Abstract
Viruses from the Flavivirus family are the causative agents of dengue fever, Zika, Japanese encephalitis, West Nile encephalitis or Yellow fever and constitute major or emerging public health problems. A better understanding of the flavivirus replication cycle is likely to offer new opportunities for the design of antiviral therapies to treat severe conditions provoked by these viruses, but it should also help reveal fundamental biological mechanisms of the host cell. During virus replication, RNA synthesis is mediated by a dynamic and membrane-bound multi-protein assembly, named the replication complex (RC). The RC is composed of both viral and host-cell proteins that assemble within vesicles composed of the endoplasmic reticulum membrane, near the nucleus. At the heart of the flavivirus RC lies NS4B, a viral integral membrane protein that plays a role in virulence and in down-regulating the innate immune response. NS4B binds to the NS2B-NS3 protease-helicase, which itself interacts with the NS5 methyl-transferase polymerase. We present an overview of recent structural and functional data that augment our understanding of how viral RNA is replicated by dengue virus. We focus on structural data that illuminate the various roles played by proteins NS2B-NS3, NS4B and NS5. By participating in viral RNA cap methylation, the NS5 methyltransferase enables the virus to escape the host cell innate immune response. We present the molecular basis for this activity. We summarize what we know about the network of interactions established by NS2B-NS3, NS4B and NS5 (their “interactome”). This leads to a working model that is captured in the form of a rather naïve “cartoon”, which we hope will be refined towards an atomic model in the near future.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Flavivirus
- Replication complex
- Protein-protein interactions
- Innate immunity evasion
- Antiviral drug discovery
9.1 Introduction
Flaviviruses such as Yellow Fever Virus (YFV), Dengue virus (DENV), West-Nile virus (WNV), Japanese Encephalitis Virus (JEV) and the recently re-emerging Zika virus (ZIKV) represent significant public-health problems. The four DENV serotypes cause hundreds of thousands cases of the severe haemorrhagic form of the disease every year and a large and increasing proportion of the world population is at risk (http://www.who.int/topics/dengue/en/). The recently marketed Sanofi tetravalent vaccine (CYD-TDV) fails to confer good protection against serotype 2 and is not recommended for children younger than 11 years old, which unfortunately represents a significant limitation of its applicability [15, 56]. While vaccination remains in principle the best way to protect large populations against infectious diseases and has indeed been extremely successful to control YFV [63], specific antiviral drugs to treat the various conditions caused by flaviviruses, such as DENV, ZIKV, WNV, YFV and JEV would also be desirable, because they would significantly expand our prophylactic and therapeutic options [29, 43]. For instance despite the availability of a very efficient YFV vaccine [63] for almost 80 years, between 30,000 and 60,000 deaths are caused every year by infections with this virus and an important outbreak was reported this year in in Angola, Uganda and Democratic Republic of the Congo, Africa. Outbreak management currently relies on controlling the mosquito vector population via the release of mosquitoes infected by the bacterium Wolbachia (see Chapter by Scott O’Neill and colleagues). Plus-strand RNA virus replication occurs in association with cytoplasmic host-cell membranes, where viral and host-cell factors cooperate within an organelle-like factory called replication complex (RC) (see refs. [3, 13, 44, 49, 64]). DENV is a well-studied enveloped virus with a positive-strand RNA genome that also serves as a prototype to understand the flavivirus structure and its replication cycle. Upon attachment to a susceptible cell, DENV viral particles about 50 nm in diameter [26] are internalized in vesicles and transported to endosomes, where major conformational changes in the envelope protein E triggered by low pH [4], lead to the release of the ribonucleocapsid into the cytoplasm. The coding region of the capped single-strand RNA genome which contains also untranslated regions (UTR) both at its 5′ and 3′ ends, is then translated into a viral polypeptide and processed into ten proteins following maturation by host proteases and also by the NS2B-NS3 viral protease (Fig. 9.1). The N-terminal part of the viral polyprotein contains three structural proteins C, prM and E and the C-terminal part seven non-structural (NS) proteins: NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5 (Fig. 9.1). Viral NS proteins and host factors assemble to form a membrane-bound RC that functions like a molecular factory by orchestrating viral RNA replication [36, 37]. Following RNA synthesis, newly copied RNA molecules are either recycled for translation and replication or alternatively, extruded from the vesicle for packaging into nascent virions. Importantly, several NS proteins of the flavivirus RC including NS2B-NS3, NS4B and NS5 constitute validated drug targets because they play crucial functions during viral replication. Today however, a major impediment in developing drugs targeting the flavivirus RC is that both its morphology and exact composition, the interplay between its molecular constituents at various stages of the replication cycle, as well as the precise molecular mechanisms for viral RNA replication are still largely elusive. One could say that the field in flavivirus research is lagging about 5–10 years behind the field of HCV research. In this respect the emergence of viruses like ZIKV is likely to provide the momentum and the level of investment needed to fill this gap.
The flavivirus polyprotein. Shown here is a gallery of structures determined for DENV, available from the PDB at www.rcsb.org. References to the original work (and respective authors) are either included in the text or can be found in the PDB. The polyprotein is depicted as blue rectangles for the three structural proteins C (capsid), Pr and M Membrane protein, E, envelope protein and as green rectangles for the seven non structural proteins NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5, expressed during the intracellular phase of the virus replication cycle. Question marks indicate NS proteins for which no high-resolution structure is available, but NMR data detailing their secondary structures and topology is available for NS2A (25), NS2B (28) and NS4B (29–30, 32) (see text)
9.2 The Flavivirus Replication Complex
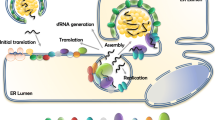
Over the last decade, several individual protein components of the RC were characterized both at the functional and structural level and also as potential antiviral drug targets [36, 37, 50]. Moreover, several human and insect proteins putatively linked to DENV replication were recently identified using proteomics and RNAi approaches [23, 58]. An interesting –although ambitious- challenge ahead is to gain a better basic understanding of the flavivirus RC as a whole, in order to picture at the atomic level molecular interactions that orchestrate the various steps of replication: The RC coordinates steps of viral cycle by spatial segregation of replicating RNA from ribosomes and from capsids undergoing assembly. In this process, an intricate and dynamic network of various RNA-RNA, RNA-protein and RNA-protein interactions must be established in the context of the physical barrier provided by ER membrane (Fig. 9.2). An immediate benefit for the replicating flavivirus is the protection that such ER perinuclear membrane vesicles confer against host cell nucleases and also the avoidance of innate immunity sensors such as RIG-I and IFIT family members, that can detect dsRNA molecules [12]. In addition, there is an increasing appreciation that intermolecular interactions between members of the RC can regulate their enzymatic activities. Catalytic activities of multifunctional proteins like the NS2B-NS3 protease NTPase/helicase or the NS5 methyltransferase-polymerase appear to be different in the context of the full-length proteins compared to isolated recombinant enzymatic domains [40,41,42]. Further modulation of enzymatic activities is expected in the context of the membrane bound RC. This suggests the existence of an extensive network of “cross-talks” where both inter-domain and intermolecular interactions regulate the various enzymatic activities harbored by each protein domain. Today, we are just beginning to understand the molecular basis of these “cross-talks”, thanks to a combination of structural and functional studies and also by using infectious clones and replicon systems to perform site-directed mutagenesis informed by structural data.
