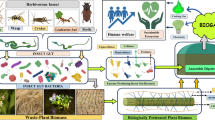
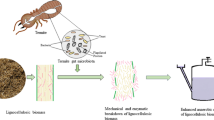
Abstract
Lignocellulosic biomass is most abundant in the environment. Enzymatic breakdown of lignocellulose, an important component of common waste materials, can be an essential step toward mitigating the wastes and generating biofuel. The diverse microbial community is maintained within the insect gut as per their food habit, ecological niche. Certain insects have shown tremendous enzymatic potential as a feed on lignocellulosic materials for their nutrition. In this context, scientific community has become interested to explore different insect gut microbial diversity through the advent of new technologies. The present manuscript encompasses the potential role of insect gut bacteria, aspects of colonization, and role in degradation of lignocellulosic biomass. Further, the significance of potential bacteria for harnessing the enzymes and appropriateness of application in lignocellulosic wastes degradation is also discussed in this review.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
The six-legged critters called insects belonging to the invertebrate group of animals are the most abundant multicellular organisms on the planet. They play incredible role to maintain the milieu, as Prof. EO Wilson, famous biologist, rightly said “if insects were to vanish, the environment would collapse into chaos.” Class Insecta of phylum Arthropoda of the Kingdom Animalia is a widely diverse brunch that includes a wide range species. The number of existing insect species is estimated from six to ten million (Chapman 2006) and potentially corresponds to over 90% of the diverse animal life forms on Earth (Erwin 1982). Insects, present in almost all inhabited condition, typically possess the basic body plan composed of three segments: head, thorax, and abdomen and having three pairs of jointed appendages (legs). Major groups of insect species belong to orders like Coleoptera (sheet winged, e.g., Beetles), Lepidoptera (paired wings, e.g., Butterflies), Diptera (e.g., Flies), and Hymenoptera (e.g., Bees, Ants, etc.) (Wheeler et al. 2001).
Due to diverse habitation, the food habits of insects vary widely. A number of diverse microorganisms play a major role in digestion, metabolism, and nutrition in insect gut (Russell and Moran 2005; Douglas 2011; Krishnan et al. 2014; Sudakaran et al. 2015). Typically, insect’s digestive tract consists of foregut, midgut (or ventriculus), and hindgut (Chapman et al. 2013) although diversified modifications of the tract are eminently related to the adaptation to different feeding modes (Chapman et al. 2013; Engel and Moran 2013a, b). Many insects utilize the lignocellulosic material as their primary food source by degrading the complex polysaccharide using inhabiting gut bacteria and subsequently converting it into glucose monomer molecule (Sun and Scharf 2010). Gut bacteria of such insects are capable of producing various cellulolytic and ligininolytic enzymes that can break down the most abundant biological macromolecule. Lignocellulosic compounds are the basic structural components of plants. Cellulose is known as the world’s most abundant organic polymer, and plants produce approximately 4 × 109 tons of cellulose per year (Irfan et al. 2012; Chatterjee et al. 2015). As they are aplenty and producing a huge biomass, having molecular complexity, are difficult to degrade (Sari et al. 2016). Due to this reason, the lignocellulosic biomass also creates environmental nuisance and pollution. However, proper utilization of the material may help in generating different products like ethanol, biogas (methane). Generally, expensive, instrument intensive various chemical and physical treatment processes are followed to convert this natural component into energy resources (Vandenbossche et al. 2014). Enzyme-based biodegradation can be a choice to develop appropriate method for proper utilization of the biomass into a productive formulation. In this context, lignocellulolytic enzymes produced by the gut bacteria of insects have been drawing interests to scientific community recently, due to its enormous scope of exploration of potential species.
The present review work encompasses the potential role of insect gut bacteria in degradation of lignocellulosic biomass. Detailed literature survey was carried out to incorporate aspects of microbial colonization in insect gut, cellulolytic enzyme production, genomic evolution, and role of insect diet in cellulase production. Further, the importance of prospective bacteria for harnessing the cellulase enzyme and appropriateness of application in lignocellulosic wastes degradation is also discussed in this review.
14.2 Insect Gut Environment
Physiochemical conditions of insect gut are important factor that affects the microbial colonization. The actively regulated lumen of different gut compartments varies in pH and oxygen availability (Dow 1992; Hyun et al. 2014). The pH of gut lumen may vary extensively in insects. Gut microbiota variation within the insect depends upon various factors like the insect order, morphological state (metamorphosis stages), gut condition that varies over life cycle of the particular insect. The aerobic or anaerobic conditions of insect gut depends upon the shape of the gut, size of insect (more anaerobic conditions in bigger insects), availability and partial pressure of oxygen in gut etc. (Elbert and Brune 1997; Hyun et al. 2014). Microbial colonization and metabolism also dynamically shape within the insect gut as per the state of different compartments.
Interestingly, the pH of lepidopteran guts is extremely alkaline (with pH around 11–12) in nature that helps them to digest tannin-rich leaves enhancing their nutrient availability within gut; however, the microbial population is very less as a consequence (Berenbaum 1980; Appel and Martin 1990; Dow 1992; Harrison 2001; Engel and Moran 2013a, b). This alkalinity is maintained through recycling of H+ into the cytoplasm by midgut electrogenic K+ pump which is energized by a H+-pumping V-ATPase and net transport of alkali metal is attained by linking it to a nH+/alkali metal exchanger; the electrical field generated by the V-type ATPase that confers high luminal pH in lepidopteran insects is explained as a model of passive (Nernstian) distribution of proton (Dow 1992). Midgut of the lepidopteran insect has the potentiality to generate pH gradient using metabolic energy and pH profiles observed along the gut is basically due to morphological difference in gut sub regions and differential acid–base transportability of midgut (Dow 1992). Similarly, Boudko et al. (2001) stated that alkaline environment in the midgut (anterior region) is dependent on V-ATPase pumps that maintain strong gradients in hydrogen ion concentrations in mosquito larvae. Less extreme pH gradients reported by Appel and Martin (1990) found in the lumens of a number of nonholometabolous insects. However, guts of few soil-feeding termite species show extreme variation with pH ranging from 5 to >12, having selective alkaline-tolerant symbiotic bacteria from Firmicutes, Clostridium, and Planctomycetes (Brune and Ohkuma 2010; Bignell 2010; Kohler et al. 2012; Engel and Moran 2013a, b). Termite guts have several hindgut compartments or paunches harboring distinct sets of microbial communities acting as bioreactors with high rates of turnover of hydrogen pools (Pester and Brune 2007; Engel and Moran 2013a, b). Microbial fermentation producing acetate, lactate, and formate are abundant in the hindgut and midgut regions in the larvae scarab beetle (Pachnoda ephippiata) (Lemke et al. 2003; Cazemier et al. 2003).
14.3 Microbial Colonization Within Insect Gut
In holometabolous insects, four phases (egg, larvae, pupa, and adult) are eminent in the life cycle, which takes place through metamorphosis of molting and demolting (Moll et al. 2001; Minard et al. 2013). During the phases of metamorphosis, the microbial habitat of insect gut is affected considerably. While, during molting, insect midgut, constantly renewing peritrophic matrix along with microbial population; however, due to peeling of exoskeleton at foregut and hindgut, they are subjected to significant changes in terms of microbial population (Fukatsu and Hosokawa 2002; Minard et al. 2013; Engel and Moran 2013a, b). Moll et al. (2001) reported that during metamorphosis in some insects (as, e.g., mosquito) total to near total eradication of the gut bacteria takes place (Moll et al. 2001; Engel and Moran 2013a, b). Habitat of insects and the source of food consumed by them also affect the gut microflora colonization (Oliver 2003; Douglas 2015). Again, many insects, in their adult stages, represent specialized crypts or paunches that support microbial habitat (Fukatsu and Hosokawa 2002; Engel and Moran 2013a, b).
