V. harveyii and V. cholerae and thereby inhibit their biofilm formation has been well documented (Bai et al. 2008; Augustine et al. 2010). AiiA enzyme have been successfully cloned into plants such as potato, tobacco, eggplant, cabbage, carrot and celery plant to produce Erwenia carotovora infection resistant crops (Dong et al. 2001). Also, Escherichia coli containing cloned Bacillus thuringiensis aiiA was identified to express greater amount of the enzyme compared to the parent strain increasing its industrial applications (Lee et al. 2002). Another AHL lactonase AttM identified from Agrobacterium tumefaciens with only 24.8% identity to AiiA was characterized to have bacterial fitness properties within the plant tumor (Haudecoeur et al. 2009).
5.1.2 Acylases
AHL-Acylases, like lactonases interfere with bacterial QS to attenuate major functions such as virulence, motility and biofilm production. Acylases have been identified to decrease 3OC12HSL and C4HSL accumulation within the bacterial cells which drives the virulence factor production machinery. Acylases have been identified both in Gram-positive and Gram- negative bacteria. AHL-acylase AhlM identified from Streptomyces strain M664 could cleave both medium and long chain AHL signal molecules. AHL acylases AiiC and Aac identified from Anabena strain PCC7120 and Shewanella respectively were proved effective in disrupting biofilm formation of fish pathogen V. anguillarum in-vitro (Morohoshi et al. 2008).
5.1.3 Oxidoreductases
A third class of AHL- degrading enzymes are the oxidoreductases that inactivates AHL molecules via oxidation or reduction of the acyl chain of the signal molecule. P450 monooxygenase and the NADH-dependent enzyme BpiB09 are the two well-studied AHL signal molecule degrading oxidoreductases. Heterologous expression of BpiB09 have been identified to decrease AHL accumulation in Pseudomonas aeruginosa subsequently inhibiting swarming motility, biofilm formation and pyocyanin production (Bijtenhoorn et al. 2011; Kumar et al. 2015).
5.2 Quorum Quenching by Inhibition of Signal Molecule Synthesis
Apart from degrading signal molecules that play important role in bacterial cell to cell communication, there have been many compounds that inhibit or decrease the signal molecule synthesis thereby interfering QS. Most of the signal molecule synthesis inhibitors work by indirectly inhibiting precursor molecules which are important for signal molecule synthesis. For example, small molecule triclosan act on enoyl-ACP reductase, an important precursor of AHL synthesis and immucillin A (ImmA) inhibits 5-MAT/S-adenosyl-homocysteine nucleosidase (MTAN) which is crucial for both AHL and AI-2 synthesis (Hoang and Schweizer 1999; Singh et al. 2005). Very low concentration of nucleoside analogues have been found effective to inhibit MTAN activity in virulent V. cholerae strains (Gutierrez et al. 2009).
Recent studies on V. cholerae QS precursor molecule (S)-4,5-dihydroxy-2,3-pentanedione (DPD) showed that nucleoside analoges of DPD delineate QS in V. cholerae, V. harveyi, V. anguillarum, V. vulnificus and S. typhimurium (Meijler et al. 2004; Lowery et al. 2008; Smith et al. 2009; Brackman et al. 2009). Adenosine analogues have been found to have potent antibiofilm activity by blocking AI-2 based QS.
5.3 Quorum Quenching by Inhibition of AHL Receptor
The CqsS receptor of V. cholerae and V. harveyi share extensive homology. However, when it comes to inhibition of CqsS receptors in both the bacteria, possess different overall stringencies for ligands. Many small molecule inhibitors that block the binding of signal molecule to the receptor thereby cutting off the QS signaling cascade has been identified recently. High throughput screening studies have identified many small molecule inhibitors of QS receptors in V. cholerae (Peach et al. 2011).
6 Antibiofilm Activity of Natural Compounds Against V. cholerae
Natural products are a good source of compounds that have various biological activities including antimicrobial and antibiofilm properties. These products are considered safe to administer and are believed to cause lower degree of antimicrobial resistance unlike antibiotics. Many of these compounds are identified to inhibit bacterial virulence or biofilm formation by interfering with the QS of bacterial pathogens. List of important QQ molecules have been listed in Table 4.1.
6.1 Antibiofilm Activity of Phytochemicals
Plant extracts and plant compounds have been used since time immemorial to treat diarrheal diseases and other stomach ailments. Traditional medicines of India and China use plant extracts for treating bronchitis, asthma, gastric ailments, phlegm, dysentery, leukorrhea, kidney trouble, urethritis, and dropsy (Mitra et al. 2007).
Resveratrol, a phytochemical present mostly in the skin of grapes, blueberries, raspberries, mulberries etc have been identified to target upstream targets of QS system, reducing V. cholerae biofilm production upto 80% (Fig. 4.1). Molecular docking studies, AphB, a LysR-type regulator important in the QS of V. cholerae was identified to be the putative target for resveratrol (Augustine et al. 2014).