The flavivirus replication complex. Schematic depiction of the membrane bound flavivirus replication complex inserted in a protective vesicle. The figure is not drawn to scale as many RCs are likely to be present in each vesicule, see for instance reference 6. Moreover, the multimerization proposed for several NS proteins such as NS4B (54) or NS5 (70) is ignored in this schematic representation. (Adapted From Pei-Yong Shi Science. 2014 Feb 21;343(6173):849–50)
Here we briefly describe ongoing work that used DENV RC as model. An interesting, yet still largely open question, pertains to whether the DENV RC is representative of all other flaviviruses, or alternatively, whether divergence in evolution has produced various answers in terms of the architecture and dynamics of say the RC from JEV, ZIKV or even between the various DENV serotypes. Initial indications given by recent 3D structures determination of the full-length NS5 protein from JEV, DENV3 and ZIKV (see PDB code 5TFR) indeed suggest variations in the architecture of their respective RC introduced during evolution. However, some clustering during the divergent evolution of flaviviruses appears likely as outlined below taking the example of the full-length NS5 protein. One immediate task therefore consists in identifying a common ancestral RC “core” shared between various flaviviruses. Another task is to check whether a simple phylogenetic analysis suffices to assign one particular RC to one group of closely related flaviviruses. In our lab, we have focused our efforts on DENV and on mapping the interactions (“interactome”) of three proteins NS2B-NS3, NS4B and NS5. These proteins play either key enzymatic roles or act as “a hub” within the flavivirus RC like NS4B -an integral membrane protein with also a role in virulence and down-regulation of the host innate immune response. NS4B binds to the NS2B-NS3 protease-helicase which itself interacts with the NS5 methyl-transferase-polymerase and we ask how are these molecular interfaces formed and what impact they have on the activities of the individual proteins?
Like other (+) strand RNA viruses such as HCV [53], DENV RNA replication is performed in close association with remodeled cell membranes inside a vesicular like compartment (Fig. 9.2). The morphology and activity of the DENV RC and TBEV was studied by purifying membrane fractions from cells transfected with replicon RNA. Such isolated RCs are able to synthesize RNA and the RdRp activity that produces dsRNA intermediates is protected from nuclease and protease digestion and also concealed from the innate immune system via vesicular structures that were recently described using elegant electron tomographic reconstructions [46, 68].
Our understanding of flavivirus NS proteins, particularly in their membrane-bound environment, has been hampered by the lack of robust in vitro expression systems and by the inherent difficulties associated with the study of integral membrane proteins. Thus, for many years, much of what was known in terms of atomic structures has made use of soluble fragments of viral replicative enzymes expressed in E. coli [50] (Fig. 9.1). However, growing evidence suggests that it is the interplay between viral enzymes, host cofactors and viral RNA that orchestrates temporally and spatially the virus RNA genome replication [17]. Thus, despite their crucial roles for viral genome replication, relatively little structural information has been available for NS2A, NS2B, NS4A, NS4B, that are integral membrane proteins (Fig. 9.1). This has recently changed and we now have a clearer picture for the topology of membrane-associated proteins NS1 [1], NS2A [73], NS2B [8, 21, 31, 32] and NS4B [31,32,33,34] by various structural and biochemical techniques including NMR and X-ray crystallography. We also have more information about how these integral membrane proteins interact with members of the RC, how they might affect membrane structure and also the assembly of new viral particles [27, 30]. In a recent study on NS2B from JEV [33, 34], the trans-membrane region of NS2B from JEV was suggested to contribute both to viral RNA replication and to the formation of new virus particles. Moreover the interaction between NS2B and NS2A may participate in modulating viral assembly [33, 34]. Remarkably, NMR structural studies performed on NS4B reconstituted in micelles [33, 34] have largely confirmed pioneering work on NS4B that was performed in the mid 2000s by the Bartenschlager group, using serial truncation and localization/labelling experiments (in order to detect ER vs cytoplasmic exposure) [45]. As said earlier, cell biology and electron tomography studies have provided in situ pictures of the complete flavivirus RC highlighting membrane rearrangements into vesicles and spherules (Fig. 9.2). However, to understand the RC at the atomic level, one must now combine these relatively low-resolution images of the RC derived by tomography with higher resolution data derived from X-ray crystallography or NMR and this task remains a major challenge. Compound screening campaigns have identified potent DENV [67] and YFV [14] inhibitors targeting NS4B. How these inhibitors interact (eventually only through a “genetic interaction”) with NS4B remains elusive in the absence of supporting biochemical and structural data. Thus structural studies of the integral membranes proteins from DENV and other flaviviruses will certainly provide crucial information required to understand their roles in membrane remodelling, in viral replication and viral particle assembly and also for the design of specific inhibitors with antiviral activity [36, 37].
