Abstract
Pyrolysis oil produced using lignocellulosic material is an alternate source of energy. Pyrolysis is an attractive option for the conversion of lignocellulosic biomass into liquid, pyrolysis oil, and gaseous hydrocarbons and is considered as an emerging and challenging research area in the current scenario of renewable energy. To ensure the production of “drop-in” liquid hydrocarbons from biomass, there is urgency of integration of pyrolysis process with conventional petroleum refinery’s trillion dollars worldwide infrastructure. This will happen into reality only after going through the necessary actions and precautions at various process development stages, such as biomass pyrolysis, pyrolysis oil upgrading, and effective integration of biomass pyrolysis with refinery. Herein, the opportunities lie in upgrading of pyrolysis oil in petroleum refinery units such as catalytic cracking and steam reforming, is described. The challenges arise ahead of pyrolysis oil upgrading in fluid catalytic cracking (FCC) approach have been reviewed. The extent of pyrolysis oil coprocessing with vacuum gas oil (VGO) in a refinery FCC unit is discussed. The advances in biomass pyrolysis process integration schemes with petroleum refinery have been revealed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The energy demand has increased due to an increase in worldwide population. The depletion of fossil fuel and concerns of environmental impact due to the usage of fossil energy resources forced the nations to take part in the development of alternative energy resources. Lignocellulosic biomass has been the potential feedstock for partial fulfillment of primary energy demand, and its demand will increase by 41% between 2012 and 2035 (Dudley 2014). Pyrolysis technology is considered to be the most promising one for getting higher (50–75%) yields of pyrolysis oil as an alternative to crude petroleum. The produced pyrolysis oil cannot be used as such because of its lower heating value, 15–20 MJ/kg, and the presence of oxygenated compounds, which are self-reactive during handling at ambient temperatures (Elliot et al. 1984).
The pyrolysis oil is a complex mixture of water, hydroxyaldehydes, hydroxyketones, phenolics, guaiacols, carboxylic acids, catechols, syringols, vanilins, sugars, and levoglucosan (Elliot et al. 1989). The composition and physicochemical properties of pyrolysis oil are different from petroleum or petroleum-derived fractions. This makes the pyrolysis oil immiscible with petroleum or petroleum-derived fractions. Besides, the pyrolysis oil is highly oxygenated (~40 wt%), acidic (pH ~ 2–3), thermally and chemically unstable; therefore, it cannot be directly utilized for fuel applications. Therefore, it requires additional upgrading to convert it into usable liquid hydrocarbons. A number of upgrading techniques have been proposed in last few decades, such as thermal treatment (Demirbas 2010), high-pressure thermal treatment (Venderbosch et al. 2010), thermal hydrotreating (Samolada et al. 1998), catalytic hydrotreating (Mercader 2010), catalytic emulsion (Zapata et al. 2012), and catalytic cracking (Graca et al. 2009). Among the aforementioned upgrading techniques, FCC seems to be a good option for effective use of refinery infrastructure as well as seeking an integration scope of pyrolysis process with refinery (Jones et al. 2009).
Hence, the chapter is aimed to discuss the key issues need to be addressed before upgrading the pyrolysis oil via refinery infrastructure, such as (i) opportunities available to process the pyrolysis oil in petroleum refinery infrastructure; (ii) challenges arise ahead of processing in catalytic cracking; (iii) upgrading of pyrolysis oil by catalytic cracking; (iv) options available for integration of biomass pyrolysis process with the petroleum refinery. This will enable to know the possibility of processing of pyrolysis oil with petroleum fraction in refinery in an effective way and also to find an application as an alternative fuel to fossil-based hydrocarbons.
2 Opportunities for Pyrolysis Oil Upgrading in Refinery
The first option available for pyrolysis oil processing in petroleum refinery infrastructure is FCC process, wherein, the straight chain hydrocarbons and the side chain of cyclic ring hydrocarbons are easily cracked and produce gasoline and olefins. The other option is the steam reforming from where the hydrogen can be produced.
2.1 Fluid Catalytic Cracking
The FCC process is extensively used for cracking hydrocarbons having high molecular weight into low molecular weight petrochemical feedstock like C3-C4 olefins, liquefied petroleum gas (LPG), gasoline, and light cycle oil (LCO). Herein, the cracking mechanism follows the carbonium ion theory. The cracking occurs over a catalyst containing hot-fluidized-microspherical particles of acidic SiO2–Al2O3 with a short contact time. The main advantages of catalytic cracking are (i) cost-effectiveness when compared to hydrocracking; (ii) enhanced product quality and selectivity over thermal cracking process; (iii) flexibility in processing of various types of feeds such as atmospheric gas oil (AGO), VGO, thermally cracked gas oil, hydrotreated VGO, hydrocracker bottom, coker gas oil, solvent deasphalted oil, reduced crude oil, and vacuum residue. The FCC process in petroleum refining has evolved over the last 60 years in fulfilling the challenges like cracking heavier and metallic contaminated feeds (with Ni and V), increasing operating flexibility, accommodating environmental legislation, and maximizing reliability.
Routinely, FCC plants have been operated on either gasoline or middle distillates modes; however, the developments in new generation catalysts led to LPG mode operation. The main reactions involved in the catalytic cracking are cracking (primary reaction) and many secondary reactions such as isomerization (double bond and skeletal), dehydrogenation, hydrogen transfer, cyclization, condensation, alkylation, and dealkylation. Besides, the non-condensable gases like methane, ethane, and ethylene are also obtained due to the cleavage of terminal bonds of hydrocarbon feedstock. Thus, ultimate composition of catalytic cracking product is a function of relative rates of different competing reactions (Scherzer 1990).
Globally, there are more than 400 FCC units operating in various capacities with fresh feed, wherein the UOP has been participated in the original nameplate design of FCC units from the scale of minimum 2000 bpsd, barrel per stream day, (Montana Refining, Great Falls, USA) to maximum 135,000 bpsd (Reliance Industries, Jamnagar, India) (Ibsen 2006). Presently, India and China have emerged as the key players for the growth of refinery FCC capacity. In the period 2005–2010, both countries reached the refinery FCC capacity of 43.0 MMTPA, which is 43.0% of the global refinery FCC capacity. In which, India alone is having the refinery FCC capacity of ~18.0 MMTPA to process fresh feed (Ghosh 2002). It helped in becoming net exporters of gasoline and other light hydrocarbons to the regions like Asia Pacific and the Middle East.
The acidic behavior of FCC catalyst is known for deoxygenation reactions, e.g., dehydration, decarboxylation, and decarbonylation, beyond the conventional FCC reactions (Sadeghbeigi 2000), wherein the β-scission is a primary reaction in FCC process, which occurs by breakage of the β C–C bond. The secondary reactions are alkylation, hydrogenation (Sedran 1994), isomerization (Mortensen et al. 2011), and condensation (Sedran 1994). According to the Whitmore (1934), the catalytic cracking of high molecular weight hydrocarbon is a chain reaction, which follows the carbonium ion theory. Adjaye and Bakhshi (1995a) proposed a reaction mechanism for FCC of pyrolysis oil, wherein it was reported that during catalytic cracking of pyrolysis oil over acidic zeolites undesirable products (such as tar and char) are produced. Corma et al. (2007) described the catalytic cracking mechanism in two stages, erstwhile one results in hydrogen production and the latter one leads to hydrogen consumption. Furthermore, a simplified mechanism proposed by Fogassy et al. (2010) for deoxygenation of pyrolysis oil is as follows:
Chen et al. (1986) reported that the effective hydrogen index (H/Ceff) is related to the amount of hydrogen available for energy production. It is defined as H/Ceff = (H–2O–3N–2S)/C; where H, O, N, S, and C correspond to the number of moles of hydrogen, oxygen, nitrogen, sulfur, and carbon present in the FCC feedstock. For energy production, the H/Ceff should be above the inflection point of 1.2, which is applicable to either processing or coprocessing of the pyrolysis oil with VGO or LCO in a FCC unit. Thus, it is very much needed to partially deoxygenate the pyrolysis oil in order to improve the H/Ceff of FCC feedstock. Otherwise, it makes the process problematic as the FCC process is originally designed and developed for petroleum fractions. In spite of several studies on coprocessing at laboratory and pilot plant level, the understanding of the blending limits and the presence of type of pyrolysis oil components on the FCC product distribution is still limited. The same holds for the understanding on the ways to optimizing the process parameters, to further obtain the limitations on coprocessing of pyrolysis oil with fraction and specifically the effect of type of pyrolysis oil compounds on FCC product distribution.