Gut epithelium of insect consists of folded membrane with either epithelial enterocyte or endocrine cells (Marianes et al. 2013). Enterocytes having microvilli secretes varied enzyme and help in assimilation of nutrients, while endocrine cells, devoid of microvilli, produce peptide and hormones (Beehler-Evans and Micchelli 2015). Other cell types such as goblet cells (in Lepidoptera), cuprophilic cells (in Dipterans) also play an important role in ion transport, with H+ pumps, that results in either alkaline or acidic conditions inside the insect gut (Huang et al. 2015). The gut epithelium functions as a selective barrier which helps in the uptake of nutrients and exchange of ions and water (Simpson et al. 2015). The transport is facilitated by two routes: transcellular route (across the epithelial cells) and paracellular route (between the epithelial cells). Beside this, water content of the body fluids is also regulated by the gut epithelium through channels known as aquaporins (Spring et al. 2009; Huang et al. 2015) and during water and heat stress condition this water moved down the osmotic gradient (from high concentration to low compartment) across biological membranes, hence, help the insects to survive in severe condition (Fig. 14.1).
14.4 Insect Gut Microbial Composition
The gut of insects has a varied group of microorganisms, which are usually mutualistic in nature and help in digestion of intractable plant polymers, supplying nutrients, stimulus of midgut self-renewal providing resistance to parasite invasion, and host fitness with different environmental conditions (Hosokawa et al. 2006; Oliver 2003; Douglas 2015). In this manner, a mutualistic association is formed between insect and intracellular microbes to play diverse metabolic roles to their host, even, working with new metabolic pathways to utilize nutrients which may otherwise be missing from their conventional food sources (Baumann 2005; Carrasco et al. 2014). Reports suggest that the extracellular symbiotic microbes associated with the alimentary tract of stinkbugs insect (Pentatomidae sp.) sustain in the uneven environment due to their potential adaptation as evident in γ-proteobacteria (Nikoh et al. 2011; Hosokawa et al. 2006; Kikuchi et al. 2009). Fukatsu and Hosokawa (2002) reported that vertical transmission of microorganisms to newly hatched nymph takes place through a symbiont capsule being ingested deposited in the midgut by the nymphs of insect. Similar observations have been reported by Kikuchi et al. (2009). During the development of midgut, the anterior portion becomes free from symbiont microorganisms, while the posterior part transformed into a baggy organ having diverse groups of symbiont cells (Hosokawa et al. 2006; Nikoh et al. 2011). However, regarding structural, functional, and evolutionary studies and their correlation on symbiont microorganisms and insect gut, the available scientific information needs to be augmented (Douglas 2015).
The insect gut bacteria also acts as an iron reservoir for the host that helps as iron sink and source for physiological activities. Pesek et al. (2011) reported that Microbacterium arborescens in the larval gut of Spodoptera exigua (Beet armyworm) possess iron reservoirs that help bacterial enzyme (Peroxidase) to inhibit the occurrence of cell-damaging oxygen radicals. Several other structurally similar enzymes (also known as DNA protecting proteins) also help the host insect during starvation (Pesek et al. 2011). Gut bacteria also contribute in maintaining biogeochemical cycle by recycling nitrogen, as members of enterobacteriace species Klebsiella, Roseateles aquatilis found in larvae of southern pine beetle accumulate nitrogen in the environment (Krishnan et al. 2014). Insect feeding on wood (lignocellulose) sources, as a biochemical catalyst, has enormous impact in carbon cycling in nature (Sun and Scharf 2010; Taggar 2015). These lignocellulosic enzymes have a wide range of potential applications including industries for various application purposes.
14.4.1 According to Diet
Anatomy and physiology of insect’s intestines differ greatly and act as the host a variety of microorganisms as per their food habit. The insect gut microbial diversity represents a large source of unexplored microbes that participate in various activities from utilization of different organic polymers, nitrogen fixation, methanogenesis, pesticide degradation, pheromone production to pathogen prevention (Nardi et al. 2002; Reeson et al. 2003; Mrázek et al. 2008). As per the report, the estimated number of bacteria ranges from 109 in honey bees, to 105 in a fruit fly, to negligible numbers in the plant sap-feeders (downloaded from http://schaechter.asmblog.org/schaechter/2013/06/). Different food habits trigger more diversity in gut bacteria. Hernández et al. (2013) reported that polyphagous Iberian geotrupid dung beetles Thorectes lusitanicus that feeds on wide range of food material from dung, acorns, fungi, fruits, to carrion, harbors assorted group of aerobic, facultative anaerobic, and aerotolerant gut bacteria. However, environment plays a critical role in acquisition of gut microbial population, which are more or less constant with respect to the habitats it shares (Wang et al. 2011). The relationships between the insect and its gut microbiome are dynamic one, and resident bacteria play a major role in colonization in the gut even by non-indigenous species. The insect gut is thus a “hot spot” for gene transfer (transfer of plasmids and transconjugation) between bacterial strains that inevitably contribute toward insect’s food habit and nutrition (Dillon and Dillon 2004). Anderson et al. (2013) reported that six of seven bacterial phylogenetic groups present in the hindgut of honey bee (Apis mellifera) play a critical role to manage important functions related to the health of host. Engel and Moran (2013a, b) suggested that high levels of genetic diversity and functional differences within gut possibly due to niche partitioning within the species during evolution. Gut bacteria also play a role in pathogen protection in insects. Reports on Wolbachia, a maternally inherited intracellular bacterium which is found in 40% of insects and other arthropod species, suggest that, it augments pathogen protection, survival against viral-infection in many insect species (Moreira et al. 2009; Bian et al. 2010; Friberg et al. 2011; Zug and Hammerstein 2012; Kuraishi et al. 2013a, b; Ye et al. 2013).
Insect gut microbial composition depends upon several biological and ecological factors (Nikoh et al. 2011; Colman et al. 2012). However, host diet plays a major role in gut bacterial diversity in insect species. Xylophagus insects feeding on decaying woods possess the abundance gut flora (102.8 ± 71.7 species-level OTUs/sample, 11.8 ± 5.9 phylogenetic diversity (PD)/sample) while the insects like bees feeding on relatively simpler food materials have low abundance in bacterial flora (11.0 species-level OTUs/sample ± 5.4, 2.6 ± 0.8 PD/sample) (Colman et al. 2012). Although insect guts can also harbor protists, fungi, archaea; however, bacterial population plays dominant role in insect physiology, food habit, nutrition, etc.; however, their maintenance depends upon the social transmission (Hongoh 2010). Wood or detritus eaters have fungi in their guts, while methanogenic archaea are present in the guts of beetles and termites that feed on dung, detritus, or wood (Brune 2010; Engel and Moran 2013a, b).
On the other hand, food components play a dominating role in microbial population having little impact on host species characterization. Pernice et al. (2014) reported that phylogenetically distantly related insect species with different gut microbial composition when feed and cultured on similar food substrates exhibited similar microbial communities in their gut. Further, Mikaelyan et al. (2015) found that diverse gut bacteria help termite to effectively digest the different wood sources through a synergistic and symbiotic effort. However, apart from bacteria, termite gut also contains other intestinal flora, like cellulolytic flagellates, prokaryotic communities, and archaeal populations (Lozupone et al. 2012; Mikaelyan et al. 2015; Brune 2010). Distinct phylogenetic pattern occurs in the termite gut microflora from different subfamilies that show the diet is the main factor which formulates the bacterial community structure having distinctive microenvironmental conditions (Mikaelyan et al. 2017).