9.3 NS2B and NS2B-NS3
NS2B-NS3 is one of the most conserved flavivirus enzymes. For instance the ZIKV NS2B-NS3 protease was found to bear high structural similarity to the homologous enzymes from DENV and WNV [51]. NS2B-NS3 possesses protease, helicase and RNA triphosphatase activities stimulated by ssRNA binding [40, 41]. The N-terminal domain of NS3 (NS3pro) is important for viral maturation because it cleaves the viral polyprotein at the junctions between NS2A/2B, NS2B/3, NS3/4A and NS4B/5. NS2B is a small (14 kDa) protein with four hydrophobic transmembrane (TM) domains formed by helices α1 (G4 to L19), α2 (L25 to M41), α3 (N90-G105) and α4 (P112-T125). A central hydrophilic region (residues 42–90), projects into the cytoplasm and acts as a necessary cofactor for the protease N-terminal domain of NS3 (NS3pro). In addition to its membrane anchoring cofactor NS2B, NS3pro from DENV has an exposed hydrophobic turn formed by residues “G29LFG32”, which is also likely to contribute to membrane association of the protein [42]. The helicase domain of NS3 (NS3hel) is involved in viral RNA replication together with the RNA-dependent RNA polymerase-NS5 [28, 76] with which it interacts [61, 80]. The linker between the NS3pro and NS3hel domains was reported to confer overall inter-domain flexibility and a switch between two conformational states was proposed to accompany the transition between polyprotein proteolysis and RNA replication [42]. However, the conformation of NS3 in the context of other RC proteins including the complete NS2B protein, NS4B and NS5 remains largely unknown. Likewise the exact role NS3 itself plays in viral replication is not clearly defined: besides its role in cap synthesis through hydrolysis of the gamma phosphate of GTP, NS3hel could participate in viral genome replication by unwinding dsRNA replication intermediates. The interaction between NS3 and NS4B is essential for dissociating the helicase from single-stranded RNA, which modulates viral replication [65]. Interestingly Dengue virus nonstructural protein 3 was found to redistributes fatty acid synthase to sites of viral replication and increases fatty acid synthesis, a result in line with the requirement for lipid membrane reorganization in the context of the RC [19].
9.4 The NS4B Protein
Both NS4A and NS4B have been implicated in membrane rearrangements and RNA replication. NS4A associates with the membrane via four TM domains and has a C-terminal “2 K fragment” that serves as a signal sequence for the translocation of the adjacent NS4B protein into the ER lumen. An N-terminal amphipathic helix in DENV NS4A mediates oligomerization and is essential for replication [60]. NS4B confers virulence through enhancing viral synthesis in a mouse model of DENV infection [18]. Likewise, NS4B from WNV is involved in virulence and a mutation resulting in an attenuated virus was identified [69]. NS4B is also implicated in the inhibition of alpha/beta interferon signalling [47] and in the regulation of immune response mediators (cytokines) associated with haemorrhagic fever [22, 52, 70]. Remarkably, NS4B influences the helicase activity of NS3 presumably via a direct protein-protein interaction [65]. NS4B was found to colocalize with NS3 and with double stranded RNA, which is an intermediate in viral RNA replication [81, 82]. A topology model initially proposed for NS4B on the basis of truncation and cytoplasmic vs ER localization experiments [45] was later essentially confirmed by NMR studies, leading however to a more precise assignment of secondary structure elements [33, 34].
For crystallization trials and interaction studies, the NS4B protein was expressed in Escherichia coli, reconstituted in dodecyl maltoside (DDM) detergent micelles, and purified to >95% homogeneity [33, 34]. Proper folding was initially checked via CD spectroscopy indicating the presence of a majority of alpha-helical structures as expected from secondary structure sequence prediction (Le Tian Lee et al., unpublished data). In vitro, the recombinant NS4B protein forms dimers, as shown by gel filtration, chemical cross-linking, and multi-angle light scattering [81]. The dimeric form of NS4B could also be detected when the protein was expressed in cells as well as in cells infected with DENV2 [81]. Mutagenesis showed that the cytosplasmic loop spanning amino acids 129–165 and the C-terminal region extending from amino acids 166–248 are important contributors driving NS4B dimerization.
We raised two mouse mAbs against the recombinant NS4B protein from DENV2 [74]. These mAbs named 10-3-7 and 44-4-7 were characterized in terms of their cross-reactivity towards other DENV serotypes and their epitopes were mapped to the available topology model for NS4B. While mAb 10-3-7 appears strictly specific for NS4B from DENV2, mAb 44-4-7 is particularly interesting as it cross-reacts with NS4B from the four DENV serotypes and also with ZIKV NS4B (Jia Huan et al., unpublished results). Using overlapping peptides spanning the whole NS4B sequence, we found that mAb 44-4-7 binds to the cytoplasmic loop of NS4B [74]). We expressed in E. coli a scFv construct containing the variable regions of mAb 44-4-7, that recapitulates binding of the parent mAb. Besides possible applications in pull-down experiments using RC extracted from infected cells, this scFv was used as a vehicle to promote the formation of well-ordered 3D crystals to increase hydrophilic surface and improve lattice order [20, 24]. However, a caveat is that the NS4B protein used for eliciting mAbs might have been presented to the mouse immune system in a partially denatured state. This could explain the lack of success in crystallizing the NS4B protein we have encountered so far. Therefore, the structural study of NS4B from DENV3 was carried out using NMR in LMPG detergent micelles [33, 34]. This work revealed the presence of a total of eleven helices with five potential TM regions (Fig. 9.3). This work has given the first detailed secondary structure assignment for this important viral protein: NS4B was found to be the site of escape mutations when DENV or YFV are grown in the presence of several antiviral inhibitors [72]. However, a direct molecular interaction between small molecule inhibitors and NS4B has not been clearly demonstrated so far and the observed evolutive pressure on NS4B brought about by incubating the virus with these drugs could be due to indirect interactions [72]. Rather surprisingly, some data suggesting a genetic and physical interaction between nonstructural proteins NS1 and NS4B with a role in modulating replication of WNV was also presented [77]. This observation brings some alteration to the currently accepted topology model in which NS1 is exposed to the lumen side of the ER whilst NS4B is exposed to the cytoplasmic side (Fig. 9.2).