2.2 Steam Reforming
Worldwide, the steam reforming process has been used for the production of hydrogen (energy carrier), wherein a methane gas reacted with steam to produce hydrogen and carbon monoxide in a catalytic tubular reactor, which operates at around 850 °C. The requirement of hydrogen is large for the (i) ammonia synthesis; (ii) methanol production; (iii) hydrotreating (including hydrodesulfurization, hydrodenitrogenation, and hydrodemetalization) and hydrocracking of petroleum fractions.
Keeping in view of the possibility to produce the hydrogen from pyrolysis oil, various techniques such as catalytic steam reforming (Czernik et al. 2002; Wang et al. 2007; Kechagiopoulos et al. 2006), aqueous phase reforming (Vispute et al. 2009), electrochemical steam reforming (Ye et al. 2009), chemical looping reforming (Lea-Langton et al. 2012), catalytic steam gasification (Dinjus et al. 2004), and sequential cracking (Iojoiu et al. 2007) have been developed. Among them, the catalytic steam reforming is one of the well-known techniques and it is a part of refinery infrastructure. It is very well understood that the biomass-derived pyrolysis oil contains both water soluble (aqueous) and water insoluble (organic) fractions. The water soluble fraction can be effectively utilized by processing in very well-established steam reforming unit for the production of hydrogen. The added advantage of this technique is that the resulted hydrogen can be utilized for the upgradation of pyrolysis oil and the whole process can be integrated. The typical status of steam reforming of pyrolysis oil and their model compounds are shown in Table 12.1.
The overall stoichiometry of steam reforming of pyrolysis oil is 17.2 g H2 per 100 g pyrolysis oil (Milne et al. 2002). The thermodynamic calculations of Kechagiopoulos et al. (2006), reported the hydrogen yield is almost 90% for the chosen mixture of compounds (acetic acid, acetone, acetaldehyde, ethylene glycol, formic acid, methanol, formaldehyde, and ethanol). Czernik et al. (2001) mentioned that the NREL group employed a fluidized bed reactor configuration and used Sud-Chemie-supplied C11-NK catalyst for the coprocessing of natural gas, containing 82.4% CH4, 6.6% C2H6, 2.6% CO2, and 8.4% N2 (by volume.), with pine sawdust derived aqueous fraction of pyrolysis oil, containing 20 wt% organics and 80 wt% water and consisted of 11.8% carbon, 9.6% hydrogen, and 78.6% oxygen. It was reported that during co-reforming the hydrogen yield was about 80% of the stoichiometric value. It was mentioned that 25% yield of hydrogen was obtained from pyrolysis oil and remaining 75% from natural gas.
3 Challenges for Pyrolysis Oil Upgrading in Refinery FCC
The pyrolysis oil is a mixture of over 300 compounds including carboxylic acids, hydroxyl ketones, hydroxyl aldehydes, lignin-derived monomers, and anhydrosugars. The typical physical properties of biomass-derived pyrolysis oils are compared with fuel oil in Table 12.2. In the present scenario with these physical properties, the pyrolysis oil cannot be used in any engine or to upgrading equipment. Therefore, there is a need for stabilization of pyrolysis oil before taking into FCC for upgrading. Currently, the available standards for pyrolysis oil are ASTM D7544, which can be specifically applied for direct use in the furnaces and automobile engines.
From the view of maintaining heat balance of the FCC unit and to limit the fresh catalyst consumption within a reasonable level, the feed to the FCC should meet certain specifications with respect to its boiling point, coking tendency, and metal and sulfur contents. In addition to the above effective hydrogen index, composition of feedstock also plays an important role for coprocessing of pyrolysis oil with VGO in FCC unit. To meet the aforementioned specifications before feeding pyrolysis oil into FCC is a real challenge for the development of process in a view of integration of pyrolysis process with refinery FCC unit.
Moreover, the quality of biomass-derived pyrolysis oil is a function of pH, heating value, viscosity, nitrogen, water, ash, and char content. The properties (physical, chemical, and combustion) of pyrolysis oil changes with time. It is reported that over a period of six months the viscosity of pyrolysis oil increases from 1127 to 2283 cP (Tiplady et al. 1991). A few of the important challenges for meeting the specifications of feed and their significance are given below.
3.1 API Gravity
The American Petroleum Institute (API) gravity refers to the specific gravity at 60 °F by the formula: API = ((141.5-Specific Gravity)-131.5). It is a measure of how petroleum is heavier or lighter in comparison with water. The higher API value indicates that the feed is more saturated and less aromatic, which helps in easy cracking and the higher gasoline yield with slightly lower octane. The API gravity of feed to the FCC unit varies from 16 to 48. Therefore, it is very much essential to maintain the API gravity within the range, so that the pyrolysis oil can be easily cracked in FCC for maximization of gasoline yield.
3.2 UOP Characterization Factor
UOP or UOP K value is a factor used to measure the paraffinicity of petroleum oils based on its boiling point and specific gravity, which is related by the relation: UOP characterization factor = UOP K = {(Cubic average boiling point)1/3/(Specific Gravity)60°F}. The K value of 12.5 would designate that a feed is highly paraffinic (saturated), whereas the value of 11.2 would show more aromatic (unsaturated cyclic). Thus, the pyrolysis oil with K value of 12.5 and above is preferable for processing or coprocessing with pyrolysis oil in a FCC unit.
3.3 Boiling Range
The boiling range of FCC hydrocarbon feed usually varies from 260 °C (initial boiling point) to 540 °C (final boiling point). However, the biomass-derived pyrolysis oil boiling range starts at room temperature and goes up to even 560 °C as it contains multicomponents lower to higher boiling points. The presence of heavy components in FCC feed would throw in the formation of undesirable coke. Hence, the heavy components (poly-nuclear aromatics, organometallic, and high sulfur compounds) are considered as coke precursors.
3.4 Carbon Residue
The terminology of carbon residue provides a measure of the carbon deposition tendencies of FCC feed when heated in a bulb under prescribed conditions. It can be determined by either Conradson or Ramsbottom methods. The petroleum-derived vacuum gas oil containing Conradson carbon residue (CCR), indicative of coke-forming potential, of 0.74 wt% can be processed through FCC unit (Naik et al. 2014b), whereas the residual fluid catalytic cracking (RFCC) process can handle up to CCR of 4.06 wt%. Therefore, it is recommended to carry out the hydrotreatment of pyrolysis oils until predetermined level of conversion (<10 wt% of CCR) is achieved (Ardiyanti et al. 2012). Typically, the CCR ranges from 18 to 23 wt% for woody (oak, eucalyptus, and pine) biomass-derived pyrolysis oil, whereas it varies from 17 to 18 wt% for pyrolysis oil from wheat straw (Oasmaa et al. 1997).
