Abstract
Dengue viruses (DENV) are mosquito-borne positive sense RNA viruses in the family Flaviviridae. The four serotypes of DENV (DENV1, DENV2, DENV3, DENV4) are widely distributed and it is estimated over a third of the world’s population is at risk of infection [4]. While the majority of infections are asymptomatic, DENV infection can cause a spectrum of disease, from mild flu-like symptoms, to the more severe DENV hemorrhagic fever and shock syndrome [24]. Over the past 20 years, there have been intense efforts to develop a tetravalent live-attenuated DENV vaccine [36]. The process of vaccine development has been largely empirical, because effective live attenuated vaccines have been developed for other flaviviruses like yellow fever and Japanese encephalitis viruses. However, recent results from phase III live attenuated DENV vaccine efficacy trials are mixed with evidence for efficacy in some populations but not others [20]. In light of unexpected results from DENV vaccine trials, in this chapter we will review recent discoveries about the human antibody response to natural DENV infection and discuss the relevance of this work to understanding vaccine performance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 DENV Structure
The DENV genome encodes a single open reading frame that is translated into a polyprotein. Viral and host proteases cleave the polyprotein into three structural and seven non-structural viral proteins. The structural envelope protein (E) contains three domains, domain I (EDI), domain II (EDII) and domain III (EDIII) [45]. Two envelope monomers come together in a head-to-tail orientation, forming the E dimer (Fig. 5.1). Three E dimers form the dimer raft, and 30 dimer rafts cover the surface of the DENV virion in icosahedral orientation with both threefold and fivefold axes of symmetry. Domain II contains the hydrophobic fusion peptide, which mediates fusion between the virus and host cell membrane. To prevent fusion with the host membrane during egress from infected cells, the pre-membrane (prM) protein covers the fusion loop. As the virus moves through the endosome, pH changes triggers the host protease furin to cleave the prM protein [60]. As the virus is released from cells, cleaved prM dissociates from the virion. This process is inefficient however, leaving a heterogeneous population of fully mature (no prM present), fully immature (containing prM), and partially mature virions [59]. While cell culture grown virus shows a spectrum of maturation states, it is now clear that the overall maturation state of virions can vary between strains and even between different preparations of the same strain [39]. As we discuss later, maturation state can influence the ability of some antibodies to bind and neutralize DENV and other flaviviruses.
5.2 Antibody Response to DENV Infection
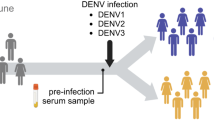
The basic kinetics of the DENV specific Ab response, the timing of IgM and IgG Ab development and the timing of neutralizing antibody (Nab) development have been well understood for many years [27, 46, 61]. In brief, individuals with no prior immunity to DENVs mount a primary antibody response that includes a specific IgM response followed by a durable IgG response. The initial IgG response contains different types of antibodies, including serotype cross-reactive neutralizing antibodies, serotype cross-reactive non-neutralizing antibodies, and serotype-specific neutralizing antibodies [6]. The serotype cross-reactive neutralizing antibodies may provide immediate protection to subsequent infection with any of the DENV serotypes, but these antibodies wane over the course of a year. DENV serotype-specific neutralizing antibodies and some cross-reactive poorly neutralizing antibodies are maintained for decades following infection and appear to protect against subsequent re-infection with the same serotype, but do not protect against the other serotypes (Fig. 5.2). Conversely, cross-reactive antibodies not only are non-protective, but can enhance subsequent infection via a mechanism known as antibody dependent enhancement (ADE) whereby non-neutralizing antibodies bind the virus and the antibody-virus complex is taken up by cells via FC-receptor mediated endocytosis [25]. Although ADE is poorly understood, the response is important in natural infection and vaccine development but will not be discussed in this review. Readers are recommended to refer to these earlier reviews for additional information on ADE and DENV [23, 25, 26].
Antibody response following DENV infection. Following primary DENV2 infection, there is an IgG response composed of neutralizing DENV2 serotype-specific antibodies, a transient population of cross-reactive neutralizing antibodies, and long-lived cross-reactive non-neutralizing antibodies. After a secondary infection, in this case with DENV3, the cross-reactive non-neutralizing antibodies become strongly neutralizing. It is also possible to generate a new population of neutralizing serotype-specific antibodies to the second infecting serotype
5.3 Methods to Study the Molecular Specificity of Human Antibodies to DENVs
A variety of techniques have been used to map the viral epitopes targeted by polyclonal antibodies in human immune sera and monoclonal antibodies (MAbs) isolated from dengue patients (Fig. 5.3). Traditionally, to study DENV-specific MAbs, peripheral blood B-cells from DENV immune donors are transformed and clones secreting DENV-reactive MAbs are fused with myeloma cell lines to generate human hybridomas producing the MAb of interest [34, 48]. Recent advances in single-cell-sequencing has allowed individual IgG heavy and light chains from the same cell to be sequenced, cloned and recombinantly expressed [44, 49]. The properties and specificity of these MAbs can then be determined using binding assays to recombinant DENV proteins (rE and rEDIII) and whole virions, and neutralization assays, as well as by solving high-resolution structures of the MAb bound to viral antigen. Once a putative MAb epitope has been identified, recombinant viruses with point mutations at the region of interest can be used to confirm and further refine the epitope. Importantly, these studies have revealed that most DENV neutralizing epitopes are created by presentation of discontinuous residues that are brought together in tertiary and quaternary structures. Additionally, our group has shown that the discontinuous residues that comprise these complex epitopes can be transplanted to a different serotype to generate chimeric DENVs that encode neutralizing epitopes from multiple DENV serotypes, and which can be used to map and confirm the binding and neutralization epitopes of individuals MAbs [17, 38].
