Abstract
The recent advances in nanotechnology open new avenues for the development of functionalized nanomaterials with wide potential application. Chitosan has become one of the most promising biopolymers with wide application in diagnostics and therapeutics. It is a linear copolymer of β-(1–4)-linked 2-acetamido-2-deoxy-β-d-glucopyranose and 2-amino-2-deoxy-β-d-glycopyranose, with a varying content of N-acetyl groups. It is obtained by deacetylation of parent polymer, chitin, and also occurs naturally in fungal species such as Absidia glauca, Absidia coerulea, Aspergillus niger, Mucor rouxii, Gongronella butleri, Phycomyces blakesleeanus, Absidia blakesleeanus, Rhizopus oryzae, Trichoderma reesei, and Lentinus edodes. Chitosan can also be directly extracted from fungi by alkaline/acid treatment and by use of microorganisms/proteolytic enzymes. Unlike chitin, chitosan is readily soluble in dilute acetic acid and widely used in preparation of gels, films, and fibers. The production of the biopolymer is generally influenced by parameters such as the nutritional factors, mode of cultivation, temperature, pH, and mineral salts. In therapeutics, chitosan and chitosan-based materials are used as antimicrobial, antitumor, antiulcer, antidiabetic, and a cholesterol-lowering agent. Being a naturally occurring polysaccharide, chitosan and its functionalized derivatives exhibit unique properties, such as biocompatibility, biodegradability, biological activity, and low toxicity. The conformational flexibility of chitosan is attributed to the presence of the free primary amino groups which makes chitosan an ideal candidate for biofabrication. Various methods, such as ionic gelation, desolvation, spray-drying, and covalent cross-linking, have been employed for functionalization of chitosan. Nanoparticles and its biofabrication impart desirable functional characteristics to chitosan. The molecular weight and the concentration of chitosan used along with an amount of cross-linking govern the physical properties of chitosan nanoparticles formed. Chitosan nanocomposites have shown to improve the dissolution rate of poorly soluble drugs and, thus, are exploited for enhancement of drug bioavailability and delivery. Various therapeutic agents, such as anticancer, anti-inflammatory, antibiotics, antithrombotic, steroids, proteins, amino acids, antidiabetic, and diuretics, have been incorporated in chitosan nanocomposites. The controlled release of therapeutic agents opened new windows in drug delivery and bio-imaging techniques using chitosan. Hence, chitosan and its nano-derivatives serve as one of the sustainable and ecofriendly alternative to synthetic polymers in biomedical applications.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Henri Braconnot (1811) discovered the first natural polysaccharide, fungine, from the cell wall of fungi almost three decades before the isolation of cellulose, and in 1823, Odier renamed fungine as chitin (Akila 2014). The discovery of chitin was based on several studies carried out on fungal material isolated from mushroom, Agaricus volvaceus, A. acris, A. cantharellus, A. piperatus, Hydnum repandum, H. hybridum, and Boletus viscidus (Muzzarelli et al. 2012). Chitin, poly-(β-(1-4)-N-acetyl-d-glucosamine), is a natural polysaccharide synthesized by an enormous number of living organisms and is the second most abundant biopolymer after cellulose. In a wide number of invertebrates, chitin serves as a structural component and provides tensile strength. The well-ordered crystalline microfibrils of chitin occur in nature as exoskeleton of arthropods and crustaceans and as a cell wall component of fungi and yeast (Jayakumar et al. 2010). However, the structural complexity, tedious extraction procedure, and insolubility in aqueous and organic solvents greatly limit its practical applications (Dash et al. 2011; Cheung et al. 2015).
In 1859, Rouget, while studying the effect of deacetylation on the thermal and chemical changes of the chitin, discovered chitosan (Akila 2014). Chitosan (CS) is a high molecular weight linear polycationic heteropolysaccharide comprising copolymers of β-1,4-linked D-glucosamine and N-acetyl-D-glucosamine. Chitosan can be obtained either by (partial) deacetylation of chitin under alkaline conditions (concentrated NaOH) or by enzymatic hydrolysis by the aid of chitin deacetylase as shown in Fig. 6.1 (Duttagupta et al. 2015). However, for commercial production, chemical deacetylation is more preferred commonly due to economic issues and feasibility for scale up (Cheung et al. 2015).
The natural polysaccharide, chitosan, is biocompatible, biodegradable, and nontoxic. It also exhibits antimicrobial activity against a wide range of pathogens including bacteria, fungi, and yeast (Kong et al. 2010). All these characteristics make CS a promising agent for fabrication of antimicrobial compounds and development of more efficient nano-therapeutics. The chemical structure and molecular weight of chitosan govern its physiochemical properties. Hence, the low solubility of chitosan in acid-free aqueous media resulting due to its high molecular weight and highly viscous nature hinders its practical application. In recent years, advances have been made on modification and functionalization of chitosan for better applicability (Zhang et al. 2010). An increase in interest and developments are made in recent years in pharmaceutical as well as biomedical applications of chitosan and its nano-derivatives. Apart from its biocompatibility, the easily controllable size, surface charge density, loading efficiency, controlled release, etc. make chitosan nanoparticles a promising tool for biomedical applications (Mazancova et al. 2017).
Chitosan possess antitumor, immunoenhancing, antimicrobial, and hypocholesterolemic properties, and numerous research works have been attempted on chitosan and its nano-derivatives for their application in tissue engineering, drug delivery, wound healing, antitumor, and antimicrobial therapy (Zhang et al. 2010; Piras et al. 2015; Cheung et al. 2015).