14.4.2 Role in Partner Selection
As discussed above, gut microbiome manipulates a number of aspects including fitness of organism that otherwise influences its mating preferences. Dodd (1989) reported the insect Drosophila pseudoobscura preferred positive assortative mating where it favored mating partners reared for more than 25 generations in the same media; whereas, the flies reared on other media (either starch-based or maltose-based media) preferred mating partner came out from their own rearing media, becoming a population of either “starch flies” or “maltose flies.” Similarly, Sharon et al. (2010) examined the mating preference of Drosophila melanogaster using molasses and starch as rearing media. It was found that the mating preference of these flies appeared only after one generation and was maintained for at least 37 generations; however, mating preference was eliminated after treating with antibiotic, which signifying the vital role of microbiota of fly gut for the phenomenon (Sharon et al. 2010). These observations triggered the scientific community to study further on the role of gut microbial population based on dietary substances. As reported, gut microbiomes have an effect on longevity and reproduction capacity to an organism. A study on antibiotic-treated termites Zootermopsis angusticollis and Reticulitermes flavipes showed the reduced diversity and decrease of useful microbes in their gut flora and subsequent malnourishment that led to the production of significantly less number of eggs (Rosengaus et al. 2011). Brucker and Bordenstein (2013) showed, in their study on the parasitic wasp Nasonia sp., that the bacteria in this insect gut of wasp species (Nasonia giraulti and Nasonia vitripennis) execute themselves as a living barrier that prevents their evolutionary trail from mating with each other and precisely preserve a different sets of gut microbiomes. However, after their forceful crossbreed, the hybrids develop an indistinct microbial population in the gut that causes their premature deaths (Brucker and Bordenstein 2013). Further, in this study, it was found that bacterial constituents and abundance are unequal in hybrids in comparison with the parental species. While Providencia sp. is the major gut bacteria in parental species, Proteus mirabilis became dominant in the hybrid one, which signifies that interbreeding between two species caused damaging modification to the gut flora; therefore, the microbiome of Nasonia helps to remain the two species separate (Brucker and Bordenstein 2013).
14.4.3 Genome Evolution
Symbiotic association with the gut microbes is believed to be one of the key factors that assist the largest phylum of the animal kingdom to be so successful over the centuries (Warnecke et al. 2007; Lize et al. 2014; Brown and Wernegreen 2016). Through the mechanism of differentiation during the process of vertical transmission, among environmentally acquired varied groups of bacteria, symbiotic microorganisms can selectively be preserved by the insects (Kiers et al. 2008). Gut environment has profound impact toward evolution of gut associates either obligate or facultative (Kikuchi et al. 2009; Nikoh et al. 2011). Transfer of gut-associated bacteria through vertical (queen to daughter) or horizontal transmission (between workers) assists the host by serving in the developmental phases (Kwong and Moran 2015). Kikuchi et al. (2009) reported the transmission of symbiotic mutualistic bacteria like Buchnera aphidicola sp. (in aphids), Wigglesworthia glossinidia sp. (in tsetse flies) take place packed in mycetocytes. This vertical transmission plays an important role in nutrition (Kikuchi et al. 2009). Further, accelerated molecular evolution, AT-biased nucleotide composition, and reduced genome size changes have been noticed conferring to major evolutionary pattern in these symbionts (Wernegreen 2002; Brown and Wernegreen 2016). The study of microorganism diversity in insect gut can be done individually or metagenomic approaches. Culture-dependent classical methods have many inadequacies in terms of species diversity study, as many of the insect gut bacteria may not be cultured in laboratory conditions. However, metagenomic approaches can provide an insight to all the microbial community presents in the gut, along with scope to ascertain inter- and intra-specific role (Brune 2010; Ellegaard and Engel 2016).
14.5 Lignocellulose as a Component: Physiological Property
“Lignocellulose” is the combination of three biopolymers: cellulose, hemicellulose, and lignin which make the natural, rigid structural component of plants. Cellulose is a polysaccharide linear chain of thousands of β(1 → 4) linked D-glucose units, with chemical formula (C6H10O5)n (Fig. 14.2). Molecular weight of cellulose ranges from 200,000 to 2,000,000, corresponding to 1250–12,500 glucose molecules per residues (Bashir et al. 2013; Chatterjee et al. 2015) molecule. The cellulose polymer is subdivided into four different categories (Cellulose I, II, III, and IV) which vary in physical and chemical properties (Zhang and Zhang 2013). Lignocellulosic biomass makes a large portion of the plant biomass, approximately 50% in the world (Sanchez and Cardona 2008). It is the most abundant component of terrestrial ecosystem and thus represents a massive source of food and energy for diverse group of microorganisms (Shaikh et al. 2013). Cellulose, a tasteless, odorless, hydrophilic, homopolysaccharide, is more crystalline in nature than starch. Cellulose requires temperature beyond 320 °C to attain the amorphous state in contrast to temperature above 60–70 °C, which converts the crystalline starch into amorphous state. Application of strong acid can break amorphous state of cellulose and produces nano-crystalline cellulose (Peng et al. 2011).
In lignocellulosic biomass, lignin comprises around 10–30% and is the second most abundant natural organic polymer that enables plants to generate rigid structures and gives protection against hydrolysis of cellulose and hemicellulose (de Gonzalo et al. 2016). Lignin is a highly cross-linked polymer, formation of which is activated by plant laccases and/or peroxidases. A range of ether and carbon–carbon bonds (β–β, β–O–4, and β–5 bonds) polymerizes the 4-hydroxyphenylpropanoid monomers (monolignols having phenolic moieties, like p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) groups), and the composition of which varies depending on the plant tissue and species (Vanholme al. 2010; de Gonzalo et al. 2016). The lignin degradation usually takes place in two stages. The first stage involves extracellular, non-specific depolymerization, forming aryl and biaryl compounds (like β-aryl ethers) following mineralization of these compounds through specific catabolic enzymes and pathways (Sainsbury et al. 2013). Most of the lignin degradation studies show that enzymes such as manganese peroxidase (MnP), lignin peroxidase (LiP), and laccase from fungi (white rot fungi) are the best enzyme that degrades the lignin component (Sainsbury et al. 2013; de Gonzalo et al. 2016).
Bacterial ligninolytic enzymes are peroxidase in nature, which belong to the family of heme-containing peroxidases, better known as dye-decolorizing peroxidases (DyPs, EC 1.11.1.19), contains a non-covalently bound heme b cofactor (Van Bloois et al. 2010; Colpa et al. 2014; Yoshida and Sugano 2015; Singh and Eltis 2015). In the year 1999, the first member of this family, DyP from Bjerkandera adusta (order: Polyporales), was isolated and characterized (Kim and Shoda 1999). Till then, different bacterial DyPs have been studied and have been reported, which suggest that putative DyP-encoding genes are amply present in bacterial genomes (Lambertz et al. 2016). Other Dyp enzymes that have been reported in bacteria are PpDyP from Pseudomonas putida MET94 (Santos et al. 2014), BsDyP from Bacillus subtilis KCTC2023 (Min et al. 2015), SviDyP from Saccharomonospora viridis DSM 43017 (Yu et al. 2014), and TfuDyP from Thermobifida fusca (Van Bloois et al. 2010). Interestingly, in the year 1988, in laboratory studies, bacterial “lignin peroxidase” has been reported from Streptomyces viridosporus (Wang et al. 1990; Thomas and Crawford 1998; de Gonzalo et al. 2016). Recently, Davis et al. (2013) reported gene encoding a putative Tat-secreted DyP in Streptomyces isolate. However, in bacteria, due to the complexity of the proteins having several disulfide bonds, integrate calcium ions and a heme cofactor, DyP is usually glycosylated, which is in contrast to the regular peroxidases present in fungi (Lambertz et al. 2016). There are some special conditions which the bacterial machinery requires for folding and processing in manufacturing protein. Although genetic engineering technique has been used to express various DyPs in E. coli, which is comparable to that of fungal peroxidases (de Gonzalo et al. 2016; Lambertz et al. 2016). Bacterial peroxidases and laccases are recently being used for large-scale recombinant enzyme development. Studies have been reported that bacterial laccases can be produced in E. coli (Ihssen et al. 2015; de Gonzalo et al. 2016). Therefore, bacterial enzymes are comparatively easier to produce and have potential application in lignin biodegradation.