Membrane topology of NS4B. (a). The topology of NS4B is represented sequence based on PRE and H–D exchange experiments. Water-soluble residues are highlighted in red, membrane interacting residues in green and residues protected from exposure to gadolinium are in blue. Residues highlighted in brown are protected from H–D exchanges. (b, c) The NS4B topology varies depending on the presence (b) or absence (c) of NS5 (that is before or after cleavage of the flavivirus polyprotein at the NS4B-NS5 junction. (Adapted from Ref. [ [30]])
9.5 Mapping the Interactions Between NS2B-NS3 and NS4B
Remarkably, NS4B regulates the helicase activity of NS3 by modulating NS4B affinity for RNA. We therefore attempted to detect a direct protein-protein interaction between these two proteins and to map their interactions on the respective proteins. Co-immuno-precipitation and in situ proximity ligation assay confirmed that NS3 co-localizes with NS4B in both DENV-infected cells and cells that co-expressed both proteins [82]. We then expressed the full-length NS3 protein and also its helicase and protease domains separately. Surface plasmon resonance showed that the helicase domain of NS3 alone was able to bind NS4B with μM affinity whilst the protease domain of NS3 gave much weaker interactions. We then took advantage of the possibility of expressing subdomains of the helicase region of NS3 spanning its subdomains 1, 2 and 2 + 3 respectively. Surface plasmon resonance demonstrated that subdomains 2 and 3 of the NS3 helicase region had the highest affinity for NS4B. Remarkably, we found that the predicted cytoplasmic loop of NS4B could be expressed in E. coli as a soluble protein. This recombinant protein is flexible, with a tendency to form a three-turn α-helix and two short beta-strands. The cytoplasmic loop of NS4B was found to be required for binding NS3, giving support to the topology model that places this segment of the protein in the cytoplasm, and therefore accessible for interaction with other members of the flavivirus RC such as NS3 and NS5. A weak interaction in the high micromolar range was shown between peptides spanning the NS4B cytoplasmic loop and the NS3 helicase domain. Using nuclear magnetic resonance (NMR), we found that upon binding to the NS3 helicase, 12 amino acids within the cytoplasmic loop of NS4B exhibited line broadening, suggesting a participation in the interaction with NS3. Sequence alignment showed that four of these twelve residues are strictly conserved across different flaviviruses. Individual mutation of these residues in the context of a DENV infectious clone showed that three residues (Q134, G140, and N144) of the four evolutionarily conserved NS4B residues are essential for DENV replication. Interestingly, these results are in agreement with a genetic complementation studies of NS4B using a replication independent expression system: Q134 from the cytosolic loop was found to be a critical determinant for NS4B-NS3 interaction and an Alanine substitution at this site completely abrogated the interaction between the two proteins and remarkably also DENV RNA replication [6]. Taken together, these results highlight the importance of some key protein-protein interactions within the RC for virus replication. In this respect, molecular interactions between both NS2B-NS3 with NS4B and between NS2B-NS3 and NS5 (via K330 from the NS5 polymerase domain) appear to play a crucial role for replication, despite a minimal impact on the individual enzymatic activities [80]. Thus, the mapping of the NS3/NS4B-interacting regions described in these two papers [6, 82] could be exploited for a compound-screening assay with the hope to design inhibitors that disrupt protein-protein interfaces for antiviral therapy [75].
9.6 RNA Recognition by the Methyltransferase Domain of NS5
Capping of the DENV RNA genome is an essential structural modification that protects the viral RNA from degradation by 5′ exoribonucleases. This modification ensures efficient expression of viral proteins and by disguising the viral RNA as a host cell mRNA, this “Trojan horse” strategy allows escape from the host innate immune response [12]. The flavivirus nonstructural protein 5 (NS5) (Mr = 105 kDa) possesses two RNA methyltransferase activities at its N-terminal region [35]. These activities are important for capping the virus RNA genome. The methyl-transfer reactions are thought to occur sequentially using the strictly conserved flavivirus 5′ RNA sequence starting with “5’AG” as substrate (GpppAG-RNA), leading to the formation of the 5′ RNA cap: G0pppAG RNA → m7G0pppAG-RNA (“cap-0”) → m7G0pppAm2′-O-G-RNA (“cap-1”) [78, 79].
Using X-ray crystallography, we elucidated how viral RNA is specifically recognized and methylated by determining the crystal structure of a ternary complex comprising the full-length NS5 protein from DENV, an 8-mer cap-0 viral RNA substrate bearing the authentic DENV genomic sequence (5′-m7G0pppA1G2U3U4G5U6U7–3′), and S-adenosyl- L-homocysteine (SAH), the by-product of the methylation reaction [9, 78, 79]. Interestingly, many attempts by several groups including ours using the isolated recombinant methyltransferase domain of NS5 were unsuccessful in providing a complex relevant to methylation. Only when using the full-length NS5 protein could a relevant complex with RNA be obtained. This suggests important differences in the energetic of RNA binding between the full-length proteins and the isolated individual domains. The structure provided for the first time a molecular basis for specific adenosine 2′-O-methylation in the flavivirus family. It also explained in molecular terms a wealth of mutagenesis studies targeting the K61-D146-K180-E216 enzymatic tetrad used for 2’O methylation, as well as residues lining the RNA binding groove. Remarkably, the RNA substrate was found to be positioned such that the 2′-O atom of residue A1 lies next to the sulfur atom of SAH and adjacent to the K180 side chain from the “K61-D146-K180-E216” enzymatic motif, poised to accept a methyl group from a SAM methyl donor. Basically, the crystal structure explains the specific recognition of the flavivirus RNA 5′ cap by NS5 and the strict requirement for an adenosine at position 1 and the preference for a guanosine at position 2 for steric reasons.