6.2 Fungal Sources of Chitosan
In fungi, chitin is an important structural component which significantly contributes to the tensile strength and integrity of the fungal cell wall. It is the major component found in the septa between mother and daughter cells of Saccharomyces cerevisiae. It is also one of the main components of the hyaline outer wall of spores of arbuscular mycorrhizal glomus species. Chitosan occurs naturally in the mucorales such as Mucor, Absidia, and Rhizopus species (Table 6.1). In four edible mushrooms, Lentinus edodes, Lycophyllum shimeji, Caju, and Volvariella volvacea, chitin is present as a minor component in mycelia, the caps and stalks of fruiting bodies. Mario et al. (2008) reported the isolation of chitosan from the mycelium of seven species of Basidiomycetes, viz., Mucor rouxii, Absidia glauca, Aspergillus niger, Gongronella butleri, P. sajor-caju, Rhizopus oryzae, L. edodes, and Trichoderma reesei. The chitin yield was estimated between 8.5% and 19.6% dry weight with <90% degree of deacetylation. However, chitin is not necessarily present in all fungi. Slime molds (Myxomycetes) and bacteria (Schizomycetes) are devoid of chitin (Akila 2014). However, chitosan is the most abundant component of both filamentous and yeast-like forms of Mucor rouxii (Tayel et al. 2010; Muzzarelli et al. 2012). In single-celled fungi-like yeast, the cell wall compose of 1–2% of chitin by dry weight, while in the case of filamentous fungi-like Neurospora and Aspergillus, chitin constitutes about 10–20% of the fungal dry weight (Bowman and Free 2008; Tayel et al. 2011). Pochanavanich and Suntornsuk (2002) investigated the ability to produce chitosan by four species of filamentous fungi, Aspergillus niger, Rhizopus oryzae, Lentinus edodes, and Pleurotus sajor-caju, and two yeast strains, Zygosaccharomyces rouxii TISTR5058 and Candida albicans TISTR5239. Fungal chitosan was produced at 10–140 mg/g cell dry weight, with the degree of deacetylation at 84–90% and a molecular weight of 2.7 × 104–1.9 × 105 Da with a viscosity of 3.1–6.2 centipoises (cP).
The physical and chemical condition of fermentation process can be manipulated to provide chitosan of more consistent physicochemical properties as compared to the ones derived chemically from chitin. Four fungal strains, Aspergillus niger TISTR3245, Rhizopus oryzae TISTR3189, Zygosaccharomyces rouxii TISTR5058, and Candida albicans TISTR5239, grown on soybean and mung bean residues resulted in the chitosan yields of 0.4–4.3 g/kg in soybean residue and 0.5–1.6 g/kg for mung bean residue. The highest amount of chitosan (4.3 g/kg) was obtained when R. oryzae was cultivated on soybean residue (Suntornsuk et al. 2002). Chitosan has been isolated from Mucor rouxii cultured in three different media, viz., molasses salt medium (MSM), potato dextrose broth (PDB), and yeast extract peptone glucose (YPG) medium under submerged condition, and their yield has been found to be almost the same, being 0.61 g/l for MSM, 0.51 g/l for PDB, and 0.56 g/l for YPG, respectively (Chatterjee et al. 2005). Kuhlmann et al. (2000) reported Absidia spp producing low molecular weight chitosan with a MW of 700 kDa and degree of deacetylation of 0.86. Niederhofer and Muller (2004) extracted chitosan with average MW 45 kDa from Absidia coerulea. Kleekayai and Suntornsuk (2011) documented the production of chitosan using Rhizopus oryzae with 86–90% degree of deacetylation, molecular weight of 80–128 kDa, and viscosity of 3.1–6.1 mPa.
6.3 Structure and Composition
Chitin serves as a fibrous structural component responsible for tensile strength and rigidity of fungal cell wall. The fungal chitin possesses predominantly the same structure to the ones present in exoskeleton of arthropods. However, a major difference is that the fungal chitin exists in association with other polysaccharides such as glucans and mannans which are absent in crustacean (Akila 2014). As shown in Fig. 6.1, Chitosan is a copolymer composed of N-acetylglucosamine and glucosamine units which are linked by β(1→4) linkage (Chattopadhyay and Inamdar 2013). In general, the individual chains of β-(1→4)-2-acetamido-D-glucose and β-(1→4)-2-amino-D-glucose assume an essentially linear structure, which undergoes one full twist every 10.1–10.5°A along the axis of the chain (Dash et al. 2011). Chitosan exist in two allomorph forms, α and β, which could be elucidated by X-ray model and NMR studies. The α-chitin isomorphs are by far the most abundant and occur in the cell walls of fungi and yeast, in krill, tendons of crab, shrimp shells, insect cuticle, etc. On the other hand, β-chitin is found in association with proteins in squid pens and in the tubes synthesized by pogonophoran and vestimentiferan worms (Younes and Rinaudo 2015). Chitosan compose of three major reactive functional groups, an amino/acetamido group along with primary and secondary hydroxyl groups at the C-2, C-3, and C-6 positions, respectively. The amino groups contribute to the structural differences and physicochemical properties of the chitosan (Dash et al. 2011). The exact molecular mass of chitin has not yet been documented; however, it was estimated that S. cerevisiae synthesizes uniform chains of chitin constituting of 120–170 GlcNAc monomer units (~24,000–34,500 Da). In S. cerevisiae terminal reducing ends of chitin chains are attached though β (1, 4) or β (1, 2) linkages to the nonreducing end of β (1, 6) glucan. Attachment of chitin to glucan is catalyzed by chitin synthase. In addition, a mannoprotein is attached to β (1, 6) glucan through a glycosylphosphatidylinositol anchors containing five α-linked mannosyl residues (Akila 2014).
6.4 Physiochemical Properties of Chitosan
Chitin constitutes the cell walls and septa of fungi class, Ascomycetes, Zygomycetes, Basidiomycetes, and Deuteromycetes. It is a structural component and helps in maintaining the shape and integrity of fungi. Although many reports are available for the preparation of chitosan-based particles, a spectrum of chemical factors like molecular weight, degree of deacetylation, pH, ionic strength, temperature, rate of stirring, etc. greatly affects the overall physicochemical and biological properties of the resultant particles. The preparation process employed ultimately leads to the formation of inherently different particles in terms of size, cross-linking, density, loading capacity, surface charge density, colloidal stability, and release kinetics. The biological properties, such as analgesic, antitumor, hemostatic, hypocholesterolemic, antimicrobial, and antioxidant properties, also govern the physical properties of chitosan (Mazancova et al. 2017).
