Abstract
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of diseases, which include simple liver steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma (HCC). It is a burgeoning health problem worldwide in line with the trend towards unhealthy diet and increased prevalence of obesity and type 2 diabetes mellitus (T2DM). Many animal models that illustrate both the histology and pathology of human NAFLD have been established. It is important to choose an animal model that best conforms to the aim of the study. This chapter presents a critical analysis of the histopathology and pathogenesis of NAFLD and the most commonly used and recently developed animal models of hepatic steatosis, NASH and NAFLD-induced hepatocellular carcinoma (NAFLD-HCC). The main mechanisms involved in the experimental pathogenesis of NAFLD in various animal models were also discussed. This chapter also includes a brief summary of recent therapeutic targets found using animal models. Although current animal models provide important guidance in understanding the pathogenesis and development of NAFLD, future study is essential to develop more precise models that better mimic the disease spectrum for both improved mechanistic understanding and identification of novel therapeutic options.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Non-alcoholic fatty liver disease (NAFLD)
- Liver cancer
- Dietary animal model
- Genetic animal model
- Disease histopathology
11.1 Introduction
Non-alcoholic fatty liver disease (NAFLD) is recognized as the hepatic exhibition of the metabolic syndrome. With the growing epidemic of obesity and insulin resistance, the worldwide prevalence of NAFLD continues to increase and is becoming the most common cause of chronic liver disease [1]. NAFLD can progresses from simple liver steatosis to non-alcoholic steatohepatitis (NASH), and even to liver fibrosis and cirrhosis. Fibrosing NASH leads to liver fibrosis and ultimately cirrhosis [2], increases risks for hepatocellular carcinoma (HCC) development [3]. Different stage of the NAFLD disease spectrum has distinctive histopathology features. Simple liver steatosis contains lipid accumulation in hepatocytes [4]. Hepatocellular injury, ballooning, and inflammation were present in NASH.
Excessive import or reduced export or oxidation of free fatty acids (FFAs) can induce hepatic steatosis. Lipid accumulation occurs when the rate of import or synthesis of FFAs by hepatocytes surpasses rate of export or catabolism [5]. Either of the following 4 can lead to triglyceride accumulation: [1] increased uptake of FFAs into hepatocytes due to excess lipolysis from adipose tissue stores or dietary intake, [2] increased de novo lipogenesis, [3] failure of FFA export through VLDL synthesis, and [4] failure of FFA elimination due to impaired β-oxidation. NAFLD occur where fat droplets accumulate in at least over 5% of hepatocytes [6]. The accumulation of fat droplets is usually macrovesicular, in which one large fat droplet or small well-defined droplets displace the nucleus from the cell center into the periphery. Microvesicular hepatic steatosis, though less common, may also occur concurrently in which very small fat droplets fill the hepatocytes without displacing the nucleus from the center of the cells. Pure microvesicular steatosis is a rare feature of NAFLD.
NASH is the resultant inflammatory response that is stimulated by various additional hits [2]. 1/3 of NAFLD patients could progress to NASH [7]. Liver steatosis, inflammatory cell infiltration and hepatocellular ballooning with or without fibrosis are the histopathology of NASH. The inflammation in NASH include lobular inflammation, which showed the infiltration of innate immune cells [8] and portal inflammation, which is usual and mild [9]. “Multiple-hit” hypothesis was reported recently for the pathogenesis of NASH, which include oxidative stress, inflammation, hyperinsulinemia, hyperleptinemia, and hypoadiponectinemia [10]. Of all these factors, oxidative stress and inflammation are two mechanisms pivotal to NASH genesis. The degree of oxidative stress is closely related with the severity of NASH [11, 12]. The imbalance of ROS generated by oxidative could induce lipid peroxidation and hepatocyte cellular damage. These damages affect plasmatic membranes, intracellular organelles, mitochondrial DNA, and respiratory chain-related proteins. Another consequence of increased ROS is that it may induce Fas ligand expression as it contains a binding site for nuclear factor-κB (NF-κB) and promote paracrine-induced apoptotic hepatocyte death. Excess FFA also leads to peroxisome proliferator-activated receptor alpha (PPAR-α)–mediated activation of the synthesis of enzymes responsible for extra-mitochondrial β-oxidation and ω-oxidation pathway. Chronic hepatic inflammation is closely related with NASH. The production of pro-inflammatory cytokines including TNF-α and interleukin-6 (IL-6) could affect adipokine levels, which induce perpetuation of the loop of chronic inflammation [13]. TNF-α increases FFA levels by inducing insulin resistance (IR), induces ROS formation by uncoupling mitochondrial respiration, and induces hepatocyte apoptosis and necrosis. Other reported pro-inflammatory cytokines that are elevated in NASH include IL-1α, IL-1β, and IL-18.
