Abstract
Oleanolic acid is extracted from Olea europaea, which is an important triterpenoid. Oleanolic acid aids in liver protection, especially increasing the amount of metallothionein and preventing glutathione emptying, and has antimicrobial and antiviral effects. Further, oleanolic acid exerts anticancer, anti-inflammatory, anti-HIV infection, hypoglycemic, and other functions. However, the poor bioavailability limited its application range. In the history of oleanolic acid’s R&D, balancing the bioavailability and the pharmacological activity in the process of drug preparation is the key to the further development of new drugs.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
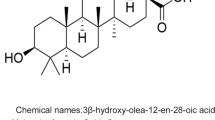
3-Hydroxy-12-oleanen-28-oic acid
-
Molecular formula, C30H48O3; MW, 456.70; CAS, 508-02-1
Properties
Appearance: Olea europaea exists in white needle crystal with ethanol or methanol. Melting point: It melts at 306–310 °C. Solubility: The ingredient is insoluble in water. Specific optical rotation: Polarized light rotates +83.3° when it passes through chloroform with concentration of 6 mol/L.
Derivatives
In recent years, there are many studies on the structure-activity relationship. Hydroxyl in the position of C-3, C-28 is the modificatory target to decrease blood glucose. The combination with glycogen phosphorylase, protein tyrosine phosphatase 1B, and α-glucosidase locus can be influenced by qualification of ring A and double bond in the position of C-12, C-13. Cyano group into the position of C-2 and modified ring A, C give rise to anti-inflammatory activity of the compound. Hydroxyl in the position of C-3, C-28 and ring A can be replaced to resist tumor [4]. People are dedicated to increase the activity of anti-HIV by modifying the hydroxyl in C-3, C-28.
Dosage Forms
Tablet and capsule
Indication
It is included in the Pharmacopoeia of the People’s Republic of China (Part 2, volume 3), the British Pharmacopoeia (2017), and the European Pharmacopoeia (8.7th ed.).
It is mainly used for the adjuvant treatment of acute infectious icteric hepatitis and acute and chronic hepatitis. It also can be utilized in psoriasis, rheumatoid arthritis, nephritic edema, ascites, stomach with turbid, metrorrhagia, bruises, carbuncle, soreness and weakness of the waist and knees, fetal irritability and so on.
Literature
The ingredient is mainly extracted from the leaves of muxilan 木樨榄 (Olea europaea). Besides, it is found in the fruit of ligustrum lucidum ait, the whole grass of swertia mileensis t.n.he et w.l.shi, s. mussotii franch, the leaves and roots of astrantia major, the root bark and stem bark of aralia chinensis, the roots of hemsleya macrosperma c.y.wu , the roots of hemsleya amabilis diels, and the roots of hemsleya chinensis cogn. There is a long history in the herb and fruit which contain oleanolic acid. They play an important role in the treatment of various diseases. Oleanolic acid may be the most effective component in various Chinese medicines.
History of R&D
The chemical formula was first identified in 1918 [1]. In 1960, Khastgir purified the compound for the first time [2]. Corey identified the structure of oleanolic acid in 1993 [3].
Now the compound has been made into a drug and is being marketed in China. As early as 2002, the tablet form of oleanolic acid was listed. In 2010, capsule preparations came into the market, and to date, there is no other listed preparation. Sanofi treated it as antiulcer and NSAIDs candidate in abroad.
Although it has anti-inflammatory and anti-ulcer effects, Sanofi stopped the study of developing oleanolic acid into a drug in 1989.
Pharmacology
Liver protection: Pretreatment of 100 mg/kg once a day for 3 days can protect the liver and decrease the activity of enzymes [5]. It achieves by increasing the amount of metallothioneins and liver glucoside transferases to prevent glutathione emptying, to inhibit several subtypes of P450 oxidase, and to prevent combination with toxic substances.
Antimicrobial and antiviral effect: Oleanolic acid may inhibit the proliferation or production of NO induced by interferon or in the macrophages by competitively binding to the enzymes necessary for the growth and reproduction of microorganisms or virus and play an anti-inflammatory and antiviral role. EC50 of HIV-1-infected H9 cells is 3.4 mol/L.