Modification of chitosan at the molecular level increases its solubility and stability (Cheung et al. 2015). The parent chitin is insoluble in most organic solvents; on the contrary due to the quaternization of the amine groups that have a pKa value of 6.3, chitosan is readily soluble in dilute acidic solutions below pH 6.0. At low pH, these amines present in chitosan get protonated making chitosan a water-soluble cationic polyelectrolyte. When the pH increases above 6, amines of chitosan gets deprotonated and the polymer loses its positive charge and becomes insoluble. The soluble-insoluble transition which occurs at its pKa value between pH 6 and 6.5 aids in the functionalization of the chitosan. As the pKa value is highly dependent on the degree of deacetylation, the solubility of chitosan is in turn dependent on the degree of deacetylation (Dash et al. 2011). The degree of deacetylation and the molecular weight of chitosan were found to influence the physiochemical properties of chitosan (Cheung et al. 2015). The molecular weight and degree of deacetylation (DD) is determined by the conditions used for deacetylation of the polymer. The treatment of chitin with an aqueous solution of 40–45% (w/v) NaOH at 90–120 °C for 4–5 h results in N-deacetylation of chitin (Dash et al. 2011).
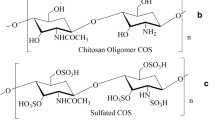
The characterization of a chitosan requires the determination of its average degree of deacetylation (DD) and molecular weight. The DD can be determined by different techniques, such as infrared spectroscopy, potentiometric titration, and NMR (Cheung et al. 2015). The DD also influences the solubility of the polymer, the interchain interactions due to H-bonds, and the hydrophobic character of the acetyl group (Zhang et al. 2010). Commercially available low molecular weight chitosan (LMWC) grade is characterized by molecular weight comprised between 20 and 190 kDa with DD <75% and that of high molecular weight chitosan (HMWC) is generally characterized by molecular weight comprised between 190 and 375 kDa with DD >75% (Dash et al. 2011). The mycelia of Rhizomucor miehei and Mucor racemosus were employed for the isolation of chitosan in which the degree of deacetylation were determined to be 98.6% and 97.1%, respectively (Tajdini et al. 2010). Production of LMWC and chitooligosaccharides (COS) from chitosan can be brought about either by chemical or enzymatic methods. The enzymatic hydrolysis of chitosan is the method of choice as it offers many advantages as compared to the energy intensive chemical method (Zhang et al. 2010). Unlike most polysaccharides, LMWC and COS possess positive charges, which allows them to bind strongly to negatively charged surfaces; this property is in turn responsible for many of the biological activities of chitosan (Cheung et al. 2015).
Crystallinity is maximum for chitin and fully deacetylated chitosan. In acidic environments, linear and high molecular chitosan acts as an excellent viscosity enhancer and a pseudoplastic material. The viscosity of chitosan solution increases with an increase in the concentration of chitosan used, decrease in temperature, and increasing DD (Dash et al. 2011). Chitosan shows different biological activities depending on its structures. Bioactive chitosan has been developed by various chemical modification and enzymatic hydrolysis (Zhang et al. 2010). The modified chitosan can be subsequently used for its potential biomedical applications.
6.5 Synthesis of Fungal Chitosan
Synthesis of chitin and chitosan from fungal mycelium has recently received increased attention due to its wide range of applications. Chitin and chitosan of crustacean may vary in the physicochemical properties, while fungal chitin and chitosan have relatively consistent properties because of the controlled fermentation conditions (Akila 2014).
The synthesis of chitin is mediated by an integral membrane enzyme; chitin synthase catalyzes the transfer of N-acetylglucosamine from uridine diphosphate (UDP)-N-acetylglucosamine to a growing chitin chain. In the solid state, chitin chains congregated by the H-bonds network which governs the solubility, swelling, and reactivity of the polymer. The hydrogen bonding between the newly formed polymers of chitin results in microfibril formation and subsequent crystallization of chitin in the extracellular space immediately adjacent to the plasma membrane (Younes and Rinaudo 2015). This process of crystalline chitin synthesis primarily occurs at sites of active growth and cell wall remodeling. For yeasts, this includes areas such as the bud tip during polarized growth and the bud neck during cytokinesis. In filamentous fungi, the synthesis occurs at the hyphal apex. So far three chitin synthases are identified in S. cerevisiae, namely, Chs1p, Chs2p, and Chs3p. Chs1p aids in cell wall repair and replenish chitin polymers lost during cytokinesis. Chs2p is involved in the formation of the primary septum within the dividing yeast cell. The Chs3p chitin synthase is majorly responsible for producing ~80 to 90% of the total cellular chitin. A. fumigatus has seven chitin synthase encoding genes, designated as CHSA through CHSF. Similarly, in N. crassa four specific chitin synthases have been reported (Bowman and Free 2008).
6.6 Methods for Preparation of Chitosan-Based Nanocomposites
Different methods have been employed for the preparation of chitosan-based nanocomposites and conjugated to different bioactive compounds (hydrophilic molecules, hydrophobic molecules, and macromolecules) either by covalent or reversible bonds. These compounds can be simply embedded through physical and irreversible interactions (hydrogen bonds, van der Waals forces, hydrophobic effects, electrostatic interactions) or can be also be loaded onto the surface of nanoparticles (Bugnicourt and Ladaviere 2016). However, the method to be employed depends on factors such as particle size, stability of the active agents and final product, loading efficiency, release kinetics, residual toxicity, the nature of the active molecule, and the delivery agent (Agnihotri et al. 2004). The lack of target specificity of bioactive species leading systemic distribution results in wastage of large doses of drugs and harmful side effects. Physiological environment such as the gastric environment at pH ∼2 can also degrade bioactive agent before reaching the targeted site.
The advances in the area of nanoparticles research bring promising solutions to overcome the abovementioned problems. The nanoparticles possess the ability to encapsulate drug molecule, thereby shielding it from the harsh physiological environment. Moreover, the controlled release and targeted delivery of the loaded drug is possible through the surface modification of nanoparticles (Bugnicourt and Ladaviere 2016). Chitosan nanoparticles (CS-NPs) have been employed to encapsulate bioactive compounds, through the following approaches (Table 6.2).