Nevertheless, the exact mechanism of NAFLD progression is still unclear. Further research for pathogenesis and therapeutic options are pivotal considering the increased incidence of NAFLD. Animal models that mirror the pathophysiology of each stage of human NAFLD progression provide important guidance in understanding the disease pathogenesis and progression. This chapter will summarize the current and most commonly used animal models. Moreover, it will briefly outline possible therapeutic options that have recently been identified using animal models.
11.2 Dietary Animal Models of NAFLD
11.2.1 High Fat Diet (HFD) Animal Model
The relationship between NAFLD and obesity induce the establishment of a high-fat diet that is similar with Western diets. In HFD animal models, 45–75% of the animals’ total calorie intake is resulted from fat.
The traditional reported HFD comprised of 71% fat, 18% protein and 11% carbohydrates for 3 weeks, whereas a standard Lieber-DeCarli diet included 35% fat, 47% carbohydrates and 18% protein. Although no weight change in rats fed with control or HFD, insulin resistance was developed as indicated by increased plasma insulin levels [14].
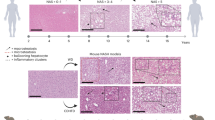
Mice fed with HFD comprised of 45% fat, 35% carbohydrates and 20% protein showed hepatic steatosis as indicated by increased lipid accumulation, hepatocyte ballooning and Mallory bodies (Fig. 11.1a). HFD could result in a higher percentage of cells enriched in lipid. For example, Wistar male rats were fed diets with same quantity (15 g/rat/day) for 16 weeks but with different composition including high-fat, moderate-fat, high-sucrose, and high-fructose groups. The HF group had the highest body and liver weight and highest percentage of liver steatosis (40%) [15].
Histopathological features of NAFLD in dietary and genetic animal models. Representative H&E staining from liver sections of (a) C57 BL/6 mice fed with control or high fat diet (HFD) for 12 weeks; (b) C57 BL/6 mice fed with control or methionine and choline-deficient (MCD) diet for 2 weeks; (c) C57 BL/6 mice fed with control or choline deficient high fat diet (CD-HFD) for 12 weeks; (d) db/db and dbm control mice fed with normal chow for 6 weeks
The advantage of HFD-fed animal model is that it mimic both the histopathology and pathogenesis of human NAFLD as they induce hallmark features observed in human NAFLD including metabolic syndrome. However, the degree of hepatic steatosis seems to depend upon various factors including rodent strain.
11.2.2 Methionine and Choline-deficient ( MCD) Dietary Model
Feeding mice a lipogenic MCD diet is a frequently used and very reproducible nutritional model of NASH. The diet is deficient in methionine and choline with moderately fat. Choline is an essential nutrient that is stored and metabolized chiefly in the liver. Choline deficient impairs hepatic VLDL secretion and results in hepatosteatosis, oxidative stress, liver cell death, and the alteration of cytokines and adipocytokines [16], but only causes mild hepatic inflammation and fibrosis. In contrast, mice fed a diet lacking both choline and amino acid methionine develop severe hepatic inflammation at 2 weeks of MCD diet feeding [5, 17] (Fig. 11.1b). Alongside with ballooning degeneration of hepatocytes, serum alanine aminotransferase (ALT) levels also increase [18]. Recent studies suggest that the progression of steatosis to steatohepatitis in MCD mice models involve significant downregulation in expressions of proteins involved in Met metabolism and oxidative stress [19].
Compared with other dietary models, MCD mouse models better mimicked pathological findings of severe human NASH. The typical features of NAFLD include inflammation, fibrosis, and hepatocyte apoptosis were much more quickly and severely than mice fed HFD or Western diets. The diet also better models the mechanisms implicated in the pathogenesis of human NASH. Endoplasmic reticulum stress, oxidative stress, and autophagocytic stress are all substantially more active in MCD models than other dietary models [20]. Thus, this model is ideal for studying histologically advanced NASH and the mechanisms of inflammation and fibrosis in NASH. It must be noted that studies have shown that different mouse strains respond differently towards the MCD diet.