Methods to dissect DENV antibody response. (a) Human DENV antibodies can be studied using a variety of approaches. PBMCs from a DENV immune donor can be EBV-transformed to generate MAb producing hybridomas, or antibody DNA sequences can be single-cell sequenced, cloned and recombinant expressed to generate MAbs. DENV polyclonal immune sera can be depleted of different populations of antibodies using beads coated with DENV antigens to determine the relative importance and neutralization capacity of these different populations. For example, a DENV2 immune sera containing polyclonal Abs (PAbs) can be depleted of all DENV cross-reactive antibodies by incubating with beads adsorbed with DENV1, DENV3, and DENV4 antigen, leaving only DENV2 serotype-specific antibodies remaining (heterotypic depletion). Conversely, all DENV antibodies can be depleted using DENV2 antigen (homotypic antigen). (b) To map the binding and neutralizing epitopes of these MAbs and PAbs, they can be evaluated for their ability to bind recombinant E domain III (rEDIII), recombinant E (rE), whole DENV, and chimeric viruses containing transplanted epitopes of multiple DENV serotypes (e.g. rDENV1/3 contains epitopes from both DENV1 and DENV3). These MAbs and PAbs can also be evaluated for their ability to neutralize these DENV and chimeric rDENV
Polyclonal sera contains a complex mixture of DENV-specific IgG antibodies, those that are neutralizing or non-neutralizing, and those that are specific to a serotype or cross-reactive to multiple serotypes (Fig. 5.2). Depletion assays can be used to determine the percentage of neutralizing serotype-specific antibodies to neutralizing cross-reactive antibodies (Fig. 5.3a). To remove cross-reactive Abs, primary infection sera can be incubated with beads coated with a heterologous serotype (e.g. a primary DENV2 sera can be incubated with DENV1/DENV3/DENV4-coated beads). Cross-reactive Abs will bind to the virus on the beads and be pelleted out, leaving only DENV2 serotype-specific Abs. Neutralization assays using depleted sera allow one to calculate the fraction of neutralization due to serotype-specific Abs, relative to the total neutralization coming from both serotype-specific and cross-reactive Abs [30, 41, 43]. These depletion techniques, in addition to use of epitope transplant chimeric rDENVs described above, has allowed us to study the amount of polyclonal antibodies targeting epitopes represented by individual MAbs [17, 41].
5.4 Molecular Specificity of Neutralizing MAbs from Primary Cases
The most striking feature of primary DENV infections is the rapid clearance of the virus and the maintenance of serotype-specific neutralizing Abs in the serum in most individuals for decades if not longer. Recent studies have only begun to define the molecular specificity of human B-cells and antibodies responsible for durable type-specific neutralization and protection. The envelope protein is the major antigenic protein and the majority of DENV-specific antibodies target E [45]. Traditionally human monoclonal antibodies (MAbs) have been screened based on their ability to bind recombinant envelope monomeric protein (rE). This has biased our study of MAbs to those that recognize epitopes contained within a single E protein. Several groups have recently used intact dengue virions as antigens in MAb screens [8, 11]. These studies have identified antibodies from each serotype that recognize unique conformations of the E monomer on the viral surface or quaternary structure epitopes that span different E proteins (dimers and rafts) on the viral envelope (Fig. 5.4). Additionally, it has been found that while antibodies using simple epitopes can be neutralizing, it is the antibodies recognizing complex epitopes that are ultimately responsible for polyclonal neutralization [9, 17, 57]. Antibodies recognizing quaternary epitopes are not unique to DENV; West Nile Virus (WNV) and Zika Virus (ZIKV) infection have also been shown to generate human MAbs recognizing similar complex epitopes [28, 31, 52, 58].