14.6 Enzymatic Breakdown of Lignocellulose
Natural degradation lignocellulosic biomass occurs through coordinated action of a set of enzymes. The conversion of lignocellulose into simpler molecule, including glucose, is essential to utilize the biomass in a productive manner. Plant biomass-derived products (like aromatic products, carbohydrates, ethanol) can be used as food and flavor compounds, polymer precursors, pharmaceutical building blocks, fuel, etc. (Asgher et al. 2014; Ragauskas et al. 2014; Kawaguchi et al. 2016; de Gonzalo et al. 2016). However, biochemical reactions for various processes use to operate at favorable pH, temperature, pressure, and other biotic and abiotic conditions. Lignocellulosic biomass hydrolysis requires a set of coordinated action of multiple enzymes. Cellulase enzyme is a complex package of three different classes of enzymes: (1) Endo-1,4-β-endoglucanase binds to non-crystalline part of cellulose, cleaves glucosidic linkages, (2) Exo-1,4-β-exoglucanase binds to crystalline part of the cellulose, and cleaves the molecule while, (3) β-glucosidase enzyme cleaves the cellobiose (a disaccharide molecule) releasing glucose monomers. These three classes of cellulase enzyme are necessary to breakdown the crystalline cellulose into simpler forms such as glucose (Willis et al. 2010; Chatterjee et al. 2015). Nature of cellulose, source of cellulolytic enzymes, optimal condition for catalytic activity, and production of enzymes also play critical role in bioconversion of cellulose (Chatterjee et al. 2015).
14.7 Cellulosomes Complex
Due to its high recalcitrant crystal structure, cellulose degradation is limited to few microorganisms and is a complicated chore. In anaerobic bacteria, cellulases are bound to scaffoldin, forming multicomponent, multienzyme cellulosome complexes that efficiently can degrade cellulosic substrates (Béguin and Lemaire 1996; Bayer et al. 2004; Bae et al. 2013). Cohesin–dockerin interaction helps the non-catalytic subunit called scaffoldin, to bind the various enzyme subunits into the complex. The interaction is highly specific between the scaffoldin-based cohesin modules and the enzyme-borne dockerin domains, which forms the assembly of the cellulosome (Bayer et al. 2004; Bae et al. 2013; Haitjema et al. 2017). This multienzyme complex facilitated by cohesin–dockerin interaction, which is the basis for newly emerging field of synthetic biology (Haitjema et al. 2017). Investigation of the growth substrate-dependent variations in cellulosomal systems has been studied with the advances in proteomics study approach. Further, deigned minicellulosomes have contributed to investigate the immediacy and targeting effects of synergistic action of cellulosomal complex. The arrangement of genes in multiple-scaffoldin or enzyme-linked group on the genome contributes toward the diversity in cellulosome structural design (Bayer et al. 2004; Haitjema et al. 2017). Chimeric cohesin-bearing scaffoldins have been used for amalgamation of recombinant dockerin-containing enzymes, for assembling the designer cellulosomes (Stern et al. 2016). Interestingly, chimeric scaffoldin, having six cohesins, has been reported to form the largest designer cellulosome. However, this has resulted in the instability of the scaffoldin polypeptide, limited numbers of available cohesin–dockerin specificities, and low expression levels (Stern et al. 2016). Again, study related to the regulation of cellulosome-related genes through genetic engineering tools and approach and promising genomics of cellulosome-producing bacteria has facilitated in examining the assembly and consequences of the multienzyme complex (Bayer et al. 2004; Bae et al. 2013). Stern et al. (2016) reported that a designer cellulosome complex having a hexavalent scaffoldin attached to adaptor scaffoldin, having a type-II cohesin forms an effective enzyme complex which is having potential capacity up to 70% as compared to that of native cellulosomes for solubilization of natural lignocellulosic substrates.
14.8 Biotechnological Application of Cellulase Enzyme
Importance and application of microbial enzymes increased rapidly in mid-1980s, where different industrial applications have also been identified. Research on insect gut micobiome has indicated the potential use of bacterial enzymes, especially cellulase for biotechnological application (Kuhad et al. 2011; Su et al. 2017). The list of insects producing cellulase has been reviewed elsewhere (Chatterjee et al. 2015). It is obvious that inside the gut of insect these cellulase enzymes help in nutrition by deconstruction of food materials. As for example, heterotermitidae and rhinotermitidae groups of termite possess highly potential cellulolytic enzymes that help in digestion of complex polysaccharide food materials including wood and wood-based products (Martin et al. 1983; Chatterjee et al. 2015; de Gonzalo et al. 2016). It has been reported that the cellulosome complex presents in the bacteria residing at hindgut area of termites that have the capacity to degrade lignocellulosic material with their cell wall by surrounding the food substrates (Bayer et al. 2004; Tokuda et al. 2005; Scharf et al. 2011; Bae et al. 2013; Chatterjee et al. 2015; Stern et al. 2016; Haitjema et al. 2017). The catalyst of this complex system together is more effective than a single enzyme unit for lignocellulosic degradation (Stern et al. 2016). However, the termite gut has a complex niche of community of bacterial, archaeal, and eukaryotic gut symbionts that synergistically break down the plant fibers into the products like acetate, hydrogen, and methane (Brune 2014; Brune and Dietrich 2015; Mikaelyan et al. 2017). It has been estimated that termite gut can digest 74–99% of cellulose and 65–87% of hemicelluloses within hours (Li et al. 2017). More than 4700 bacterial phylotypes have been detected in the lower termite Reticulitermes, where Bacteriodetes, Proteobacteria, Spirochetes, Firmicutes, and Eubacteria are prominent members of this microbiota that help in biomass degradation (Cragg et al. 2015). Some Archaeal species present in the termite gut can degrade lignocellulose at higher temperature. Reports suggest that endoglucanase GH12 gene presents in archaeon Pyrococcus, and genes encoding laccase enzymes from Halobacteriales, and Thermoproteales are potential element that caters lignocellulosic degradation in archaea (Graham et al. 2011; de Gannes et al. 2013; Tian et al. 2014; Cragg et al. 2015).
14.8.1 In Waste Management
Most of the agricultural and household wastes contain lignocellulose as major components. The waste amelioration process can easily be achieved by treating wastes using bacterial cellulases and lignin-degrading enzymes (Kuhad et al. 2011; Gupta et al. 2011; Brune and Dietrich 2015; Chatterjee et al. 2015). Composition and dynamics of microflora play a major role during this process of enzymatic degradation; however, a detailed study of microbial succession and selection to accelerate the process is important for effective and appropriate management of biowastes (Kuhad et al. 2011; Gupta et al. 2011).
14.8.2 Food and Brewage Industry
Increase demand of fruit and vegetable juice has drawn the attention of food and brewage industries toward macerating enzymes like cellulase, pectinase, and related enzymes to ease in processing of fruit and vegetable juice. The conventional system involves multistep processing like maceration, extraction, clarification, and stabilization. Using cellulase for macerating fruit pulp can yield better in starch and protein extraction (Ventorino et al. 2015). In addition, the better maceration helps in color and carotenoid extraction of fruits and vegetables which in turn help in improved texture, quality, flavor, aroma, and viscosity of fruit purees (Kuhad et al. 2011; Chatterjee et al. 2015). Further using enzymes’ mixture or in combination like pectinases, cellulases, and hemicellulases improves extraction, malaxation, and quality of olive-based oil and paste (Wongputtisin et al. 2014). It has also been observed that infusion of enzymes such as pectinases and β-glucosidases reduces bitterness of citrus fruits by some extent (Sharada et al. 2014). Similarly, these microbial enzymes have a key role in alcoholic beverages production also. Brewing of beer initiates with barley malt or malted sorghum which contains raw starch and protein material which require enzymes’ to convert it into simpler form like sugars, amino acids, and peptides. Enzymes help in improving skin maceration, color extraction, clarification, filtration, and stability (Singh et al. 2007). Further, to produce liquor controlled fermenting conditions, along with microbial enzymes play a major role in deciding the quality and yields of the fermented products.