Isolation of active compounds from natural sources and analysis of antibiofilm activity of the compound against V. cholerae. CLSM images of V. cholerae biofilm inhibition using resveratrol. (a) untreated culture. (b, c) Biofilm treated with 15 & 20 μg/ml of resveratrol. (d) 3D view of biofilm thickness of untreated. (e, f) 3D view of biofilm thickness of cultures treated with 15 and 20 μg/ml resveratrol
Vikram et al. (2011) demonstrated that flavonoid compounds such as naringenin and quercetin isolated from citrus fruits have been identified to reduce biofilm formation in V. harveyii and V. cholerae by acting as antagonists of AHL and AI-2 (Vikram et al. 2011). Also, several studies are available showing the anti-virulence activity of plants like ‘neem’, apple, hop, green tea and elephant garlic via inhibition of bacterial growth or the secreted cholera toxin (CT). Mangrove plant extracts have been known for their mosquito larvicidal, antifungal, antiviral, anti-cancer and anti-diabetic activity. Leaves and bark extracts of mangrove plants Ceriops tagal and Pemphis acidula revealed to have potent antibiofilm ability against a wide set of microorganisms such as P. aeruginosa, Klebsiella pneumonia, V. parahaemolyticus, S. aureus and V. cholerae (Arivuselvan et al. 2011). Cinnamaldehyde and its derivatives have been found effective as an antibiofilm compound against all Vibrio sp., by interfering AI-2 mediated QS pathway by decreasing the DNA-binding ability of LuxR (Brackman et al. 2008). Also, in V. cholerae, cinnamaldehyde analogues have been proved to have antibiofilm (Niu et al. 2006; Brackman et al. 2011). Water soluble extract of Cranberry has found to have potent antibiofilm property against V. cholerae acting via down-regulating the vps operon by modulating the level of c-di-GMP in the QS pathway. Also it has been shown that Cranberry extracts can block the initial attachment of V. cholerae into the host enterocytes during an infection and also inhibit cholera toxin production by regulating LuxO-HapR pathway (Dinh et al. 2014).
6.2 Antibiofilm Activity of Marine Compounds
Compounds from marine sources have been a rich source of bioactive compounds. Marine organisms, plants and even sediment compounds have shown antibacterial and antibiofilm activity against a wide array of bacterial pathogens. Quorum quenching from marine sources gained its importance from early discoveries of antibiofilm activity of these compounds against biofouling bacteria (Kalia et al. 2014). Marine bacterial exopolysaccharaides have been able to disrupt Vibrio sp., QS and thereby inhibit virulence gene expression and biofilm formation. Many marine bacteria such as Oceanobacillus and Halomonas have been identified to produce small molecule QQ enzymes that are able to disrupt V. cholerae and V. parahaemolyticus biofilm. Bacteria such as Bacillus indicus, B. pumilus and Bacillus sp. SS4 isolated from Palk Bay (Bay of Bengal) were shown to cause substantial inhibition of QS based biofilm formation in Gram-negative bacteria such as Vibrio species, Serratia marcescens and P. aeruginosa PAO1 (Nithya et al. 2010, 2011; Musthafa et al. 2011). Other major sources of antibiofilm compounds are halogenated furanones produced by Delisea pulchra, secretions from Chlamydomonas reinhardtii which mimic bacterial QS signals, bromoperoxidase produced by algae Laminaria digitata which deactives AHL molecules, Ahnfeltiopsis flabelliformis, red algae that produces α-D-galactopyranosyl-(1 → 2)-glycerol (Floridoside), betonicine and isoethionic acid which produces QS analogues (Gram et al. 1996; Manefield et al. 2000; Borchardt et al. 2001; Teplitski et al. 2004; Kim et al. 2007; Musthafa et al. 2011).
6.3 Activity of Synthetic QS Analogues
Many synthetic analogues have been identified to target QS molecules of Gram negative bacteria including V. cholerae. Though most of the QQ studies of synthetic quorum sensing inhibitors have been carried out in V. fischeri, the compound being translated to target QS systems of V. cholerae is not very difficult. Probable QQ compounds that could be used against V. cholerae are 4-hydroxy cis or trans analogs of HSL ring of signal molecule 3OC8HSL which have been found effective in inhibiting V. fischeri LuxR based QS reporter system, N-(heptyl-sulfanyl acetyl)-L-HSL (HepS-AHL) identified as potent LuxR inhibitor in V. fischeri, N-sulfonyl-HSL (with a pentyl chain), identified as an potent LuxR analogue in V. fischeri, Hexyl-4,5-dihydroxy-2,3-pentaedione identified to interfere QS in V. harveyi and thereby reduce its bioluminescence and Furanone C-30 which reduce virulence gene expression of V. anguillarum (Schaefer et al. 1996; Olsen et al. 2002; Castang et al. 2004; Rasch et al. 2004; Persson et al. 2005; Lowery et al. 2009). Nanoparticle therapy has also been proved to be effective against bacterial biofilms (Dobrucka and Długaszewska 2015; Szweda et al. 2015; Ahiwale et al. 2017). Quest for a single quorum quenching molecule that can limit quorum sensing in multiple pathogens is underway (Koul and Kalia 2017).
7 Advantage of Antibiofilm Drugs Over Antibiotics
Biofilms are generally insensitive to antibiotics. However, use of antibiotics or antimicrobial agents is effective against planktonic bacteria. Planktonic bacterial cells are known to be 1000- times sensitive to antibiotics compared to their biofilm counterparts (Rasmussen and Givskov 2006). Antibiotics and other antimicrobial agents generally act by inhibiting the growth of bacterial cells by interfering with their major metabolic or biosynthesis pathway killing them. Biofilm mode of life helps bacteria to survive the harsh antibiotic treatment. Within the biofilm, bacteria have the ability to exist as heterogeneous population and have a reduced metabolic activity (Mah et al. 2003). Bacterial cells with reduced metabolic activity have been identified to be inherently more resistant to antimicrobial therapies (Coates and Hu 2008).
Prolonged persistence of such antimicrobial compounds can induce toxicity to not just the target microorganism but also to non-target beneficial microbes existing in the particular environment. Another major problem in using antimicrobial agents over long period of time is the emerging antibiotic resistance. Multidrug resistant bacteria have now days become a global problem aggravating mortality due to infections (Neu 1992). This escalation of antimicrobial resistance among bacterial pathogens worldwide has necessitated an urgent need to look for alternative strategies to combat bacterial infections by reducing virulence rather than by killing the bacteria.
8 Combinatorial Therapy of Antibiofilm Agents and Antibiotics
Since the discovery of persistent antibiotic resistant cells in bacterial biofilms, alternative approaches to control bacterial diseases by using a combination of quorum sensing inhibitors that inhibit or disperse the biofilm and antibiotics that kill the dispersed bacterial cells have gained importance. Co-dosing of QSI/biofilm inhibitors with sub-inhibitory concentration of an antibiotic that can kill the bacteria that become sensitive to antibiotics upon release from biofilms in turn reduces the chance of antimicrobial resistance as these compounds target biofilm/QS pathways and does not affect bacterial growth. A pictorial representation of the combinatorial therapy that could be used to treat cholera has been depicted in Fig. 4.2.