5.5 Differences in Neutralizing MAb Epitopes Across Serotypes
While the E protein is structurally similar between DENV serotypes (~80% conservation of amino acids), the location of type-specific epitopes targeted by human antibodies appear to be different between serotypes (Fig. 5.4). Unlike anti-DENV mouse MAbs that predominantly target EDIII [19, 53], many human MAbs recognize EDI, EDII, and the EDI/II hinge region. For example, DENV1 type-specific human MAbs 1F4 and 14C10 recognize epitopes centered on EDI [14, 54]. The 14C10 epitope includes amino acids on EDI and EDIII on the adjacent dimer. Interestingly, the DENV1 14C10 epitope is quite similar to an epitope on WNV recognized by human MAb CR4354 [31]. The DENV3 MAb 5J7 targets an epitope centered around the EDI/II hinge region and the footprint of this epitope includes amino acids from three different E molecules within a single raft [16]. Recent work has identified human DENV4 MAbs that target epitopes near the EDI/II hinge although further studies are required to precisely map the DENV4 epitopes [41]. Interestingly, DENV2 MAbs appear to use an epitope distinct to the EDI/EDII region, instead centering on EDIII [15, 17]. Our understanding of immunodominant epitopes for each serotype is informed by only a handful of monoclonal antibodies from a few immune individuals. To fully define the boundaries of the polyclonal neutralizing epitopes against each serotype, additional antibodies from more individuals will need to be studied.
5.6 Cryptic Epitopes
The majority of human epitopes studied are present on the surface of the intact virion. Some studies have identified mouse MAbs that target cryptic epitopes not readily accessible on the surface of the virus. However, at elevated temperature E proteins on the viral surface can flex/move and these cryptic epitopes are transiently displayed, allowing antibody binding and neutralization [13]. Recent studies suggest that there are antibodies present in human immune sera that also target these cryptic epitopes, potentially allowing the virus to be neutralized when it is under specific conditions exposing these epitopes [13]. Further studies are needed to evaluate the importance of cryptic epitopes in human antibody neutralization and protective immunity.
5.7 Other Flaviviruses – Zika Virus MAbs
With the emergence of Zika virus (ZIKV), approaches developed for DENV have been extended to isolate MAbs and map the human antibody response to ZIKV [28, 52, 58]. Multiple groups have generated human ZIKV MAbs. Similarly to DENV, the strongest neutralizing MAbs target quaternary epitopes only present on the intact ZIKV virion. These quaternary ZIKV epitopes are similar to previously identified quaternary DENV epitopes that are centered around the EDI/II hinge region, span across E monomers within the dimer, or span across dimers [28, 52, 58].
5.8 Mapping the Molecular Specificity of the Polyclonal Serum Neutralizing Antibody Response
While MAbs are isolated or generated from memory B-cells, circulating polyclonal antibodies come from plasma cells [33]. The memory B-cell derived human MAbs can be used as tools to interrogate the properties and specificity of the more complex polyclonal serum antibody response (Fig. 5.5). Work by multiple groups have shown that individual MAbs can be representative of the anti-DENV B-cell repertoire, polyclonal Abs from the same individual, and polyclonal Abs across other naturally infected and vaccinated individuals, confirming the importance of studying individual monoclonals [17, 22, 41]. Importantly, depletion assays have revealed that after primary DENV infections, the majority of polyclonal neutralization comes from serotype-specific antibodies, not cross-reactive ones [30, 41, 43]. Additionally, we have found that epitopes defined by individual MAbs that are complex and quaternary, are representative of the polyclonal epitopes targeted by neutralizing serotype-specific antibodies [9, 57]. With the rapid emergence of ZIKV, similar techniques as described above were applied to dissecting the antibody response to ZIKV infection. Multiple groups have found that strongly neutralizing ZIKV MAbs target complex quaternary epitopes [28, 52, 58]. Additional work using depletion assays, has identified that primary ZIKV infections can results in ZIKV specific Abs, despite populations of Abs that cross-neutralize DENV [5].
From MAbs to polyclonal serum Abs. Complex host generic diversity, exposure history, and immune differences can make it challenging to study DENV polyclonal antibody responses across a population. Studying DENV antibody immunity in a single individual can simplify these analyses, however there is still the polyclonal nature of the adaptive immune response. Conversely, we can characterize the properties of individual MAbs from DENV immune donors. Information learned from MAbs can then be used to inform study of the B-cell repertoire from that, and other donors. Additionally, it can be determined whether the individual MAbs represent the polyclonal antibodies in that donor, and in a larger DENV immune population
5.9 Molecular Specificity of Neutralizing Antibodies Following Secondary DENV Infection
Individuals experiencing secondary DENV infections with a new serotype develop a neutralizing and protective antibody response that is fundamentally different from a primary infection-induced response. People with known sequential infections with two different DENV serotypes have type-specific antibodies to serotypes of infection and a new population of durable serotype-cross neutralizing antibodies that are also effective against serotypes not encountered by the person [7]. Human cohort studies in dengue-endemic countries have also established that tertiary infections are nearly always mild or inapparent, implicating a protective role for these broadly cross-neutralizing antibodies that develop after a second DENV infection [42]. Figure 5.6 presents a model to explain the evolving antibody response following sequential DENV infections with different serotypes. The model is based on the premise that low affinity DENV cross-reactive memory B-cells derived from primary infections undergo antibody somatic hypermutation and each subsequent DENV exposure selects and expands rare affinity matured clones with greater neutralization breadth and potency [43]. The model is supported by recent studies demonstrating that serotype cross-reactive antibodies derived from secondary infections had stronger neutralization potencies and higher binding avidities than those derived from patients with primary infections [10, 37, 55, 56, 62].