The disadvantage of MCD model is obvious. Instead of being obese, mice fed an MCD diet exhibit significant weight loss, cachexia, without metabolic profile of human NASH, and low serum insulin, fasting glucose, leptin and triglyceride levels [21]. Hence, MCD diets are often fed to db/db or ob/ob mice in order to better mimic human NASH. MCD diet fed db/db mice are obese and showed marked hepatic inflammation and fibrosis [22].
11.2.3 High Cholesterol Diet Model
Dietary cholesterol is an important factor in the progression of steatohepatitis and hepatic inflammation in both animal models [23,24,25] and humans [26]. Mice fed a high-cholesterol diet (HCD) (1%) alone show striking increases in serum insulin levels but only slight increases in liver weight, triglyceride, FFAs, and serum ALT [26]. However, when high-cholesterol is given in conjunction with high-fat or high-cholate, features of NASH are much more pronounced. Mice fed a high-fat (15%), high-cholesterol (1%) diet (HFHC) showed greater weight gain, greater hepatic lipid accumulation, elevated serum ALT level, decreased adiponectin, adipose tissue inflammation, and fibrosis, the features of which were much more severe in HFHC mice than HFD or HCD mice [26]. Similarly, mice fed a high-cholesterol (1.25%), high-cholate (0.5%) diet also showed greater steatosis, inflammation, hepatocellular ballooning and fibrosis [23, 27]. Mice fed with 23% fat, 424 g/Kg sucrose and1.9 g/kg cholesterol diet or choline-deficient high fat diet (CD-HFD) for 3 months developed pronounced steatohepatitis (Fig. 11.1c). Several studies suggest dietary cholesterol reduces VLDL synthesis and β-oxidation of fatty acids and increases apoptosis and hepatic oxidative stress [25, 26].
11.2.4 High Fructose Diet Model
Epidemiologic data suggests that humans consume a significant number of calories from fructose rich foods and this has been paralleled with the development of obesity and NASH in humans [28]. C57BL/6 mice a HFD or high-fat high-fructose (HFHF) diet showed similar fructose consumption [28]. In a study from our group, CXCR3-knockout and C57BL/6 wild-type mice were fed a similar HFHF diet comprising of a HFHC diet supplemented with 23 g/L of fructose in drinking water. Results showed that CXCR3-knockout mice had improved liver histology, significantly lower necroinflammation, and reduced lipid peroxidation. This suggests that CXCR3 plays a pivotal role in NASH development in HFHF mouse models [29].
11.3 Genetic Animal Models of NAFLD
11.3.1 db/db and ob/ob Genetic Animal Model
db/db mice are homozygous for the autosomal recessive diabetic gene (db). The db gene encodes for a point mutation of the leptin receptor (Ob-Rb), which leads to defective signaling of leptin hormone [30]. Thus, db/db mice have normal or elevated levels of leptin but are resistant to its effects. Leptin is responsible for regulating feeding behaviour by promoting satiety. These mice have persistent hyperphagia and are obese and diabetic [31]. They show severe hyperglycaemia, hyperinsulinemia, elevated serum leptin, and develop macrovesicular hepatic steatosis [5, 22, 32] (Fig. 11.1d). Prolonged calorie overconsumption (>1 month) may lead to slightly aggravated hepatic inflammation [30]. Nevertheless, db/db mice when fed a control diet rarely display features of NASH. Thus, db/db mice alone are good models of NAFLD but not of NASH.
Unlike db/db mice, ob/ob mice have functional leptin receptors but have truncated and non-functional leptin. Similarly, these mice are grossly overweight, hyperphagic, hyperinsulimenic, hyperglycemic, and resistant to insulin, and develop spontaneous marked liver steatosis [30] but not steatohepatitis. Secondary insults are also required to trigger steatohepatitis. This may be provided through exposure to MCD diet, HFD, small doses of lipopolysaccharide endotoxin [31], ethanol or hepatic ischaemia-reperfusion challenge [5]. ob/ob mice are essentially resistant to fibrosis because leptin is essential for hepatic fibrosis [32].