6.6.1 Ionotropic Gelation
The ionic gelation technique is by far the most simple and widely used methods used to prepare chitosan nanoparticles (CS-NPs). The polymeric nanoparticles are formed by electrostatic cross-links between the positively charged amino groups in CS molecules and the negatively charged sodium TPP (Zhang et al. 2016). For ionic gelation, chitosan is dissolved in aqueous acidic solution which quaternizes the chitosan amino groups making it soluble; this solution is then added dropwise under constant stirring to polyanionic TPP solution. The complexation between oppositely charged species causes the chitosan to undergo ionic gelation and precipitate as spherical particles. Various formulations of chitosan nanoparticles produced by the ionic gelation of TPP and chitosan were studied by Xu and Du (2003). As the CS reacts with TPP by simple electrostatic interaction, there is no permanent chemical cross-linking. In addition, the use of toxic chemicals is also avoided throughout the preparation and loading procedure (Jamil et al. 2016). The chitosan/TPP nanoparticles have also been used to incorporate metal ions, such as silver, copper, and zinc, to enhance their antimicrobial activity (Qi et al. 2005; Du et al. 2009; Potara et al. 2011) or iron oxide (Sanjai et al. 2014) and gadolinium (Jahanbin et al. 2015). Zhang and Zhao (2015) prepared TP-loaded β-chitosan (CS) nanoparticles (NPs) based on the principle of ionic gelation between CS and sodium tripolyphosphate (TPP). The tea polyphenol (TP)-Zn complex-loaded β-CS NPs had an encapsulation efficacy of 97.33% and average particle size of 84.55 nm. Further, TP-Zn complex-loaded β-CS NPs exhibited higher antioxidant activity than that of TP-loaded β-CS NPs (Zhang and Zhao 2015).
6.6.2 Coprecipitation
This method is based on the principle that chitosan is insoluble in alkaline pH and hence precipitates/coacervates when it in contact with alkaline solution. In this method, chitosan solution is added into an alkali solution, for example, sodium hydroxide, NaOH-methanol, or ethanediamine, by means of a compressed air nozzle to form nanoparticles (Mao et al. 2001). Under the action of emulsified solvent, the water phase containing chitosan is dispersed in the organic phase encapsulating the drug, where turbulence appears between the interfaces of the two phases and chitosan is precipitated, resulting in the generation of nanoparticles (Wang et al. 2011). The size can be controlled by regulating the compressed air pressure or spray-nozzle diameter. The release of drug is controlled by using appropriate cross-linking agent (Madureira et al. 2015). This technique has been used to prepare chitosan-DNA nanoparticles by Mao et al. to encapsulate and protect the plasmid DNA from nuclease degradation. The particle size was successfully optimized to 100–250 nm with a narrow distribution by keeping the amino to phosphate group ratio between 3 and 8 and chitosan concentration of 100 μg/ml (Mao et al. 2001). Liu et al. (2011) prepared magnetic Fe3O4-chitosan nanoparticles by coprecipitation using glutaraldehyde as a cross-linking agent. The synthesized nanoparticles were used subsequently used to immobilize lipase (Liu et al. 2011).
6.6.3 Emulsion Cross-Linking
This method exploits the reactive functional amine group of chitosan to interact with the available reactive groups of the cross-linking agent. In this method water-in-oil (w/o) emulsion is obtained by emulsifying the chitosan aqueous solution in the oil phase. A suitable surfactant is used to stabilize the aqueous droplets and obtain the final particles. Consequently, a stable emulsion is cross-linked using a suitable cross-linking agent to solidify the particles. This method is useful to control the dimension of the NPs, because the dimension of the final product is dependent on the amount of cross-linking agent used. The emulsion cross-linking method involves a few drawbacks, such as the use of organic solvents which affects the proteins and cell viability. Moreover, complete removal of the unreacted cross-linking agent may be a challenge (Shi et al. 2011; Wang et al. 2011; Madureira et al. 2015). Sankar et al. (2001) used this method to prepare chitosan-based pentazocine microspheres for intranasal delivery. The formulation parameters such as drug loading, polymer concentration, stirring speed during cross-linking, and oil phase were controlled to develop the desired microspheres. The in vivo and in vitro studies indicated a significant enhancement in bioavailability of pentazocine and diffusion-controlled release kinetics, respectively (Sankar et al. 2001). Majithiya and Murthy (2005) developed chitosan-based mucoadhesive microspheres of clarithromycin using glutaraldehyde as a cross-linking agent. The prepared microspheres exhibited an entrapment up to 74% along with sustained release of the drug. In another study, oleic acid-coated Fe3O4 nanoparticles are absorbed by chitosan and cross-linked with glutaraldehyde, resulting in Fe3O4-chitosan nanoparticles of average size of 10.5 nm with a narrow size distribution. These nanoparticles have highly saturated magnetization effect, superparamagnetic properties, and a sufficiently high temperature to induce hyperthermia (Qu et al. 2010).
6.6.4 Droplet Coalescence Method
Developed by Tokumitsu et al. (1999), in this method precipitation is caused by enabling coalescence of chitosan particles with NaOH, rather than cross-linking stable particles. Two separate emulsions are prepared, one containing aqueous solution of chitosan along with drug and another containing chitosan aqueous solution on NaOH in liquid paraffin oil. When the emulsions are blended under high-speed stirring, the particles of each emulsion collide at random and coalesce to generate small-sized particles that precipitate (Tokumitsu et al. 1999). This method was employed to prepare gadolinium-loaded chitosan nanoparticles (Gd-nanoCPs) with different particle sizes by using Gd-DTPA and chitosan with varying molecular weight. The nanoparticles formed exhibited significant tumor growth suppression (Shikata et al. 2001; Ichikawa et al. 2014).
6.6.5 Reverse Micellar Method
Reverse micelles are thermodynamically stable liquid mixtures of water, oil, and surfactant. The size, polydispersity, and thermodynamic stability of these droplets are maintained by the rapid dynamic equilibrium. Ultrafine polymeric nanoparticles with narrow size distribution could be achieved by using this method (Leong and Candau 1982). To prepare reverse micelles, the surfactant is dissolved in an organic solvent followed by the addition of chitosan and drug with constant agitation. To the transparent solution obtained, a cross-linking agent is added and incubated overnight with constant stirring. The maximum amount of drug that can be dissolved in reverse micelles varies from drug to drug and has to be determined by gradually increasing the amount of drug until the clear microemulsion turns translucent (Agnihotri et al. 2004; Mitra and Dey 2011). Anticancer drug, doxorubicin (DXR), coupled with dextran (DEX) encapsulated in chitosan nanoparticles was prepared by this method. The antitumor effect of these DEX-DXR nanoparticles (100 ± 10 nm diameter) when evaluated in J774A.1 macrophage tumor cells implanted in Balb/c mice resulted in the enhanced permeability and retention effect (EPR) in solid tumors and further reduction in undesirable side effects such as cardiotoxicity (Mitra et al. 2001).