Model of B-cell maturation following sequential DENV infections. With each successive DENV infection, the ratio of serotype-specific (TS) and cross-reactive (CR) antibodies that contribute to DENV neutralization changes. During a primary infection (DENV2 in this example), dengue-specific naïve B-cells are activated and these cells give rise to both memory B-cells (MBCs) and antibody secreting long lived plasma cells (LLPCs). This primary response is dominated MBC and LLPCs clones producing low affinity, weakly neutralizing serotype CR antibodies. The primary response also contains rare MBC and LLPCs producing TS antibodies that strongly neutralize DENV2. Following a secondary infection with a new serotype (DENV3 in this example), the overall DENV-specific B-cell response will be dominated by the activation and expansion of DENV2 and 3 cross-reactive MBCs induced by the primary infection. MBCs producing CR antibodies that bind to the second infecting serotype with high affinity will be preferentially activated. These activated cells will reenter germinal centers and undergo further rounds of somatic hypermutation. CR B-cells with high affinity for the second serotype will be selectively expanded to give rise to cross-reactive MBC and LLPCs that strongly cross-neutralize multiple serotypes. In Fig. 5.6., this increase in affinity and neutralization is depicted by an increase in the color gradient (light pink to bright pink) of CR B-cells. Following a tertiary infection (DENV4 in this example), this process is repeated again and results in a population of CR MBCs and LLPCs that dominate the neutralizing antibody response. While the B-cell clones producing TS strongly neutralizing antibodies are also likely to be maintained through each successive round of infection, the TS response will account for only a small fraction of the total neutralizing response
While we know a lot about epitopes targeted by DENV serotype-specific neutralizing and protective antibodies, less is known about the targets of durable serotype-cross neutralizing antibodies. Several cross-neutralizing human MAbs that bind to an epitope near the bc-loop on domain II of the E protein monomer have been recently described (Fig. 5.7) [50, 55]. Another class of serotype cross-reactive and strongly cross-neutralizing MAbs, which bind to quaternary epitopes on the E homodimer, was recently isolated from acute-phase plasmablasts in the peripheral blood of secondary DENV cases [11, 47]. These MAbs, which have been designated E dimer epitope (EDE) antibodies, bind to epitopes that span domains I or III of one monomer and domain II of the adjacent monomer (Fig. 5.7). It is unclear if the strongly cross-neutralizing MAbs isolated from acute-phase plasmablasts are maintained as MBCs and LLPCs and responsible for the durable cross-neutralizing antibodies observed in people after recovery from secondary infections. Additionally, there are still unknowns regarding if the order of infecting serotypes is important for the epitopes of strongly cross-neutralizing MAbs. The molecular mechanisms leading to the evolution of cross-neutralizing antibodies from the memory B-cell pool from a primary infection are also unclear.
EDE and other cross-reactive epitopes. Envelope dimer epitope 1 (EDE1) targets EDIII of one monomer and spans over the fusion loop region of EDII of the neighboring monomer. EDE2 uses a similar epitope, but is shifted to also expand into EDI of the first monomer. Another class of cross-reactive antibodies targets the highly conserved bc-loop region of EDII
5.10 NS1 and prM MAbs
While E is the major antigenic protein of DENV, antibodies are also generated targeting the viral proteins NS1 and prM. The host sees prM protein in several configurations. As mature viruses are released from infected cells, prM protein dissociates from the virus and is released as an antigen. Additionally, immature viruses have prM present on their surface, allowing the immune system to recognize them as part of the virus. prM antibodies are predominantly non-neutralizing enhancing antibodies; they allow non-infectious immature viruses to be taken up into cells via FC-receptor-mediated endocytosis [51].
DENV NS1 protein has many roles depending on its interactions and location [1, 40]. NS1 can exist as a monomer, dimer or hexamer, and is important in viral RNA replication, viral assembly and release, and immune evasion. NS1 is secreted from infected cells primarily as a hexamer, which can bind to endothelial cells, triggering hyperpermeability, suggesting a role in the vascular leakage seen in severe DENV disease [3]. Clinically, levels of circulating NS1 are correlated with disease severity [2, 35]. People infected with DENV make antibodies directed against NS1, but it is unclear if these are an important part of the protective immune response, or are merely a consequence of high levels of circulating viral antigen [40].