Unlike dietary models, db/db and ob/ob mouse models exhibit features of human metabolic syndrome. When fed a standard diet without an additional hit, these mice are useful models of NAFLD as they develop pronounced hepatic steatosis. With the addition of a second-hit like MCD diet, db/db mice can also be used to study the progression of steatosis to NASH. However, congenital leptin deficiency and leptin resistance caused by gene mutation in obese humans are extremely rare [33], so db/db and ob/ob mice models are limited in reflecting the aetiology of human obesity, insulin resistance and hepatic steatosis.
11.3.2 foz/foz Genetic Model
foz/foz mice have a mutated Alms1 which have a possible role in intracellular transport and appetite regulation [34]. foz/foz mice are morbidly obese, hyperphagic, and develop IR, significantly reduced adiponectin levels, increased cholesterol levels, and steatosis. A HFD promotes the transition of steatosis to NASH with severe fibrosis in these mice by attenuating metabolic complications, resulting in further decreased adiponectin levels and elevated cholesterol levels. However, the severity of diet-induced NASH in foz/foz mice depends on the strain. When foz/foz BALB/c and C57BL6/J mice were fed a HFD, weight gain was equivalent, suggesting that the appetite defect in foz/foz mice is independent of strain, however NAFLD was much more severe in foz/foz C57BL6/J mice than in foz/foz BALB/c mice. IR, hyperinsulinaemia, obesity, and substantial NAFLD-related liver fibrosis were exhibited in foz/foz C57BL6/J mice but not in foz/foz BALB/c mice. These findings suggest that although obesity in foz/foz mice is equal, the responses to obesity including features of NASH are dependent on strain [35].
11.3.3 db/db Genetic Supplemented Dietary NASH Model
In addition to MCD diet, a recent study found that iron overload in db/db mice can also cause progression of NAFLD to NASH and fibrosis. Unlike db/db mice fed a normal chow diet, db/db mice fed a chow diet supplemented with high iron showed hepatocellular ballooning, fibrogenesis increased hepatic oxidative stress, inflammasome activation, hepatic inflammatory immune cell activation and impaired hepatic mitochondrial fatty acid β-oxidation [36].
11.4 Animal Models of NAFLD-Induced HCC
HCC is the third most common cause of cancer-related death worldwide. There is a weighty connection between NASH and HCC. Liver cirrhosis is the most important risk factor for HCC although HCC could occur in non-cirrhotic NASH [8]. Increased fat uptake, hepatic steatosis, and NASH are all incremental risk factors for HCC. 4–27% of patients with NASH-related cirrhosis ultimately progress to HCC [3]. Long-term follow up studies reveal that the prevalence of HCC in NASH patients is 0–2.8% [3, 37, 38].
Current mouse models of NAFLD and NASH do not replicate pathological process from fatty liver, NASH, and fibrosis to HCC. Various experimental mouse models for HCC are present but only a few of them represent NAFLD-induced HCC [39].
11.4.1 Diet NAFLD-Induced HCC Model
Models fed with only one type of diet have distinctive limitations. C57BL/6 mice fed MCD or CD diets is lean without insulin resistance. HFD-fed mice do not exhibit NASH-like pathology whereas mice fed a MCD or CD diet do. To solve this problem, Wolf et al. proposed a mixed diet model combining choline deficient diet and HFD for the investigation of NAFLD-induced HCC development. Liver steatosis, features of metabolic syndrome and liver damage reflected by elevated serum ALT and AST levels were present concurrently in this novel model. Features of liver damage were reminiscent of human NASH including oxidative stress, hepatocyte ballooning, immune cell infiltration, glycogeneated nuclei, and MDB. Liver analysis of HFD versus CD-HFD mice found that tumor incidence in HFD mice is only 2.5% while incidence in CD-HFD mice is 25% [40].
In another diet model, C57BL/6 mice are fed a choline-deficient L-amino-acid-defined-diet (CDAA). The mice develop liver injury that mimic NASH features that lead to HCC. Treatment of mice with CDAA induced insulin resistance, increase in hepatic steatosis, modifications of enzymes of carbohydrate and lipid metabolism, liver damage, and fibrosis. HCC developed after 9 months of feeding [41].
Asgharpour et al. recently reported a diet-induced animal model that recapitulates the key human NASH-HCC features. They generated an isogenic mouse strain B6/129 by repeating brother-sister mating of the C57BL/6 J and 129S1/SvlmK mice for over 4-years. B6/129 mice fed with high fat high carbohydrate diet will sequentially develop steatosis in 4–8 weeks, NASH in 16–24 weeks and HCC at week 52, which may be an ideal preclinical model of NASH-HCC investigation [42].