9 Conclusion
The escalation of antimicrobial resistance among bacterial pathogens worldwide is becoming a critical concern. This necessitates an urgent need to look for alternative strategies to combat bacterial infections by reducing virulence rather than by killing the bacteria. In this context, scientists from all over the world are trying to develop novel therapeutic strategies that give importance to bacteriostatic compounds rather than bacteriocidal drugs. Similar to many opportunistic pathogens V.cholerae also rely on Quorum sensing, a bacterial cell to cell communication system for biofilm formation and virulence character expression. Quorum quenching or antipathogenic approach and biofilm inhibition will be the promising alternative strategies to contain the disease in future. Vibrios that reside within mature biofilms are highly resistant to antibiotics and host immune response due to the complex architecture and composition of the extracellular matrix. Recent studies have shown the key role played by the biofilm mode of life adapted by vibrios in the emergence of resistant strains, pathogenicity, host colonization and survival in the natural as well as human niches of vibrio species. Small molecules that can inhibit biofilm formation of V.cholerae and/or disrupt biofilms will greatly reduce transmission potential of the bacteria especially in epidemic situations. Inhibiting quorum sensing mechanism appears to be an ideal alternative to conventional therapy as V.cholerae suppresses both virulence and biofilm formation at high cell densities.
10 Opinion
Previous studies have strongly associated biofilm formation to the virulence of V.cholerae. Thus targeting biofilm formation is considered as a potential anti-virulent strategy to treat infections caused by bacterial pathogens. To the best of our knowledge, there is no antibiofilm compound that is clinically approved thus far. In this context, discovering potential Quorum Quenching/anti-biofilm compounds against vibrios is highly warranted. This can be achieved by employing two strategies: (1) Screening natural sources especially from anti-diarrheal plants (formulation of herbal extracts), actinomycetes (small molecules) and well characterised phytochemicals and peptides. (2) Identification of conserved biofilm inhibiting/quorum quenching targets by employing multi-omic approaches such as transcriptomics and proteomics. The identified targets could be used in modern computational based drug discovery approaches for accelerating the development of broad spectrum anti-biofilm compounds against the major pathogens in the Vibrionaceae family. It has also been suggested that targeting the quorum sensing system and biofilm forming ability, which could disarm the bacteria may offer a affirming avenue to fray both pathogenesis as well as antibiotic resistance. Hence, by taking it as a top priority research agenda will definitely help to move forward to control the disease. Studies in some bacterial species have successfully demonstrated that these quorum sensing inhibitors can specifically bind to target proteins and inhibit virulence gene expression. However, the efficacy of these anti-quorum sensing drug targets for the inhibition of V.cholerae quorum sensing has not been extensively evaluated. A strengthened international networking and improved coordination among researchers active in cholera research program will accelerate the program at large.
References
Ahiwale SS, Bankar AV, Tagunde S, Kapadnis BP (2017) A bacteriophage mediated gold nanoparticle synthesis and their anti-biofilm activity. Indian J Microbiol 57:188–194. https://doi.org/10.1007/s12088-017-0640-x
Alam M, Hasan NA, Sadique A, Bhuiyan NA, Ahmed KU, Nusrin S, Nair GB, Siddique AK, Sack RB, Sack DA, Huq A, Colwell RR (2006) Seasonal cholera caused by Vibrio cholerae serogroups O1 and O139 in the coastal aquatic environment of Bangladesh. Appl Environ Microbiol 72:4096–4104. https://doi.org/10.1128/AEM.00066-06
Ali M, Nelson AR, Lopez AL, Sack DA (2015) Updated global burden of Cholera in endemic countries. PLoS Negl Trop Dis 9:e0003832. https://doi.org/10.1371/journal.pntd.0003832
Amy TM, cai T, Lui Z, Zhu J, Kulkarni RV (2009) Regulatory targets of Quorum sensing in Vibrio cholerae: evidence for two distict HapR- binding motifs. Nucleic Acids Res 8:2747–2756. https://doi.org/10.1093/nar/gkp121
Arivuselvan N, Silambarasan D, Govindan T, Kathiresan K (2011) Antibacterial activity of Mangrove Leave and bark extracts against Human pathogens. Adv Biol Res 5:251–254. doi: Not Available
Augustine N, Kumar P, Thomas S (2010) Inhibition of Vibrio cholerae biofilm by AiiA enzyme produced from Bacillus spp. Arch Microbiol 192:1019–1022. https://doi.org/10.1007/s00203-010-0633-1