5.11 Mechanisms of Neutralization
MAbs can neutralize viruses through a variety of mechanisms. MAbs have been shown to neutralize DENV by blocking attachment to host cell receptors, binding directly to the fusion-loop, binding across E proteins preventing conformational changes required for fusion, as well as via opsonization. Anti-DENV MAbs have been shown to neutralize using many of these mechanisms [15, 16, 29, 47]. DENV maturation state (amount of prM present) and virus breathing are important factors for virus neutralization. A fully immature virus (i.e. 180 copies of prM present) is non-infectious, and therefore cannot be neutralized, but a partially mature virus, can still be infectious [12]. Alternatively, under certain temperature conditions, some DENV strains can undergo reversible conformational changes where the E proteins expand and contract analogous to “breathing”. These expansion and contraction changes can reveal or hide epitopes, limiting neutralization by antibodies recognizing these epitopes to specific conditions [13, 18, 32, 63]. While DENV maturation and “breathing” have been studied in cell culture systems, the importance of these phenomenon in natural infection, and therefore the potential impact on antibody neutralization, is not well understood.
5.12 Implications for Evaluating Antibodies to DENV Live Attenuated Vaccines (LAVs)
Recently we have learned important lessons from DENV tetravalent vaccine clinical trials. The leading tetravalent vaccine had variable efficacy depending on DENV serotype and vaccinated population [20]. The vaccine had higher efficacy in DENV-primed individuals compared to DENV naïve individuals who received the vaccine, establishing the impact of immunological memory on vaccine performance [21]. The population with the greatest need for a DENV vaccine is young children, the majority of whom will be DENV-naïve at vaccination. As discussed above, in people exposed to primary natural DENV infections, the neutralizing and protective antibody response is dominated by type-specific antibodies to quaternary epitopes. Therefore, in this population the success of tetravalent vaccination is likely to require balanced replication of the four vaccine viruses leading to type-specific antibodies that target quaternary epitopes in each serotype.
As discussed above, secondary DENV infections result in activation of memory B-cells and development and expansion of cross-reactive antibodies that broadly neutralize multiple DENV serotypes, driven by the sequential infection and robust replication of two different serotypes of DENV [43]. A similar mechanism is likely to be responsible for the superior performance of tetravalent LAVs in DENV-primed individuals. In a subject with pre-existing DENV-specific MBCs, even unbalanced replication of one or two vaccine components is likely to activate MBCs and expand somatically mutated higher-affinity cross-reactive clones with capacity to broadly neutralize multiple serotypes.
Immune correlates of protection and vaccine efficacy are urgently needed. For the leading DENV vaccine, the mere presence of in vitro neutralizing antibodies was not sufficient for protection because many individuals experienced breakthrough infections despite having neutralizing antibodies to the breakthrough serotype [21]. The lessons we have learned from natural infections studies about the molecular specificity of human antibodies to DENV infection may also lead to more robust correlates of vaccine efficacy than mere levels of total neutralizing antibodies [36]. Certainly, the reagents and tools are now available to interrogate vaccine responses in a manner similar to that we have described here for natural DENV infections.
References
Amorim JH, Alves RP, Boscardin SB, Ferreira LC (2014) The dengue virus non-structural 1 protein: risks and benefits. Virus Res 181:53–60
Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, Jairungsri A, Kanlaya R, Tangthawornchaikul N, Puttikhunt C, Pattanakitsakul SN, Yenchitsomanus PT, Mongkolsapaya J, Kasinrerk W, Sittisombut N, Husmann M, Blettner M, Vasanawathana S, Bhakdi S, Malasit P (2006) Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis 193:1078–1088
Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E (2015) Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 7:304ra141
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature 496:504–507
Collins MH, Mcgowan E, Jadi R, Young E, Lopez CA, Baric RS, Lazear HM, De Silva AM (2017) Lack of durable cross-neutralizing antibodies against Zika virus from dengue virus infection. Emerg Infect Dis 23:773–781
Coloma J, Harris E (2015) Broad and strong: the ultimate antibody to dengue virus. Nat Immunol 16:135–137
Corbett KS, Katzelnick L, Tissera H, Amerasinghe A, De Silva AD, De Silva AM (2015) Preexisting neutralizing antibody responses distinguish clinically inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J Infect Dis 211:590–599
De Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brien JD, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, De Silva AM (2011) In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis 5:e1188
De Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE Jr, De Silva AM (2012) Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 109:7439–7444