9.7 The Full-Length NS5 Protein
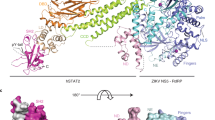
NS5 is the largest protein component within the flavivirus RC. NS5 plays key enzymatic roles through its N-terminal methyltransferase (MTase) and C-terminal RNA-dependent-RNA polymerase (RdRp) domains, and these two enzymatic activities constitute major targets for the design of antivirals. Crystal structures of the full length NS5 from JEV [39] and from DENV3 [78, 79] have been determined using X-ray crystallography. Moreover, small angle X-ray scattering (SAXS) solution studies have complemented these crystallographic studies [5, 7, 57]. We determined a crystal structure of the full-length NS5 protein from Dengue virus serotype 3 (DENV3) at a resolution of 2.3 Å in the presence of bound SAH and GTP [78, 79]. Although the overall molecular shape of NS5 from DENV3 resembles that of NS5 from Japanese Encephalitis Virus (JEV), the relative orientation between the MTase and RdRp domains differs between the two structures (Fig. 9.4). This observation (which was confirmed by another group who crystallized the DENV NS5 protein in a different crystal form where the protein forms crystallographic dimers, but where NS5 displays the same interdomain arrangement [25]) can be interpreted in essentially two mutually non exclusive ways: (i) it could suggest the existence of a set of discrete stable molecular conformations that may be required for NS5 function and crystallization has trapped one of these discrete possible conformations. (ii) it could also suggest that the full-length NS5 proteins from various flaviviruses have diverged during evolution such that their interdomain interfaces are not identical, leading to a variety of “cross-talks” or allosteric interactions between their two enzymatic domains (Fig. 9.4). While the inter-domain region is mostly disordered in NS5 from JEV, the NS5 structure from DENV3 reveals a well-ordered linker region comprising a short 310 helix that may act as a swivel. Solution Hydrogen/Deuterium Exchange Mass Spectrometry (HDX-MS) analysis revealed an increased mobility of the thumb subdomain of RdRp in the context of the full length NS5 protein. This observation correlated well with the analysis of the crystallographic temperature factors of DENV3 NS5. Site-directed mutagenesis targeting the mostly polar interface between the MTase and RdRp domains of DENV3 NS5 led to the identification of several evolutionarily conserved residues that are important for viral replication. This suggested that inter-domain cross-talk in NS5 regulates virus replication. A possible evolutionary pathway for the full length NS5 protein is summarized in Fig. 9.4: the MTase domain and RdRp domain probably originally existed as two separate smaller proteins. These two proto-domains became linked together to form the NS5 protein from an ancestral Flavivirus through gene fusion. This fusion promoted co-localization of both enzymatic activities and increased the effective concentration of the proteins with respect to each other (middle panel of Fig. 9.4). Following further divergent evolution, NS5 acquired different adaptive mutations giving rise to the full length NS5 protein structures now observed for various viruses such as DENV, JEV and also ZIKV (see PDB code) (Fig. 9.4). Thus NS5 proteins from DENV on one hand, and JEV and ZIKV on the other hand, appear to have different conformations and different allosteric mechanisms, in which the MTase and RdRp domain cross-talk to each other through unique interfaces specific to either DENV or JEV/ZIKV. Remarkably, a phylogenetic analysis of various flaviviruses correlates with the evolutionary hypothesis presented in this section: the four DENV serotypes cluster in a separate branch, whilst JEV, WNV and ZIKV originate from the same node suggesting a common NS5 full length structure for these three viruses.
A model for the divergent evolution of Flaviviridae and the flavivirus full length NS5 protein.
Methyltransferase (MTase) domain of NS5 is in yellow, the polymerase (RdRp) fingers in green, palm in blue, thumb in salmon. The linker region 310 helix (residues 263–266) between the two domains is in orange. Active sites for MTase and RdRp are labelled with dotted tetragon and pentagon respectively. Linker residues and interface residues are labeled. (see text) Modified from reference 66
9.8 A “Cartoon Model” of the Interactions between NS5, NS2B-NS3 and NS4B
The NS3 protease-helicase plays a central role in viral replication by interacting both with the integral membrane protein NS4B and with NS5, a soluble protein consisting in two domains. Moreover, both NS2B-NS3 and NS5 interact with RNA. Interestingly, the RNA triphosphatase activity of NS3 implies that NS3 must interact with the 5′ end of the RNA genome before AND after RNA synthesis. This conundrum might be resolved by the presence of cyclization sequences identified in the genome. The interaction between NS3 and NS5 was mapped to the NLS region of the NS5 protein with residue K330 crucial for the interaction and to the helicase domain of NS3 [65]. We summarize in Fig. 9.5 what we know about these interactions. Disrupting either NS3–NS5, NS3–NS4B or NS3–NS2B interactions constitute possible approaches to identify compounds with antiviral activity.
The current model for NS2B-NS3-NS4B interactions and RNA + strand synthesis
Cartoon model of the membrane-bound NS2B–NS3 protein and its interactions with NS4B and NS5. NS3pro is colored in brown, NS3hel in green, cyan (RecA like subdomains 1 and 2, and ssRNA is depicted as a black line. NS5 and NS4B, are drawn across the ER membrane and their intermolecular interfaces are depicted schematically with the subdomain and residues involved in the interactions labeled
9.9 Conclusions
Work by several groups over more than 20 years have led to our current understanding of RNA replication by flaviviruses. While a vaccine against DENV has already reached the market, there is a consensus that this vaccine can be improved in terms of safety and the protection it provides to large populations affected by DENV. Other vaccines from Takeda and other manufacturers are also making their way to the market. Immunotherapeutic strategies have also been proposed [55] but it remains to be seen how practical these could be given the relatively small window available for intervention and the associated cost inherent to such strategies. Likewise, it also remains to be seen how effective will antiviral strategies be to treat such acute diseases. Target-based strategies have produced promising compounds against the NS5 polymerase domain that in our opinion should be developed further [38, 48]. Unfortunately, the financial investment to bring these compounds to the market is very high and is testing the patience of funding agencies, government bodies and private pharmas alike, as shown recently by the closure of the Novartis Institute for Tropical Diseases in Singapore, who has pioneered this approach against DENV. In this respect it would be interesting to compare the funding invested in DENV research compared to the cost associated with the development of efficient drugs to treat HCV like sofosbuvir [59]. Aside from practical vaccinology and antiviral drug discovery aspects, one fascinating aspect of flaviviruses is their ability to down-regulate the innate immune response of the host [2, 11, 16] with only a very limited set of proteins, providing opportunities to dissect the set of interactions between viral and host immune proteins in more details. In this respect the many interactions established by NS5 including with the importin/exportin system of the host cell appears also worth to study [62] (see Chapter by Subhash G. Vasudevan and colleagues), keeping in mind the existence of divergent groups that is started to be revealed between DENV on one hand and ZIKV, WNV, JEV on the other hand (Fig. 9.4). A further challenge remaining is to provide a more complete description of the interactome underlying the formation and functioning of the RC within membrane bilayers, a challenging “multi-scale” task that is likely to use a combination of structural and biochemical techniques ranging from Cryo-EM tomography to nano-discs to mass spectrometry aided by replicon and infectious clones systems [10, 54, 66, 71].