14.8.3 Ethanol Production from Lignocellulosic Biomasses
Nowadays, through the use of starch or sucrose as provided by agricultural crops such as corn, wheat, or sugarcane, fermentation of ethanol is being carried out on a larger scale. The biological conversion of the lignocellulosic wastes produces either ethanol, methanol, or hydrogen, which depends upon the process (biochemical or thermochemical) and ideal microorganism (Dutta et al. 2014). In nature, degradation of organic matter, i.e., lignocelluloses leads to methane generation, whereas ethanol and hydrogen are the intermediates by-products in anaerobic degradation (Ahring and Westermann 2007). Ethanol is an environmentally safe liquid transportation fuel as it does not contribute toward greenhouse gas emissions because ethanol produced from the renewable plant materials and CO2 generated from the ethanol burning is recycled by the plant body in their photosynthesis process (Limayem and Ricke 2012; Saini et al. 2015). The fermentation of lignocellulosic substrates is an eminent and collective process. This tough process of conversion of lignocellulose to ethanol has several steps, such as (i) detaching (or delignification) lignin from other molecules to release free cellulose and hemicellulose from the lignocellulosic material; (ii) depolymerization of carbohydrate to release sugars from cellulose and hemicelluloses; (iii) fermentation of hexose and pentose sugars to produce ethanol (Lee 1997; De Souza 2013) (Tables 14.1 and 14.2).
14.8.4 Pulp and Paper Industry
Annually, massive amount lignocellulosic biomass is being processed in pulp and paper industry. Use of cellulase enzyme for efficient conversion of lignocellulosic waste into quality paper is an eco-friendly and appropriate approach. Enzymes-based process includes pre-bleaching of pulp and deinking process that helps in pulp freeness and cleanliness, as a result, improves fiber brightness and strength properties (Kuhad et al. 2011; Chatterjee et al. 2015).
14.8.5 Textile Industry
Cellulase has been employed widely in textile industries for biostoning of jeans, biopolishing of cotton, and other cellulosic fabrics. Earlier biostoning was performed mechanically by pumice stone which use to cause damage to the fiber; however, after the introduction of cellulase enzyme, this process becomes easy with less damage to fiber (Kuhad et al. 2011; Chatterjee et al. 2015). Further, acidic cellulase takes care of biopolishing process as a result soft, smooth, and bright color fabric obtain.
14.9 Conclusion
Insect gut microbiota varies widely. As for examples, complex gut microbial communities can be found in termite gut, while, little or no gut microbiota are present in sap-feeding insects (Colman et al. 2012; Chapman et al. 2013). As a whole, less complexity of microbial community structure in insect gut can be found, which may be due to varied reasons like, the simple gut structure (that can afford fewer ecological niches), smaller retention time (due to their tiny structure), lacking a “classical” adaptive immune system etc. (Colman et al. 2012; Chapman et al. 2013; Engel and Moran 2013a, b). However, bacterial consortia in insect gut specifically help the host in digestion of food and various other activities (Engel and Moran 2013a, b). Lignocellulose eaters (scarab beetles and termite), however, retain food longer having fermentative guts with diverse gut microbial communities (Colman et al.2012). Recent developments of omics technologies have led the researchers to know more about the insect gut bacteria and their potential enzymatic activities upon lignocelluloses degradation. Further, several applications like production of biofuels from such wastes have pushed the researchers to understand different aspects related to biotechnological applications, like lignocellulose-active genes, substrate binding paradigms, oxidation of polysaccharides, architecture of enzyme domain, enzymatic synergies, and lignin bond breakdown (Brunecky et al. 2013; Agger et al. 2014; Cragg et al. 2015). However, there are several aspects like comprehensive individual enzyme-based sequence–structure–function relationships or synergistic action of cocktails of enzymes that work together within the insect gut are yet to be ascertained (Cragg et al. 2015). It is essential to explore these unknown aspects to optimize the enzyme activities in different industrial applications. In coming years, the relevant findings will enormously help to understand about the diversity of insect gut microbial community, in one hand, and various applications to develop sustainable eco-friendly technologies for generation of wealth (e.g., biofuel) from lignocellulosic wastes.
References
Agger JW, Isaksen T, Varnai A, Vidal-Melgosa S, Willats WGT, Ludwig R, Horn SJ, Eijsink VGH, Westereng B (2014) Discovery of LPMO activity on hemicelluloses shows the importance of oxidative processes in plant cell wall degradation. Proc Natl Acad Sci USA 111:6287–6292
Ahring BK, Westermann P (2007) Coproduction of bioethanol with other biofuels. Adv Biochem Eng Biotechnol 108:289–302
Anderson KE, Sheehan TH, Mott BM, Maes P, Snyder L, Schwan MR, Walton A, Jones BM, Corby-Harris V (2013) Microbial ecology of the hive and pollination landscape: bacterial associates from floral nectar, the alimentary tract and stored food of honey bees (Apis mellifera). PLoS ONE8:e83125. https://doi.org/10.1371/journal.pone.0083125
Appel HM, Martin MM (1990) Gut redox conditions in herbivorous lepidopteran larvae. J Chem Ecol 16 (12):3277–3290. https://doi.org/10.1007/BF00982098
Asgher M, Bashir F, Iqbal HMN (2014) A comprehensive ligninolytic pre-treatment approach from lignocellulose green biotechnology to produce bio-ethanol. Chem Eng Res Des 92:1571–1578
Bae J, Morisaka H, Kuroda K, Ueda M (2013) Cellulosome complexes: natural biocatalysts as arming microcompartments of enzymes. J Mol Microbiol Biotechnol 2:370–378
Bashir Z, Kondapalli VK, Adlakha N, Sharma A, Bhatnagar RK, Chandel G, Yazdani SS (2013) Diversity and functional significance of cellulolytic microbes living in termite, pill-bug and stem-borer guts. Sci Rep 3:2558. https://doi.org/10.1038/srep02558
Baumann P (2005) Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol 59:155–189
Bayer EA, Belaich JP, Shoham Y, Lamed R (2004) The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu Rev Microbiol 58:521–554
Beehler-Evans R, Micchelli CA (2015) Generation of enteroendocrine cell diversity in midgut stem cell lineages. Development 142:654–664 [PubMed: 25670792]
Béguin P, Lemaire M (1996) The cellulosome: an exocellular, multiprotein complex specialized in cellulose degradation. Crit Rev Biochem Mol Biol 31:201–236
Berenbaum M (1980) Adaptive significance of midgut pH in larval Lepidoptera. Am Nat 115:38–146
Bian G, Xu Y, Lu P, Xie Y, Xi Z (2010) The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6:e1000833. https://doi.org/10.1371/journal.ppat.1000833
Bignell DE (2010) Morphology, physiology, biochemistry and functional design of the termite gut: an evolutionary wonderland. In: Bignell DE, Roisin Y, Lo N (eds) Biology of termites: a modern synthesis. Springer, Dordrecht, pp 375–412
Boudko DY, Moroz LL, Linser PJ, Trimarchi JR, Smith PJ, Harvey WR (2001) In situ nalysis of pH gradients in mosquito larvae using non-invasive, self-referencing, pH-sensitive microelectrodes. J Exp Biol 204:691–699