De Souza VA, Tateno AF, Oliveira RR, Domingues RB, Araujo ES, Kuster GW, Pannuti CS (2007) Sensitivity and specificity of three ELISA-based assays for discriminating primary from secondary acute dengue virus infection. J Clin Virol 39:230–233
Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NT, Duangchinda T, Grimes JM, Tsai WY, Lai CY, Wang WK, Malasit P, Farrar J, Simmons CP, Zhou ZH, Rey FA, Mongkolsapaya J, Screaton GR (2015) A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 16:170–177
Dowd KA, Mukherjee S, Kuhn RJ, Pierson TC (2014) Combined effects of the structural heterogeneity and dynamics of flaviviruses on antibody recognition. J Virol 88:11726–11737
Dowd KA, Demaso CR, Pierson TC (2015) Genotypic differences in dengue virus neutralization are explained by a single amino acid mutation that modulates virus breathing. MBio 6:e01559–e01515
Fibriansah G, Tan JL, Smith SA, De Alwis AR, Ng TS, Kostyuchenko VA, Ibarra KD, Wang JQ, Harris E, De Silva A, Crowe JE, Lok SM (2014) A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. Embo Molecular Medicine 6:358–371
Fibriansah G, Ibarra KD, Ng TS, Smith SA, Tan JL, Lim XN, Ooi JS, Kostyuchenko VA, Wang J, De Silva AM, Harris E, Crowe JE, Lok SM (2015a) DENGUE VIRUS. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 349:88–91
Fibriansah G, Tan JL, Smith SA, De Alwis R, Ng TS, Kostyuchenko VA, Jadi RS, Kukkaro P, De Silva AM, Crowe JE, Lok SM (2015b) A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat Commun 6:6341
Gallichotte EN, Widman DG, Yount BL, Wahala WM, Durbin A, Whitehead S, Sariol CA, Crowe JE, De Silva AM, Baric RS (2015) A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies. MBio 6:e01461–e01415
Goo L, Vanblargan LA, Dowd KA, Diamond MS, Pierson TC (2017) A single mutation in the envelope protein modulates flavivirus antigenicity, stability, and pathogenesis. PLoS Pathog 13:e1006178
Gromowski GD, Barrett AD (2007) Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology 366:349–360
Guy B, Jackson N (2016) Dengue vaccine: hypotheses to understand CYD-TDV-induced protection. Nat Rev Microbiol 14:45–54
Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, Tran HN, Bouckenooghe A, Chansinghakul D, Cortes M, Fanouillere K, Forrat R, Frago C, Gailhardou S, Jackson N, Noriega F, Plennevaux E, Wartel TA, Zambrano B, Saville M, CYD-TDV Dengue Vaccine Working Group (2015) Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 373:1195–1206
Hadjilaou A, Green AM, Coloma J, Harris E (2015) Single-cell analysis of B cell/antibody cross-reactivity using a novel multicolor FluoroSpot assay. J Immunol 195:3490–3496
Halstead SB (2003) Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60:421–467
Halstead SB (2007) Dengue. Lancet 370:1644–1652
Halstead SB (2014) Dengue antibody-dependent enhancement: knowns and unknowns. Microbiol Spectr 2
Halstead SB (2015) Pathogenesis of dengue: Dawn of a new era. F1000Res 4
Halstead SB, Rojanasuphot S, Sangkawibha N (1983) Original antigenic sin in dengue. Am J Trop Med Hyg 32:154–156
Hasan SS, Miller A, Sapparapu G, Fernandez E, Klose T, Long F, Fokine A, Porta JC, Jiang W, Diamond MS, Crowe JE Jr, Kuhn RJ, Rossmann MG (2017) A human antibody against Zika virus crosslinks the E protein to prevent infection. Nat Commun 8:14722
He RT, Innis BL, Nisalak A, Usawattanakul W, Wang S, Kalayanarooj S, Anderson R (1995) Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. J Med Virol 45:451–461
Henein S, Swanstrom J, Byers AM, Moser JM, Shaik SF, Bonaparte M, Jackson N, Guy B, Baric R, De Silva AM (2017) Dissecting antibodies induced by a chimeric yellow fever-dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in naive and dengue-exposed individuals. J Infect Dis 215:351–358
Kaufmann B, Vogt MR, Goudsmit J, Holdaway HA, Aksyuk AA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG (2010) Neutralization of West Nile virus by cross-linking of its surface proteins with fab fragments of the human monoclonal antibody CR4354. Proc Natl Acad Sci U S A 107:18950–18955
Kuhn RJ, Dowd KA, Beth Post C, Pierson TC (2015) Shake, rattle, and roll: impact of the dynamics of flavivirus particles on their interactions with the host. Virology 479–480:508–517
Kurosaki T, Kometani K, Ise W (2015) Memory B cells. Nat Rev Immunol 15:149–159
Kwakkenbos MJ, Diehl SA, Yasuda E, Bakker AQ, Van Geelen CM, Lukens MV, Van Bleek GM, Widjojoatmodjo MN, Bogers WM, Mei H, Radbruch A, Scheeren FA, Spits H, Beaumont T (2010) Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat Med 16:123–128
Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL (2002) High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 186:1165–1168
Liu Y, Liu J, Cheng G (2016) Vaccines and immunization strategies for dengue prevention. Emerg Microbes Infect 5:e77
Matheus S, Deparis X, Labeau B, Lelarge J, Morvan J, Dussart P (2005) Discrimination between primary and secondary dengue virus infection by an immunoglobulin G avidity test using a single acute-phase serum sample. J Clin Microbiol 43:2793–2797
Messer WB, Yount BL, Royal SR, De Alwis R, Widman DG, Smith SA, Crowe JE Jr, Pfaff JM, Kahle KM, Doranz BJ, Ibarra KD, Harris E, De Silva AM, Baric RS (2016) Functional transplant of a dengue virus serotype 3 (DENV3)-specific human monoclonal antibody epitope into DENV1. J Virol 90:5090–5097