3.5 Metal Content
Specifically, in the refinery FCC unit the presence of metals (Ni, V, Cu, and Fe) causes undesirable dehydrogenation reactions resulting in an increase of hydrogen, coke, olefins, and lighter hydrocarbons in the cracked products at the expense of LPG or gasoline. Nickel and copper are more effective in promoting these hydrogen-producing reactions. Thus, an increase in the volume ratio of hydrogen/methane (which is a measure of the extent of metallic poisoning) or the olefinic content of C3 stream is all indications of contaminations of the catalyst. The ratio of H2/CH4 from 0.3 to 0.8 is preferred, whereas the value >1.0 specifies significant degree of poisoning.
Further, the contamination of a feedstock to FCC like unit is measured by a metal factor (Fm), i.e., Fm = Fe + V + 10 (Ni + Cu); where Fe, V, Ni, Cu are the concentration in ppm of iron, vanadium, nickel, and copper. A metal factor of 1.0 is considered as safe and feedstock with metal factor of above 3.0 may result in poisoning the catalyst. The presence of sodium weakens the molecular structure and decreases the hydrothermal stability of catalyst resulting in damage due to sintering at lower temperatures and subsequent loss of surface area. Thus, the control of ash content before pyrolysis oil processing in refinery units is very much required to control the side reactions and poisoning of FCC catalyst. In regard to biomass, the inorganic materials, especially the potassium and calcium, catalyze biomass decomposition and char-forming reactions (Garcia-Perez et al. 2009).
3.6 Nitrogen
Nitrogen acts as a temporary catalyst poison which reduces the catalyst activity by neutralizing the acid sites of cracking catalyst available for promoting reactions. The presence of nitrogen compounds in the FCC feeds is undesirable as the organic nitrogen converts into basic nitrogen compounds (like ammonia, pyridine, quinoline) in FCC riser, which act as a poison by neutralizing the active acid sites of the cracking catalysts, which results in rapid loss of activity (Fu et al. 1985). The loss of activity results in changes in product selectivity of FCC. A FCC feed with total nitrogen concentration of <1000 ppm is not detrimental to activity, whereas it is detrimental on above 1500 ppm.
In case of woody biomass-derived pyrolysis oil, the nitrogen content is less than 0.4 wt% (wet basis) (Oasmaa and Peacocke 2010), whereas corn-derived pyrolysis oil is 1% (Elliott et al. 2009), which are highly dependent on type of biomass feedstock. The basic and nonbasic nitrogen species in petroleum also falls in the range of 0.1–0.9% (Abdel-A et al. 2003), whereas the conventional FCC feedstock, gas oil, contains the nitrogen in the range of 0.1–0.8 wt% (Scherzer 1987).
3.7 Hydrogen Effective Index
Typically, the number of hydrogen molecules available for the production of energy is related by the index called effective hydrogen index, which can be defined by the equation (H/Ceff = (H–2O–3N–2S)/C) as proposed (Chen et al.1986), wherein, H, O, N, S, and C are the number of moles of hydrogen, oxygen, nitrogen, sulfur, and carbon present in the FCC feedstock. The effective hydrogen index is less than 1 for highly oxygenated compounds, close to 1.5 for triglyceride-based biomass, whereas for hydrocarbons it varies from 2 (liquid alkanes) to 1 (for benzenes) (Melero et al. 2012); while it is 0, 1/3, and 2/3 for glucose, sorbitol, and glycerol, respectively (Corma et al. 2007). Zhang et al. (2011) reported that on conversion of lignocellulosic biomass-derived feedstocks over ZSM-5, the feedstocks with H/Ceff less than 0.15 produce more coke and it is suggested to have a minimum of H/Ceff of 1.2 (as an inflection point) to produce optimum aromatics and olefins in refinery setups.
3.8 Composition
It is very difficult to describe the overall composition of pyrolysis oil as it depends on many factors like feed structural composition, type of pyrolysis reactor, pyrolysis operating conditions, liquid collection systems that have been used for condensing vapors, and by the storage stability of pyrolysis oil. Earlier, the typical range of pyrolysis oil composition is reported by Diebold (2005). Further, Brown (2009) also reported the composition of pyrolysis oil obtained from auger pyrolyzer, however, which is out of the range for some compounds as mentioned by Diebold (2005). Branca et al. (2003) compared the pyrolysis oil composition (with the identified 40–43% of compounds) of four major commercial pyrolyzers: bubbling fluidized bed (Dynamotive), rotating cone (BTG), circulating fluidized bed (ENSYN), and vacuum pyrolyzer. However, the recent ASTM D7544-12 covers the specification of pyrolysis liquid biofuel (obtained from biomass) as a fuel for industrial burner, whereas the same is not applicable to home heaters, small-scale boilers, and engines applications.
3.9 Acid Value
The acidity of pyrolysis oil is derived mainly from the presence of low volatile acids (60–70%) with lowest pKa values as compared to hydroxy acids in sugar (20%) (Oasmaa et al. 2010). These acids do not react with pyrolysis oil components at a moderate temperature of ≤ 80 °C. The acidity of pyrolysis oil causes corrosion in pipelines and process equipment of processing units. Hence, the selection of material for transportation and reactions becomes most vital in order to see the feasibility of integration of pyrolysis process with refinery. Table 12.3 shows that pyrolysis oil derived from different types of pyrolyzers, the pH value varies from 2 to 3.
In the case of FCC, the feedstock with high total acid number (TAN) would not affect the stability of FCC catalyst as the large number of acidic compounds decomposes rapidly at FCC riser operating temperature. However, naphthenic acid is highly active at its boiling point, and hence it causes severe corrosion in condensation equipments. Thus, the TAN of crude oil fractions >1.5 is believed to be significantly corrosive in the temperature range of 232–398 °C.
3.10 Water
Typical formation of water during the pyrolysis reaction is due to the presence of bound moisture in feed biomass (>10 wt% leads to phase separation aqueous and oily viscous) and alkali metals, especially potassium (which catalyze secondary pyrolysis reaction (Agblevor and Besler 1996). In addition to this, the aging reactions (like etherification and esterification between hydroxyl and carbonyl compounds) also take part for the formation of water as a by-product (Lehto et al. 2013). This cannot be separated from pyrolysis oil by means of centrifugation (Oasmaa et al. 2010). From NREL report (Ringer et al. 2006), it has been observed that even if the bone-dry biomass is subjected to pyrolysis, the resulting pyrolysis oil still contains a minimum of 12–15 wt% water.
The formation of high water content in pyrolysis oil will lead to decrease in the heating value, adiabatic flame temperature, and viscosity. Further, the high water content will lead to phase separation, leading to non-homogeneous mixture (Bardalai et al. 2015). This can be analyzed by Karl Fischer volumetric titration technique as per ASTM Standard E 203 (Oasmaa et al. 2010). The water content should be low (<0.2 vol.%) in order to prevent shocks and vibrations, resulting from flash vaporization of water droplets in FCC operation. Free water in bulky quantities is dangerous as it can upset the pressure balance of the reactor regenerator system in FCC process. The updated list of recommended analysis methods along with properties is summarized in Table 12.3 as reported by Oasmaa (2005). In addition to the above-mentioned typical challenges of pyrolysis oil properties, the role of proper modeling of biomass decomposition is very much needed in order to get high quality of pyrolysis oil (Sadhukhan et al. 2008).