6.6.6 Spray-Drying
This method is employed to create powders, granules, or agglomerates by the combination of drug and carrier solutions. The process is based on drying of atomized particles in a flow of hot air. For a short time, chitosan is diluted in aqueous acetic acid solution, and then drug is diluted or dispersed in the solution, followed by the addition of an appropriate cross-linking agent. This solution or dispersion is atomized in a flow of hot air that results in the formation of small particles, from which the solvent evaporates and immediately leads to the formation of nanoparticles. Various process parameters, e.g., the size of nozzle, spray flow rate, atomization pressure, inlet air temperature, and extent of cross-linking, are controlled to get the desired dimension of particles (He et al. 1999). Huang et al. (2002) prepared betamethasone disodium phosphate chitosan microspheres by this method using type-A gelatin and ethylene oxide-propylene oxide block copolymer poloxamer as modifiers. A good drug stability (less 1% hydrolysis product), high entrapment efficiency (95%), and positive surface charge (37.5 mV) was achieved. The gelatin/chitosan ratio of 0.4–0.6 (w/w) showed a fairly prolonged drug release up to 12 h (Huang et al. 2002). Similarly, Huang et al. (2010) prepared chitosan-iron oxide nanoparticles with various chitosan: iron oxide ratios by spray-drying. These nanoparticles were stable in water with strong superparamagnetic.
6.6.7 Sieving Method
It is a simple method developed by Agnihotri and Aminabhavi (2004) to produce chitosan microparticles containing the drug clozapine. In this method, the microparticles are prepared by cross-linking 4% acetic acid chitosan solution to form glassy hydrogels that are passed through a sieve. Clozapine (C18H19ClN4) was incorporated into chitosan gel before cross-linking with 99% efficiency. Irregular shaped microparticles (540–700 nm) were formed on sieving, and in vivo studies indicated a slow release of clozapine (Agnihotri and Aminabhavi 2004).
6.7 Chemical Modification and Functionalization of Chitosan
Chitosan usually reacts with other bioactive molecules or polymers and changes into derivatives or composites. The presence of free hydroxyl and amino groups on the chitosan chains allows chemical modifications and provides sites for a variety of side group attachment under mild conditions (Cheung et al. 2015; Zhang et al. 2010). In addition, the characteristic features of chitosan, such as being cationic, hemostatic, and insoluble at high pH, can be modified by sulfating the amine group which makes the nano-derivative anionic and water-soluble (Dash et al. 2011). The mucoadhesive property of chitosan aids in transport of molecules across mucosal membrane and subsequent delivery of vaccines (Sawaengsak et al. 2014; Del Guidice and Baudner 2015). Chitosan nanoparticles are used as drug excipients as they are (i) biocompatible and biodegradable, (ii) soluble in water, (iii) available in a wide range of molecular weights, (iv) easy in derivatization, (v) good loading efficiency, and (vi) controlled drug release (Liu et al. 2014; Lu et al. 2014; Ragelle et al. 2014).
Generally, the process of drug loading in chitosan systems are achieved either (i) by incorporating the drug simultaneously during the preparation of the particles or (ii) by loading the drug to the preformed particles by incubating with them. The first method is employed to incorporate water-soluble drugs with chitosan, while in case of water-insoluble drugs, the loading of drug involves incubation of preformed particles in a saturated solution of drug (Kumbar et al. 2002). Chitosan microspheres were loaded with tetracycline using two different methods, i.e., cross-linking and precipitation, by Hejazi and Amiji (2002). The loading efficacy was found to be 69% (w/w), and the release of about 30% of tetracycline in solution at pH 1.2 in 12 h was achieved (Hejazi and Amji 2002).
6.8 Biomedical Applications of Chitosan-Based Systems
6.8.1 Chitosan-Based Systems for Antibiotics
Drug delivery systems are designed to reduce drug side effects and to allow a specific drug to be delivered to the targeted tissue in a controlled manner. Chitosan nanoparticles (CS-NPs) have been widely used as drug carriers in diagnosis and therapeutics. Because of its nanosize, these nanoparticles can easily penetrate the targeted tissues (Jamil et al. 2016). Aranaz et al. (2016) synthesized ciprofloxacin hydrochloride (CFX)-loaded chitosan films to achieve controlled drug delivery of CFX. It was found that the amount of drug release was influenced by the thickness of the film and the degree of cross-linking. The antimicrobial effect of CFX and AgNPs-CFX-loaded chitosan films against P. aeruginosa was tested, and it was observed that the antimicrobial loaded films exhibited higher antimicrobial efficacy than the chitosan films alone (Aranaz et al. 2016). Jamil et al. (2016) reported the synthesis of cefazolin-fabricated CS-NPs by ionic gelation method. It was demonstrated that cefazolin-loaded CS-NPs possess excellent antimicrobial potential against multidrug-resistant K. pneumoniae and P. aeruginosa and extended spectrum beta-lactamase (ESBL)-positive E. coli.
Limited cellular penetration reduces the effectiveness of many antimicrobial treatments and also results in various side effects. Ibrahim et al. (2015) explored the possible improvement in cellular penetration and antimicrobial activity of the antibiotics (ciprofloxacin, chlortetracycline hydrochloride, and gentamicin sulfate) when incorporated into chitosan-based nanoparticles. Chitosan nanoparticles were prepared via the ionic gelation of chitosan with tri-polyphosphate anions. The nanoparticles exhibited higher antibacterial activity against gram-positive (S. aureus) bacteria than gram-negative bacteria (E. coli) (Ibrahim et al. 2015).