11.4.2 Combined Chemical & Dietary NAFLD- Induced HCC Model
CDAA diet C57BL/6 mice subjected to low dose intraperitoneal injections of Carbon Tetrachloride (CCl4) have more marked features of NASH and HCC. Mice had greater steatosis, lobular inflammation, and fibrogenesis when compared with CDAA diet alone. In addition, CDAA C57BL/6 mice showed presence of HCC only in 35% of cases but CDAA + CCl4 group showed presence of HCC in all mice and with a significantly higher average tumour diameter. Thus the CDAA+CCl4 model better represents NASH and its progression to HCC than CDAA diet alone model [41].
In another combined chemical and dietary model, Mice fed a HFD and treated with Streptozotocin (STZ), a glucosamine-nitrosourea compound, is toxic towards pancreatic β cells and induces hypoinsulinemia, hyperglycaemia, and diabetes in mice. STZ-primed mice stimulated with HFD induced histological changes including steatosis, lobular inflammation, fibrosis and, at 20 weeks, tumor protrusion. Male STZ-HFD mice developed significant proliferation of hepatocytes at 16 weeks and eventually HCC. The model provides insight into the mechanism linking metabolic disorder, NASH and HCC [43].
11.4.3 Genetic NAFLD-Induced HCC Model
Phosphatase and tensin homolog (PTEN) is a tumor suppressor gene through its lipid phosphatase activity and is mutated in many human cancers [44]. PTEN is a putative tumor suppressor in liver and PTEN loss could promote cell proliferation, inhibit apoptosis and induce tumor formation. Mice with PTEN loss in hepatocytes develop features similar to human NASH and NASH-induced HCC [45]. Hepatocarcinogenesis is evident in PTEN-deficient mice as liver tumors were present in 66% of male and 30% of female PTEN-deficient mice at 40–44 weeks and pathological examinations showed that HCC was present in 83% of male and 50% of females at 74–78 weeks [45]. Thus, this model is useful for not only the understanding of pathogenesis of NASH but also the progression to HCC.
11.4.4 Combined Genetic and Chemical NAFLD-Induced HCC Model
Shen et al. found that genetic obesity in db/db mice is a direct promoter of NASH-HCC development. Compared to wild-type lean mice, db/db mice treated with carcinogen diethylnitrosamine (DEN) had higher body weight, higher liver weight, hepatic steatosis, higher HCC incidence, and tumor nodules significantly higher in number and larger in size. Results also found that these mice had genetic alterations in inflammation-related pathways and mutations in Cel, which leads to endoplasmic reticulum (ER) stress and cell proliferation. Findings from this mouse model suggest that obesity and NASH increases susceptibility of HCC development [46].
11.5 Usage of Animal Models
Animal models are crucial in elucidating the mechanisms and pathways involved in the pathogenesis of the NAFLD progression. Often studies using the aforementioned animal models may provide encouraging results for future treatments for NAFLD and NASH.