Effects on metabolism of glucose and lipid: The treatment of 50–100 mg/kg for 4 days achieves the glucosidase activity inhibition, reduction of glucose absorption, inhibition of glycogen phosphorylase, activation of glycogen synthesis, inhibition of protein tyrosine phosphatase 1B, regulation of insulin signaling pathway, and increase of insulin sensitivity, and it may also be achieved by inhibiting the transport of glucose from the stomach to the intestine and transport activity of intestinal villi. 200 mg/kg suspension administrated by gavage for 4 weeks decreases the level of triglycerides, cholesterol, and β-lipoprotein in high-fat animal, and it contributes to increased platelet swimming rate and decreased blood viscosity.
Effects on immunologic function: 60 mg/kg intraperitoneal injection may regulate the immune system by inhibiting type I allergic reaction, promoting proliferation of lymphocyte and inhibiting enzyme C-3 in the traditional complement pathway.
Antioxidant effect: It fights against lipid peroxidation by scavenging free radicals.
Other functions: 10–5 mol/L oleanolic acid may inhibit the activity of DNA ligase I active site. It exerts anticancer and anti-mutation effects, and IC50 value is 2.2 × 105 mol/L in inhibiting ornithine decarboxylase (ODC) induced by external TPA at the transcriptional level. Oleanolic acid in polyvinylpyrrolidone suspension at the dose of 16 mg/kg by intragastric administration for 30 days also reversibly decreases quality and exercise capacity of mouse sperms [6].
It is reported that it is consistent with the single compartment model, or non- compartmental model, and may be distributed in the compound in accordance with the two compartment models [7]. Eventually, it will be eliminated by P450 oxidase in the liver.
Clinical Application
It is reported that contrast with Western medicine, the 60~90 mg/d oleanolic acid tablets supplemented with vitamin B can treat acute and chronic hepatitis, with effective rates of 94.4% and 69.81%, respectively, in clinical results [8]. It has been listed on the drug manual as adjuvant therapy for acute and chronic hepatitis.
Discussion
According to the results of current research, oleanolic acid is effective in protecting the liver. It also exerts anticancer, anti-inflammatory, anti-HIV infection, and hypoglycemic functions. Due to its poor bioavailability, balancing the bioavailability and the pharmacological activity in the process of drug preparation is the key to the further development of new drugs.
References
Dodge FD. The isomeric lactones, caryophyllin add urson. J Am Chem Soc. 1918;40:1917–39.
Khastgir H, Sengupta S, Sengupta P. Note on the constituents of the Indian medicinal plant Oldenlandia corymbosa Linn. J Am Pharm Assoc. 1960;49(8):562–3.
Corey E, Lee J. Enantioselective total synthesis of oleanolic acid, erythrodiol, beta.-amyrin, and other pentacyclic triterpenes from a common intermediate. J Am Chem Soc. 1993;115(19):8873–4.
Tang C, Chen Y, Bai S, et al. Advances in the study of structural modification and biological activities of oleanolic acid. Chin J Org Chem. 2012;33(01):46–65.
Tian LT, Ma L, Du NS. Survey of pharmacology of oleanolic acid. China J Chin Mater Med. 2002;27(12):884–6.
Mdhluli M, Horst GVD. The effect of oleanolic acid on sperm motion characteristics and fertility of male Wistar rats. Lab Anim. 2002;36(4):432–7.
Pei D, OuYang Z, Zhao M, et al. Pharmacokinetics studies on ursolic acid and oleanolic acid from total terpenes of Longchai recipe in rats. Tradit Chin Drug Res Clin Pharmacol. 2013;3:017.
Wang LX, Han GX, et al. Survey of pharmacology and chemistry of oleanolic acid. J Pharm Pract. 2001;19(2):104–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd. and People's Medical Publishing House, PR of China
About this chapter
Cite this chapter
Zhou, QM., Du, GH. (2018). Oleanolic Acid. In: Natural Small Molecule Drugs from Plants. Springer, Singapore. https://doi.org/10.1007/978-981-10-8022-7_72
Download citation
DOI: https://doi.org/10.1007/978-981-10-8022-7_72
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8021-0
Online ISBN: 978-981-10-8022-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)