6.8.2 Chitosan-Based Systems for Metals
Chitosan hybrid metal and metal oxide nanoparticles have been developed with excellent properties and synergistic effects. Currently, the research on the combination of CS and metal oxide has focused on titanium dioxide (TiO2), as it possesses excellent photocatalytic performance and is stable in acidic and alkaline solvents. In addition to TiO2, CS has tremendous ability to form metal complexes with Zn metal (Dhillon et al. 2014). The ZnO-CS NPs were synthesized by nano-spray-drying and precipitation significantly inhibited biofilm formation and growth in both M. luteus and S. aureus at a concentration ranging from 0.625 to 0.156 mg/ml. Hebeish et al. (2014) prepared chitosan-grafted-poly acrylonitrile silver nanocomposites (Cs-g-PAN/Ag) via in situ chemical reduction of Ag ions in graft copolymerization of acrylonitrile onto chitosan. The nanoparticles with an average of 15–20 nm showed excellent antimicrobial performance toward both E. coli and S. aureus (Hebeish et al. 2014). Synergistic antibacterial activity of chitosan-silver nanocomposites on S. aureus results in the changes in morphology of S. aureus cells due to disruption of bacterial cell wall integrity (Potara et al. 2011). Chitosan nanoparticles and copper(II)-loaded chitosan nanoparticles were prepared by ionic gelation of chitosan with tripolyphosphate anions and copper ion sorption. It was observed that chitosan nanoparticles and copper(II)-loaded chitosan nanoparticles exerts dose-dependent cytotoxic effects on the proliferation of tumor cell lines, BEL7402, BGC823, and Colo320 tumor cells, while having little effect on the growth of L-02 human normal liver cells (Qi et al. 2005). Metal ions, Ag+, Cu2+, Zn2+, and Mn2+, loaded onto chitosan nanoparticles were prepared based on ionic gelation between chitosan and sodium tripolyphosphate. The metal ion-loaded nanoparticles showed enhanced antibacterial activity against both gram-positive and gram-negative bacteria (E. coli 25922, S. choleraesuis ATCC 50020, and S. aureus 25923) (Du et al. 2009).
6.8.3 Chitosan-Based Systems for Protein and Peptides
Antimicrobial protein and peptides have received growing interest due to their broad spectrum of activities. It is well known that lysozyme can inhibit some gram-positive bacteria due to its unique ability to damage bacterial cell wall by hydrolyzing 1,4-β-linkage between N-acetyl-muramic acid and N-acetyl-d-glucosamine of cell wall peptidoglycan (Yuan et al. 2013). CS-NPs and chitosan-lysozyme nanoparticles (CS-Lys-NPs) were prepared according to the ionic gelation technique with tripolyphosphate anions. The integration of lysozyme into CS-NPs enhanced the antibacterial activity against negative bacteria, E. coli, and gram-positive bacteria, B. subtilis. The antibacterial action may be attributed to the ability of CS-NPs/CS-Lys-NPs to penetrate the cell membrane, which results in the leakage of the cytoplasm components and eventual cell death. CS-NPs/CS-Lys-NPs also influence metabolism enzyme activities and interfere with bacterial metabolism (Wu et al. 2017). In another report, Piras et al. (2014) explored the use of CS-NPs as delivery systems for lysozyme (LZ). The chitosan nanoparticles loaded with lysozyme (LZ-NPs) were successfully prepared by means of a mild ionic gelation technique. LZ loading in the NPs was up to 8% and the release up to 20% over 3 weeks, in a controlled and sustained manner. The nanoparticles showed in vitro cytocompatibility and a good antimicrobial activity on S. epidermidis (Piras et al. 2015). The encapsulation of the frogskin-derived antimicrobial peptide, AMP temporin B (TB), into chitosan nanoparticles(CS-NPs) resulted in enhanced activity of the antimicrobial peptide, while reducing its toxic potential. TB-loaded CS-NPs were prepared, based on the ionotropic gelation between CS and sodium tripolyphosphate. The encapsulation efficiency of TB in the formulation was up to 75%. The encapsulation of TB in CS-NPs significantly reduces the peptide’s cytotoxicity against mammalian cells. Moreover, the nanocarrier evidenced a sustained antibacterial action against various strains of S. epidermidis (Piras et al. 2015).
6.8.4 Chitosan-Based Systems for Dye
Immobilization of photosensitizers on polymeric supports prevents the toxic side effects of residual photosensitizers and also provides an added advantage of stability in the physiological environment. Nanoparticles either encapsulated or surface modified with photosensitizer have been studied for enhanced antimicrobial photodynamic therapy (PDT). Shrestha et al. (2014) reported the synthesis of photoactivated rose Bengal (RB) dye-functionalized CS nanoparticles (CSRBnp) by conjugating CS-NPs with RB via chemical cross-linking. CSRBnp exerted antibacterial mechanism by adhering to bacterial cell surface, permeabilizing the membrane and lysing the cells subsequent to photodynamic treatment. CSRBnp combined with PDT showed complete disruption of the biofilm structure and reduced viability of E. faecalis biofilms (Shrestha et al. 2014).
6.9 Conclusion
Chitosan nanoparticles have attracted increasing attention because of their biocompatible, biodegradable, and nontoxic nature. In the area of therapeutics, biocidal and biostatic natural polymers are incorporated into fibers, membrane, or hydrogel and used for various biomedical applications, including wound dressing, tissue engineering, drug delivery, cancer diagnosis, etc. Functionalized nanoparticles decorated with various bioactive molecules and peptides have opened up new avenues in therapeutics and diagnosis. These modified nanomaterials offer unique physicochemical properties, such as ultrasmall sizes, large surface area/mass ratio, and increased chemical reactivity. Developing targeted chitosan carriers is an area of future development for sustained/controlled release drugs and targeted delivery. The absorption and bioavailability of drug encapsulated into chitosan nanoparticles renders improved delivery of bioactive molecules/drugs and also protect them from enzymatic degradation. Great progress has been achieved in the application of chitosan nanoparticles as drug carriers. However, further investigation is required on the biocompatibility of modified chitosan and its derivatives for wider applications.