References
Akey DL et al (2014) Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 343:881–885
Ashour J, Laurent-Rolle M, Shi PY, García-Sastre A (2009) NS5 of dengue virus mediates STAT2 binding and degradation. J Virol 83(11):5408–5418
Belov GA, van Kuppeveld FJM (2012) (+) RNA viruses rewire cellular pathways to build replication organelles. Current Opin Virol 2:740–747
Bressanelli S, Stiasny K, Allison S, Stura EA, Duquerroy S, Lescar J, Heinz FX, Rey FA (2004) Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J 23:728–738
Bussetta C, Choi KH (2012) Dengue virus nonstructural protein 5 adopts multiple conformations in solution. Biochemistry 51:5921–5931
Chatel-Chaix L, Fischl W, Scaturro P, Cortese M, Kallis S, Bartenschlager M, Fischer B, Bartenschlager R (2015) A combined genetic-proteomic approach identifies residues within dengue virus NS4B critical for interaction with NS3 and viral replication. J Virol 89(14):7170–7186
Choi KH, Morais M (2016) Use of small-angle X-ray scattering to investigate the structure and function of dengue virus NS3 and NS5. Methods Mol Biol 1138:241–252
Choksupmanee O, Hodge K, Katzenmeier G, Chimnaronk S (2012) Structural platform for the autolytic activity of an intact NS2B-NS3 protease complex from dengue virus. Biochemistry 51:2840–2851
Chung KY, Dong H, Chao A, Shi P-Y, Lescar J, Lim SP (2010) Higher catalytic efficiency of N7 methylation is responsible for processive N7 and 2’-O methyltranferase activity in dengue virus. Virology 402:52–60
Cyrklaff M et al (2013) Cryo-electron tomography of vaccinia virus. PNAS 102:2772–2777
Dalrymple NA, Cimica V, Mackow ER (2015) Dengue virus NS proteins inhibit RIG-I/MAVS signaling by blocking TBK1/IRF3 phosphorylation: dengue virus serotype 1 NS4A is a unique interferon-regulating virulence determinant. MBio 6(2015 May):e00553
Decroly E, Ferron F, Lescar J, Canard B (2012) Conventional and unconventional mechanisms for capping of virus mRNA. Nat Rev Microbiol 10:51–65
Den Boon JA, Ahlquist P (2010) Organelle-like membrane compartmentalization of positive strand RNA virus replication factories. Ann Rev Microbiol 64:241–256
Fang G et al (2016) A novel benzodiazepine compound inhibits yellow fever virus infection by specifically targeting NS4B protein. J Virol 90(23):10774–10788
Ferguson NM, Rodríguez-Barraquer I, Dorigatti I, Mier-Y-Teran-Romero L, Laydon DJ, Cummings DA (2016) Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science 353(6303):1033–1036
Fernandez-Garcia M-D, Mazzon M, Jacobs M, Amara A (2009) Pathogenesis of flaviviruses: using and abusing the host cell. Cell Host Microbe 5:318–328
Fischl W & Bartenschlager R (2011) Exploitation of cellular pathways by Dengue virus 14, 470–475
Grant D, Tan GK, Qing M, Ng JKW, Yip A, Zou G, Xie X, Yuan Z, Schreiber MJ, Schul W, Shi P-Y, Alonso S (2011) A single amino-acid in non-structural protein NS4B confers virulence to dengue virus in AG129 mice though enhancement of viral synthesis. J Virol 85:775–7787
Heaton NS, Perera R, Berger KL, Khadka S, Lacount DJ, Kuhn RJ, Randall G (2010) Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases fatty acid synthesis. PNAS 107:17345–17350
Hino T, Iwata S, Murata T. (2013) Generation of functional antibodies for mammalian membrane protein crystallography. Curr Opin Struct Biol. Aug;23(4):563–8.
Huang Q, Li Q, Joy J, Chen AS, Ruiz-Carrillo D, Hill J, Lescar J, Kang C (2013) Lyso-myristoyl phosphatidylcholine micelles sustain the activity of dengue non structural protein 3 protease domain fused with the full-length NS2B. Protein Expr Purif 92:156–162
Kelley JF, Kaufusi PH, Volper EM, Nerurkar VR (2011) Mutation of dengue virus NS4B in monocytes enhances production of haemorrhagic fever associated chemokines and cytokines. Virology 418:27–39
Khadka S et al (2011) A Physical Interaction Network of Dengue Virus and Human Proteins. Molecular & Cellular Proteomics 10 (12) 1–16 https://doi.org/10.1074/mcp.M111.012187-1
Kim AR, Dobransky T, Rylett RJ, Shilton BH. (2005) Surface-entropy reduction used in the crystallization of human choline acetyltransferase. Acta Crystallogr D Biol Crystallogr. Sep;61(Pt 9):1306–10
Klema VJ, Ye M, Hindupur A, Teramoto T, Gottipati K, Padmanabhan R, Choi KH (2015) Dengue virus nonstructural protein 5 (NS5) assembles into a dimer with a unique methyltransferase and polymerase interface. Plos Pathogens 12(2):e1005451. https://doi.org/10.1371/journal.ppat.1005451
Kuhn et al (2002) Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717–725
Kummerer BM, Rice CM (2002) Mutations in the yellow fever virus non-structural protein NS2A selectively block production of infectious particles. J Virol 76:4773–4784
Lescar J, Canard B (2009) RNA-dependent RNA polymerases from flaviviruses and picornaviridae. Curr Opin in Struct Biol 19:759–767
Lescar J, Luo D, Xu T, Lim SP, Canard B, Vasudevan SG (2008) Towards the design of antiviral inhibitors against flaviviruses: the case for the multifunctional NS3 protein from dengue virus as a target. Antivir Res 80:94–101
Leung JY, Piljman GP, Kondratieva N, Hyde J, Mackenzie JM, Khromykh AA (2008) Role of non structural protein NS2A in flavivirus assembly. J Virol 82:4731–4474
Li Y, Li Q, Wong YL, Liew LS, Kang C (2015) Membrane topology of NS2B of dengue virus revealed by NMR spectroscopy. Biochim Biophys Acta 1848(10 Pt A):2244–2252. https://doi.org/10.1016/j.bbamem.2015.06.010
Li Y, Kim YM, Zou J, Wang QY, Shovanlal Gayen S, Wong YL, Lee LT, Xie X, Huang Q, Lescar J, Shi PY, Kang C (2015) Secondary structure and membrane topology of dengue virus NS4B N-terminal 125 amino acids. Biochim Biophys Acta 1848(12):3150–3157
Li Y, Wong YL, Lee MY, Li Q, Wang QY, Lescar J, Shi PY, Kang CB (2016) Secondary structure and membrane topology of the full length dengue NS4B in micelles. Angew Chem Int Ed 55:12068–12072
Li XD, Deng CL, Ye HQ, Zhang HL, Zhang QY, Chen DD, Zhang PT, Shi PY, Yuan ZM, Zhang B (2016) Transmembrane domains of NS2B contribute to both viral RNA replication and particle formation in Japanese encephalitis virus. J Virol 90(12):5735–5749. https://doi.org/10.1128/JVI.00340-16
Lim SP, Wen D, Yap TL, Yan CK, Lescar J, Vasudevan SG (2008) A scintillation proximity assay for dengue virus NS5 2’O-methyltransferase: kinetic and inhibition analysis. Antivir Res 80:360–369
Lim SP, Wang Q-Y, Noble CG, Chen Y-L, Dong H, Zou B, Yokokawa F, Nilar S, Smith P, Beer D, Lescar J, Shi P-Y (2013) Ten years of dengue drug discovery: progress and prospects. Antivir Res 100:500–519
Lim SP, Hong J, Seh CC, Liew CW, Davidson AD, Chua LS, Chandrasekaran R, Cornvik T, Shi P-Y, Lescar J (2013) A crystal structure of the dengue virus NS5 polymerase delineates inter-domain amino acids residues that enhance its thermostability and de novo initiation activities. J Biol Chem 288:31105–31114
Lim SP, Noble CG, Seh CC, Soh TS, El Sahili A, Chan GK, Lescar J, Arora R, Benson T, Nilar S Manjunatha U, Wan KF, Dong H, Xie X, Shi PY, Yokokawa F. (2016) Potent allosteric dengue virus NS5 polymerase inhibitors: mechanism of action and resistance profiling. PLoS Pathog. 12(8):e1005737. doi: https://doi.org/10.1371/journal.ppat.1005737.