Mukherjee S, Sirohi D, Dowd KA, Chen Z, Diamond MS, Kuhn RJ, Pierson TC (2016) Enhancing dengue virus maturation using a stable furin over-expressing cell line. Virology 497:33–40
Muller DA, Young PR (2013) The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antivir Res 98:192–208
Nivarthi UK, Kose N, Sapparapu G, Widman D, Gallichotte E, Pfaff JM, Doranz BJ, Weiskopf D, Sette A, Durbin AP, Whitehead SS, Baric R, Crowe JEJ, De Silva AM (2017) Mapping the human memory B cell and serum neutralizing antibody responses to dengue virus serotype 4 infection and vaccination. J Virol 91:e02041–e02016
Olkowski S, Forshey BM, Morrison AC, Rocha C, Vilcarromero S, Halsey ES, Kochel TJ, Scott TW, Stoddard ST (2013) Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis 208:1026–1033
Patel B, Longo P, Miley MJ, Montoya M, Harris E, De Silva AM (2017) Dissecting the human serum antibody response to secondary dengue virus infections. PLoS Negl Trop Dis 11:e0005554
Robinson WH (2015) Sequencing the functional antibody repertoire—diagnostic and therapeutic discovery. Nat Rev Rheumatol 11:171–182
Roehrig JT (2003) Antigenic structure of flavivirus proteins. Adv Virus Res 59:141–175
Rothman AL (2011) Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol 11:532–543
Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney MC, Kikuti CM, Navarro Sanchez ME, Dejnirattisai W, Wongwiwat W, Haouz A, Girard-Blanc C, Petres S, Shepard WE, Despres P, Arenzana-Seisdedos F, Dussart P, Mongkolsapaya J, Screaton GR, Rey FA (2015) Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 520:109–113
Smith SA, Crowe JE Jr (2015) Use of human Hybridoma technology to isolate human monoclonal antibodies. Microbiol Spectr 3, AID-0027-2014
Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, Wilson PC (2009) Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc 4:372–384
Smith SA, De Alwis AR, Kose N, Harris E, Ibarra KD, Kahle KM, Pfaff JM, Xiang X, Doranz BJ, De Silva AM, Austin SK, Sukupolvi-Petty S, Diamond MS, Crowe JE Jr (2013) The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. MBio 4:e00873–e00813
Smith SA, Nivarthi UK, De Alwis R, Kose N, Sapparapu G, Bombardi R, Kahle KM, Pfaff JM, Lieberman S, Doranz BJ, De Silva AM, Crowe JE Jr (2015) Dengue virus prM-specific human monoclonal antibodies with virus replication-enhancing properties recognize a single Immunodominant antigenic site. J Virol 90:780–789
Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D (2016) Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353:823–826
Sukupolvi-Petty S, Austin SK, Purtha WE, Oliphant T, Nybakken GE, Schlesinger JJ, Roehrig JT, Gromowski GD, Barrett AD, Fremont DH, Diamond MS (2007) Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol 81:12816–12826
Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, Macary PA (2012) The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med 4:139ra83
Tsai WY, Lai CY, Wu YC, Lin HE, Edwards C, Jumnainsong A, Kliks S, Halstead S, Mongkolsapaya J, Screaton GR, Wang WK (2013) High-avidity and potently neutralizing cross-reactive human monoclonal antibodies derived from secondary dengue virus infection. J Virol 87:12562–12575
Tsai WY, Durbin A, Tsai JJ, Hsieh SC, Whitehead S, Wang WK (2015) Complexity of neutralizing antibodies against multiple dengue virus serotypes after heterotypic immunization and secondary infection revealed by in-depth analysis of cross-reactive antibodies. J Virol 89:7348–7362
Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, De Silva AM (2009) Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 392:103–113
Wang Q, Yang H, Liu X, Dai L, Ma T, Qi J, Wong G, Peng R, Liu S, Li J, Li S, Song J, Liu J, He J, Yuan H, Xiong Y, Liao Y, Li J, Yang J, Tong Z, Griffin BD, Bi Y, Liang M, Xu X, Qin C, Cheng G, Zhang X, Wang P, Qiu X, Kobinger G, Shi Y, Yan J, Gao GF (2016) Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci Transl Med 8:369ra179
Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J (2008) Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 319:1834–1837
Yu IM, Holdaway HA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J (2009) Association of the pr peptides with dengue virus at acidic pH blocks membrane fusion. J Virol 83:12101–12107
Zompi S, Harris E (2013) Original antigenic sin in dengue revisited. Proc Natl Acad Sci U S A 110:8761–8762
Zompi S, Montoya M, Pohl MO, Balmaseda A, Harris E (2012) Dominant cross-reactive B cell response during secondary acute dengue virus infection in humans. PLoS Negl Trop Dis 6:e1568
Zybert IA, Van Der Ende-Metselaar H, Wilschut J, Smit JM (2008) Functional importance of dengue virus maturation: infectious properties of immature virions. J Gen Virol 89:3047–3051
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Discussion of Chapter 5 in Dengue and Zika: Control and Antiviral Treatment Strategies
Discussion of Chapter 5 in Dengue and Zika: Control and Antiviral Treatment Strategies
This discussion was held at the 2nd Advanced Study Week on Emerging Viral Diseases at Praia do Tofo, Mozambique.Transcribed by Hilgenfeld R and Vasudevan SG (Eds); approved by Dr. Aravinda de Silva.