Augustine N, Wilson Peter A, Kerkar S, Thomas S, (2012) Arctic actinomycetes as potential inhibitors of Vibrio cholerae biofilm. Curr Microbiol 64(4):338–342
Augustine N, Goel AK, Sivakumar KC, Kumar RA, Thomas S (2014) Resveratrol—a potential inhibitor of biofilm formation in Vibrio cholerae. Phytomedicine 21:286–289. https://doi.org/10.1016/j.phymed.2013.09.010
Bai F, Han Y, Chen J, Zhang XH (2008) Disruption of quorum sensing in Vibrio harveyi by the AiiA protein of Bacillus thuringiensis. Aquaculture 274:36–40. https://doi.org/10.1007/s12088-013-0415-y
Baldry M, Kitir B, Frøkiær H, Christensen SB, Taverne N, Meijerink M, Franzyk H, Olsen CA, Wells JM, Ingmer H (2016) The agr inhibitors Solonamide B and analogues alter immune responses to Staphylococccus aureus but do not exhibit adverse effects on immune cell functions. PLoS One 11:e0145618. https://doi.org/10.1371/journal.pone.0145618
Bari SMN, Roky MK, Mohiuddin M, Kamruzzaman M, Mekalanos JJ, Faruque SM (2013) Quorum-sensing autoinducers resuscitate dormant Vibrio cholerae in environmental water samples. Proc Natl Acad Sci U S A 110:9926–9231. https://doi.org/10.1073/pnas.1307697110
Bassler BL, Greenberg EP, Stevens AM (1997) Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol 179:4043–4045. https://doi.org/10.1128/jb.179.12.4043-4045.1997
Bengtsson-Palme J, Larsson DGJ (2016) Concentrations of antibiotics predicted to select for resistant bacteria: proposed limits for environmental regulation. Environ Int 86:140–149. https://doi.org/10.1016/j.envint.2015.10.015
Bijtenhoorn P, Mayerhofer H, Muller-Dieckmann J, Utpatel C, Schipper C, Hornung C, Szesny M, Grond S, Thurmer A, Brzuszkiewicz E, Daniel R, Dierking K, Schulenburg H, Streit WR (2011) A novel metagenomic short-chain dehydrogenase/reductase attenuates Pseudomonas aeruginosa biofilm formation and virulence on Caenorhabditis elegans. PLoS One 6:e26278. https://doi.org/10.1371/journal.pone.0026278
Bilecen K, Fong JCN, Cheng A, Jones CJ, Zamorano-Sánchez D, Yildiz FH (2015) Polymyxin B resistance and biofilm formation in Vibrio cholerae are controlled by the response regulator CarR. Infect Immun 83:1199–1209. https://doi.org/10.1128/IAI.02700-14
Borchardt SA, Allain EJ, Michels JJ, Stearns GW, Kelly RF, McCoy WF (2001) Reaction of acylated homoserine lactone bacterial signaling molecules with oxidized halogen antimicrobials. Appl Environ Microbiol 67:3174–3179. https://doi.org/10.1128/AEM.67.7.3174-3179.2001
Brackman G, Defoirdt T, Miyamoto C, Bossier P, Van Calenbergh S, Nelis H, Coeyne T (2008) Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol 8:149. https://doi.org/10.1186/1471-2180-8-149
Brackman G, Celen S, Baruah K, Bossier P, Van Calenbergh S, Nelis HJ, Coenye T (2009) AI-2 quorum-sensing inhibitors affect the starvation response and reduce virulence in several Vibrio species, most likely by interfering with LuxPQ. Microbiology 155:4114–4122. https://doi.org/10.1099/mic.0.032474-0
Brackman G, Celen S, Hillaert U, Calenbergh SV, Cos P, Maes L, Nelis HS, Coenye T (2011) Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. PLoS One 6:e16084. https://doi.org/10.1371/journal.pone.0016084
Bruhn JB, Dalsgaard I, Nielsen KF, Buchholtz C, Larsen JL, Gram L (2005) Quorum sensing signal molecules (acylated homoserine lactones) in gram-negative fish pathogenic bacteria. Dis Aquat Org 65:43–52. https://doi.org/10.3354/dao065043
Buchholtz C, Nielsen KF, Milton DL, Larsen JL, Gram L (2006) Profiling of acylated homoserine lactones of Vibrio anguillarum in vitro and in vivo: influence of growth conditions and serotype. Syst Appl Microbiol 29:433–445. https://doi.org/10.1016/j.syapm.2005.12.007
Cao X, Wang Q, Liu Q, Rui H, Liu H, Zhang Y (2011) Identification of a luxO-regulated extracellular protein Pep and its roles in motility in Vibrio alginolyticus. Microb Pathog 50:123–131. https://doi.org/10.1016/j.micpath.2010.12.003
Castang S, Chantegrel B, Deshayes C, Dolmazon R, Gouet P, Haser R, Reverchon S, Nasser W, Hugouvieux- Cotte- Pattat N, Doutheau A (2004) N-Sulfonyl homoserine lactones as antagonists of bacterial quorum sensing. Bioorg Med Chem Lett 14:5145–5149. https://doi.org/10.1016/j.bmcl.2004.07.088
Chang C, Jing-Jing Z, Chun-Hua R, Chao-Qun H (2010) Deletion of valR, a homolog of Vibrio harveyis luxR generates an intermediate colony phenotype between opaque/rugose and translucent/smooth in Vibrio alginolyticus. Biofouling 26:595–601. https://doi.org/10.1080/08927014.2010.499511
Coates AR, Hu Y (2008) Targeting non-multiplying organisms as a way to develop novel antimicrobials. Trends Pharmacol Sci 29:143–150. https://doi.org/10.1016/j.tips.2007.12.001