4 Upgrading of Pyrolysis Oil in FCC Approach
4.1 Upgrading of Pyrolysis Oil Model Compounds
The order of reactivity and reaction pathways of model pyrolysis oil compounds (such as 1- and 2-propanol, 1- and 2-butanol, acetic acid, acetone, acetaldehyde, phenol, and 2-methoxy phenol) over an acid zeolite (H-ZSM-5) catalyst in an isothermal fixed bed reactor at different temperatures ranging from 200 to 450 °C were ascertained by Gayubo et al. (2005). The coke formation order is as follows: aldehyde > acetone (or) acetic acid > alcohols. It indicates that the deoxygenation is favored by decarboxylation and decarbonylation at around 400 °C temperature, and deoxygenation is favored by dehydration at around 250 °C temperatures on catalytic transformations of acetone and acetic acid. Alcohols have higher reactivity over acid zeolite catalysts leads to the formation of light olefins (~200 °C) then to higher olefins (at 250 °C) followed by the considerable amount of C5+ paraffin and the smaller amount of aromatics at temperatures above 400 °C. Acetone was less reactive as compared to alcohols; however, they also produced C5+ paraffins and aromatics (more than obtained from alcohols).
The reactivity of acetic acid was very low up to the temperature of 400 °C, whereas due to autocatalytic effect the acetic acid was converted into acetone at a temperature more than 400 °C. Once the acetone is formed from acetic acid, it follows similar path of acetone on catalytic transformations. The phenol has low reactivity to produce hydrocarbons over an acid HZSM-5 zeolite catalyst; they produce small amount of light olefins and thermal coke. Aldehyde also has the lower reactivity over HZSM-5 catalyst and resulted higher amount of thermal coke. Therefore, the recommended presence of aldehydes in pyrolysis oil is 3.6 wt% excluding water (Aguado et al. 2000). The ethers and phenolic compounds have low reactivity, while high conversions can be achieved with the compounds of acids, esters, alcohols, aldehydes, and ketones (Adjaye and Bakhshi 1995a, b; Adjaye et al. 1996). However, the catalyst deactivation by coke formation is particularly important with aldehydes and phenols.
4.2 Upgrading of Pyrolysis Oil Model Compounds with VGO
The FCC of mixtures of gas oil and pyrolysis oil representative model compounds (acetic acid, hydroxyacetone, and phenol) in a laboratory scale unit using an E-CaT and a mixture of E-CaT and ZSM-5 additive was carried out by Graca et al. (2009). It has been reported that the coprocessing of oxygenated compounds with vacuum gas oil results in (i) increase of gasoline, gaseous products (CO, CO2, fuel gas, LPG); (ii) decrease of coke yield except for phenol; (iii) decrease of hydrogen yield (follows the order of hydroxyacetone > acetic acid > phenol) in the product and it is confirmed by increase in C2–4 olefins/C2–4 paraffins ratio. Further, it was concluded that these oxygenated compounds can be processed in a FCC unit up to 10 wt%. However, the presence of phenol might be critical due to the limitations in benzene content specification in the gasoline (max. 1 vol.%). The typical product distribution pattern on catalytic cracking of pyrolysis oil model representative compounds with vacuum gas oil is shown in Table 12.4. The catalytic cracking of pyrolysis oil model compounds using zeolite catalyst resulted in the decrease of hydrogen yield for two carbon-oxygenate (e.g., acetic acid) and three carbon-oxygenates (e.g., hydroxyacetone), wherein the hydrogen is consumed during the deoxygenation reactions like decarbonylation and decarboxylation. These observations indicate to further focus on catalytic pyrolysis reactions instead of typical pyrolysis reaction to get the product having lower carbon number based oxygenates, which can be easily converted into products in similar to FCC distillates without changing much the existing FCC infrastructure.
To further understand the coprocessing of pyrolysis oil with VGO in FCC unit, the studies have been extended with the model compounds such as C2-C3 carbonyls (hydroxyacetone and glycolaldehyde dimer), acetic acid, and 2-methoxy phenol (Naik et al. 2014a, b). For the case of coprocessing of C2-C3 carbonyls and VGO, the blending ratios were varied within the range of 5–20 wt%. From the experimental investigations, it was observed that the presence of acetol increased the FCC conversion from 68 to 78% with blending ratio. It was due to the increase in the yield of LPG from 21 to 47 wt.%. It happens at the cost of gasoline from 39 to 23 wt.% followed by LCO from 18 to 12 wt.% and HCO from 11 to 7 wt.%, respectively. It was observed that the yield of LPG increased linearly on increasing the blending ratio. Further, the presence of acetol reduced the coke formation in comparison to pure VGO catalytic cracking over equilibrium FCC catalyst at a constant C/O ratio (with 5).
While on coprocessing the glycolaldehyde dimer with VGO, the FCC conversion increased from 69 to 75% with an increase in blending ratio from 5 to 10%, whereas beyond that the conversion decreased to 65% for the blending ratio of 20%. The dry gas and LPG yield first increased from 1.8 to 2.4 wt% and 35 to 43 wt%, respectively, with an increase in blending ratio. The further increase of blending ratio to 20% the yields of dry gases and LPG decreased to 1.8 and 27 wt%, respectively. Furthermore, it was observed that the gasoline yield first decreased from 27 to 25 wt% and then increased to 32 wt% with an increase in blending ratio, while the LCO yield first decreased from 17 to 15 wt% and then increased to 20 wt%, whereas the yield of HCO first decreased from 11 to 9 wt% and then increased to 13 wt% with an increase in blending ratio from 5 to 10 wt%. The yield of ethylene and propylene also followed the same trend with an increase of glycolaldehyde blending ratio up to 10 wt% blending, and thereon the yields were decreased with further increase in blending ratio. The increase in coke formation was observed beyond the blending ratio of 10%, which is due to the increase in poly-aromatics formation.
The simulation distillation-based product analysis shows the presence of guaiacol increased the product selectivity of gasoline fraction, whereas the presence of acetic acid clearly increased the yield of light olefins, CO, and CO2 (Naik et al. 2014b). The FCC conversion was higher on coprocessing of guaiacol followed by acetic acid with VGO as compared to FCC of VGO. An increase in coke and aromatics formation was observed in the order of guaiacol + VGO feed > acetic acid + VGO feed > VGO. The higher yields of light olefins, CO, and CO2 were observed during FCC of acetic acid and VGO feed over E-Cat. Subsequently, the light olefins were reduced for the case of guaiacol and VGO feed as compared to other feeds.
The cracking pattern of acetic acid and guaiacol with VGO were supported by FTIR analysis, by observing carboxylic acid peaks in the range of 1650–1720 cm−1. The analysis shows that the acetic acid and VGO feed were completely absent in their distillates, which gives a clear indication of complete conversion of acetic acid, while the formation of phenolic peaks was observed in the distillate of guaiacol and VGO feed. Therefore, it is preferable to separate the aromatic oxygenated compounds from pyrolysis oil before coprocessing it with VGO in refinery FCC unit by keeping in mind the limitations of total aromatics and the benzene percentages in gasoline. Moreover, an increase in coke and aromatics content was observed with the insertion of guaiacol while coprocessing with VGO in FCC.
Likewise, the acetol can be co-processed with VGO up to a blending ratio of 5:95 without major changes in the original FCC product slate; beyond that, the LPG range products were increased (Naik et al. 2014b). However, there is a limit for the coprocessing of glycolaldehyde with VGO in refinery FCC unit because of the increase in poly-aromatics formation.