References
Agnihotri SA, Aminabhavi TM (2004) Controlled release of clozapine through chitosan microparticles prepared by a novel method. J Control Release 96:245–259
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM (2004) Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release 100(1):5–28
Akila RM (2014) Fermentative production of fungal Chitosan, a versatile biopolymer (perspectives and its applications). Adv Appl Sci Res 5(4):157–170
Aranaz I, Harris R, Navarro-Garcia F, Heras A, Acosta N (2016) Chitosan based films as supports for dual antimicrobial release. Carbohydr Polym 146:402–410
Bowman SM, Free SJ (2008) The structure and synthesis of the fungal cell wall. Bioassays 28(8):799–808
Bugnicourt L, Ladaviere C (2016) Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Prog Polym Sci 60:1–17
Chatterjee S, Adhya M, Guha KA, Chatterjee BP (2005) Chitosan from Mucor rouxii: production and physico-chemical characterization. Process Biochem 40:395–400
Chattopadhyay D, Inamdar M (2013) Improvement in properties of cotton fabric through synthesized nano-chitosan application. Indian J Fibre Text Res 38:14–21
Cheung RC, Ng TB, Wong JH, Chan WY (2015) Chitosan: an update on potential biomedical and pharmaceutical applications. Mar Drugs 13(8):5156–5186
Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Chitosan-a versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci 36:981–1014
Del Guidice G, Baudner B (2015) Mucosal vaccines with chitosan adjuvant and meningococcal antigens. Patent
Dhillon GS, Kaur S, Brar SK (2014) Facile fabrication and characterization of chitosan-based zinc oxide nanoparticles and evaluation of their antimicrobial and antibiofilm activity. Int Nano Lett 4:107
Di Mario F, Rapana P, Tomati U, Galli E (2008) Chitin and chitosan from Basidiomycetes. Int J Biol Macromol 43(1):8–12
Du WL, Niu SS, Xu YL, Xu ZR, Fan CL (2009) Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polymer 75:385–389
Duttagupta DS, Jadhav VM, Kadam VJ (2015) Chitosan: a propitious biopolymer for drug delivery. Curr Drug Deliv 12(4):369–381
He P, Davis SS, Illum L (1999) Chitosan microspheres prepared by spray drying. Int J Pharm 187:53–65
Hebeish AA, Ramadan MA, Montaser AS, Farag AM (2014) Preparation, characterization and antibacterial activity of chitosan-g-poly acrylonitrile/silver nanocomposite. Int J Biol Macromol 68:178–184
Hejazi R, Amiji M (2002) Stomach-specific anti-H pylori therapy. I: preparation and characterization of tetracyline-loaded chitosan microspheres. Int J Pharm 235:87–94
Huang Y, Yeh M, Chiang C (2002) Formulation factors in preparing BTM-chitosan microspheres by spray drying method. Int J Pharm 242:239–242
Huang HY, Shieh YT, Shih CM, Twu YK (2010) Magnetic chitosan/iron (II, III) oxide nanoparticles prepared by spray-drying. Carbohydr Polym 81(4):906–910
Ibrahim HM, El-Bisi MK, Taha GM, El-Alfy EA (2015) Chitosan nanoparticles loaded antibiotics as drug delivery bio material. J Appl Pharm Sci 5(10):085–090
Ichikawa H, Uneme T, Andoh T, Arita Y, Fujimoto T, Suzuki M, Sakurai Y, Shinto H, Fukasawa T, Fujii F, Fukumori Y (2014) Gadolinium-loaded chitosan nanoparticles for neutron-capture therapy: influence of micrometric properties of the nanoparticles on tumor-killing effect. Appl Radiat Isot 88:109–113
Jahanbin TH, Sauriat-Dorizon P, Spearman S, Benderbous H, Korri-Youssoufi (2015) Development of Gd(III) porphyrin-conjugated chitosan nanoparticles as contrast agents for magnetic resonance imaging. Mater Sci Eng C 52:325–332
Jamil B, Habib H, Abbasi S, Nasir H, Rahman A, Rehman A, Bokhari H, Imran M (2016) Cefazolin loaded chitosan nanoparticles to cure multi drug resistant Gram-negative pathogens. Carbohydr Polym 20(136):682–691
Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H (2010) Biomedical applications of chitin and chitosan based nanomaterials-a short review. Carbohydr Polym 82:227–232
Kleekayai T, Suntornsuk W (2011) Production and characterization of chitosan obtained from Rhizopus oryzae grown on potato chip processing waste. World J Microbiol Biotechnol 27(5):1145–1154
Kong M, Chen XG, Xing K, Park HJ (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 144:51–63
Kuhlmann KA, Czupala J, Haunhorst Weiss T, Prasch U, Schorken A (2000) Preparation and characterization of chitosan from Mucorales. In: Peter M, Domard A, Muzzarelli RAA (eds) Advances in chitin science. Druckhaus Schmergow, Germany, pp 7–14
Kumbar SG, Kulkarni AR, Aminabhavi TM (2002) Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: effect of crosslinking agent. J Microencapsul 19:173–180
Leong YS, Candau F (1982) Inverse microemulsion polymerization. J Phys Chem 86:2269–2271
Liu Y, Jia SY, Wu QA, Ran JY, Zhang W, Wu SH (2011) Studies of Fe3O4-chitosan nanoparticles prepared by co-precipitation under the magnetic field for lipase immobilization. Catal Commun 12:717
Liu L, Dong X, Zhu D, Song L, Zhang H, Leng XG (2014) TATLHRH conjugated low molecular weight chitosan as a gene carrier specific for hepatocellular carcinoma cells. Int J Nanomedicine 9:2879
Lu Y, Cheng D, Lu S, Huang F, Li G (2014) Preparation of quaternary ammonium salt of chitosan nanoparticles and their textile properties on Antheraea pernyi silk modification. Text Res J 84(19):2115–2124
Madureira AR, Pereira A, Pintado M (2015) Current state on the development of nanoparticles for use against bacterial gastrointestinal pathogens. Focus on chitosan nanoparticles loaded with phenolic compounds. Carbohydr Polymer 130:429–439
Majithiya RJ, Murthy RS (2005) Chitosan-based mucoadhesive microspheres of clarithromycin as a delivery system for antibiotic to stomach. Curr Drug Deliv 2(3):235–242
Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW (2001) Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release 70:399–421