Using HFD mice models, Jin et al. reported that Cyclin D3-cyclin-dependent kinase 4 (CDK4) activation is a crucial event in NAFLD progression [47]. C/EBPα and C/EBPβ are members of the C/EBP protein family, which control multiple functions in different tissues and are involved in the development of NAFLD. C/EBPα is a strong inhibitor of liver proliferation. Its functions and biological activities on the liver are controlled by post-translational modification at the Ser193 amino acid site. Cyclin D3-cyclin-dependent kinase 4 (cdk4) phosphorylates C/EBPα on Ser193 causing it to form a complex with chromatin remodeling protein p300. C/EBPα-p300 causes C/EBPα-dependent growth arrest. HFD activates cdk4 in wild-type mice, leading to an increase in C/EBPα-p300 complexes. Similarly, HFD-mediated steatosis, fibrosis, and liver injury are inhibited in Cdk-4 resistant C/EBPα-S193A mice. These findings suggest that elevation of cdk4 is a key event in the development of NAFLD and cdk4 inhibition can be considered as a possible treatment for NAFLD. Using db/db mice model, Li et al. demonstrated that Carboxylesterase 2 (CES2) is a novel triglyceride hydrolase in lipid regulation and NAFLD [48]. Glucagon-like peptide-1 (GLP-1) is a neuropeptide that induces pancreatic β-cells to release insulin in response to glucose, restores glucose sensitivity of β-cells, and promotes β-cells proliferation. Exendin-4 is a GLP-1 analogue that is resistant to such inactivation and is hence a target for the treatment of type 2 diabetes mellitus. Using MCD-fed db/db mice model, Yamamoto et al. showed that exendin-4 treatment prevented MCD-induced steatohepatitis with decreased lipid accumulation and FFA content. Results found that exendin-4 exerted such effects via three mechanisms. Firstly, exendin-4 could suppress SREBP-1c-related hepatic de novo lipogenesis. Secondly, it was observed that exendin-4 attenuated the MCD-diet induced decrease in levels of peroxisomal acyl-coenzyme A oxidase 1 (ACOX1) mRNA. This suggests that exendin-4 induces lipid oxidation, as ACOX1 is an enzyme involved in hepatic β-oxidation. Lastly, fatty acid transport protein 4 (FATP4) plays an important role in hepatic fatty acid uptake and exendin-4 administration attenuated the MCD-diet induced increase in liver FATP4 mRNA, thus suggesting a decrease in hepatic FFA influx. With regards to hepatic inflammation, exendin-4 reduced hepatic inflammation score, levels of a hepatic ROS marker namely MDA, and levels of pro-inflammatory cytokines and chemokines such as TNF-α and monocyte chemoattractant protein-1 (MCP-1). These data found using a MCD mice model shed light on the possible use of exendin-4 for the treatment of non-obese patients with NASH [49].
11.6 Conclusion
The animal models aforementioned in this chapter are useful tools in studying the pathogenesis of NAFLD and the identification of possible therapeutic options. However, they are not perfect to characterize all the features of NAFLD. Some replicate the histopathology of NAFLD remarkably but not the physiological properties, and others vice versa. Therefore, more accurate animal models that better mimic the disease spectrum are still essential and need further study.
References
Alexander Wree LB, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis—new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–36.
Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83.
Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–32.
Hubscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49:450–65.
Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1–16.
Tandra S, Yeh MM, Brunt EM, Vuppalanchi R, Cummings OW, Unalp-Arida A, et al. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol. 2011;55:654–9.
Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112.
Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:5286–96.
Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–20.
Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–46.
Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92.
George J, Pera N, Phung N, Leclercq I, Yun Hou J, Farrell G. Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. J Hepatol. 2003;39:756–64.
Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis. 2010;28:179–85.
Nieto-Vazquez I, Fernandez-Veledo S, Kramer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008;114:183–94.
Fakhoury-Sayegh N, Trak-Smayra V, Khazzaka A, Esseily F, Obeid O, Lahoud-Zouein M, et al. Characteristics of nonalcoholic fatty liver disease induced in wistar rats following four different diets. Nutr Res Pract. 2015;9:350–7.
Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 2012;28:159–65.
Yamada T, Obata A, Kashiwagi Y, Rokugawa T, Matsushima S, Hamada T, et al. Gd-EOB-DTPA-enhanced-MR imaging in the inflammation stage of nonalcoholic steatohepatitis (NASH) in mice. Magn Reson Imaging. 2016;34:724–9.
Larter CZ, Yeh MM, Williams J, Bell-Anderson KS, Farrell GC. MCD-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J Hepatol. 2008;49:407–16.
Lee SJ, Kang JH, Iqbal W, Kwon OS. Proteomic analysis of mice fed methionine and choline deficient diet reveals marker proteins associated with steatohepatitis. PLoS One. 2015;10:e0120577.
Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, et al. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One. 2015;10:e0127991.
Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40:47–51.
Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, et al. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035–43.
Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–403.
Zheng S, Hoos L, Cook J, Tetzloff G, Davis H Jr, van Heek M, et al. Ezetimibe improves high fat and cholesterol diet-induced non-alcoholic fatty liver disease in mice. Eur J Pharmacol. 2008;584:118–24.
Subramanian S, Goodspeed L, Wang S, Kim J, Zeng L, Ioannou GN, et al. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res. 2011;52:1626–35.
Savard C, Tartaglione EV, Kuver R, Haigh WG, Farrell GC, Subramanian S, et al. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57:81–92.
Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2300–8.
Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934–44.
Zhang X, Han J, Man K, Li X, Du J, Chu ES, et al. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J Hepatol. 2016;64:160–70.