Colwell RR, Brayton P, Herrington D, Tall B, Huq A, Levine MM (1996) Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World J Microbiol Biotechnol 12:28–31. https://doi.org/10.1007/BF00327795
Cotter PA, Stibitz S (2007) c-di-GMP-mediated regulation of virulence and biofilm formation. Curr Opin Microbiol 10:17–23. https://doi.org/10.1016/j.mib.2006.12.006
Croxatto A, Chalker VJ, Lauritz J, Jass J, Hardman A, Williams P, Cámara M, Milton DL (2002) VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J Bacteriol 184:1617–1629. https://doi.org/10.1128/JB.184.6.1617-1629.2002
De Spiegeleer B, Verbeke F, D’Hondt M, Hendrix A, Van De Wiele C, Burvenich C, Peremans K, De Wever O, Bracke M, Wynendaele E (2015) The quorum sensing peptides PhrG, CSP and EDF promote angiogenesis and invasion of breast cancer cells in vitro. PLoS One 10:e0119471. https://doi.org/10.1371/journal.pone.0119471
Dharmaprakash A, Reghunathan D, Sivakumar KC, Prasannakumar M, Thomas S (2016) Insights into the genome sequences of an N-acyl homoserine lactone molecule producing two Pseudomonas spp. isolated from the Arctic. Genome Announc 4:e00767–e00716. https://doi.org/10.1128/genomeA.00767-16
Dinh J, Angeloni JT, Pederson DB, Wang X, Cao M, Dong Y (2014) Cranberry extract standardized for Proanthocyanidins promotes the immune response of Caenorhabditis elegans to Vibrio cholerae through the p38 MAPK pathway and HSF-1. PLoS ONE 9:e103290. https://doi.org/10.1371/journal.pone.0103290
Dobrucka R, Długaszewska J (2015) Antimicrobial activities of silver nanoparticles synthesized by using water extract of Arnicae anthodium. Indian J Microbiol 55:168–174. https://doi.org/10.1007/s12088-015-0516-x
Dong YH, Zhang LH (2005) Quorum sensing and quorum-quenching enzymes. J Microbiol 43:101–109. doi: Not Available
Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH (2001) Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813–817. https://doi.org/10.1038/35081101
Dutta D, Chowdhury G, Pazhani GP, Guin S, Dutta S, Ghosh S, Rajendran K, Nandy RK, Mukhopadhyay AK, Bhattacharya MK, Mitra U, Takeda Y, Nair GB, Ramamurthy T (2013) Vibrio cholerae non-O1, non-O139 serogroups and cholera-like diarrhea, Kolkata, India. Emerg Infect Dis 19:464–467. https://doi.org/10.3201/eid1903.121156
Emerenini BO, Hense BA, Kuttler C, Eberl HJ (2015) A mathematical model of quorum sensing induced biofilm detachment. PLoS One 10:e0132385. https://doi.org/10.1371/journal.pone.0132385
Eppinger M, Pearson T, Koenig SSK, Pearson O, Hicks N, Agrawal S, Sanjar F, Galens K, Daugherty S, Crabtree J, Hendriksen RS, Price LB, Upadhyay BP, Shakya G, Fraser CM, Ravel J, Keim PS (2014) Genomic epidemiology of the Haitian cholera outbreak: a single introduction followed by rapid, extensive, and continued spread characterized the onset of the epidemic. MBio 5:e01721–e01714. https://doi.org/10.1128/mBio.01721-14
Faruque SM, Biswas K, Udden SMN, Ahmad QS, Sack DA, Nair GB, Mekalanos JJ (2006) Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc Natl Acad Sci U S A 103:6350–6355. https://doi.org/10.1073/pnas.0601277103
Flodgaard LR, Dalgaard P, Andersen JB, Nielsen KF, Givskov M, Gram L (2005) Non-bioluminescent strains of Photobacterium phosphoreum produce the cell-to-cell communication signal N-(3-Hydroxyoctanoyl) homoserine lactone. Appl Environ Microbiol 71:2113–2120. https://doi.org/10.1128/AEM.71.4.2113-2120.2005
Fong JC, Syed KA, Klose KE, Yildiz FH (2010) Role of Vibrio polysaccharide (vps) genes in VPS production, biofilm formation and Vibrio cholerae pathogenesis. Microbiology 156:2757–2769. https://doi.org/10.1099/mic.0.040196-0
Frerichs RR, Keim PS, Barrais R, Piarroux R (2012) Nepalese origin of cholera epidemic in Haiti. Clin Microbiol Infect 18:E158–E163. https://doi.org/10.1111/j.1469-0691.2012.03841.
Galperin MY (2004) Bacterial signal transduction network in a genomic perspective. Environ Microbiol 6:552–567. https://doi.org/10.1111/j.1462-2920.2004.00633.x
Gram L, de Nys R, Maximilien R, Givskov M, Steinberg P, Kjelleberg S (1996) Inhibitory effects of secondary metabolites from the red alga Delisea pulchra on swarming motility of Proteus mirabilis. Appl Environ Microbiol 62:4284–4287. doi: Not Available
Gupta PK, Pant ND, Bhandari R, Shrestha P (2016) Cholera outbreak caused by drug resistant Vibrio cholerae serogroup O1 biotype El Tor serotype Ogawa in Nepal; a cross-sectional study. Antimicrob Resist Infect Control 5:23. https://doi.org/10.1186/s13756-016-0122-7
Gutierrez JA, Crowder T, Rinaldo-Matthis A, Ho M-C, Almo SC, Schramm VL (2009) Transition state analogs of 5′-methylthioadenosine nucleosidase disrupt quorum sensing. Nat Chem Biol 5:251–257. https://doi.org/10.1038/nchembio.153.