4.3 Upgrading of Pyrolysis Oils with VGO
Directly, one cannot upgrade or catalytically crack the pyrolysis oil with VGO in FCC approach due to the challenges mentioned in Sect. 12.3. Therefore, one has to stabilize or partially upgrade the pyrolysis oil before coprocessing it with petroleum fraction in FCC. Among the various stabilization techniques, the hydrodeoxygenation or catalytic pyrolysis route is considered to be the best choices before coprocessing.
A mixture of hydrodeoxygenated (over Ru/C catalyst) pyrolysis oil with VGO in a ratio of 80:20 was processed in a fixed bed reactor under FCC conditions and compared the results with the processing of pure VGO (Fogassy et al. 2010). It was observed that during coprocessing major part of the oxygen is removed in the form of carbon dioxide and water by means of decarboxylation and dehydration reactions. Cracking of this particular 80:20 mixture produces more dry gas and coke yields, lower LPG yield, while gasoline and light cycle oil yields were comparable to those of the products obtained on cracking of VGO. Their results indicate that C-C bond cleavage takes place before decarboxylation and decarbonylation reactions while cracking hydrodeoxygenated pyrolysis oil as it contains unsaturated hydrocarbons, whereas, Osmont et al. (2007) mentioned that C–O bond breaking reactions proceeds faster than carbon-to-carbon (C–C) bond cleavage for saturated hydrocarbons. It was also emphasized that hydrogen-consuming reactions dominate by water formation and hydrogenation reactions besides some hydrogen-elimination reactions which leads to favor higher yields aromatics (continuation of reaction which leads to coke formation) in the products while cracking VGO/HDO oil, wherein the decrease of LPG may be due to the well-known fact that HDO oil containing more aromatics. It is known that aromatics are difficult to crack than aliphatic hydrocarbons over an E-cat. Similarly, Ng et al. (2006) also mentioned that the aromatic-rich feedstock side chains could be detached and fragmented using acid catalysts but the cracking of refractory aromatic rings needs the presence of hydrogen at higher pressure.
Samolada et al. (1998) performed the catalytic cracking experiments on heavy fraction of hydrotreated pyrolysis oil (80% deoxygenated) in a modified microactivity test (MAT) fixed bed reactor system (ASTM D3907-80), with the LCO in a ratio of 15:85 by wt%. It was reported that both saturated naphthenes (from 3.8 to 4.2%) and aromatics (from 50.5 to 53.8%) were increased on using ReUSY2 catalyst. Lappas et al. (2009) extended the work of Samolada et al. (1998) and carried out the experiments with thermally hydrotreated pyrolysis oil fraction with conventional VGO in the Chemical Process and Energy Resources Institute (CPERI) FCC pilot plant. In order to avoid the feeding nozzle plugging difficulty of FCC unit with such feed, the upgraded oil was diluted in LCO in a portion of 15:75 by wt% and the mixture was blended with VGO. Their experimental result showed that the VGO/upgraded pyrolysis oil co-feed produces about 1 wt% more gasoline, about 0.5 wt% more coke, and more LCO as compared to the VGO feed expected. Their PIONA analysis of liquid product also indicated more aromatics and less olefins and paraffins in comparison to pure vacuum gas oil cracking. It was suggested that the option of co-feeding VGO with upgraded oil is technically viable for refinery FCC unit running with quality feedstock.
The high amounts of oxygen can be allowed in upgraded HDO oil (i.e., up to ≈28 wt%) during coprocessing in FCC unit without any deterioration of the yield pattern (Mercader 2010). It was also mentioned that the coprocessing of HDO oil (20 wt%) with a long residue, a promising yields of FCC gasoline (44–46 wt%) and LCO (23–25 wt%) were obtained without an increase of coke and dry gas yields, as compared to the base feed only.
The tar fraction of Jatropha curcas cake pyroloysis oil was hydrodeoxygenated over Pd/Al2O3 catalyst in an autoclave reactor at 300 °C temperature and 80 bar pressure (Naik et al. 2015). It resulted into the reduction of oxygen content of pyrolysis oil from 32 to 10 wt% and becomes hydrodeoxygenated pyrolysis oil (HDO). The HDO was catalytically cracked with VGO by varying blending ratios from 5 to 20%. On coprocessing of pyrolysis oil with VGO indicated that the yields of gasoline and LCO increased from 29 to 35 wt% and 14.8 to 20.4 wt%, respectively, whereas the yields of dry gas and LPG decreased from 2.1 to 1.4 wt% and 38.8 to 23.7 wt%, respectively, with an increase in blending ratio from 5 to 20%. Their product distribution was compared with the pure VGO and pyrolysis oil with VGO at similar conversion (Table 12.5). The iso-conversion results on coprocessing of HDO with VGO shows a higher yield of LPG, whereas lower yields of gasoline and LCO have been observed as compared to pyrolysis oil coprocessing with VGO and coprocessing of pure VGO.
Thegarid et al. (2013) performed the coprocessing studies of catalytic pyrolysis oil obtained from commercial lignocellulosic biomass (i.e., Lignocel HBS 150–500 originated from beech wood) with VGO in a fixed bed quartz reactor with FCC catalyst in a ratio of 10:90. Their results indicated higher yields of coke, dry gas, and gasoline; lower yields of hydrogen, LPG, LCO, and bottoms as compared to pure vacuum gas oil processing. Further, their observations were (i) the presence of more oxygen (27 wt%) in CPO resulted in hydrogen consumption reactions via water formation; (ii) the presence of alkyl phenols already in the CPO and narrow pore size limitation of ZSM-5 catalyst lead to significant amount of alkyl phenols in the gasoline fraction; (iii) the aromatic-rich gasoline fraction leads to less reactive for further cracking and thereby decreases LPG yield; (iv) P-NMR data showed that all oxygenated compounds (except the phenolic fraction) has been converted. It was also mentioned that the overall yield of organic oil is 30% as compared to 24% on coprocessing of HDO oil with VGO.
5 Schemes for Integration of Pyrolysis with Refinery FCC
It is clear that the pyrolysis oil cannot be processed directly or co-processed with fossil hydrocarbons in order to convert into fuel range hydrocarbons. The option of coprocessing of biomass-derived fractions with petroleum fraction is relatively inexpensive and also helps in increase of profitability of present low margins of petroleum refineries (Alhajri et al. 2014). A list of petroleum refinery units like FCC, hydrotreating, hydrocracking, and steam reforming units can be utilized for the specific applications by approaching the specific pathways. In this scenario, several options have been pointed out by various research groups for the effective use of a refinery infrastructure for upgrading of biomass-derived pyrolysis oil into liquid hydrocarbons, chemicals, and materials. Moreover, this kind of option is more viable in countries like India, wherein the demand of petroleum-derived fraction is extremely high. It also helps the refineries with a safe and secured domestic feedstock source. With this scenario, it has been tried to put some of the schemes for integration of pyrolysis process with petroleum refinery in and another approaches in the following sections.
5.1 Biorefinery and Refinery Integration
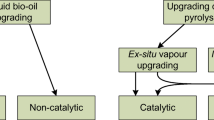
Zacher et al. (2014) proposed a way for the conversion of biomass into companionable petroleum-derived hydrocarbons by the pyrolysis and HDO route, as shown in Fig. 12.1. The pathway has been divided into a series of stages, wherein the scope of insertion into the hydrocarbon economy has been considered. The stages of pyrolysis oil upgrading have been classified into the pyrolysis oil purification by physical and chemical modifications, heteroatom removal by mild hydro treating, cracking into small hydrocarbons, and separations into various products by various unit operations. The well-treated pyrolysis oil (after purification and heteroatom removal) can be utilized in the refinery units wherever it is suited.