Lu G, Gong P (2013) Crystal structure of the full-length Japanese encephalitis virus NS5 reveals a conserved methyltransferase-polymerase interface. PLoS Pathog 9(8):e1003549
Luo D, Xu T, Hunke C, Gruber G, Vasudevan SG, Lescar J (2008) Crystal structure of the NS3 protease-helicase from dengue virus. J Virol 82:173–183
Luo D, Xu T, Watson RP, Scherer-Becker D, Sampath A, Jahnke W, Yeong SS, Wang CH, Lim SP, Strongin A, Vasudevan SG, Lescar J (2008) Insights into RNA unwinding and ATP hydrolysis by the flavivirus NS3 protein. EMBO J 27:3209–3219
Luo D, Wei N, Doan D, Paradkar P, Chong Y, Davidson A, Kotaka M, Lescar J, Vasudevan SG (2010) Flexibility between the protease and helicase domains of the dengue virus NS3 protein conferred by the linker region and its functional implications. J Biol Chem 285:18817–18827
Malet H, Massé N, Selisko B, Romette J-L, Alvarez K, Guillemot J-C, Tolou H, Yap TL, Vasudevan SG, Lescar J, Canard B (2008) The flavivirus polymerase as a target for drug discovery. Antivir Res 80:23–35
Miller S, Krinjse-Locker J (2008) Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol 6:363–374
Miller S, Sparacio S, Bartenschlager R (2006) Subcellular localization and membrane topology of the dengue virus type 2 non-structural protein 4B. J Biol Chem 281(13):8854–8863
Miorin L, Romero-Brey I, Maiuri P, Hoppe S, Krinjse-Locker J, Bartenschlager R, Marcello A (2013) Three-dimensional architecture of tick-borne encephalitis virus replication sites and trafficking of the replicated RNA. J Virol 87:6469–6481
Munoz Jordan JL, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin WI, A G-S (2005) Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol 79:8004–8013
Noble CG, Lim SP, Chen Y-L, Liew CW, Yap L, Lescar J, Shi P-Y (2013) Conformational flexibility of the dengue virus RNA-dependent RNA polymerase revealed by a complex with an inhibitor. J Virol 87:5291–5295
Paul D, Bartenschlager R (2013) Architecture and biogenesis of plus-strand RNA virus replication factories. World J Virol 2(2):32–48
Perera R, Kuhn RJ (2008) Structural proteomics of dengue virus. Curr Opin Microbiol 11(4):369–377. https://doi.org/10.1016/j.mib.2008.06.004. Epub 2008 Jul 31. Review. PMID:18644250
Phoo WW, Li Y, Zhang Z, Lee MY, Loh YR, Tan YB, Ng EY, Lescar J, Kang CB, Luo D (2016) Structure of the NS2B-NS3 protease form Zika virus caught after self- cleavage. Nat Commun 7:13410. https://doi.org/10.1038/ncomms13410
Puig-Basagoiti F, Tilgner M, Bennett CJ, Zhou Y, Munoz-Jordan JL, Garcia-Sastre A, Bernard KA, Shi P-Y (2007) A mouse cell adapted NS4B mutation attenuates West-Nile virus RNA synthesis. Virology 361:229–241
Quinkert D, Bartenschlager R, Lohmann V (2005) Quantitative analysis of the hepatitis C virus replication complex. J Virol 79:13594–13605
Ritchie TK, Grinkova YV, Bayburt TH, Denisov IG, Zolnerciks JK, Atkins WM, Sligar SG (2009) Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol 464:211–231
Robinson L, Tharakaraman K, Rowley K, Costa VV, Chan KR, Wong YH, Ong LC, Tan HC, Koch T, Cain D, Kirloskar R, Viswanathan K, Liew CW, Tissire H, Ramakrishnan B, Myette J, Babcock GJ, Sasisekharan V, Alonso S, Chen J, Lescar J, Shriver Z, Ooi EE, Sasisekharan R (2015) Structure-guided design of an anti-dengue antibody directed to a non-immunodominant epitope. Cell 162:493–504
Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J (2012) Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomized, controlled phase 2b trial. Lancet 380(9853):1559–1567
Saw WG, Tria G, Grüber A, Subramanian Manimekalai MS, Zhao Y, Chandramohan A, Srinivasan Anand G, Matsui T, Weiss TM, Vasudevan SG, Grüber G (2015) Structural insight and flexible features of NS5 proteins from all four serotypes of dengue virus in solution. Acta Crystallogr D Biol Crystallogr 71(Pt 11):2309–2327
Sessions OM et al (2009) Discovery of insect and human dengue virus host factors. Nature 458:1047–1050
Sofia MJ (2016) Enter Sofosbuvir: the path to curing HCV. Cell 167(1):25–29
Stern O, Hung YF, Valdau O, Yaffe Y, Harris E, Hoffmann S, Willbold D, Sklan EH (2013) An N-terminal amphipathic helix in dengue virus nonstructural protein 4A mediates oligomerization and is essential for replication. J Virol 87:4080–4085
Tay M, Saw WG, Zhao YQ, Chan WK, Singh D, Chong Y, Forwood JK, Ooi EE, Gruber G, Lescar J, Luo D, Vasudevan SG (2015) The C-terminal 50 amino acid residues of dengue NS3 protein are important for NS3-NS5 interaction and viral replication. J Biol Chem 290:2379–2394
Tay MYF, Smith K, Ng IHW, Chan KWK, Zhao Y, Lescar J, Luo D, Jans DA, Forwood JK, Vasudevan SG (2016) The C-terminal 18 amino acid region of dengue virus NS5 regulates its subcellular localization and contains a conserved arginine residue essential for Infectious Virus Production. PLoS Pathog 12(9):e1005886. https://doi.org/10.1371/journal.ppat.1005886