Mazancova P, Nemethova V, Lacik I, Razga F (2017) Chitosan-based particles: the (forgotten) interplay between process, properties and performance. Mater Sci Eng C Mater Biol Appl 71:570–571
Mitra A, Dey B (2011) Chitosan microspheres in novel drug delivery systems. Indian J Pharm Sci 73(4):355–366
Mitra S, Gaur U, Ghosh PC, Maitra AN (2001) Tumor targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release 74:317–323
Muzzarelli RAA, Boudrant J, Meyer D, Manno N, DeMarchis M, Paoletti MG (2012) Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: a tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr Polymer 87:995–1012
Niederhofer A, Muller BW (2004) A method for direct preparation of chitosan with low molecular weight from fungi. Eur J Pharm Biopharm 57(1):101–105
Piras AM, Maisetta G, Sandreschi S, Esin S, Gazzarri M, Batoni G, Chiellini F (2014) Preparation, physical-chemical and biological characterization of chitosan nanoparticles loaded with lysozyme. Int J Biol Macromol 67:124–131
Piras AM, Maisetta G, Sandreschi S, Gazzarri M, Bartoli C, Grassi L, Esin S, Chiellini F, Batoni G (2015) Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front Microbiol 6:372
Pochanavanich P, Suntornsuk W (2002) Fungal chitosan production and its characterization. Lett Appl Microbiol 35(1):17–21
Potara M, Jakab E, Damert A, Popescu O, Canpean V, Astilean S (2011) Synergistic antibacterial activity of chitosan-silver nanocomposites on Staphylococcus aureus. Nanotechnology 22(13):135101
Qi L, Xu Z, Jiang X, Li Y, Wang M (2005) Cytotoxic activities of chitosan nanoparticles and copper-loaded nanoparticles. Bioorg Med Chem Lett 15(5):1397–1399
Qu J, Liu G, Wang Y, Hong R (2010) Preparation of Fe3O4-chitosan nanoparticles used for hyperthermia. Adv Powder Technol 21(4):461–467
Ragelle H, Riva R, Vandermeulen G, Naeye B, Pourcelle V, Le Duff CS, D’Haese C, Nysten B, Braeckmans K, De Smedt SC (2014) Chitosan nanoparticles for siRNA delivery: optimizing formulation to increase stability and efficiency. J Control Release 176:54–63
Sanjai C, Kothan S, Gonil P, Saesoo S, Sajomsang W (2014) Chitosan-triphosphate nanoparticles for encapsulation of super-paramagnetic iron oxide as an MRI contrast agent. Carbohydr Polym 104:231–237
Sankar C, Rani M, Srivastava AK, Mishra B (2001) Chitosan based pentazocine microspheres for intranasal systemic delivery: development and biopharmaceutical evaluation. Pharmazie 56:223–226
Sawaengsak C, Mori Y, Yamanishi K, Mitrevej A, Sinchaipanid N (2014) Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS Pharm SciTech 15(2):317–325
Shi LE, Fang XJ, Xing LY, Chen M, Zhu DS, Zhu L, Guo XF, Zhao LM, Tang ZX (2011) Chitosan nanoparticles as drug delivery carriers for biomedical engineering. J Chem Soc Pak 33:929–934
Shikata F, Tokumitsu H, Ichikawa H, Fukumori Y (2001) In vitro cellular accumulation of gadolinium incorporated into chitosan nanoparticles designed for neutron-capture therapy of cancer. Eur J Pharm Biopharm 53(1):57–63
Shrestha A, Hamblin MR, Kishen A (2014) Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomedicine 10(3):491–501
Suntornsuk W, Pochanakanich P, Suntornsuk L (2002) Fungal chitosan production on food processing by-products. Process Biochem 37:727–729
Tajdini F, Amini MA, Nafissi-Varcheh N, Faramarzi MA (2010) Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucor miehei and Mucor racemosus. Int J Biol Macromol 47(2):180–183
Tayel AA, Moussa S, Opwis K, Knittel D, Schollmeyer E, Nickisch-Hartfiel A (2010) Inhibition of microbial pathogens by fungal chitosan. Int J Biol Macromol 47(1):10–14
Tayel AA, Moussa SH, El-Tras WF, Elguindy NM, Opwis K (2011) Antimicrobial textile treated with chitosan from Aspergillus niger mycelial waste. Int J Biol Macromol 49(2):241–245
Tokumitsu H, Ichikawa H, Fukumori Y (1999) Chitosan-gadopentetic acid complex nanoparticles for gadolinium neutron-capture therapy of cancer: preparation by novel emulsion-droplet coalescence technique and characterization. Pharm Res 16:1830–1835
Wang JJ, Zeng ZW, Xiao RZ, Xie T, Zhou GL, Zhan XR, Wang SL (2011) Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine 6:765–774
Wu T, Wu C, Fu S, Wang L, Yuan C, Chen S, Hu Y (2017) Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr Polym 155:192–200
Xu Y, Du Y (2003) Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm 250:215–226
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13(3):1133–1174
Yuan S, Yin J, Jiang W, Liang B (2013) Enhancing antibacterial activity of surface-grafted chitosan with immobilized lysozyme on bioinspired stainless steel substrates. Colloids Surf B Biointerf 106:11–21
Zhang HC, Zhao YY (2015) β-Chitosan nanoparticles encapsulated tea polyphenol–Zn complex as a potential antioxidant substances delivery carrier. Food Hydrocoll 48:260–273
Zhang J, Xia W, Liu P, Cheng Q, Tahirou T, Gu W, Li B (2010) Chitosan modification and pharmaceutical/biomedical applications. Mar Drugs 8(7):1962–1987
Zhang H, Jung J, Zhao Y (2016) Preparation, characterization and evaluation of antibacterial activity of catechins and catechins-Zn complex loaded β-chitosan nanoparticles of different particle sizes. Carbohydr Polym 137:82–91
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rajkumari, J., Busi, S. (2018). Advances in Biomedical Application of Chitosan and Its Functionalized Nano-derivatives. In: Prasad, R., Kumar, V., Kumar, M., Wang, S. (eds) Fungal Nanobionics: Principles and Applications. Springer, Singapore. https://doi.org/10.1007/978-981-10-8666-3_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-8666-3_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8665-6
Online ISBN: 978-981-10-8666-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)