Biorefinery and refinery integration (Zacher et al. 2014)
5.2 BIOCOUP Biorefinery
Venderbosch et al. (2010) proposed a different pathway against overall biorefinery concept for the conversion of biomass residues into conventional energy, fuels, and chemicals. This follows hydrothermal liquefaction followed by separation of various fractions like hydrocarbon-rich pyrolysis oil, lignin-rich pyrolysis oil, and derivatives of holocelluloses and process residues, as shown in Fig. 12.2, in which they have emphasized to use the hydrocarbon-rich pyrolysis oil with petroleum-derived fraction for coprocessing in refinery processes. They quoted that hydrodeoxygenation is very much suitable for decreasing the oxygen and acidity of pyrolysis oil before coprocessing in refinery processes. Further, it was reported that the 20% of HDO oil can be co-processed with VGO with slight decrease in the yield of gasoline and the presence of more unsaturated compounds and oxygenates (Way et al. 2011).
BIOCOUP consortium’s overall biorefinery concept (Venderbosch et al. 2010)
5.3 Biomass Feedstocks Integration with Refinery
Melero et al. (2012) proposed a biomass feedstock integration pathway for the conversion of feedstock’s like sugar-rich, starch-rich, and lignocellulosic biomasses into biofuels using standard petroleum refinery processes, as shown in Fig. 12.3. This pathway has been critically described on the basis of feedstock’s effective hydrogen index, energy density, and specific chemistry involved in the conversion or upgrading processes. With this approach, the other refinery process (hydro treating) can also be effectively utilized for the processing of biomass-derived feeds. Hydrotreating processes (HDO, HDS, and HDN) are aimed for higher selectivity toward the liquid fraction (diesel) by minimizing the lighter hydrocarbons, gases, and coke as compared to the catalytic cracking units. Herein, this process can be used for the mild hydrotreating of pyrolysis oil or triglycerides. Further, the hydrogen required for hydrotreating process can be produced from aqueous phase reforming (APR) process with sugar-rich biomass feedstock, which helps in reducing the cost of overall biorefinery integration with petroleum refinery and to produce more energy-dense products.
Integration of biomass-derived feedstocks in conventional refinery processes (Melero et al. 2012)
5.4 A Stand-Alone Refinery Process
Jones et al. (2009) proposed a design case for the conversion of hybrid poplar into gasoline and diesel, and its block diagram is shown in Fig. 12.4 in terms of stand-alone refinery for fast pyrolysis-hydrotreating-hydrocracking approach. This approach has tried to use the refinery’s hydrocracker unit for the upgrading of pyrolysis oil into drop-in liquid hydrocarbons like gasoline and diesel by avoiding the challenges (like the presence of oxygen and a high intensity of aromatic content in diesel cut of hydrotreated pyrolysis oil, which mismatches the specifications of fossil-derived diesel fuels) on using hydrotreating process. Hence, the hydrotreatment process can be used for getting stable pyrolysis oil and then further crack its heavy stable pyrolysis oil into drop-in diesel fuel in a hydrocracker, which can be a more viable option in terms better economy.
A design case stand-alone refinery for fast pyrolysis-hydrotreating-hydrocracking process to produce hydrocarbons (Jones et al. 2009)
Their process economics analysis suggested that the production of transport fuels range hydrocarbons from biomass via the pyrolysis route is potentially economically attractive. Further, the cost becomes even more economical if the integration of conversion and upgrading processes are brought together under one roof with an existing refinery to take the advantage of infrastructure. The catalytic steam reforming is one of the techniques which can utilize the refinery equipment. It is very well understood that the pyrolysis oil contains both aqueous and organic fractions. The aqueous fraction can be effectively utilized for the production of hydrogen by processing in very well-established steam reforming unit. The added advantage of this technique is that the resulted hydrogen can be utilized for the hydrodeoxygenation of pyrolysis oil and as a result the whole process can be integrated.
5.5 Cascading Catalytic Upgrading
The complex nature and multicomposition of pyrolysis oil leads to difficulties in direct processing in refinery processes. Anh (2014) proposed a different kind of biorefinery integration with thermochemical conversion of biomass route, i.e., torrefaction, as shown in Fig. 12.5. The motive of this scheme is to collect the pyrolysis vapors on torrefaction of biomass by condensation in the form of oil at three different pyrolysis temperature zones.
Integration of multistage biomass torrefaction with catalytic upgrading (Anh 2014)
The first zone temperatures varies in between 220 and 315 °C wherein the pyrolysis oil fraction is enriched with light oxygenates and water. The second zone temperatures would be from 315 to 400 °C in which the oil fraction contains cellulose-derived compounds, whereas the third zone is from 400 °C in which the oil fraction contains lignin-derived compounds. Then it has proposed several chemical synthesis techniques like ketonization, aldol condensation, and alkylation for converting zone-1 and zone-2 fractions into C8-C13 oxygenates and C10-C13 phenolics, as shown in Fig. 12.5. Further, it has been emphasized to use the refinery process units like hydro treating, hydrodesulphurization, and FCC unit for final upgrading into fuel range hydrocarbons.
5.6 Kior Process
The KIOR group proposed a scheme for FCC of biomass-derived oxygenates like glycerol and pyrolysis oil with VGO in traditional refinery FCC unit at various levels (Cantu et al. 2012). The first option is to feed the biomass separately into the riser reactor bottom from where the pyrolysis vapors and char particles move toward the cyclone separator, from where the char and FCC catalyst particles are separated and moves into regeneration reactor wherein the char and the deposited coke on FCC catalyst are combusted with air to produce process heat. In alternative to first option, the three other options have been encouraged to co-process the biomass along with VGO either just before VGO feeding or along with VGO or just after VGO feeding.
5.7 Pyrolysis Oil Processing in Refinery
Based on the results (Naik et al. 2014a, b) obtained from the coprocessing of pyrolysis oil model compounds, such as acetic acid, 2-methoxy phenol, hydroxyacetone and glycoldehyde dimer, with VGO in FCC unit, it has been observed that the aliphatic oxygenates are easily crackable in FCC process at lower blending ratios, whereas the aromatic hydrocarbons are not easily cracked. Therefore, Naik et al. (2014b) proposed to first separate the aliphatic and aromatic oxygenates from the pyrolysis oil either by solvent extraction or other techniques. Accordingly, an approach for processing of pyrolysis oil in refinery infrastructure is proposed in Fig. 12.6. This kind of approach may help petroleum refineries to integrate it with fast pyrolysis process to increase the yield of liquified petroleum gas and also the valuable and most demanded petrochemical feedstock, i.e., propylene.
6 Summary
This chapter sets out to be a comprehensive review of pyrolysis oil upgrading through refinery processes. The refinery infrastructure like FCC, steam reforming, hydrotreating, and hydrocracking unit can be utilized for upgrading of biomass-derived pyrolysis oil in effective way. The FCC catalyst is able to crack straight chain hydrocarbons or oxygenates of pyrolysis oil. Therefore, it is better to co-process the aliphatic oxygenates with VGO in FCC process after separating the compounds of lignin-derived monomers, whereas the steam reforming unit is able to crack the aqueous fraction of pyrolysis oil and the produced hydrogen can be utilized for the refinery utilization and make it economical. While the lignin-derived monomers or aromatic fraction of pyrolysis oil can be cracked in hydrocracking unit along with petroleum-derived fractions. The scope of integration of pyrolysis process with refinery units opens up the opportunity in reducing the processing and transportation costs of biomass-derived oxygenates while coprocessing. The concept of coprocessing of pyrolysis oil with petroleum fraction is feasible by means of effective utilization of refinery’s infrastructure without major modifications.