Theiler M, Smith HH (1937) The use of yellow fever virus modified by in vitro cultivation for human immunization. J Exp Med 65:787–800
Uchil PD, Satchidanandam V (2003) Architecture of the flaviviral replication complex. J Biol Chem 278:24388–24398
Umareddy I, Chao A, Sampath A, Gu F, Vasudevan SG (2006) Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. J Gen Virol 87(Pt 9):2605–2614
Waltzthoeni T, Leitner A, Stengel F, Aebersold R (2013) Mass spectrometry supported determination of protein complex structure. Current Opin Struct Biol 23:252–260
Wang QY, Dong H, Zou B, Karuna R, Wan KF, Zou J, Susila A, Xu H, Ding M, Chan WL, Gu F, Seah PG, Liu W, Lakshminarayana SB, Kang C, Lescar J, Blasco F, Smith P, Shi P-Y (2015) Discovery of dengue virus NS4B inhibitors. J Virol 89:8233–8244
Welsch S et al (2009) Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host & Microbes 5:365–375
Wicker J, Whiteman MC, Beasley DWC, Davis CT, Zhang S, Schneider BS, Higgs S, Kinney RM, Barrett ADT (2006) A single amino-acid substitution in the central portion of the West-Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology 349:245–253
Wicker JA, Whiteman MC, Beasley DWC, Davis CT, McGee CE, Lee C-JC, Higgs S, Kinney RM, CYH H, Barrett ADT (2012) Mutational analysis of the West-Nile virus NS4B protein. Virology 426:22–33
Xia Y, Yu H, Jansen R, Seringhaus M, Baxter S, Greenbaum D, Zhao H, Gerstein M (2004) Analyzing cellular biochemistry in terms of molecular networks. Annu Rev Biochem 73:1051–1087
Xie X, Wang QY, Xu HY, Qing M, Kramer L, Yuan Z, Shi PY (2011) Inhibition of dengue virus by targeting viral NS4B protein. J Virol 85(21):11183–11195
Xie X, Gayen S, Kang C, Yuan Z, Shi PY (2013) Membrane topology and function of dengue virus NS2A protein. J Virol 87(8):4609–4622
Xie X, Zou J, Wang Q-Y, Noble CG, Lescar J, Shi P-Y (2014) Generation and characterization of two mouse monoclonal antibodies against NS4B protein of dengue virus. Virology 450–451:250–257
Xie X, Zou J, Wang QY, Shi PY (2015) Targeting dengue virus NS4B protein for drug discovery. Antivir Res 118:39–45. https://doi.org/10.1016/j.antiviral.2015.03.007
Yap TL, Xu T, Chen YL, Malet H, Egloff M-P, Canard B, Vasudevan SG, Lescar J (2007) The crystal structure of the dengue virus RNA-dependent RNA polymerase at 1.85 Å resolution. J Virol 81:4753–4765
Youn S, McCune BT, Edeling MA, Fremont DH, Cristea IM, Diamond MS (2012) Evidence for a genetic and physical interaction between nonstructural proteins NS1 and NS4B that modulate replication of West Nile virus. J Virol 86:7360–7371
Zhao Y, Soh S, Chung KY, Lim SP, Swaminathan K, Vasudevan SG, Shi PY, Lescar J, Luo D (2015) Molecular basis for viral RNA recognition and 2’O ribose methylation by the dengue virus NS5 protein. Proc Natl Acad Sci U S A 112(48):14834–14839
Zhao Y, Soh S, Zheng J, Chan KWK, Phoo WW, Swaminathan K, Cornvik TC, Lim SP, Shi PY, Lescar J, Vasudevan SG, Luo D (2015) A crystal structure of the dengue virus NS5 protein reveals a novel inter-domain interface essential for protein flexibility and virus replication. PLoS Pathog 11(3):e1004682. https://doi.org/10.1371/journal.ppat.1004682
Zou G, Chen Y-L, Dong H, Lim CC, Yap LJ, Yau YH, Geifman Shochat S, Lescar J, Shi P-Y (2011) Functional analysis of two cavities in flavivirus NS5 polymerase. J Biol Chem 286:14362–14372
Zou J, Xie X, Lee LT, Chandrasekaran R, Reynaud A, Yap L, Wang Q-Y, Dong HP, Kang C, Yuan Z, Lescar J, Shi P-Y (2014) Dimerization of flavivirus NS4B protein. J Virol 88:3379–3391
Zou J, Lee LT, Wang QY, Xie X, Lu S, Yau YH, Yuan Z, Geifman Shochat S, Kang C, Lescar J, Shi P-Y (2015) Mapping the interactions between the NS4B and NS3 proteins of dengue virus. J Virol 89:3471–3483
Acknowledgements
This work was supported by grant NRF2016NRF-CRP001-063 to the laboratory of JL. We acknowledge the contributions of many coworkers (some now PIs active in the field) whose names are listed in the references. We also apologize for omitting some relevant primary litterature due to space limitations.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lescar, J., Soh, S., Lee, L.T., Vasudevan, S.G., Kang, C., Lim, S.P. (2018). The Dengue Virus Replication Complex: From RNA Replication to Protein-Protein Interactions to Evasion of Innate Immunity. In: Hilgenfeld, R., Vasudevan, S. (eds) Dengue and Zika: Control and Antiviral Treatment Strategies. Advances in Experimental Medicine and Biology, vol 1062. Springer, Singapore. https://doi.org/10.1007/978-981-10-8727-1_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-8727-1_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8726-4
Online ISBN: 978-981-10-8727-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)