Trak-Smayra V, Paradis V, Massart J, Nasser S, Jebara V, Fromenty B. Pathology of the liver in obese and diabetic ob/ob and db/db mice fed a standard or high-calorie diet. Int J Exp Pathol. 2011;92:413–21.
Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–62.
Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–13.
Paz-Filho G, Mastronardi C, Delibasi T, Wong ML, Licinio J. Congenital leptin deficiency: diagnosis and effects of leptin replacement therapy. Arq Bras Endocrinol Metabol. 2010;54:690–7.
Bell-Anderson KS, Aouad L, Williams H, Sanz FR, Phuyal J, Larter CZ, et al. Coordinated improvement in glucose tolerance, liver steatosis and obesity-associated inflammation by cannabinoid 1 receptor antagonism in fat Aussie mice. Int J Obes (Lond). 2011;35:1539–48.
Farrell GC, Mridha AR, Yeh MM, Arsov T, Van Rooyen DM, Brooling J, et al. Strain dependence of diet-induced NASH and liver fibrosis in obese mice is linked to diabetes and inflammatory phenotype. Liver Int. 2014;34:1084–93.
Handa P, Morgan-Stevenson V, Maliken BD, Nelson JE, Washington S, Westerman M, et al. Iron overload results in hepatic oxidative stress, immune cell activation, and hepatocellular ballooning injury, leading to nonalcoholic steatohepatitis in genetically obese mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G117–27.
Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–8.
Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–12.
Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–86.
Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–64.
De Minicis S, Agostinelli L, Rychlicki C, Sorice GP, Saccomanno S, Candelaresi C, Giaccari A, Trozzi L, Pierantonelli I, Mingarelli E, Marzioni M, Muscogiuri G, Gaggini M, Benedetti A, Gastaldelli A, Guido M, Svegliati-Baroni G. HCC development is associated to peripheral insulin resistance in a mouse model of NASH. PLoS One. 2014;9:e97136.
Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol. 2016;65:579.
Fujii M, Shibazaki Y, Wakamatsu K, Honda Y, Kawauchi Y, Suzuki K, Arumugam S, Watanabe K, Ichida T, Asakura H, Yoneyama H. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol. 2013;46:141–52.
Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–83.
Watanabe S, Horie Y, Kataoka E, Sato W, Dohmen T, Ohshima S, et al. Non-alcoholic steatohepatitis and hepatocellular carcinoma: lessons from hepatocyte-specific phosphatase and tensin homolog (PTEN)-deficient mice. J Gastroenterol Hepatol. 2007;22(Suppl 1):S96–S100.
Shen J, Tsoi H, Liang Q, Chu ES, Liu D, Yu AC, et al. Oncogenic mutations and dysregulated pathways in obesity-associated hepatocellular carcinoma. Oncogene. 2016;35:6271.
Jin J, Valanejad L, Nguyen TP, Lewis K, Wright M, Cast A, et al. Activation of CDK4 triggers development of non-alcoholic fatty liver disease. Cell Rep. 2016;16:744.
Li Y, Zalzala M, Jadhav K, Xu Y, Kasumov T, Yin L, et al. Carboxylesterase 2 prevents liver steatosis by modulating lipolysis, endoplasmic reticulum stress, and lipogenesis and is regulated by hepatocyte nuclear factor 4 alpha in mice. Hepatology. 2016;63:1860–74.
Yamamoto T, Nakade Y, Yamauchi T, Kobayashi Y, Ishii N, Ohashi T, et al. Glucagon-like peptide-1 analogue prevents nonalcoholic steatohepatitis in non-obese mice. World J Gastroenterol. 2016;22:2512–23.
Acknowledgment
This chapter was modified from the paper published by our group in Journal of Pathology (Lau, Zhang and Yu 2017; 241:36–44). The related contents are re-used with the permission.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lau, J.K.C., Zhang, X., Yu, J. (2018). Animal Models of Non-alcoholic Fatty Liver Diseases and Its Associated Liver Cancer. In: Yu, J. (eds) Obesity, Fatty Liver and Liver Cancer. Advances in Experimental Medicine and Biology, vol 1061. Springer, Singapore. https://doi.org/10.1007/978-981-10-8684-7_11
Download citation
DOI: https://doi.org/10.1007/978-981-10-8684-7_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8683-0
Online ISBN: 978-981-10-8684-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)