References
Abdel-A HK, Mohamed A, Fahim MA (2003) Petroleum and gas field processing handbook. Marcel Dekker Inc., New York, Basel, pp 317–329
Adjaye JD, Bakhshi NN (1995a) Production of hydrocarbons by catalytic upgrading of a fast pyrolysis bio-oil, Part II: comparative catalyst performance and reaction pathways. Fuel Process Technol 45:185–202
Adjaye JD, Bakhshi NN (1995b) Production of hydrocarbons by catalytic upgrading of a fast pyrolysis bio-oil. Fuel Process Technol 45:161–183
Adjaye JD, Katikaneni SPR, Bakhshi NN (1996) Catalytic conversion of a biofuel to hydrocarbons: effect of mixtures of HZSM-5 and silica-alumina catalysts on product distribution. Fuel Process Technol 48:115–143
Agblevor FA, Besler S (1996) Inorganic compounds in biomass feedstocks, 1, effect on the quality of fast pyrolysis oils. Energy Fuels 10:293–298
Aguado R, Olazar M, San Jose MJ, Aguirre G, Bilbao J (2000) Pyrolysis of sawdust in a conical spouted bed reactor: yields and product composition. Ind Eng Chem Res 39:1925–1933
Alhajri I, Alper E, Is G, Fung J, Lo J, Yanez K, Elkamel A (2014) Optimization model for the integration of biomass into a conventional oil refinery. In: Proceedings of international conference on industrial engineering and operations management, Indonesia, January
Anh (2014) Simultaneous cracking of phenolic compounds and hydrocarbons over zeolites. PhD Thesis, Norman, Oklahoma
Ardiyanti AR, Ermakov D, Heeres HJ, Khromova S, Parmon V, Venderbosch RH (2012) Process for the hydrotreatment of vegetal materials. Patent: WO 2012030215 A1
Bardalai M, Mahanta DK (2015) A review of physical properties of biomass pyrolysis oil. Int J Renew Energy Res 5:277–286
Branca C, Giudicianni P, Blasi CD (2003) GC/MS characterization of liquids generated from low-temperature pyrolysis of wood. Ind Eng Chem Res 42:3190–3202
Brown JN (2009) Development of a lab-scale auger reactor for biomass fast pyrolysis and process optimization using response surface methodology. Graduate Thesis and Dissertations. Paper 10996
Cantu TS, Daugaard DE, Gong K, Platon A, (2012) Integrated FCC biomass pyrolysis/upgrading. Patent No. WO2012091815 A1
Chen NY, Degnan JTF, Koenig LR (1986) Liquid fuel from carbohydrates. Chem Technol 16:506–511
Corma A, Huber GW, Sauvanaud L, Connor PO (2007) Processing biomass-derived oxygenates in the oil refinery: Catalytic cracking (FCC) reaction pathways and role of catalyst. J Catal 247:307–327
Czernik S, French R, Feik C, Chornet E (2001) Production of hydrogen from biomass derived liquids. In: Proceedings of the 2001 DOE Hydrogen Program Review. NREL/CP-570-30535
Czernik S, French R, Feik C, Chornet E (2002) Hydrogen by catalytic steam reforming of liquid byproducts from biomass thermoconversion processes. Ind Eng Chem Res 41:4209–4215
Demirbas A (2010) Biorefineries: for biomass upgrading facilities. Green Energy Technol :75–92
Diebold JP (2005) A review of the chemical and physical mechanisms of the storage stability of fast pyrolysis bio-oils. In: Fast pyrolysis of biomass: a handbook. CPL Press, Newbury, UK
Dinjus E, Henrich E, Weirich F (2004) A two stage process for synfuel from biomass. In: IEA bioenergy agreement task 33: thermal gasification of biomass, task meeting, Vienna, May 3–5
Dudley B (2014) BP Energy Outlook 2035. http://www.bp.com/content/dam/bp/pdf/Energy-economics/Energy-Outlook/EnergyOutlook2035_booklet.pdf. Accessed 23 May 2014, January
Elliott DC, Baker EG (1984) Upgrading biomass liquefaction products through hydrodeoxygenation. Biotechnol Bioenergy Symp 14:159–174
Elliott DC, Schiefelbein GF (1989) Liquidhydrocarbon fuels from biomass. Abstr Papers Am Chem Soc 34:1160–1166
Elliott DC, Hart TR, Neuenschwander GG, Rotness LJ, Zacher AH (2009) Catalytic hydroprocessing of biomass fast pyrolysis bio-oil to produce hydrocarbon products. Environ Progress Sustain Energy 28:441–449
Fogassy G, Thegarid N, Toussaint G, van Veen AC, Schuurman Y, Mirodatos C (2010) Biomass derived feedstock coprocessing with vacuum gas oil for second-generation fuel production in FCC units. Appl Catal B 96:476–485
Fu CM, Schaffer AM (1985) Effect of nitrogen compounds on cracking catalysts. Ind Eng Chem Prod Res Dev 1:68–75
Garcia-Perez M, Chen S, Zhou S, Wang Z, Lian J, Johnson RL, Liaw S, Das O (2009) New bio-refinery concept to convert soft bark wood to transport fuels. Washington State University, Ecology Publication. 09-07-061
Gayubo AG, Aguayo AT, Atutxa A, Valle B, Bilbao J, Gayubo AG, Aguayo AT, Atutxa A, Valle B, Bilbao J (2005) Undesired components in the transformation of biomass pyrolysis-oil into hydrocarbons on a HZSM-5 zeolite. J Chem Technol Biotechnol 80:1244–1251
Ghosh S (2002) Fluid catalytic cracking: an overview and future scenario. Indian Chem Eng (Special Issue) 1:27
Graça I, Ribeiro FR, Cerqueira HS, Lam YL, de Almeida MBB (2009) Catalytic cracking of mixtures of model bio-oil compounds and gasoil. Appl Catal B 90:556–563
Graça I, Lopes JM, Ribeiro MF, Ribeiro FR, Cerqueira HS, de Almeida MBB (2011) Catalytic cracking in the presence of guaiacol. Appl Catal B 101:613–621
Ibsen K (2006) Equipment design and cost estimation for small modular biomass systems, synthesis, gas cleanup, and oxygen separation equipment. Contract report NREL/SR-510-39943, NREL Technical Monitor, May
Iojoiu EE, Domine ME, Davidian T, Guilhaume N, Mirodatos C (2007) Hydrogen production by sequential cracking of biomass-derived pyrolysis oil over noble metal catalysts supported on ceria-zirconia. Appl Catal A 323:147–161
Jones SB, Holladay JE, Valkenburg C, Stevens DJ, Walton CW, Kinchin C, Elliott DC, Czernik S (2009) Production of gasoline and diesel from biomass via fast pyrolysis, hydrotreating and hydrocracking: a design case. PNNL Report No-18284, US Department of Energy, February
Kechagiopoulos PN, Voutetakis SS, Lemonidou AA, Vasalos IA (2006) Hydrogen production via steam reforming of the aqueous phase of bio-oil in a fixed bed reactor. Energy Fuels 20:2155–2163
Lan P, Xu Q, Zhou M, Lan L, Zhang S, Yan Y (2010) Catalytic steam reforming of fast pyrolysis bio-oil in fixed bed and fluidized bed reactors. Chem Eng Technol 33:2021–2028
Lappas AA, Bezergianni S, Vasalos IA (2009) Production of biofuels via coprocessing in conventional refining processes. Catal Today 145:55–62