Brown BP, Wernegreen JJ (2016) Deep divergence and rapid evolutionary rates in gut associated Acetobacteraceae of ants. BMC Microbiol 16:140. https://doi.org/10.1186/s12866-016-0721-8
Brucker RM, Bordenstein SR (2013) The hologenomic basis of speciation: gut bacteria cause hybrid lethality in the genus Nasonia. Science 9(341):667–669
Brune A (2010) Methanogens in the digestive tract of termites. (Endo)symbiotic methanogenic archaea. In: Hackstein JHP (ed) Microbiology monographs. Springer, pp 81–100
Brune A (2014) Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol 12:168–180. https://doi.org/10.1038/nrmicro3182
Brune A, Dietrich C (2015) The gut microbiota of termites: digesting the diversity in the light of ecology and evolution. Annu Rev Microbiol 69:145–166
Brune A, Ohkuma M (2010) Role of the termite gut microbiota in symbiotic digestion. In: Bignell DE, Roisin Y, Lo N (eds) Biology of termites: a modern synthesis. Springer, Dordrecht, pp 439–475
Brunecky R, Alahuhta M, Xu Q, Donohoe BS, Crowley MF, Kataeva IA, Yang S-J, Resch MG, Adams MWW, Lunin VV et al (2013) Revealing nature’s cellulase diversity: the digestion mechanism of Caldicellulosiruptor bescii CelA. Science 342:1513–1516
Carrasco P, Perez-Cobas AE, van de PC, Baixeras J, Moya A, Latorre A (2014) Succession of the gut microbiota in the cockroach Blattella germanica. Int Microbio 17:99–109. https://doi.org/10.2436/20.1501.01.212
Cazemier AE, Verdoes JC, Reubsaet FAG, J. Hackstein HP, Drift C van der, Camp Op den HJM (2003) Promicromonospora pachnodae sp. nov., a member of the (hemi) cellulolytic hindgut flora of larvae of the scarab beetle Pachnoda marginata. Antonie Leeuwenhoek 83:135–148
Chapman AD (2006) Numbers of living species in Australia and the World. Canberra: Aus Biol Res Study. ISBN 978-0-642-56850-2
Chapman RF, Simpson SJ, Douglas AE (2013) The insects: structure and function, 5th edn. Cambridge University Press, Cambridge
Chatterjee S, Sharma S, Prasad RK, Datta S, Dubey D, Meghvansi MK, Vairale MG, Veer V (2015) Cellulase enzyme based biodegradation of cellulosic materials: an overview. South Asian J Exp Biol 5:271–282
Colman DR, Toolson EC, Takacs-Vesbach CD (2012) Do diet and taxonomy influence insect gut bacterial communities? Mol Ecol 21:5124–5137
Colpa DI, Fraaije MW, Van BE (2014) DyP-type peroxidases: a promising and versatile class of enzymes. J Ind Microbiol Biotechnol 41:1–7
Cragg SM, Beckham GT, Bruce NC, Bugg TDH, Distel DL, Dupree P, Etxabe AG, Goodell BS, Jellison J, McGeehan JE, McQueen-Mason SJ, Schnorr K, Walton PH, Watts JEM, Zimmer M (2015) Lignocellulose degradation mechanisms across the tree of life. Curr Opin Chem Biol 29:108–119
Davis JR, Goodwin L, Teshima H, Detter C, Tapia R, Han C, Huntemann M, Wei CL, Han J, Chen A, Kyrpides N, Mavrommatis K, Szeto E, Markowitz V, Ivanova N, Mikhailova N, Ovchinnikova G, Pagani I, Pati A, Woyke T, Pitluck S, Peters L, Nolan M, Land M, Sello JK (2013) Genome sequence of Streptomyces viridosporus strain T7A ATCC 39115, a lignin-degrading actinomycete. Genome Announc.1:pii:e00416-13
de Gannes V, Eudoxie G, Hickey WJ (2013) Prokaryotic successions and diversity in composts as revealed by 454-pyrosequencing. Bioresour Technol 133:573–580
de Gonzalo G, Colpa DI, Habib MHM, Fraaije MW (2016) Bacterial enzymes involved in lignin degradation. J Biotechnol 236:110–119
De Souza WR (2013) Microbial degradation of lignocellulosic biomass. https://doi.org/10.5772/54325
Dillon RJ, Dillon VM (2004). The gut bacteria of insects: nonpathogenic interactions. Ann Rev Entomol 49:71–92. https://doi.org/10.1146/annurev.ento.49.061802.123416
Dodd DMB (1989) Reproductive isolation as a consequence of adaptive divergence in Drosophila pseudoobscura. Evolution 43:1308–1311
Douglas AE (2011) Lessons from studying insect symbioses. Cell Host Microbe 10(4):359–367. https://doi.org/10.1016/j.chom.2011.09.001
Douglas AE (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 60:17–34. https://doi.org/10.1146/annurev-ento-010814-020822
Dow JA (1992) pH gradients in lepidopteran midgut. J Exp Biol 172:355–375
Dutta K, Daverey A, Lin JG (2014) Evolution retrospective for alternative fuels: first to fourth generation. Renew Energy 69:114–122
Elbert A, Brune A (1997) Hydrogen concentration profiles at the oxicanoxic interface: a microsensor study of the hindgut of the wood-feeding lower termite Reticulitermes flavipes (Kollar). Appl Environ Microbiol 63:4039–4046
Ellegaard KM, Engel P (2016) Beyond 16S rRNA community profiling: intra-species diversity in the gut microbiota. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01475
Engel P, Moran NA (2013a) The gut microbiota of insects diversity in structure and function. FEMS Microbiol Rev 37:699–735
Engel P, Moran NA (2013b) Functional and evolutionary insights into the simple yet specific gut microbiota of the honey bee from metagenomic analysis. Gut Microbes 4:60–65
Erwin TL (1982) Tropical forests: their richness in Coleoptera and other arthropod species. Coleopt Bull 36:74–75
Fischer R, Ostafe R, Twyman RM (2013) Cellulases from insects. Adv Biochem Eng Biotechnol. https://doi.org/10.1007/10_2013_206
Friberg U, Miller PM, Stewart AD, Rice WR (2011) Mechanisms promoting the long-term persistence of a Wolbachia infection in a laboratory-adapted population of Drosophila melanogaster. PloS One6:e16448. https://doi.org/10.1371/journal.pone.0016448
Fukatsu T, Hosokawa T (2002) Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug, Megacopta punctatissima. Appl Environ Microbiol 68:389–396. https://doi.org/10.1128/AEM.68.1
Graham JE, Clark ME, Nadler DC, Huffer S, Chokhawala HA, Rowland SE, Blanch HW, Clark DS, Robb FT (2011) Identification and characterization of a multidomain hyperthermophilic cellulase from an archaeal enrichment. Nat Commun 2:375
Gupta R, Khasa YP, Kuhad RC (2011) Evaluation of pretreatment methods in improving the enzymatic saccharification of cellulosic materials. Carbohyd Polym 84:1103–1109
Haitjema CH, Gilmore SP, Henske JK, Solomon KV, de Groot R, Kuo A, Mondo SJ, Salamov AA, LaButti K, Zhao Z, Chiniquy J, Barry K, Brewer HM, Purvine SO, Wright AT, Hainaut M, Boxma B, van Alen T, Hackstein JHP, Henrissat B, Baker SE, Grigoriev IV, O’Malley MA (2017) A parts list for fungal cellulosomes revealed by comparative genomics. Nat Microbiol 2:17087. https://doi.org/10.1038/nmicrobiol.2017.87
Harrison JF (2001) Insect acid-base physiology. Annu Rev Entomol 46:221–250 [PubMed:11112169]
Hernández N, Escudero JA, Millán AS, González-Zorn B, Lobo JM, Verdú JR, Suárez M (2013) Culturable aerobic and facultative bacteria from the gut of the polyphagic dung beetle Thorectes lusitanicus Jeckel. Insect Sci. https://doi.org/10.1111/1744-7917.12094
Hongoh Y (2010) Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosci Biotechnol Biochem 74:1145–1151
Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T (2006) Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol 4(10):e337
Huang JH, Jinga X, Douglas AE (2015) The multi-tasking gut epithelium of insects. Insect Biochem Mol Biol 67:15–20. https://doi.org/10.1016/j.ibmb.2015.05.004
Hyun JH, Woon RS, Woong WT, Ja JM, Soo KM, Sang PD, Changmann Y, Do NY, Ji KH, Hye CJ, Yong KJ, Ri SN, Hee KS, Jae LW, Woo BJ (2014) Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl Env Microbiol 80(17):5254–5264