Hang L, John M, Asaduzzaman M, Bridges EA, Vanderspurt C, Kirn TJ, Taylor RK, Hillman JD, Progulske-Fox A, Handfield M, Ryan ET, Calderwood SB (2003) Use of in vivo-induced antigen technology (IVIAT) to identify genes uniquely expressed during human infection with Vibrio cholerae. Proc Natl Acad Sci U S A 100:8508–8513. https://doi.org/10.1073/pnas.1431769100
Haudecoeur E, Tannieres M, Cirou A, Raffoux A, Dessaux Y, Faure D (2009) Different regulation and roles of lactonases AiiB and AttM in Agrobacterium tumefaciens C58. Mol Plant Microbe Interact 22:529–537. https://doi.org/10.1094/MPMI-22-5-0529
Hoang TT, Schweizer HP (1999) Characterization of Pseudomonas aeruginosa enoyl-acyl carrier protein reductase (FabI): a target for the antimicrobial triclosan and its role in acylated homoserine lactone synthesis. J Bacteriol 181:5489–5497. doi: Not Available
Huma N, Shankar P, Kushwah J, Bhushan A, Joshi J, Mukherjee T, Raju SC, Purohit HJ, Kalia VC (2011) Diversity and polymorphism in AHL-lactonase gene (aiiA) of Bacillus. J Microbiol Biotechnol 21:1001–1011. https://doi.org/10.4014/jmb.1105.05056
Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR (1983) Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 45:275–283. doi: Not Available
Jutla A, Whitcombe E, Hasan N, Haley B, Akanda A, Huq A, Alam M, Sack RB, Colwell RR (2013) Environmental factors influencing epidemic cholera. Am J Trop Med Hyg 89:597–607. https://doi.org/10.4269/ajtmh.12-0721
Kalia VC, Raju SC, Purohit HJ (2011) Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-homoserine lactone-acylase and –lactonase. Open Microbiol J 5:1–13. https://doi.org/10.2174/1874285801105010001
Kalia VC, Kumar P, Pandian SK, Sharma P (2014) Biofouling control by quorum quenching. In: Kim SK (ed) Hb_25 Springer handbook of marine biotechnology chapter 15. Springer, Berlin, pp 431–440. https://doi.10.1007/978-3-642-53971-8_15
Ke X, Miller LC, Ng WL, Bassler BL (2014) CqsA-CqsS quorum-sensing signal-receptor specificity in Photobacterium angustum. Mol Microbiol 91:821–833. https://doi.org/10.1111/mmi.12502
Kim JS, Kim YH, Seo YW, Park S (2007) Quorum sensing inhibitors from the red alga, Ahnfeltiopsis flabelliformis. Biotechnol Bioprocess Eng 12:308–311. https://doi.org/10.1007/s12257-008-0131-3
Koul S, Kalia VC (2017) Multiplicity of quorum quenching enzymes: a potential mechanism to limit quorum sensing bacterial population. Indian J Microbiol 57:100–108. https://doi.org/10.1007/s12088-016-0633-1
Kumar P, Koul S, Patel SKS, Lee JK, Kalia VC (2015) Heterologous expression of quorum sensing inhibitory genes in diverse organisms. In: Kalia VC (ed) Quorum sensing vs quorum quenching: a battle with no end in sight. Springer, New Delhi, pp 343–356. https://doi.org/10.1007/978-81-322-1982-8_28
Lee SJ, Park SY, Lee JJ, Yum DY, Koo BT, Lee JK (2002) Genes encoding the N-acyl homoserine lactone-degrading enzyme are wide spread in many subspecies of Bacillus thuringiensis. Appl Environ Microbiol 68:3919–3924. https://doi.org/10.1128/AEM.68.8.3919-3924.2002
Liu H, Wang Q, Liu Q, Cao X, Shi C, Zhang Y (2011) Roles of Hfq in the stress adaptation and virulence in fish pathogen Vibrio alginolyticus and its potential application as a target for live attenuated vaccine. Appl Microbiol Biotechnol 91:353–364. https://doi.org/10.1007/s00253-011-3286-3
Lowery CA, Park J, Kaufmann GF, Janda KD (2008) An unexpected switch in the modulation of AI-2-based quorum sensing discovered through synthetic 4,5-Dihydroxy-2,3-pentanedione analogues. J Am Chem Soc 130:9200–9201. https://doi.org/10.1021/ja802353
Lowery CA, Abe T, Park J, Eubanks LM, Sawada D, Kaufmann GF, Janda KD (2009) Revisiting AI-2 quorum sensing inhibitors: direct comparison of alkyl-DPD analogues and a natural product fimbrolide. J Am Chem Soc 131:15584–15585. https://doi.org/10.1021/ja9066783
Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA (2003) A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306–310. https://doi.org/10.1038/nature02122
Manefield M, Harris L, Rice SA, de Nys R, Kjelleberg S (2000) Inhibition of luminescence and virulence in the black tiger prawn (Penaeus monodon) pathogen Vibrio harveyi by intercellular signal antagonists. Appl Environ Microbiol 66:2079–2084. doi: Not Available
Marques JC, Lamosa P, Russell C, Ventura R, Maycock C, Semmelhack MF, Miller ST, Xavier KB (2011) Processing the interspecies quorum-sensing signal autoinducer-2 (AI-2): characterization of phospho-(S)-4,5-dihydroxy-2,3-pentanedione isomerization by LsrG protein. J Biol Chem 286:18331–18343. https://doi.org/10.1074/jbc.M111.230227
Martínez JL (2012) Natural antibiotic resistance and contamination by antibiotic resistance determinants: the two ages in the evolution of resistance to antimicrobials. Front Microbiol 3:1. https://doi.org/10.3389/fmicb.2012.00001
Matz C, McDougald D, Moreno AM, Yung PY, Yildiz FH, Kjelleberg S (2005) Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae. Proc Natl Acad Sci U S A 102:16819–16824. https://doi.org/10.1073/pnas.0505350102
Meibom KL, Blokesch M, Dolganov NA, Wu C-Y, Schoolnik GK (2005) Chitin induces natural competence in Vibrio cholerae. Science 310:1824–1827. https://doi.org/10.1126/science.1120096
Meijler MM, Hom LG, Kaufmann GF, McKenzie KM, Sun CZ, Moss JA, Matsushita M, Angew JKD (2004) Synthesis and biological validation of a ubiquitous quorum-sensing molecule. Chem Int Ed 43:2106–2108. https://doi.org/10.1002/anie.200353150