Lea-Langton A, Md Zin R, Dupont V, Twigg MV (2012) Biomass pyrolysis oils for hydrogen production using chemical looping reforming. Int J Hydrogen Energy 37:2037–2043
Lehto J, Oasmaa A, Solantausta Y, Power M, Chiaramonti D (2013) Fuel oil quality and combustion of fast pyrolysis bio-oils. VTT Technol 87, Espoo
Magrini-Bair K, Czernik S, French R, Parent Y, Ritland M, Chornet E (2002) Fluidizable catalysts for producing hydrogen by steam reforming biomass pyrolysis liquids. In: Proceedings of the 2002 U.S. DOE hydrogen program review NREL/CP-610-32405
Melero JA, Iglesias J, Garcia A (2012) Biomass as renewable feedstock in standard refinery units. Feasibility, opportunities and challenges. Energy Environ Sci 5:7393
Mercader FM (2010) Pyrolysis oil upgrading for coprocessing in standard refinery units. Ph.D. Thesis, University of Twente, Netherlands
Milne TA, Elam CC, Evans RJ (2002) Hydrogen from biomass: state of the art and research challenges. NREL-IEA/H2/TR-02/001
Mortensen PM, Grunwaldt JD, Jensen PA, Knudsen KG, Jensen AD (2011) A review of catalytic upgrading of bio-oil to engine fuels. Appl Catal A 407:1–19
Naik DV, Kumar V, Prasad B, Behera B, Atheya N, Singh KK, Adhikari DK, Garg MO (2014a) Catalytic cracking of pyrolysis oil oxygenates (aliphatic and aromatic) with vacuum gas oil and their characterization. Chem Eng Res Des 92:1579–1590
Naik DV, Kumar V, Prasad B, Behera B, Atheya N, Adhikari DK, Nigam KDP, Garg MO (2014b) Catalytic cracking of C2-C3 carbonyls with vacuum gas oil. Ind Eng Chem Res 53:18816–18823
Naik DV, Kumar V, Prasad B, Behera B, Poddar MK, Bal R, Khatri OP, Adhikari DK, Garg MO (2015) Catalytic cracking of jatropha-derived fast pyrolysis oil with VGO and their NMR characterization. RSC Adv 5:398–409
Ng SH, Nakajima N, Fairbridge C, Kuehler C (2006) Production of light olefins through gas oil cracking. http://www.nt.ntnu.no/users/skoge/prost/proceedings/aiche-2006/data/papers/P61373.pdf
Oasmaa A (2005) Norms and standards for pyrolysis liquids: end-user requirements and specifications. Energy Fuels 19:2155–2163
Oasmaa A, Peacocke C (2010) Properties and fuel use of biomass-derived fast pyrolysis liquids: a guide. VTT Publications 731, Espoo
Oasmaa A, Leppamaki E, Levander PKJ, Tapola E (1997) Physical characterization of biomass-based pyrolysis liquids application of standard fuel oil analyses. VTT PUBLICATIONS 306
Oasmaa A, Elliott DC, Korhonen J (2010) Acidity of biomass fast pyrolysis bio-oils. Energy Fuels 24:6548–6554
Osmont A, Catoire L, Gökalp I, Swihart MT (2007) Thermochemistry of C–C and C–H bond breaking in fatty acid methyl esters. Energy Fuels 21:2027–2032
Ringer M, Putsche V, Scahill J (2006) Large-scale pyrolysis oil production: a technology assessment and economic analysis. National Renewable Energy Laboratory Technical Report, NREL/TP-510–37779, November
Sadeghbeigi R (2000) Fluid catalytic cracking handbook: design, operation, and troubleshooting of FCC facilities, 2nd edn. Gulf Professional Publishing, Houston
Sadhukhan AK, Gupta P, Saha RK (2008) Modeling and experimental studies on pyrolysis of biomass particles. J Anal Appl Pyrol 81:183–192
Samolada MC, Baldauf W, Vasalos IA (1998) Production of a bio-gasoline by upgrading biomass flash pyrolysis liquids via hydrogen processing and catalytic cracking. Fuel 77:1667–1675
Scherzer J (1987) Process for the catalytic cracking of feedstock containing high levels of nitrogen. Patent US4810369, May 7
Scherzer J (1990) Octane-enhancing zeolitic FCC catalysts: scientific and technical aspects. CRC Press
Sedran UA (1994) Laboratory testing of FCC catalysts and hydrogen transfer properties evaluation. Catal Rev Sci Eng 36:405–431
Thegarid N, Fogassy G, Schuurman Y, Mirodatos C, Stefanidis S, Iliopoulou EF, Kalogiannis K, Lappas AA (2013) Second-generation biofuels by coprocessing catalytic pyrolysis oil in FCC units. Appl Catal B 145:161–166
Tiplady IR, Peacocke GVC, Bridgwater AV (1991) Physical properties of fast pyrolysis liquids from the Union Fenosa pilot plant. Bio-oil production & utilization. In: Bridgwater AV; Grassi G (eds) Elsevier Applied Science, London, pp 164–174
Venderbosch RH, Prins W (2010) Review: fast pyrolysis technology development. Biofuels Bioprod Biorefin 4:178–208
Vispute TP, Huber GW (2009) Production of hydrogen, alkanes and polyols by aqueous phase processing of wood-derived pyrolysis oils. Green Chem 11:1433–1445
Wang D, Czernik S, Chornet E (1998) Production of hydrogen from biomass by catalytic steam reforming of fast pyrolysis oils. Energy Fuels 12:19–24
Wang C, Du Z, Pan J, Li J, Yang Z (2007) Direct conversion of biomass to bio-petroleum at low temperature. J Anal Appl Pyrol 78:438–444
Way N, Meesala L, Moppi A, Fogassy G, Toussaint G, Schuurman Y, Geantet C, Mirodatos C (2011) Coprocessing of upgraded pyrolysis oils in lab scale standard refinery units. In: NWBC 2011 Conference Proceedings, NWBC 2011, Stockholm, March 22–24
Whitmore FC (1934) Mechanism of the polymerization of olefins by acid catalysts. Ind Eng Chem 26:94–95
Ye T, Yuan L, Chen Y, Kan T, Tu J, Zhu X, Torimoto Y, Yamamoto M, Li Q (2009) High efficient production of hydrogen from bio-oil using low-temperature electrochemical catalytic reforming approach over NiCuZn–Al2O3 catalyst. Catal Lett 127:323–333
Zacher AH, Olarte MV, Santosa DM, Elliott DC, Jones SB (2014) A review and perspective of recent bio-oil hydrotreating research. Green Chem 16:491–515
Zapata PA, Faria J, Ruiz MP, Resasco DE (2012) Condensation/hydrogenation of biomass-derived oxygenates in water/oil emulsions stabilized by nanohybrid catalysts. Top Catal 55:38–52
Zhang H, Cheng Y, Vispute TP, Xiao R, Huber GW (2011) Catalytic conversion of biomass-derived feedstocks into olefins and aromatics with ZSM-5: the hydrogen to carbon effective ratio. Energy Environ Sci 4:2297–2307
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Naik, D.V., Kumar, V., Prasad, B. (2018). Pyrolysis Oil Upgrading to Fuels by Catalytic Cracking: A Refinery Perspective. In: Singh, A., Agarwal, R., Agarwal, A., Dhar, A., Shukla, M. (eds) Prospects of Alternative Transportation Fuels. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-10-7518-6_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-7518-6_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7517-9
Online ISBN: 978-981-10-7518-6
eBook Packages: EnergyEnergy (R0)