-
Félix Rey: So you will be calling to question the fact that antibodies against Dengue would neutralize Zika?
-
Aravinda de Silva: No. But what I am saying is that in people who have recovered from Dengue – when they are in the late convalescent stage – they do not have circulating antibodies that neutralize Zika. I think in people who have secondary Dengue, when you isolate antibodies from their plasmablast, you can certainly find monoclonals that cross-neutralize Zika or even cross-protective against Zika, but it looks like they are not persisting into memory.
-
Félix Rey: How do you know that?
-
Aravinda de Silva: So in those people who have repeated Dengue infections – when we bleed them 6 months out from their infection, there is no neutralizing antibody against Zika. And I think that even in some of the other studies that are coming out to say that Zika and Dengue cross-neutralize, many of these studies have been done with samples within the first 3 or 4 weeks of an acute secondary Dengue infection. We know that one of the hallmarks of Dengue is that soon after they recover from Dengue during the convalescence period, there are very high levels of cross-neutralizing antibodies. This is even the case with primary Dengue infection, where we get a lot of cross-neutralizing antibodies during the convalescence period. But that is transient and it goes down and the response becomes more monotypic.
-
Paul Young: Can I just explore that further, because we have known that for a very long time. Why does the cross-neutralizing activity go down yet the serotype-specificity stays on. What is driving it?
-
Aravinda de Silva: So one of the obvious things is that IgM plays a role in cross-neutralization. But the second possibility is that there is an extrafollicular reaction. These cells are activated but they don’t get into the germinal centers and differenciate into plasmablast. They make a transcient antibody response but the cells do not persist. So a lot of the cross-neutralizing antibody is coming from extrafollicular reactions.
-
Paul Young: But why? I’m still a little confused. But I understand that’s why it happened. But why are those selectively lost?
-
Aravinda de Silva: Yes. That’s a good question. What is it about those epitopes that are getting lost, why are type-specific ones being maintained?
-
George Gao: Can we have a big picture for those three domains [of the envelope protein]? Which domain contributes the most to neutralizing antibodies? Can we say that now?
-
Aravinda de Silva: I think you have to really ask that question in the context of primary infection. In someone who has only had Dengue once or Zika once and no other flavivirus exposures, then what epitope is responsible for durable neutralization? We find in these cases there are defined epitopes responsible and they are the quaternary structure type-specific epitopes. But in someone with repeated infections – at least repeated Dengue infections – it could be ADE antibodies, it could be other antibodies that we haven’t discovered. But after natural infection, I don’t think that there is evidence that there are these long-lasting memory responses that are cross-neutralizing multiple flaviviruses.
-
Félix Rey: You would say that if it does not bind recombinant E protein, it has to bind some super-organization between dimers or something, but the recombinant E is monomeric unless you have it at a very high concentration.
-
Aravinda de Silva: Yes I agree that it could be binding dimers because the recombinant E protein test would not pick dimers.
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gallichotte, E.N., Baric, R.S., de Silva, A.M. (2018). The Molecular Specificity of the Human Antibody Response to Dengue Virus Infections. In: Hilgenfeld, R., Vasudevan, S. (eds) Dengue and Zika: Control and Antiviral Treatment Strategies. Advances in Experimental Medicine and Biology, vol 1062. Springer, Singapore. https://doi.org/10.1007/978-981-10-8727-1_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-8727-1_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8726-4
Online ISBN: 978-981-10-8727-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)