Milton DL (2006) Quorum sensing in vibrios: complexity for diversification. Int J Med Microbiol 296:61–71. https://doi.org/10.1016/j.ijmm.2006.01.044
Milton DL, Hardman A, Camara M, Chhabra SR, Bycroft BW, Stewart GS, Williams P (1997) Quorum sensing in Vibrio anguillarum: characterization of the vanI/vanR locus and identification of the autoinducer N-(3-oxodecanoyl)-L-homoserine lactone. J Bacteriol 179:3004–3012. https://doi.org/10.1128/jb.179.9.3004-3012.1997
Mireille Aye A, Bonnin-Jusserand M, Brian-Jaisson F, Ortalo-Magne A, Culioli G, Koffi Nevry R, Rabah N, Blache Y, Molmeret M (2015) Modulation of violacein production and phenotypes associated with biofilm by exogenous quorum sensing N-acylhomoserine lactones in the marine bacterium Pseudoalteromonas ulvae TC14. Microbiology 161:2039–2051. https://doi.org/10.1099/mic.0.000147
Mitra R, Orbell J, Muralitharan MS (2007) Agriculture- medicinal plants of Malaysia. Asia-Pac Biotech News 11:105–110. https://doi.org/10.1142/S0219030307000110
Morohoshi T, Nakazawa S, Ebata A, Kato N, Ikeda T (2008) Identification and characterization of N-acylhomoserine lactone-acylase from the fish intestinal Shewanella sp. strain MIB015. Biosci Biotechnol Biochem 72:1887–1893. https://doi.org/10.1271/bbb.80139
Musthafa KS, Saroja V, Pandian SK, Ravi AV (2011) Antipathogenic potential of marine Bacillus sp. SS4 on N-acyl-homoserine-lactone-mediated virulence factors production in Pseudomonas aeruginosa (PAO1). J Biosci 36:55–67. https://doi.org/10.1007/s12038-011-9011-7
Narendrakumar L, Suryaletha K, Reghunathan D, Prasannakumar M, Thomas S (2017) Insights into the draft genome sequence of a Haitian variant Vibrio cholerae strain isolated from a clinical setting in Kerala, South India. Genome Announc 5:e00843–e00817. https://doi.org/10.1128/genomeA.00843-17
Neu HC (1992) The crisis in antibiotic resistance. Science 257:1064–1073. https://doi.org/10.1126/science.257.5073.1064
Ng WL, Bassler BL (2009) Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197–222. https://doi.org/10.1146/annurev-genet-102108-134304
Nithya C, Arvindraja C, Pandian SK (2010) Bacillus pumilus of Palk Bay origin inhibits quorum-sensing-mediated virulence factors in gram-negative bacteria. Res Microbiol 161:293–304. https://doi.org/10.1016/j.resmic.2010.03.002
Nithya C, Devi MG, Pandian SK (2011) A novel compound from the marine bacterium Bacillus pumilus S6-15 inhibits biofilm formation in gram-positive and gram-negative species. Biofouling 27:519–528. https://doi.org/10.1080/08927014.2011.586127
Niu C, Afre S, Gilbert ES (2006) Subinhibitory concentrations of cinnamaldehyde interfere with quorum sensing. Lett Appl Microbiol 43:489–494. https://doi.org/10.1111/j.1472-765X.2006.02001.x
Okutsu N, Morohoshi T, Xie X, Kato N, Ikeda T (2015) Characterization of N-acyl-homoserine lactones produced by bacteria isolated from industrial cooling water systems. Sensors 16:E44. https://doi.org/10.3390/s16010044
Olsen JA, Severinsen R, Rasmussen TB, Hentzer M, Givskov M, Nielsen J (2002) Synthesis of new 3- and 4-substituted analogues of acyl homoserine lactone quorum sensing autoinducers. Bioorg Med Chem Lett 12:325–328. https://doi.org/10.1016/S0960-894X(01)00756-9
Peach KC, Bray WM, Shikuma NJ, Gassner NC, Lokey RS, Yildiz FH, Linington RG (2011) An image-based 384-well high-throughput screening method for the discovery of biofilm inhibitors in Vibrio cholerae. Mol BioSyst 7:1176–1184. https://doi.org/10.1039/c0mb00276c
Pereira CS, Thompson JA, Xavier KB (2013) AI-2-mediated signalling in bacteria. FEMS Microbiol Rev 37:156–181. https://doi.org/10.1111/j.1574-6976.2012.00345.x
Persson T, Givskov M, Nielsen J (2005) Quorum sensing inhibition: targeting chemical communication in gram-negative bacteria. Curr Med Chem 12:3103–3115. https://doi.org/10.2174/092986705774933425
Rasch M, Buch C, Austin B, Slierendrecht WJ, Ekmann KS, Larsen JL, Johansen C, Riedel K, Ebery L, Givskov M, Gram L (2004) An inhibitor of bacterial quorum sensing reduces mortyalities caused by Vibriosis in Rainbow trout (Oncorhynchus mykiss, Walbaum). Syst Appl Microbiol 27:350–359. https://doi.org/10.1078/0723-2020-00268
Rasmussen TB, Givskov M (2006) Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol 296:149–161. https://doi.org/10.1016/j.ijmm.2006.02.005
Rasmussen BB, Nielsen KF, Machado H, Gram L, Sonnenschein E (2014) Global and phylogenetic distribution of quorum sensing signals, acyl homoserine lactones, in the family of Vibrionaceae. Mar Drugs 12:5527–5546. https://doi.org/10.3390/md12115527
Ruby EG (1996) Lessons from a cooperative, bacterial- animal association: the Vibrio fischeri- Euprymna scolopes light organ symbiosis. Annu Rev Microbiol 50:591–624. https://doi.org/10.1146/annurev.micro.50.1.591
Schaefer AL, Hanzelka BL, Eberhard A, Greenberg EP (1996) Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J Bacteriol 178:2897–2901. doi: Not Available
Sharma R, Jangid K (2015) Fungal quorum sensing inhibitors. In: Kalia VC (ed) Quorum sensing vs quorum quenching: a battle with no end in sight. Springer, New Delhi, pp 237–257
Shiva Krishna P, Sudheer Kumar B, Raju P, Murty MSR, Prabhakar Rao T, Singara Charya MA, Prakasham RS (2015) Fermentative production of pyranone derivate from marine Vibrio sp. SKMARSP9: isolation, characterization and bioactivity evaluation. Indian J Microbiol 55:292–301. https://doi.org/10.1007/s12088-015-0521-0