Ihssen J, Reiss R, Luchsinger R, Thöny-Meyer L, Richter M (2015) Biochemical properties and yields of diverse bacterial laccase-like multicopper oxidases expressed in Escherichia coli. Sci Rep 5:10465
Irfan M, Safdar A, Syed Q, Nadeem M (2012) Isolation and screening of cellulolytic bacteria from soil and optimization of cellulase production and activity. Turk J Biochem 37:287–293
Kohler T, Dietrich C, Scheffrahn RH, Brune A (2012) High resolution analysis of gut environment and bacterial microbiota reveals functional compartmentation of the gut in wood-feeding higher termites (Nasutitermes spp.). Appl Environ Microbiol 78:4691–4701
Kawaguchi H, Hasunuma T, Ogino C, Kondo A (2016) Bioprocessing of bio-based chemicals produced from lignocellulosic feedstocks. Curr Opin Biotechnol 42:30–39
Kiers ET, Denison RF (2008) Sanctions, cooperation, and the stability of plant-rhizosphere mutualisms. Annu Rev Ecol Evol Syst 39:215–236
Kikuchi Y, Hosokawa T, Nikoh N, Meng XY, Kamagata Y, Fukatsu T (2009) Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol 7:2. http://www.biomedcentral.com/1741-7007/7/2
Kim SJ, Shoda M (1999) Purification and characterization of a novel peroxidase from Geotrichum candidum Dec 1 involved in decolorization of dyes. Appl Environ Microbiol 65:1029–1035
Krishnan M, Bharathiraja C, Pandiarajan J, Prasanna VA, Rajendhran J, Gunasekaran P (2014) Insect gut microbiome—an unexploited reserve for biotechnological application. Asian Pacific J Trop Biomed 4(Suppl 1):S16–S21
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzyme Res Article ID 280696. https://doi.org/10.4061/2011/20696
Kuraishi T, Hori A, Kurata S (2013a) Host-microbe interactions in the gut of Drosophila melanogaster. Front Physiol 4:375
Kuraishi T, Hori A, Kurata S (2013b) Host-microbe interactions in the gut of Drosophila melanogaster. Front Physiol 17. https://doi.org/10.3389/fphys.2013.00375
Kwong WK, Moran NA (2015) Evolution of host specialization in gut microbes: the bee gut as a model. Gut Microbes 6(3):214–220
Lambertz C, Ece S, Fischer R, Commandeur U (2016) Progress and obstacles in the production and application of recombinant lignin-degrading peroxidases. Bioengineered 7:145–154
Lee J (1997) Biological conversion of lignocellulosic biomass to ethanol. J Biotechnol 56:1–24 PubMed PMID: 9246788
Lemke T, Stingl U, Egert M, Friedrich MW, Brune A (2003) Physicochemical conditions and microbial activities in the highly alkaline gut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl Environ Microbiol 69(11):6650–6658
Li H, Yelled DJ, Lie C, Yang M, Ke J, Zhang R, Liu Y, Zhu N, Mo X, Ralph J, Currie CR, Mo J, Liang S (2017). www.pnas.org/lookup/suppl. https://doi.org/10.1073/pnas.1618360114/-/DCSupplemental
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energy Combust Sci 38:449–467
Lize A, McKay R, Lewis Z (2014) Kin recognition in Drosophila: the importance of ecology and gut microbiota. ISME J 8:469–477
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230
Marianes A, Spradling AC, Brand A (2013) Physiological and stem cell compartmentalization within the Drosophila midgut. Elife 2:e00886 [PubMed: 23991285]
Martin M (1983) Cellulose digestion in insects. Comp Biochem Physiol 75 A:313–324
Mikaelyan A, Dietrich C, Kohler T, Poulsen M, Sillam DD, Brune A (2015) Diet is the primary determinant of bacterial community structure inthe guts of higher termites. Mol Ecol 24(20):5284–5295. https://doi.org/10.1111/mec.13376
Mikaelyan A, Meuser K and Brune A (2017) Microenvironmental heterogeneity of gut compartments drives bacterial community structure in wood- and humus-feeding higher termites. FEMS Microbiol Ecol 93:fiw210. https://doi.org/10.1093/femsec/fiw210
Min K, Gong G, Woo HM, Kim Y, Um Y (2015) A dye-decolorizing peroxidase from Bacillus subtilis exhibiting substrate-dependent optimum temperature for dyes and β-ether lignin dimer. Sci Rep 5:8245
Minard G, Mavingui P, Moro CV (2013) Diversity and function of bacterial microbiota in the mosquito holobiont. Parasites Vectors 6:146
Moll RM, Romoser WS, Modrzakowski MC, Moncayo AC, Lerdthusnee K (2001) Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J Med Entomol 38:29–32
Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den HAF, Ryan PA, O’Neill SL (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue; Chikungunya; and Plasmodium. Cell 139:1268–1278
Mrázek J, Strosová L, Fliegerová K, Kott T, Kopecný J (2008) Diversity of insect intestinal microflora. Folia Microbiol (Praha) 53:229–233. https://doi.org/10.1007/s12223-008-0032-z
Nardi JB, Mackie RI, Dawson JO (2002) Could microbial symbionts of arthropod guts contribute significantly to nitrogen fixation in terrestrial ecosystems? J Ins Physiol 48:751–763. https://doi.org/10.1016/S0022-1910(02)00105-1
Nikoh N, Hosokawa T, Oshima K, Hattori M, Fukatsu T (2011) Reductive evolution of bacterial genome in insect gut environment. Genome Biol Evol 3:702–714. https://doi.org/10.1093/gbe/evr064
Oliver KM, Russell JA, Moran NA, Hunter MS (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA 100:1803–1807
Peng BL, Dhar N, Liu HL, Tam KC (2011) Chemistry and applications of nannocrystalline cellulose and its derivatives: a nanotechnology prospective. Can J Chem Eng 89(5):1191–1206
Pernice M, Simpson JS, Ponton F (2014) Towards an integrated understanding of gut microbiota using insects as model systems. J Insect Physiol 69:12–18
Pesek J, Buchler R, Albrecht R, Boland W, Zeth K (2011) Structure and mechanism of iron translocation by a Dps protein from Microbacterium arborescens. J Biol Chem 286. https://doi.org/10.1074/jbc.M111.246108
Pester M, Brune A (2007) Hydrogen is the central free intermediate during lignocellulose degradation by termite gut symbionts. ISME J 1(6):551–565
Ragauskas AJ, Beckham GT, Biddiy MJ, Chandra R, Chen F, Davis MF, Davison BH, Dixon RA, Gilna P, Keller P, Langan P, Naskar AK, Saddler JN, Tschaplinski TJ, Tuskan GA, Wyan CE (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344:1246843
Reeson AF, Jankovic T, Kasper ML, Rogers S, Austin AD (2003) Application of 16S rDNA-DGGE to examine the microbial ecology associated with a social wasp Vespula germanica. Insect Mol Biol 12:85–91. https://doi.org/10.1046/j.1365-2583.2003.00390.x
Rosengaus RB, Zecher CN, Schultheis KF, Brucker RM, Bordenstein SR (2011) Disruption of the termite gut microbiota and its prolonged consequences for fitness. Appl Environ Microbiol 77:4303–4312
Russell JA, Moran NA (2005) Horizontal transfer of bacterial symbionts: heritability and fitness effects in a novel aphid host. Appl Environ Microbiol 71:7987–7994
Saini JK, Tewari L, Saini R (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5:337–353. https://doi.org/10.1007/s13205-014-0246-5
Sainsbury PD, Hardiman EM, Ahmad M, Otani H, Seghezzi N, Eltis LD, Bugg TDH (2013) Breaking down lignin to high-value chemicals: the conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcus jostii RHA1. ACS Chem Biol 8:2151–2156
Sanchez OJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol 99(13):5270–5295
Santos A, Mendes S, Brissos B, Martins LO (2014) New dye-decolorizing peroxidases from Bacillus subtilis and Pseudomonas putida MET94: towards biotechnological applications. Appl Microbiol Biotechnol 98:2053–2065