Singh V, Evans GB, Lenz DH, Mason JM, Clinch K, Mee S, Painter GF, Tyler PC, Furneaux RH, Lee JE, Howell PL, Schramm VL (2005) Femtomolar transition state analogue inhibitors of 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli. J Biol Chem 280:18265–18273. https://doi.org/10.1074/jbc.M414472200
Smith JAI, Wang J, Nguyen-Mau S-M, Leeb V, Sintim HO (2009) Biological screening of a diverse set of AI-2 analogues in Vibrio harveyi suggests that receptors which are involved in synergistic agonism of AI-2 and analogues are promiscuous. Chem Commun 45:7033–7035. https://doi.org/10.1039/b909666c
Szweda P, Gucwa K, Kurzyk E, Romanowska E, Dzierżanowska-Fangrat K, Jurek AZ, Kuś PM, Milewski S (2015) Essential oils, silver nanoparticles and propolis as alternative agents against fluconazole resistant Candida albicans, Candida glabrata and Candida krusei clinical isolates. Indian J Microbiol 55:175–183. https://doi.org/10.1007/s12088-014-0508-2
Tashiro Y, Kimura Y, Furubayashi M, Tanaka A, Terakubo K, Saito K, Kawai-Noma S, Umeno D (2016) Directed evolution of autoinducer selectivity of Vibrio fischeri LuxR. J Gen Appl Microbiol 62:240–247. https://doi.org/10.1093/nar/gkq1070
Teplitski M, Chen H, Rajamani S, Gao M, Merighi M, Sayre RT, Robinson JB, Rolfe BG, Bauer WD (2004) Chlamydomonas reinhardtii Secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol 134:137–146. https://doi.org/10.1104/pp.103.029918
Teschler JK, Zamorano-Sánchez D, Utada AS, Warner CJA, Wong GCL, Linington RG, Yildiz FH (2015) Living in the matrix: assembly and control of Vibrio cholerae biofilms. Nat Rev Microbiol 13:255–268. https://doi.org/10.1038/nrmicro3433
Tseng BS, Majerczyk CD, Passos da Silva D, Chandler JR, Greenberg EP, Parsek MR (2016) Quorum sensing influences Burkholderia thailandensis biofilm development and matrix production. J Bacteriol 198:2643–2650. https://doi.org/10.1128/JB.00047-16
Valiente E, Bruhn JB, Nielsen KF, Larsen JL, Roig FJ, Gram L, Amaro C (2009) Vibrio vulnificus produces quorum sensing signals of the AHL-class. FEMS Microbiol Ecol 69:16–26. https://doi.org/10.1111/j.1574-6941.2009.00691.x
van Sinderen D, Luttinger A, Kong L, Dubnau D, Venema G, Hamoen L (1995) comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol Microbiol 15:455–462. https://doi.org/10.1111/j.1365-2958.1995.tb02259.x
Vikram A, Jesudhasan PR, Jayaprakasha GK, Pillai SD, Patil BS (2011) Citrus limonoids interfere with Vibrio harveyi cell-cell signaling and biofilm formation by modulating the response regulator LuxO. Microbiology 157:99–110. https://doi.org/10.1099/mic.0.041228-0
Vinoj G, Vaseeharan B, Thomas S, Spiers AJ, Shanthi S (2014) Quorum-quenching activity of the AHL-lactonase from Bacillus licheniformis DAHB1 inhibits Vibrio biofilm formation in vitro and reduces shrimp intestinal colonisation and mortality. Mar Biotechnol 16:707–715. https://doi.org/10.1007/s10126-014-9585-9
Wang L, Ling Y, Jiang H, Qiu Y, Qiu J, Chen H, Yang R, Zhou D (2013) AphA is required for biofilm formation, motility, and virulence in pandemic Vibrio parahaemolyticus. Int J Food Microbiol 160:245–251. https://doi.org/10.1016/j.ijfoodmicro.2012.11.004
Williams P, Camara M (2009) Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol 12:182–191. https://doi.org/10.1016/j.mib.2009.01.005
World Health Organization (2016) Weekly epidemiological record, vol 91, 38:433–440. doi: Not Available
Xavier KB, Bassler BL (2005) Interference with AI-2-mediated bacterial cell-cell communication. Nature 437:750–753. https://doi.org/10.1038/nature03960
Yang Q, Han Y, Zhang XH (2011) Detection of quorum sensing signal molecules in the family Vibrionaceae. J Appl Microbiol 110:1438–1448. https://doi.org/10.1111/j.1365-2672.2011.04998.x
Yang K, Meng J, Huang YC, Ye LH, Li GJ, Huang J, Chen HM (2014) The role of the QseC quorum-sensing sensor kinase in epinephrine-enhanced motility and biofilm formation by Escherichia coli. Cell Biochem Biophys 70:391–398. https://doi.org/10.1007/s12013-014-9924-5
Yildiz FH, Dolganov NA, Schoolnik GK (2001) VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPSETr-associated phenotypes in Vibrio cholerae O1 El Tor. J Bacteriol 183:1716–1726. https://doi.org/10.1128/JB.183.5.1716-1726.2001
Zhao X, Kuipers OP (2016) Identification and classification of known and putative antimicrobial compounds produced by a wide variety of Bacillales species. BMC Genomics 17:882. https://doi.org/10.1186/s12864-016-3224-y
Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ (2002) Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A 99:3129–3134. https://doi.org/10.1073/pnas.052694299
Acknowledgement
Lekshmi. N is obliged to Department of Science and Technology, Govt. of India for providing INSPIRE fellowship (Fellow code: IF 140851). Authors from NIT Rourkela acknowledge the PhD scholar institute fellowship given by NIT Rourkela, Odisha, India.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Narendrakumar, L., Das, B., Paramasivan, B., Rasu, J., Thomas, S. (2018). Quorum Quenching and Biofilm Inhibition: Alternative Imminent Strategies to Control the Disease Cholera. In: Kalia, V. (eds) Biotechnological Applications of Quorum Sensing Inhibitors. Springer, Singapore. https://doi.org/10.1007/978-981-10-9026-4_4
Download citation
DOI: https://doi.org/10.1007/978-981-10-9026-4_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-9025-7
Online ISBN: 978-981-10-9026-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)