Sari SLA, Pangastuti A, Susilowati A, Purwoko T, Mahajoeno E, Hidayat W, Mardhena I, Panuntun DF, Kurniawati D, Anitasari R (2016) Cellulolytic and hemicellulolytic bacteria from the gut of Oryctes rhinoceros larvae. Biodiversitas 17:78–83
Scharf ME, Karl ZJ, Sethi A, Boucias DG (2011) Multiple levels of synergistic collaboration in termite lignocellulose digestion. PloS One 6:e21709. https://doi.org/10.1371/journal.pone.0021709
Shaikh NM, Patel AA, Mehta SA, Patel ND (2013) Isolation and screening of cellulolytic bacteria in habiting different environment and optimization of cellulase production. Univ J Environ Res Technol 3(1):39–49
Sharada R, Venkateswarlu G, Venkateswar S, Anand RM (2014) Applications of cellulases—review. Int J Pharmac Chem Biolog Sci 4(2):424–437
Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E (2010) Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci USA 107:20051–20056
Shil RK, Mojumder S, Sadida FF, Uddin M, Sikdar D (2014) Isolation and identification of cellulolytic bacteria from the gut of three Phytophagus insect species. Braz Arch Biol Tech 57(6):927–932. https://doi.org/10.1590/S1516-8913201402620
Simpson SJ, Clissold FJ, Lihoreau M, Ponton F, Wilder SM, Raubenheimer D (2015) Recent advances in the integrative nutrition of arthropods. Annu Rev Entomol 60:293–311 [PubMed: 25341097]
Singh R, Eltis LD (2015) The multihued palette of dye-decolorizing peroxidases. Arch Biochem Biophys 574:56–65
Singh A, Kuhad RC, Ward OP (2007) Industrial application of microbial cellulases. In: Kuhad RC, Singh A (eds) Lignocellulose biotechnology: future prospects. I.K. International Publishing House, New Delhi, India, pp 345–358
Spring JH, Robichaux SR, Hamlin JA (2009) The role of aquaporins in excretion in insects. J Exp Biol 212:358–362 [PubMed: 19151210]
Stern J, Moraïs S, Lamed R, Bayer EA (2016) Adaptor scaffoldins: an original strategy for extended designer cellulosomes, inspired from nature. M Bio 7(2):e00083. https://doi.org/10.1128/mBio.00083-16
Su LH, Zhao S, Jiang SX, Liao XZ, Duan CJ, Feng JX (2017) Cellulase with high β-glucosidase activity by Penicillium oxalicum under solid state fermentation and its use in hydrolysis of cassava residue. World J Microbiol Biotechnol 33(2):37
Sudakaran S, Retz F, Kikuchi Y, Kost C, Kaltenpoth M (2015) Evolutionary transition in symbiotic syndromes enabled diversification of phytophagous insects on an imbalanced diet. ISME J 9:2587–2604
Sun JZ, Scharf ME (2010) Exploring and integrating cellulolytic systems of insects to advance biofuel technology. Insect Sci 17:163–165
Taggar MS (2015) Insect cellulolytic enzymes: novel sources for degradation of lignocellulosic biomass. J Appl and Nat Sci 7(2):625–630
Thomas L, Crawford DL (1998) Cloning of clustered Streptomyces viridosporus T7A lignocellulose catabolism genes encoding peroxidase and endoglucanase and their extracellular expression in Pichia pastoris. Can J Microbiol 44:364–372
Tian JH, Pourcher AM, Bouchez T, Gelhaye E, Peu P (2014) Occurrence of lignin degradation genotypes and phenotypes among prokaryotes. Appl Microbiol Biotechnol 98:9527–9544
Tokuda G, Lo N, Watanabe H (2005) Marked variations in patterns of cellulase activity against crystalline- vs. carboxymethyl-cellulose in the digestive systems of diverse, wood-feeding termites. Physiol Entomol 30:372–380
Van Bloois E, DE Torres Pazmi, Winter RT, Fraaije MW (2010) A robust and extracellular heme-containing peroxidase from Thermobifida fusca as prototype of a bacterial peroxidase superfamily. Appl Microbiol Biotechnol 86:1419–1430
Vandenbossche V, Brault J, Vilarem Gérard, Hernández-Meléndez Oscar, Vivaldo-Lima Eduardo, Hernández-Luna Martín, Barzana Eduardo, Duque Aleta, Manzanares Paloma, Ballesteros Mercedes, Mata Julio, Castellón Erick, Rigal Luc (2014) A new lignocellulosic biomass deconstruction process combining thermo-mechano chemical action and bio-catalytic enzymatic hydrolysis in a twin-screw extruder. Ind Crops Prod 55:258–266
Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Physiol 153:895–905
Ventorino V, Aliberti A, Faraco V, Robertiello A, Giacobbe S, Ercolini D, Amore A, Fagnano M, Pepe O (2015) Exploring the microbiota dynamics related to vegetable biomasses degradation and study of lignocellulose-degrading bacteria for industrial biotechnological application. Sci Rep 5:8161. https://doi.org/10.1038/srep08161
Wang ZM, Bleakley BH, Crawford DL, Hertel G, Rafii F (1990) Cloning and overexpression of a lignin peroxidase gene from Streptomyces viridosporus in Streptomyces lividans. J Biotechnol 13:131–144
Wang Y, Gilbreath TM, Kukutla P, Yan G, Xu J (2011) Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS One 6:e24767. https://doi.org/10.1371/journal.pone.0024767
Warnecke F, Luginbuhl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, Cayouette M, McHardy AC, Djordjevic G, Aboushadi N (2007) Metagenomic and functional analysis of hindgut microbiota of a wood feedinghigher termite. Nature 450:560–565
Wernegreen JJ (2002) Genome evolution in bacterial endosymbionts of insects. Nat Rev Genet 3:850–861
Wheeler WC, Whiting M, Wheeler QD, Carpenter JM (2001) The phylogeny of the extant hexapod orders. Cladistics 17:113–169
Willis JD, Oppert C, Jurat-Fuentes JL (2010) Methods for discovery and characterization of cellulolytic enzymes from insects. Insect Sci 17:184–198
Wongputtisin P, Khanongnuch C, Kongbuntad W, Niamsup P, Lumyong S, Sarkar PK (2014) Use of Bacillus subtilis isolates from Tua-nao towards nutritional improvement of soya bean hull for monogastric feed application. Lett Appl Microbiol. 59(3):328–333. https://doi.org/10.1111/lam.12279
Ye YH, Woolfit M, Rancès E, O’Neill SL, McGraw EA (2013) Wolbachia-associated bacterial protection in the mosquito Aedes aegypti. PLoS Negl Trop Dis 7:e2362
Yoshida T, Sugano Y (2015) A structural and functional perspective of DyP-type peroxidase family. Arch Biochem Biophys 574:49–55
Yu W, Liu W, Huang H, Zheng F, Wang X, Wu Y, Li K, Xie X, Jin Y (2014) Application of a novel alkali-tolerant thermostable DyP-type peroxidase from Saccharomonospora viridis DSM 43017 in biobleaching of eucalyptus kraft pulp. Plos One 9:e110319-e
Zhang XZ, Zhang YHP (2013) Cellulases: characteristics, sources, production, and applications. Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers. Wiley
Zug R, Hammerstein P (2012) Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 7:e38544
Acknowledgements
Authors thankfully acknowledge Director, DRL, and DRDO for fellowship to RKP for kind support to carry out the work. Authors’ sincerely apologies many colleagues and their scientific publications that could not been referred here due to space limitation. Further, authors declare there is no conflict of interest present.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Prasad, R.K., Chatterjee, S., Sharma, S., Mazumder, P.B., Vairale, M.G., Raju, P.S. (2018). Insect Gut Bacteria and Their Potential Application in Degradation of Lignocellulosic Biomass: A Review. In: Varjani, S., Agarwal, A., Gnansounou, E., Gurunathan, B. (eds) Bioremediation: Applications for Environmental Protection and Management. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-10-7485-1_14
Download citation
DOI: https://doi.org/10.1007/978-981-10-7485-1_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7484-4
Online ISBN: 978-981-10-7485-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)